A Comparison of the Transglycosylation Capacity between the Guar GH27 Aga27A and Bacteroides GH36 BoGal36A α-Galactosidases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Expression and Purification of BoGal 36A and Confirmation of Identity for Aga27A
2.3. Enzyme Activity Determination
2.4. Screening for Transglycosylation with pNP-Gal and MALDI-TOF MS Analysis
2.5. Screening for Transglycosylation with Raffinose or LBG and MALDI-TOF MS Analysis
2.6. Enzyme Stability in the Presence of Acceptors
2.7. Screening of Transglycosylation Products with Raffinose and LBG using HPLC
2.8. Evaluation of Transglycosylation Capacity with Raffinose or LBG and Methanol using HPLC
3. Results and Discussion
3.1. Enzyme Preparation
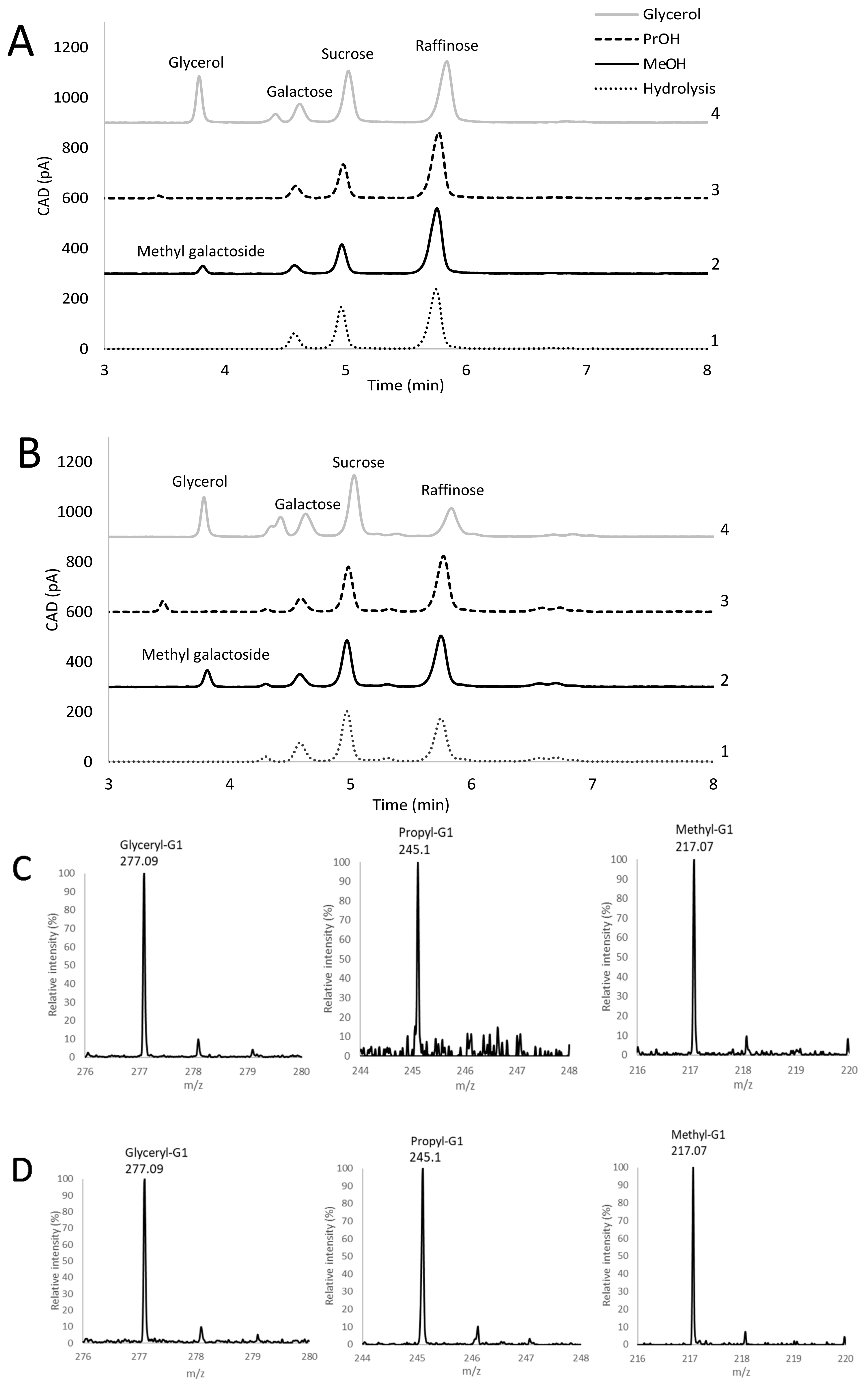
3.2. Initial Screening for Transglycosylation Ability Using pNP-Gal as Donor
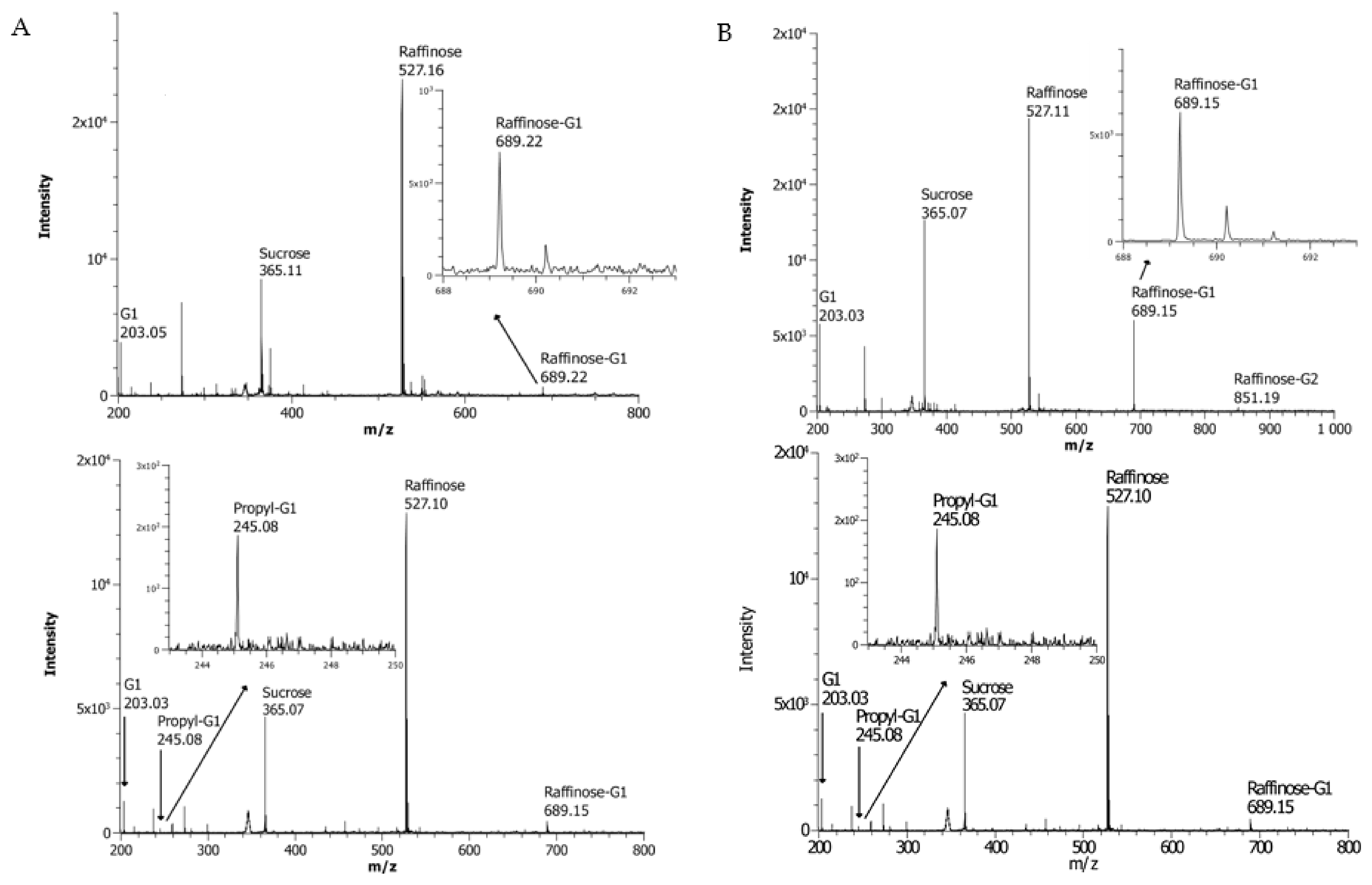
3.3. Screening for the Ability to Utilzse Raffinose as the Donor Substrate
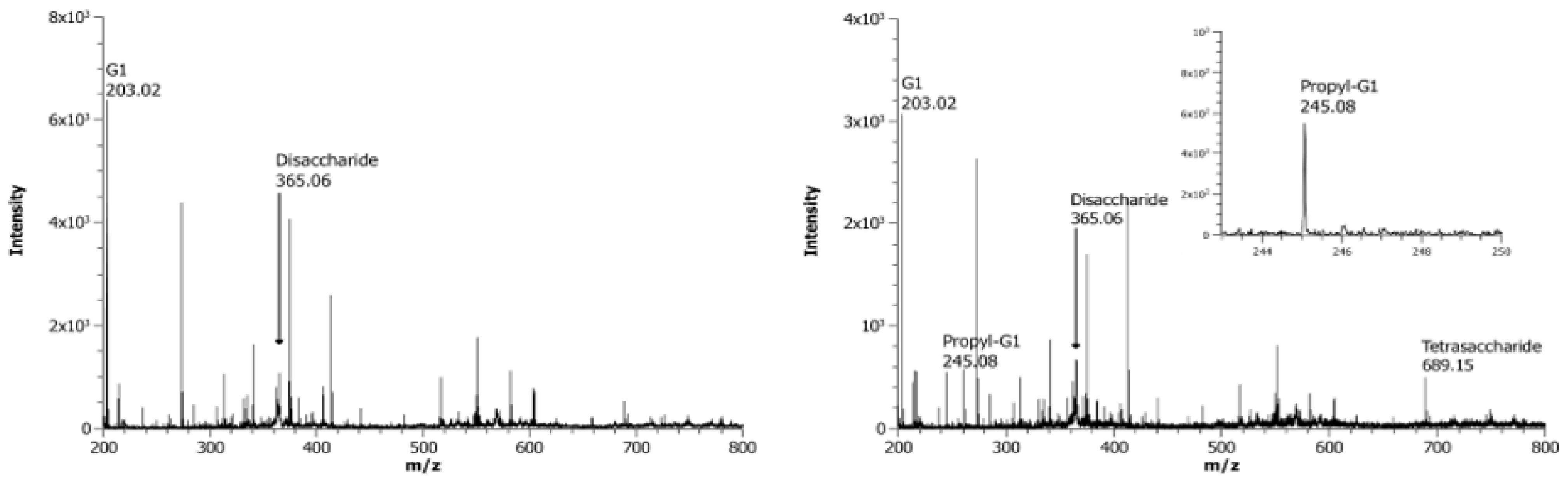
3.4. Screening for the Ability to Utilize Locust Bean Gum as the Donor Substrate
3.5. Analysis of Transglycosylation Products of BoGal36A and Aga27A Using HPLC
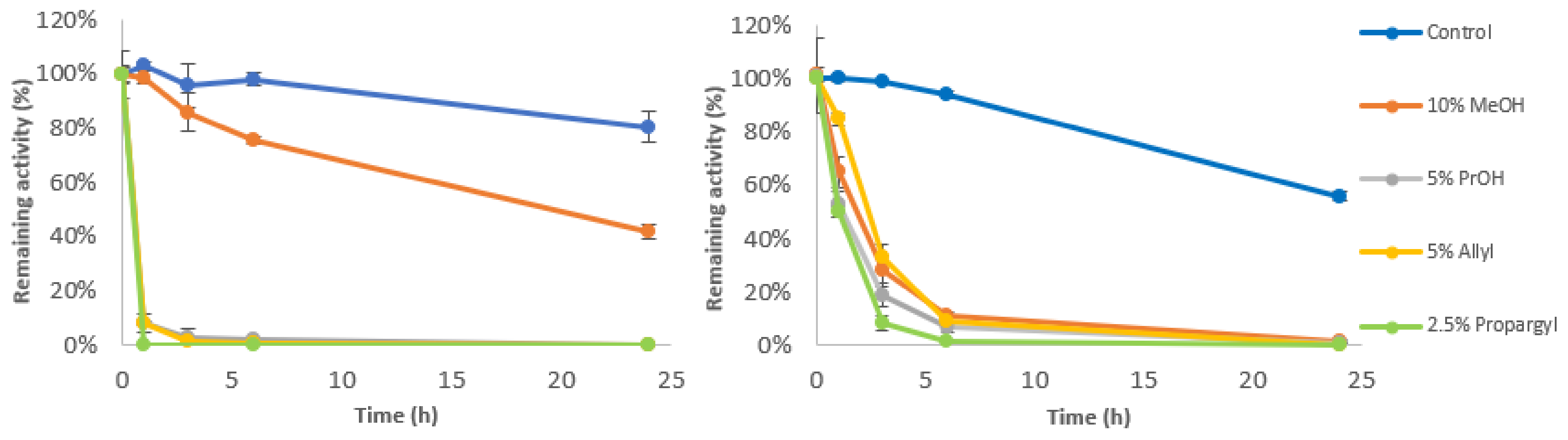
3.6. Stability in the Presence of Acceptor Molecules
3.7. Evaluation of Transglycosylation Capacity with Natural Substrates
3.7.1. Comparison of Transglycosylation Properties with Raffinose and Locust Bean Gum
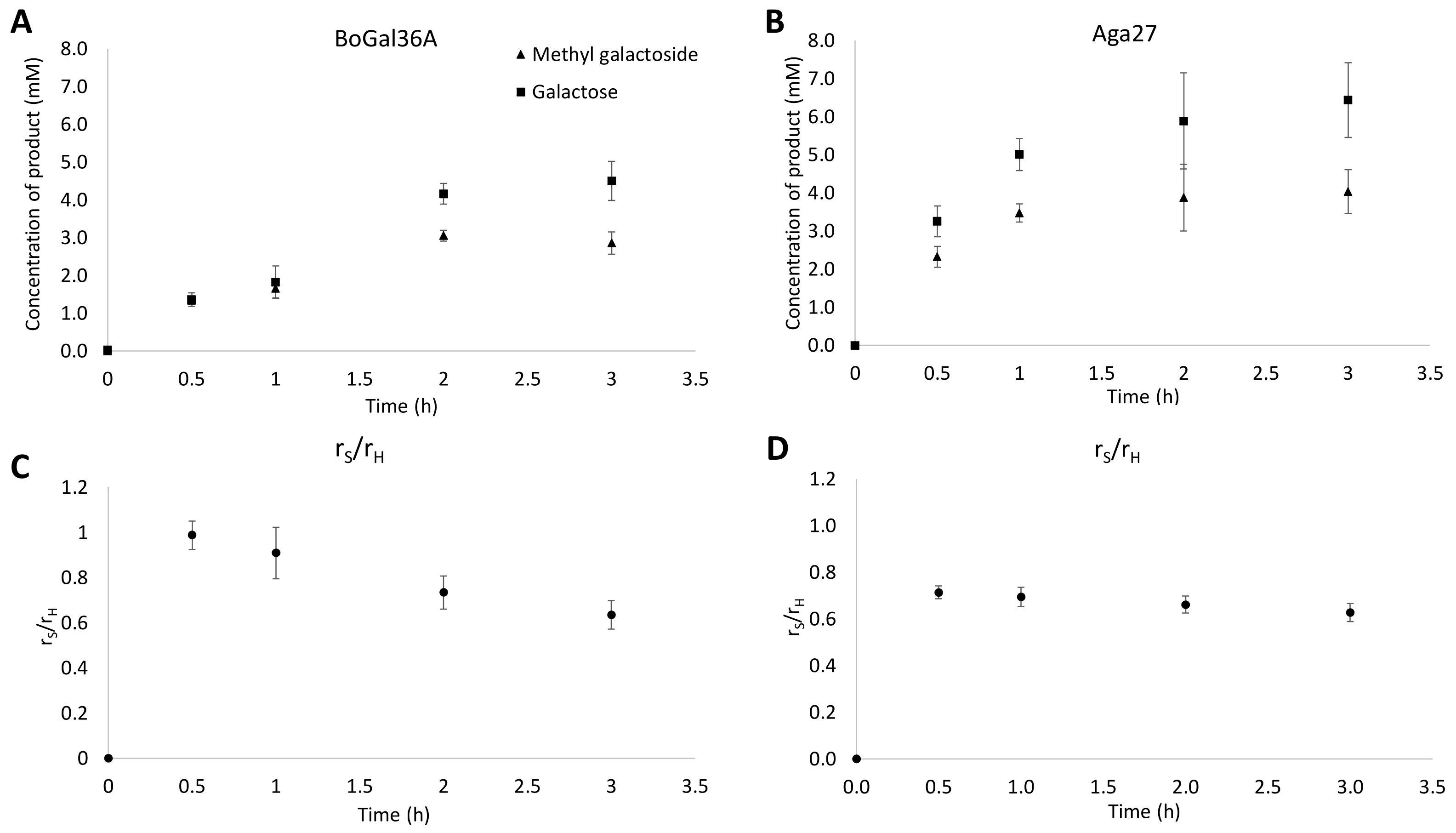
3.7.2. Determination of Initial rS/rH Ratios and Assessment of Secondary Hydrolysis in Raffinose and Methanol Reactions
