Pyrolysis Process for the Recycling of Cork Dust Waste from the Processing of Cork Agglomerate Caps in Lightweight Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pyrolysis Test and Char Characterization
2.2. Lightweight Aggregate (LWA) Preparation and Characterization
- Char 7.5%:
- 85 wt% clay (<1000 μm);
- 7.5 wt% char from pyrolysis at 420 °C of the cork scrap (<1000 μm);
- 7.5 wt% powder from exhausted, dried and sieved spent coffee grounds (<250 μm).
- Char 15%:
- 85 wt% clay (<1000 μm);
- 15 wt% char from pyrolysis at 420 °C of the cork scrap (<1000 μm).
- Shrinkage after firing (SF%): this was calculated based on two measurements of diameter, post-drying (Dd) and post-firing (Df), for ten LWA samples per formulation. Equation (1) is used:SF% = (Dd − Df)/Dd ∗ 100
- Water absorption after 24 h (WA%): this test is governed by the UNI EN 772-21: 2011 [24] standard, and it involved the execution of a water absorption test at room temperature in which the aggregates (ten beads for each formulation) are immersed in distilled water, in such a quantity as to cover them entirely (about 200 mL of water in a beaker), and left in a static condition for 24 h. The following day, they were removed from the water, dried and weighed. The sample, after drying, was weighed before (Wi, initial weight) and after testing (Wf, final weight) with the immersion in water. The water absorption percentage WA (%) was quantified using Equation (2):WA% = (Wf − Wi)/Wi ∗ 100
- Apparent density: for the calculation of the bulk density, a GeoPyc 1360 was used in which a first “tare” analysis was carried out with graphite powder (Dryflow). The test was carried out on both samples, for each of which two aggregates were sampled in order to obtain data redundancy and greater accuracy. Some preset values were kept constant in the instrument, such as the force = 28 N and the conversion factor (in our case for spherical samples) = 0.12840. The instrument carried out five measurement cycles for each sample so that the data obtained were for the mean values and gave a standard deviation.
- True Density: the true density of the aggregates was measured with a He pycnometer, which calculates the volume of a porous solid (Micrometrics Accupyc 1340). Previously, ten LWA spheres were ground into a powder with a small agate mortar. The total porosity percentage (TP (%)) was obtained by processing the absolute (Mycrometrics Accupyc 1340) and apparent (Enveloped Density Micrometrics Geopyc 1360) density data, indicated as ρabs and ρapp, using Equation (3):Total Porosity (%) = (True density − Bulk density)/(True density) ∗ 100
- pH and conductivity measurements: pH and electrical conductivity measurements were carried out as reported in UNI EN 13,037:2012 (pH rule standard) [25] and UNI EN 13,038:2012 (conductivity rule standard) [26]. Bulk specimens (10 g) were placed in distilled water with a solid/liquid ratio of 1:5 under stirring conditions (360 rpm) for 1 h at room temperature. The liquid was filtered in order to obtain a transparent liquid fraction; with this eluate, the pH and electric conductivity were measured.
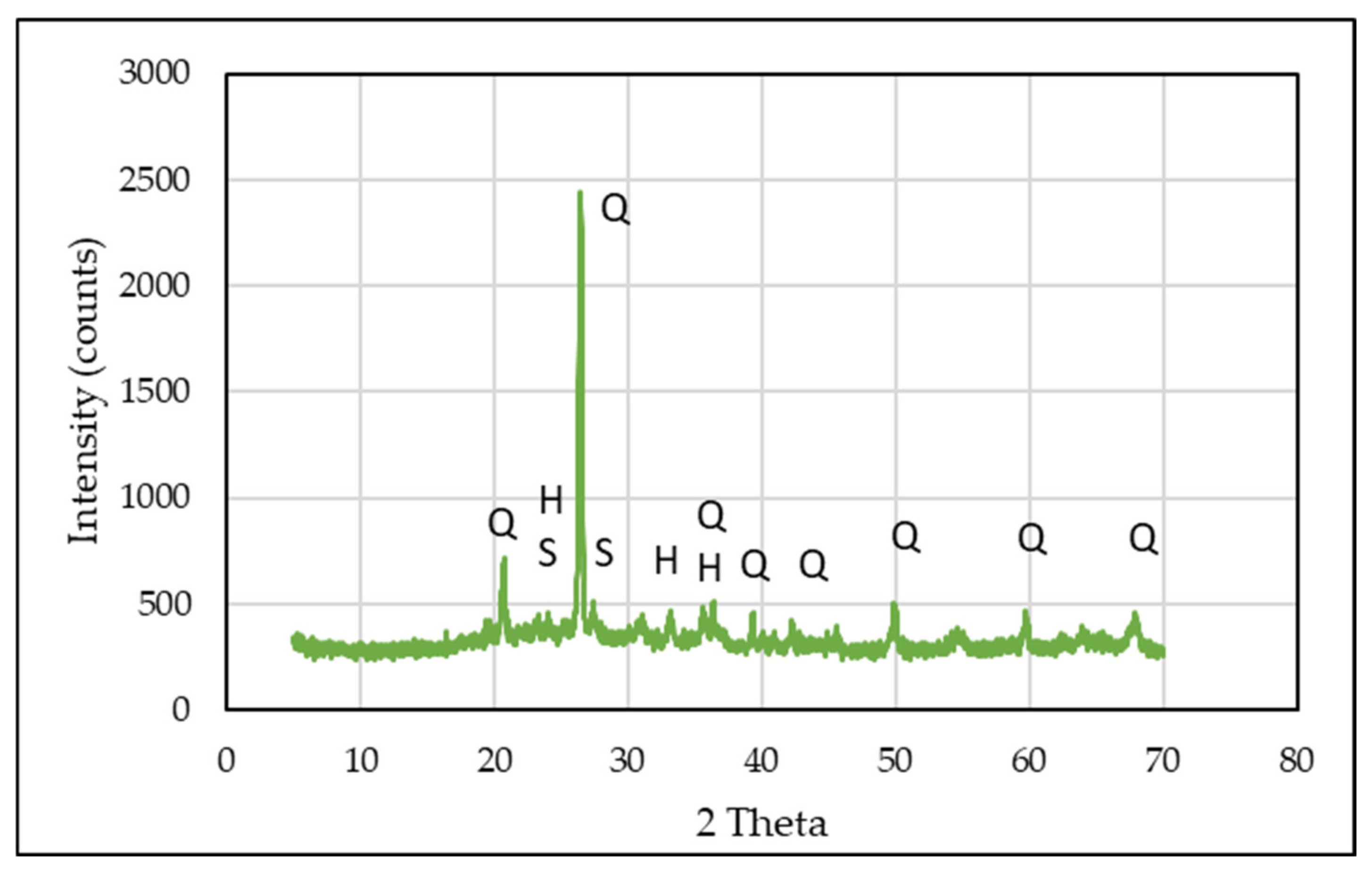
- Mineralogical analysis: mineralogical analysis was carried out with an X-ray powder diffraction analyser (PW 3710, Philips Research Laboratories, Eindhoven, the Netherlands) with Ni-filtered CuKa radiation in the 5–70° 2θ range and a speed of 1°/min, operating at 40 mA and 40 keV. Highscore Plus software version 3.0 coupled to the International Centre for Diffraction Data (ICDD) cards database was used to identify the crystalline phases with a qualitative method.