3.8. Evaluation of Transglycosylation Capacity Aga27A and BoGal36A
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhatia, S.; Singh, A.; Batra, N.; Singh, J. Microbial production and biotechnological applications of α-galactosidase. Int. J. Biol. Macromol. 2020, 150, 1294–1313. [Google Scholar] [CrossRef] [PubMed]
- Bakunina, I.Y.; Balabanova, L.A.; Pennacchio, A.; Trincone, A. Hooked on α-d-galactosidases: From biomedicine to enzymatic synthesis. Crit. Rev. Biotechnol. 2016, 36, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Ademark, P.; Varga, A.; Medve, J.; Harjunpää, V.; Torbjörn, D.; Tjerneld, F.; Stålbrand, H. Softwood hemicellulose-degrading enzymes from Aspergillus niger: Purification and properties of a β-mannanase. J. Biotechnol. 1998, 63, 199–210. [Google Scholar] [CrossRef]
- Malgas, S.; van Dyk, J.S.; Pletschke, B.I. A review of the enzymatic hydrolysis of mannans and synergistic interactions between β-mannanase, β-mannosidase and α-galactosidase. World J. Microbiol. Biotechnol. 2015, 31, 1167–1175. [Google Scholar] [CrossRef]
- Reddy, S.K.; Bågenholm, V.; Pudlo, N.A.; Bouraoui, H.; Koropatkin, N.M.; Martens, E.C.; Stålbrand, H. A β-mannan utilization locus in Bacteroides ovatus involves a GH36 α-galactosidase active on galactomannans. FEBS Lett. 2016, 590, 2106–2118. [Google Scholar] [CrossRef]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Moving towards the second generation of lignocellulosic biorefineries in the EU: Drivers, challenges, and opportunities. Renew. Sustain. Energy Rev. 2019, 101, 590–599. [Google Scholar] [CrossRef] [Green Version]
- Qaseem, M.F.; Shaheen, H.; Wu, A.-M. Cell wall hemicellulose for sustainable industrial utilization. Renew. Sustain. Energy Rev. 2021, 144, 110996. [Google Scholar] [CrossRef]
- Rye, C.S.; Withers, S.G. Glycosidase mechanisms. Curr. Opin. Chem. Biol. 2000, 4, 573–580. [Google Scholar] [CrossRef]
- Bissaro, B.; Monsan, P.; Fauré, R.; O’Donohue, M.J. Glycosynthesis in a waterworld: New insight into the molecular basis of transglycosylation in retaining glycoside hydrolases. Biochem. J. 2015, 467, 17–35. [Google Scholar] [CrossRef]
- van Rantwijk, F.; Woudenberg-van Oosterom, M.; Sheldon, R.A. Glycosidase-catalysed synthesis of alkyl glycosides. J. Mol. Catal. B Enzym. 1999, 6, 511–532. [Google Scholar] [CrossRef]
- Zeuner, B.; Teze, D.; Muschiol, J.; Meyer, A.S. Synthesis of Human Milk Oligosaccharides: Protein Engineering Strategies for Improved Enzymatic Transglycosylation. Molecules 2019, 24, 2033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moulis, C.; Guieysse, D.; Morel, S.; Séverac, E.; Remaud-Siméon, M. Natural and engineered transglycosylases: Green tools for the enzyme-based synthesis of glycoproducts. Curr. Opin. Chem. Biol. 2021, 61, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Edmunds, G.; Gibbons, C.; Zhang, J.; Gadi, M.R.; Zhu, H.; Fang, J.; Liu, X.; Kong, Y.; Wang, P.G. Toward Automated Enzymatic Synthesis of Oligosaccharides. Chem. Rev. 2018, 118, 8151–8187. [Google Scholar] [CrossRef] [PubMed]