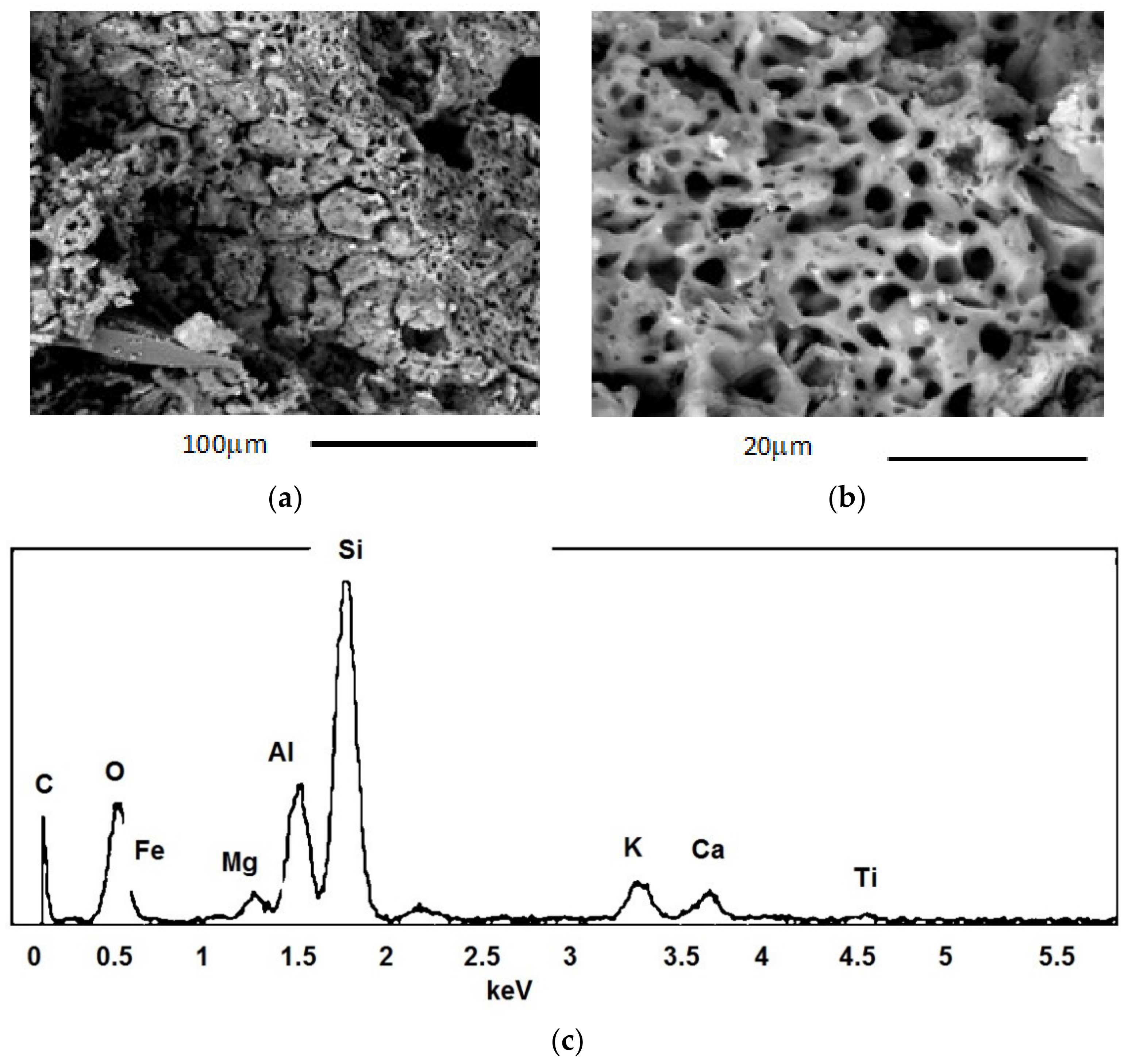
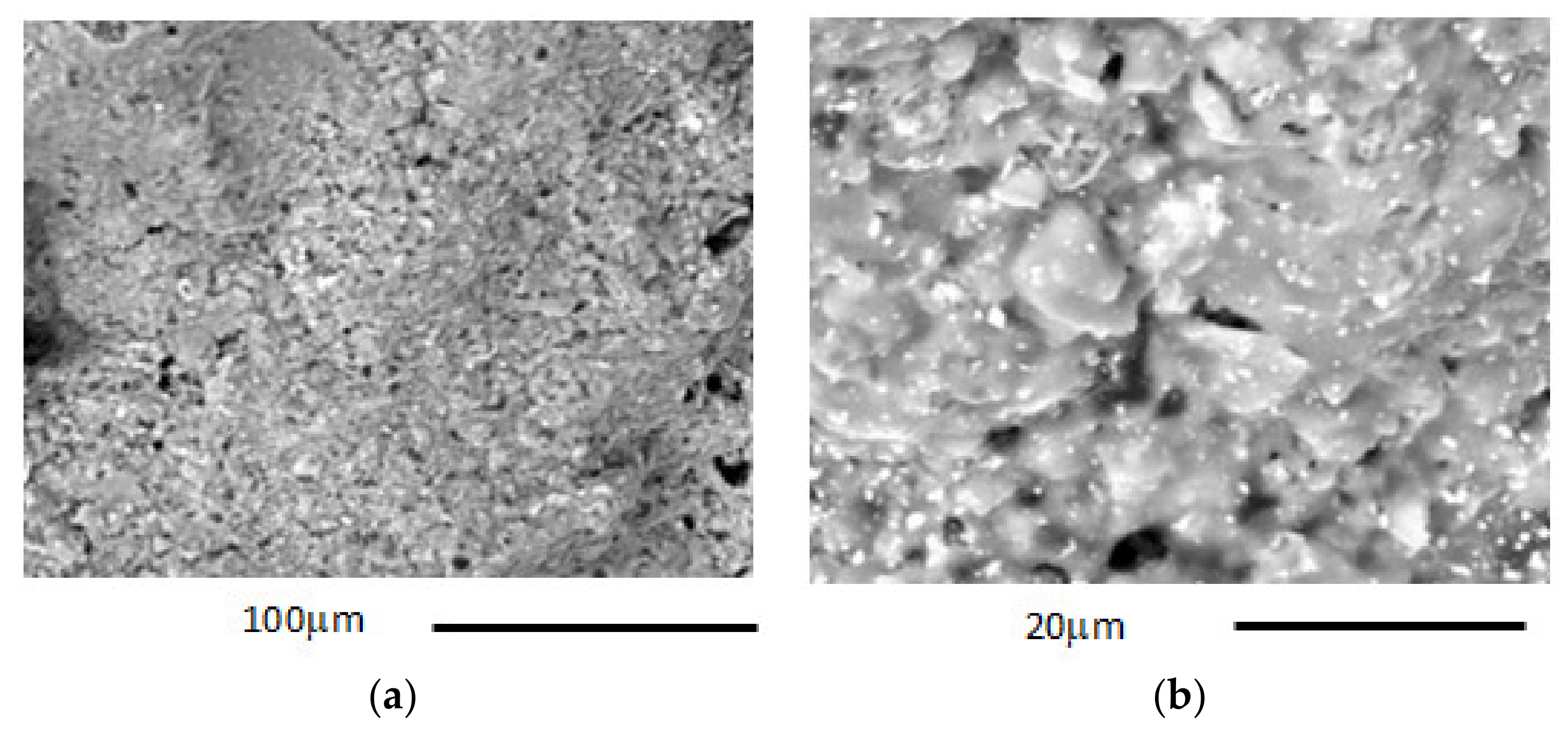
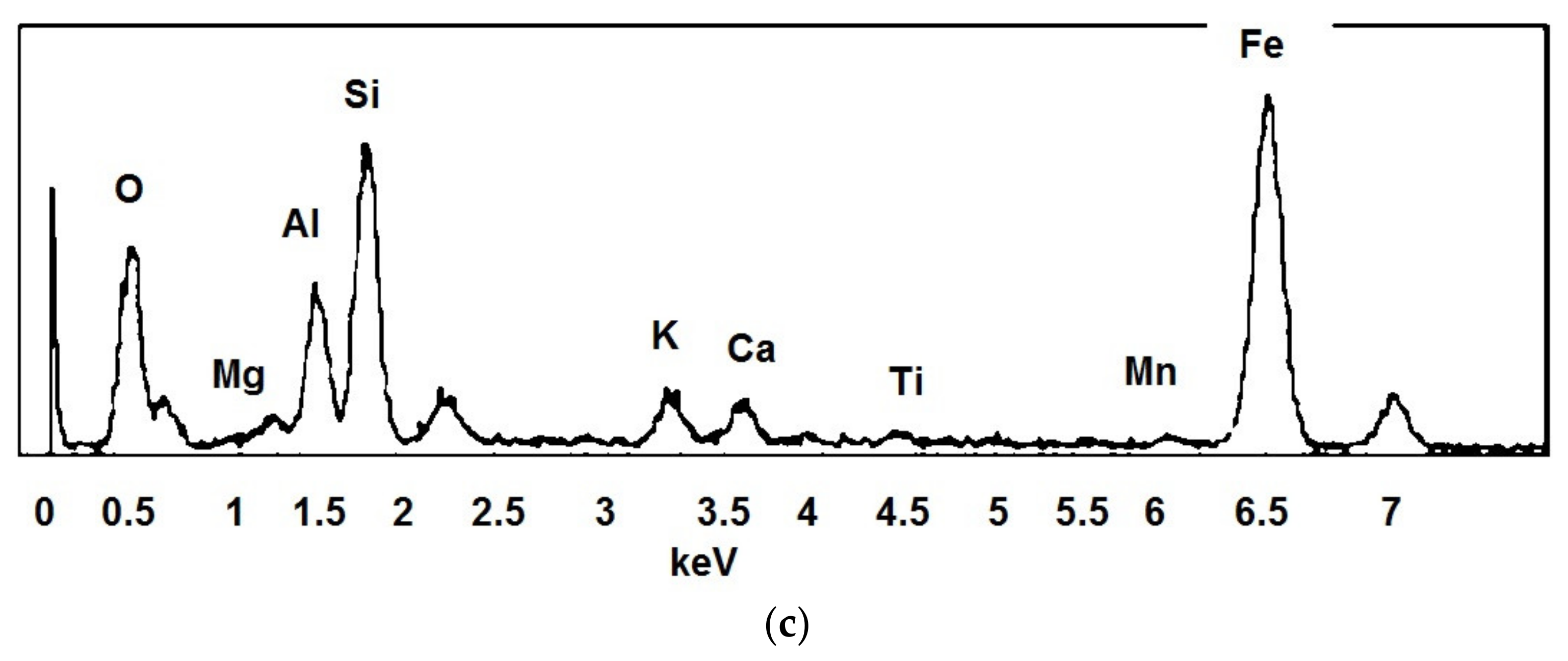
- Microstructural analysis (SEM): microstructural analysis was performed using SEM (Model XL40, Philips Research Laboratories, the Netherlands) coupled with X-EDS equipment (Model QUANTAX-200, Bruker, MA, USA) and the following beam voltage operative conditions: 25 KW; spot size: 5.0; pressure: 0.60 Torr; working distance: 12–13 mm. Thanks to the scanning electron microscope, it was possible to carry out investigations relating to the morphology and microstructure of the materials in order to analyse the shape and size of the grains, the porosity and the defects and inclusions present. It was also possible to perform mineralogical characterizations to identify the phases within a material, determine their concentrations and search for the presence of heavy metals. The following samples were subjected to microstructural analysis: (i) char sample produced by pyrolysis at 420 °C; (ii) char sample produced by pyrolysis at 640 °C; (iii) LWA char 7.5% internally and externally; (iv) LWA char 15% internally and externally.