- Rosengren, A.; Butler, S.J.; Arcos-Hernandez, M.; Bergquist, K.-E.; Jannasch, P.; Stålbrand, H. Enzymatic synthesis and polymerisation of β-mannosyl acrylates produced from renewable hemicellulosic glycans. Green Chem. 2019, 21, 2104–2118. [Google Scholar] [CrossRef] [Green Version]
- Eneyskaya, E.V.; Golubev, A.M.; Kachurin, A.M.; Savel’ev, A.N.; Neustroev, K.N. Transglycosylation activity of α-d-galactosidase from Trichoderma reesei An investigation of the active site. Carbohydr. Res. 1997, 305, 83–91. [Google Scholar] [CrossRef]
- Puchart, V.; Biely, P. Glycosylation of internal sugar residues of oligosaccharides catalyzed by alpha-galactosidase from Aspergillus fumigatus. Biochim. Biophys. Acta 2005, 1726, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Simerská, P.; Kuzma, M.; Monti, D.; Riva, S.; Macková, M.; Křen, V. Unique transglycosylation potential of extracellular α-d-galactosidase from Talaromyces flavus. J. Mol. Catal. B Enzym. 2006, 39, 128–134. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Ma, R.; Shi, P.; Niu, C.; Luo, H.; Yang, P.; Yao, B. Biochemical characterization of a novel thermophilic α-galactosidase from Talaromyces leycettanus JCM12802 with significant transglycosylation activity. J. Biosci. Bioeng. 2016, 121, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Ma, R.; Shi, P.; Xue, X.; Luo, H.; Huang, H.; Bai, Y.; Yang, P.; Yao, B. A new α-galactosidase from thermoacidophilic Alicyclobacillus sp. A4 with wide acceptor specificity for transglycosylation. Appl. Biochem. Biotechnol. 2014, 174, 328–338. [Google Scholar] [CrossRef]
- Delgado-Fernandez, P.; Plaza-Vinuesa, L.; Hernandez-Hernandez, O.; de las Rivas, B.; Corzo, N.; Muoz, R.a. Unravelling the carbohydrate specificity of MelA from Lactobacillus plantarum WCFS1: An α-galactosidase displaying regioselective transgalactosylation. Int. J. Biol. Macromol. 2020, 153, 1070–1079. [Google Scholar] [CrossRef]
- Kurakake, M.; Okumura, T.; Morimoto, Y. Synthesis of galactosyl glycerol from guar gum by transglycosylation of α-galactosidase from Aspergillus sp. MK14. Food Chem. 2015, 172, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Kong, L.; Xia, C.; Zeng, X.; Wu, Z.; Guo, Y.; Pan, D. Sources, Processing-Related Transformation, and Gut Axis Regulation of Conventional and Potential Prebiotics. J. Agric. Food Chem. 2022, 70, 4509–4521. [Google Scholar] [CrossRef]
- Panwar, D.; Shubhashini, A.; Chaudhari, S.R.; Prashanth, K.V.H.; Kapoor, M. GH36 α-galactosidase from Lactobacillus plantarum WCFS1 synthesize Gal-α-1,6 linked prebiotic α-galactooligosaccharide by transglycosylation. Int. J. Biol. Macromol. 2020, 144, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Marín-Manzano, M.d.C.; Hernandez-Hernandez, O.; Diez-Municio, M.; Delgado-Andrade, C.; Moreno, F.J.; Clemente, A. Prebiotic properties of non-fructosylated α-galactooligosaccharides from pea (Pisum sativum L.) using infant fecal slurries. Foods 2020, 9, 921. [Google Scholar] [CrossRef] [PubMed]
- Butler, S.J.; Birgersson, S.; Wiemann, M.; Arcos-Hernandez, M.; Stålbrand, H. Transglycosylation by β-mannanase TrMan5A variants and enzyme synergy for synthesis of allyl glycosides from galactomannan. Process Biochem. 2022, 112, 154–166. [Google Scholar] [CrossRef]