2.3. Preparation and Characterization of Porous Ceramics
- A series obtained by substituting 5, 10 and 15 wt% of clay for char produced by pyrolysis at 420 °C and sieved below 1000 μm in order to keep the particle size as close as possible to that of the clay matrix;
- A series based on finer char, below 250 μm, in order to investigate whether reducing the cork grain size would make it possible to improve the compaction and density of the brick created. In this case, 5 and 10 wt% of the total clay was substituted for char.
- Linear shrinkage (LS%)which was calculated after drying using the diameters of the fresh and dried specimens and after firing using the dried and the fired specimensLS% = (dmean initial − dmean final)/dmean initial ∗ 100
- Weight loss (WL%):WL% = (Wi − Wf)/Wi ∗ 100
- Volume of the fired cylinder;
- Bulk density of fired ceramics (which took into account the internal porosity).
3. Results
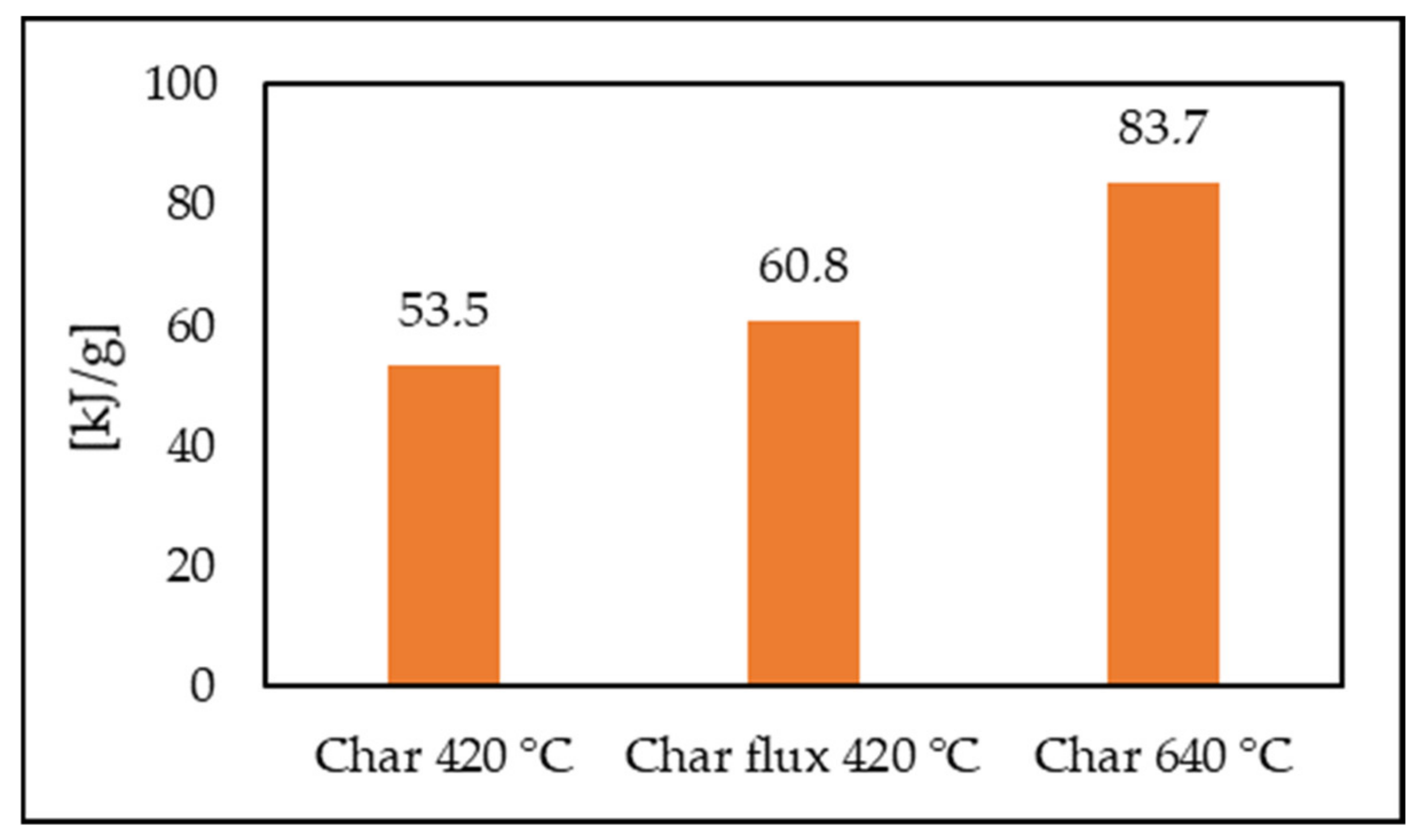
3.1. Pyrolysis Test and Char Characterization
3.2. LWA Characterization
3.2.1. Physical and Chemical Properties
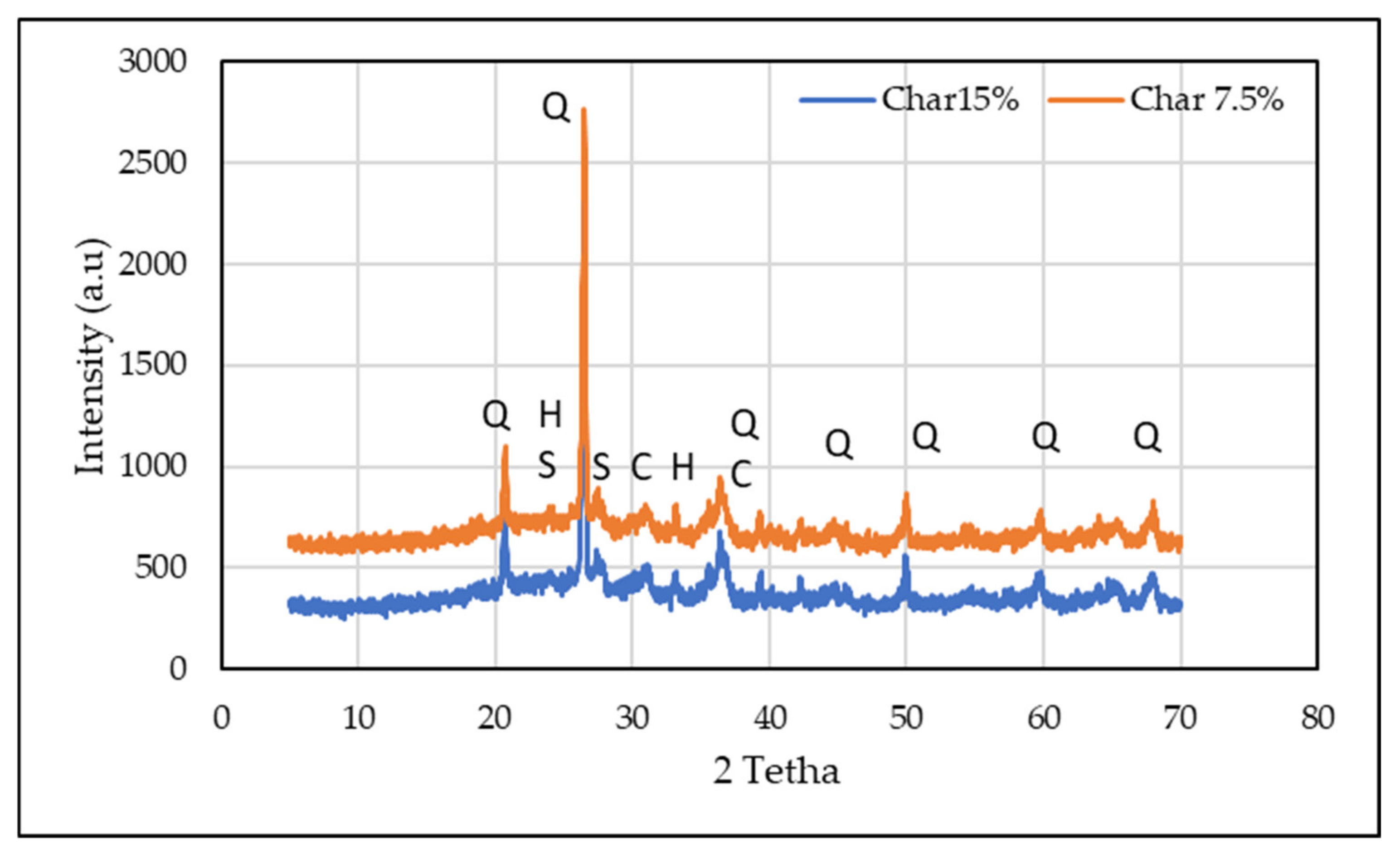
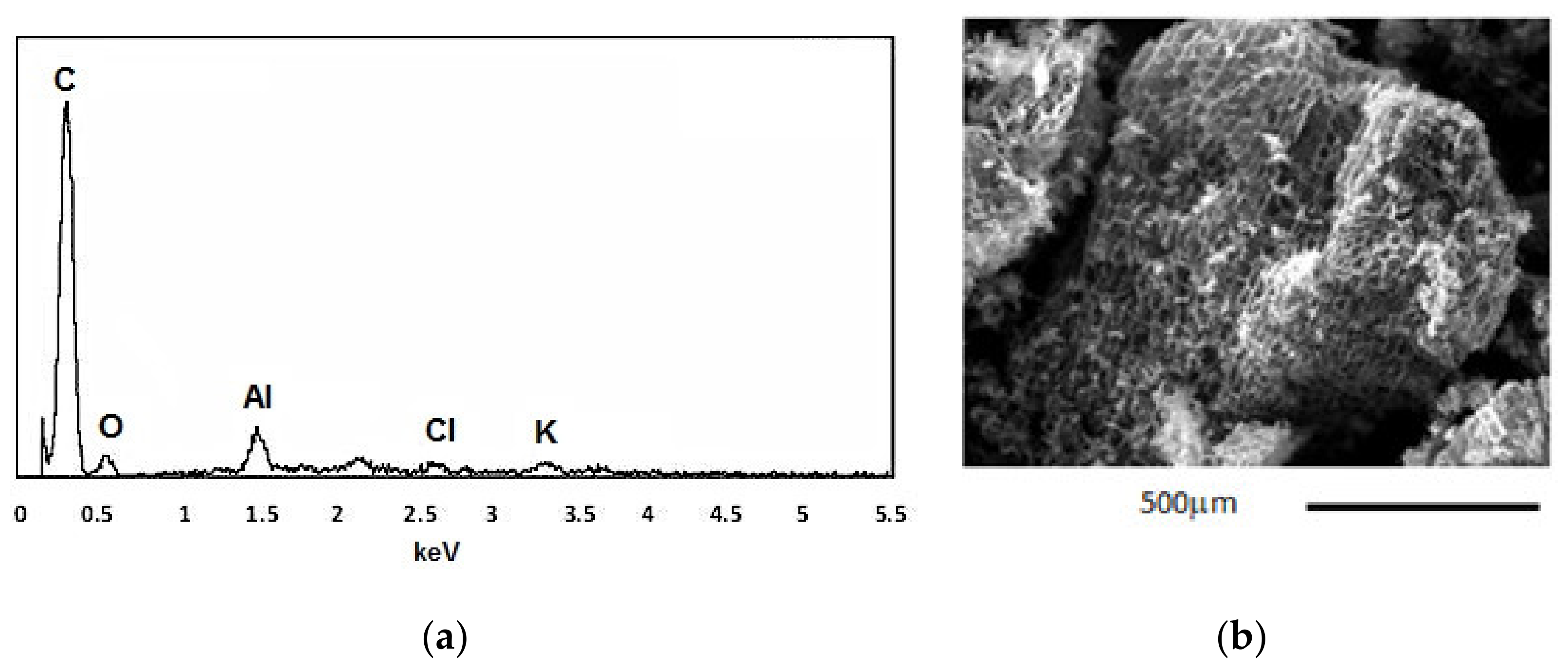
3.2.2. Mineralogical and Microstructural Analysis
3.3. Characterization of Porous Ceramics
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dos Santos, R.G.; Carvalho, R.; Ribeiro Silva, E.; Bordado, J.C.; Cardoso, A.C.; do Rosário Costa, M.; Mateus, M.M. Natural polymeric water-based adhesive from cork liquefaction. Ind. Crops Prod. 2016, 84, 314–319. [Google Scholar] [CrossRef]
- I Vantaggi del Tappo di Sughero Naturale. Available online: https://www.montefioralle.wine/it/blog/dettaglio/14 (accessed on 10 April 2022).