- Malgas, S.; van Dyk, S.J.; Pletschke, B.I. β-Mannanase (Man26A) and α-galactosidase (Aga27A) synergism—A key factor for the hydrolysis of galactomannan substrates. Enzym. Microb. Technol. 2015, 70, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Overbeeke, N.; Fellinger, A.J.; Toonen, M.Y.; van Wassenaar, D.; Verrips, C.T. Cloning and nucleotide sequence of the α-galactosidase cDNA from Cyamopsis tetragonoloba (guar). Plant Mol. Biol. 1989, 13, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Teze, D.; Zhao, J.; Wiemann, M.; Kazi, Z.G.A.; Lupo, R.; Zeuner, B.; Vuillemin, M.; Rønne, M.E.; Carlström, G.; Duus, J.; et al. Rational Enzyme Design without Structural Knowledge: A Sequence-Based Approach for Efficient Generation of Transglycosylases. Chem. A Eur. J. 2021, 27, 10323–10334. [Google Scholar] [CrossRef]
- Casali, M.; Tarantini, L.; Riva, S.; Hunkova, Z.; Weignerova, L.; Kren, V. Exploitation of a library of α-galactosidases for the synthesis of building blocks for glycopolymers. Biotechnol. Bioeng. 2002, 77, 105–110. [Google Scholar] [CrossRef]
- Stevenson, D.E.; Stanley, R.A.; Furneaux, R.H. Oligosaccharide and alkyl β-galactopyranoside synthesis from lactose with Caldocellum saccharolyticum β-glycosidase. Enzym. Microb. Technol. 1996, 18, 544–549. [Google Scholar] [CrossRef]
- Ademark, P.; Larsson, M.; Tjerneld, F.; Stlbrand, H. Multiple α-galactosidases from Aspergillus niger: Purification, characterization and substrate specificities. Enzym. Microb. Technol. 2001, 29, 441–448. [Google Scholar] [CrossRef]