- Rives, J.; Fernandez-Rodriguez, I.; Gabarrell, X.; Rieradevall, J. Environmental analysis of cork granulate production in Catalonia-Northern Spain, Resources. Conserv. Recycl. 2012, 58, 132–142. [Google Scholar] [CrossRef]
- Matos, A.M.; Nunes, S.; Sousa-Coutinho, J. Cork waste in cement based materials. Mater. Des. 2015, 85, 230–239. [Google Scholar] [CrossRef]
- Rodriguez, S.; Almeida, A.; Ribeiro, A.; Neto, P.; Ramalho, E.; Pilão, R. Influence of temperature on the gasification of cork wastes. Energy Procedia 2017, 136, 127–132. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Matias, J.C.O.; Catalão, J.P.S. Energy recovery from cork industrial waste: Production and characterisation of cork pellets. Fuel 2013, 113, 24–30. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction-Practical Design and Theory, 3rd ed.; Academic Press: London, UK, 2018; pp. 155–187. [Google Scholar]
- Marchetti, R. Preliminary Study of Valorisation of Cork Waste. Master’s Thesis, University of Modena and Reggio Emilia, Modena, Italy, 2021. [Google Scholar]
- Lim, T.C.; Cuellar, A.; Langseth, K.; Waldon, J.L. Techno Economic Analysis of Negative Emissions Bioenergy with Carbon Capture and Storage through Pyrolysis and Bioenergy District Heating Infrastructure. Environ. Sci. Technol. 2022, 56, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- EBC (2012–2022) ‘European Biochar Certificate—Guidelines for a Sustainable Production of Biochar’. European Biochar Foundation (EBC), Arbaz, Switzerland. Version 10.1 from 10 January 2022. Available online: http://european-biochar.org (accessed on 25 May 2022).
- IBI (2015) Standardized Product Definition and Product Testing Guidelines for Biochar That Is Used in Soil, International Biochar Initiative. Available online: http://www.biochar-international.org/characterizationstandard (accessed on 25 May 2022).
- Uceda-Rodríguez, M.; Moreno-Maroto, J.M.; Cobo-Ceacero, C.J.; López-García, A.B.; Cotes-Palomino, T.; Martínez-García, C. Comparative Life Cycle Assessment of Lightweight Aggregates Made from Waste—Applying the Circular Economy. Appl. Sci. 2022, 12, 1917. [Google Scholar] [CrossRef]
- Luik, H.; Luik, L.; Tiikma, L.; Vink, N. Parallels between slow pyrolysis of Estonian oil shale and forest biomass residues. J. Anal. Appl. Pyrolysis 2007, 79, 205–209. [Google Scholar] [CrossRef]
- Hoffman, D.A.; Fitz, R.A. Batch Retort Pyrolysis of Solid Municipal Wastes. Environ. Sci. Technol. 1968, 2, 1023–1026. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Rasul, M.G.; Chowdhury, A.A.; Ashwath, N. Biofuels Production through Biomass Pyrolysis—A Technological Review. Energies 2012, 5, 4952–5001. [Google Scholar] [CrossRef]
- Neeft, J.P.A.; Knoef, H.A.M.; Zielke, U.; Sjoestroem, K.; Hasler, P.; Simell, P.A.; Dorrington, M.A.; Thomas, L.; Abatzoglou, N.; Deutch, S.; et al. Guideline for Sampling and Analysis of Tar and Particles in Biomass Producer Gases, Version 3.3.; Energy Project ERK6-CT1999-20002 (Tar Protocol). 2002. Available online: http://www.tarweb.net/results/pdf/guideline-3.3-v2.pdf (accessed on 25 May 2022).
- Chen, Y.; Wang, Y.; Pezzola, L.; Mussi, R.; Bromberg, L.; Heywood, J.; Kasseris, E. A novel low-cost tar removal technology for small-scale biomass gasification to power. Biomass Bioenergy 2021, 149, 106085. [Google Scholar] [CrossRef]
- Gellert, R. Inorganic mineral materials for insulation in buildings (Cap. 8). In Woodhead Publishing Series in Energy, Materials for Energy Efficiency and Thermal Comfort in Buildings; Hall, M.R., Ed.; Woodhead Publishing: Cambridge, UK, 2010; pp. 193–228. ISBN 9781845695262. [Google Scholar] [CrossRef]
- Farias, R.; Garcia, C.; Palomino, T.; Andreola, F.; Lancellotti, I.; Barbieri, L. Valorization of agro-industrial wastes in lightweight aggregates for agronomic use: Preliminary study. Environ. Eng. Manag. J. 2017, 16, 1691–1699. [Google Scholar] [CrossRef]
- European Standard UNI EN 206-1:2006; Calcestruzzo—Parte 1: Specificazione, Prestazione, Produzione e Conformità. (Concrete—Part 1: Specification, Performance, Production and Conformity). Ente Nazionale Italiano di Unificazione (UNI): Milan, Italy, 2006.
- Andreola, F.; Barbieri, L.; Lancellotti, I.; Pozzi, P.; Vezzali, V. Char valorization in construction materials. In Proceedings of the 5th International Conference Wastes: Solutions, Treatments and Opportunities III, Lisbon, Portugal, 4–6 September 2019; Vilarinho, C., Castro, F., Gonçalves, M., Fernando, A.L., Eds.; Taylor & Francis Group: London, UK, 2019. ISBN 978-0-367-2577-4. [Google Scholar]
- Andreola, F.; Borghi, A.; Pedrazzi, S.; Allesina, G.; Tartarini, P.; Lancellotti, I.; Barbieri, L. Spent Coffee Grounds in the Production of Lightweight Clay Ceramic Aggregates in View of Urban and Agricultural Sustainable Development. Materials 2019, 12, 3581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-García, C.; Andreola, F.; Lancellotti, I.; Farías, R.D.; Cotes-Palomino, M.T.; Barbieri, L. Cleaner Design and Production of Lightweight Aggregates (LWAs) to Use in Agronomic Application. Appl. Sci. 2021, 11, 800. [Google Scholar] [CrossRef]
- European Standard UNI EN 772-21:2011; Metodi di Prova per Elementi per Muratura—Parte 21: Determinazione Dell’assorbimento D’acqua di Elementi per Muratura di Laterizio e di Silicato di Calcio per Assorbimento di Acqua Fredda. (Methods of Test for Masonry Units Determination of Water Absorption of Clay and Calcium Silicate Masonry Units by Cold Water Absorption). Ente Nazionale Italiano di Unificazione (UNI): Milan, Italy, 2011.
- European Standard UNI EN 13037:2012; Ammendanti e Substrati di Coltivazione-Determinazione del pH (Soil Improvers and Growing Media-Determination of pH). Ente Nazionale Italiano di Unificazione (UNI): Milan, Italy, 2012.
- European Standard UNI EN 13038:2012; Soil Improvers and Growing Media-Determination of Electrical Conductivity. Ente Nazionale Italiano di Unificazione (UNI): Milan, Italy, 2012.
- Tang, F.; Fudouzi, H.; Uchikoshi, T.; Sakka, Y. Preparation of porous materials with controlled pore size and porosity. J. Eur. Ceram. Soc. 2004, 24, 341–344. [Google Scholar] [CrossRef]
- Kim, P.; Weaver, S.; Labbé, N. Effect of sweeping gas flow rates on temperature-controlled multistage condensation of pyrolysis vapors in an auger intermediate pyrolysis system. J. Anal. Appl. Pyrolysis 2016, 118, 325–334. [Google Scholar] [CrossRef]
- Akhtar, J.; Amin, N.S. A review on operating parameters for optimum liquid oil yield in biomass pyrolysis. Renew. Sustain. Energy Rev. 2012, 16, 5101–5109. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soil, 14th ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2008; ISBN 13: 9780132279383. [Google Scholar]
- Flint, A.L.; Flint, L.E. Particle Density. In Methods of Soil Analysis, Part (4), Physical Methods, 3rd ed.; Dane, J.H., Topp, G.C., Eds.; SSSA: Madison, WI, USA, 2002; pp. 229–241. [Google Scholar]
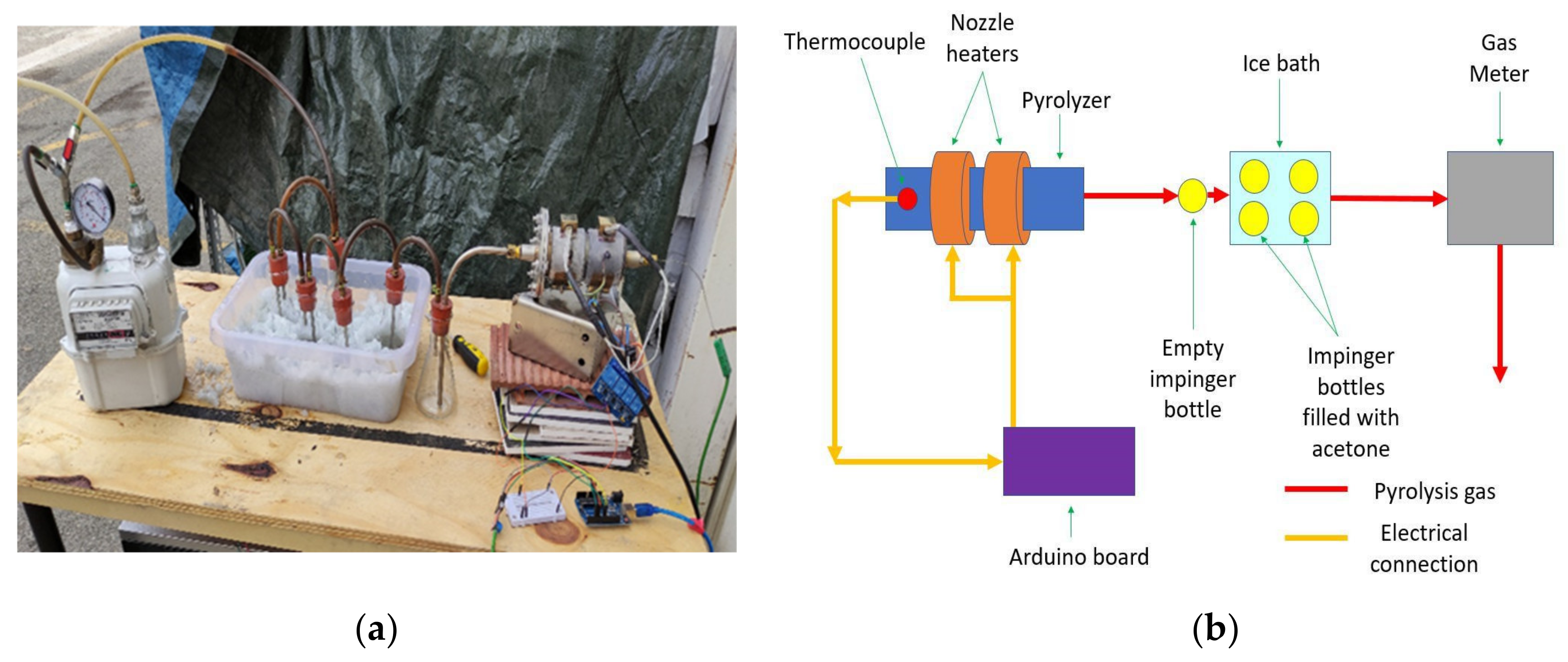




| Test | T-Const (°C) | T-Max (°C) | Initial Mass (g) | Final Mass (g) | Mass Loss (%) | Gas Production (L) |
|---|---|---|---|---|---|---|
| Pyrolysis T = 420 °C | 420 | 517 | 19.44 | 8.31 | 57 | 1.8 |
| Flux pyrolysis T = 420 °C | 415 | 475 | 20.81 | 10.42 | 50 | - |
| Pyrolysis T = 640 °C | 638 | 650 | 18.60 | 6.88 | 63 | 1.5 |
| Sample | Particulates (g) | Tars (g) |
|---|---|---|
| Sample pyrolysed at 420 °C | 0.0077 | 1.8090 |
| Sample pyrolysed at 640 °C | 0.0103 | 1.4241 |
| Sample pyrolysed at 420 °C (fluxed) | 0.0771 | 2.5664 |
| Material | N% | C% | H% | S% | ASH% |
|---|---|---|---|---|---|
| Dry cork powder | 0.88 | 63.33 | 8.53 | - | 0.52 |
| Dry cork powder pyrolysed at 420 °C | 0.76 | 73.95 | 9.95 | - | 0.76 |
| Dry cork powder pyrolysed at 640 °C | 1.32 | 76.75 | 7.84 | - | 1.71 |
| Dry cork powder pyrolysed at 415 °C (fluxed) | 1.27 | 71.11 | 9.09 | - | 1.10 |
| Property | Char 420 °C | Char 640 °C |
|---|---|---|
| pH | 7.05 | 7.70 |
| Electrical conductivity (mS/cm) | 0.396 | 0.340 |
| Property | Char 0% (Only Clay) | Char 7.5% | Char 15% |
|---|---|---|---|
| Water absorption (%) | 7.27 | 24.14 | 26.26 |
| Weight loss (%) | 16.50 | 23.40 | 20.40 |
| Shrinkage after firing (%) | 7.50 | 7.19 | 6.75 |
| True density (kg/m3) | 2690 ± 1.1 | 2725 ± 1 | 2715 ±0.4 |
| Apparent density (kg/m3) | 1330 ± 2.0 | 951.7 ± 2.4 | 924.0 ± 1.7 |
| Porosity (%) | 50.55 | 66.10 | 64.94 |
| pH | 7.16 | 7.22 | 7.41 |
| Electrical conductivity (mS/cm) | 1.15 | 0.345 | 0.311 |
| Sample | Water Absorption (%) | Weight Loss (%) | Shrinkage after Firing (%) | Apparent Density (kg/m3) | Total Porosity (%) |
|---|---|---|---|---|---|
| PC 0% | 15.60 | 12.00 | 1.15 | 1550.0 | 40.80 |
| PC 5% | 19.26 | 12.92 | 2.42 | 1576.8 | 42.15 |
| PC 10% | 29.01 | 17.88 | 2.96 | 1134.5 | 58.35 |
| PC 15% | - | 18.25 | 3.88 | 918.6 | 66.2 |
| Sample | Water Absorption (%) | Weight Loss (%) | Shrinkage after Firing (%) | Apparent Density (kg/m3) | Porosity (%) |
|---|---|---|---|---|---|
| PC 5% | 18.07 | 12.67 | 2.73 | 1653.2 | 39.3 |
| PC 10% | 31.36 | 17.11 | 2.68 | 1266.4 | 53.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coppelli, P.; Pedrazzi, S.; Puglia, M.; Morselli, N.; Allesina, G.; Andreola, F.; Lancellotti, I.; Barbieri, L. Pyrolysis Process for the Recycling of Cork Dust Waste from the Processing of Cork Agglomerate Caps in Lightweight Materials. Appl. Sci. 2022, 12, 5663. https://doi.org/10.3390/app12115663
Coppelli P, Pedrazzi S, Puglia M, Morselli N, Allesina G, Andreola F, Lancellotti I, Barbieri L. Pyrolysis Process for the Recycling of Cork Dust Waste from the Processing of Cork Agglomerate Caps in Lightweight Materials. Applied Sciences. 2022; 12(11):5663. https://doi.org/10.3390/app12115663
Chicago/Turabian StyleCoppelli, Paride, Simone Pedrazzi, Marco Puglia, Nicolò Morselli, Giulio Allesina, Fernanda Andreola, Isabella Lancellotti, and Luisa Barbieri. 2022. "Pyrolysis Process for the Recycling of Cork Dust Waste from the Processing of Cork Agglomerate Caps in Lightweight Materials" Applied Sciences 12, no. 11: 5663. https://doi.org/10.3390/app12115663
APA StyleCoppelli, P., Pedrazzi, S., Puglia, M., Morselli, N., Allesina, G., Andreola, F., Lancellotti, I., & Barbieri, L. (2022). Pyrolysis Process for the Recycling of Cork Dust Waste from the Processing of Cork Agglomerate Caps in Lightweight Materials. Applied Sciences, 12(11), 5663. https://doi.org/10.3390/app12115663