- Coconi Linares, N.; Dilokpimol, A.; Stålbrand, H.; Mäkelä, M.R.; de Vries, R.P. Recombinant production and characterization of six novel GH27 and GH36 α-galactosidases from Penicillium subrubescens and their synergism with a commercial mannanase during the hydrolysis of lignocellulosic biomass. Bioresour. Technol. 2020, 295, 122258. [Google Scholar] [CrossRef] [PubMed]
- Limnios, D.; Kokotos, C.G. Photoinitiated Thiol-Ene “Click” Reaction: An Organocatalytic Alternative. Adv. Synth. Catal. 2017, 359, 323–328. [Google Scholar] [CrossRef]
- Morrill, J.; Månberger, A.; Rosengren, A.; Naidjonoka, P.; von Freiesleben, P.; Krogh, K.B.R.M.; Bergquist, K.-E.; Nylander, T.; Karlsson, E.N.; Adlercreutz, P.; et al. β-Mannanase-catalyzed synthesis of alkyl mannooligosides. Appl. Microbiol. Biotechnol. 2018, 102, 5149–5163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shabalin, K.A.; Kulminskaya, A.A.; Savel’ev, A.N.; Shishlyannikov, S.M.; Neustroev, K.N. Enzymatic properties of α-galactosidase from Trichoderma reesei in the hydrolysis of galactooligosaccharides. Enzym. Microb. Technol. 2002, 30, 231–239. [Google Scholar] [CrossRef]
- Kurakake, M.; Moriyama, Y.; Sunouchi, R.; Nakatani, S. Enzymatic properties and transglycosylation of α-galactosidase from Penicillium oxalicum SO. Food Chem. 2011, 126, 177–182. [Google Scholar] [CrossRef]
- Rosengren, A.; Hägglund, P.; Anderson, L.; Pavon-Orozco, P.; Peterson-Wulff, R.; Nerinckx, W.; Stålbrand, H. The role of subsite+ 2 of the Trichoderma reesei β-mannanase TrMan5A in hydrolysis and transglycosylation. Biocatal. Biotransformation 2012, 30, 338–352. [Google Scholar] [CrossRef]
- Rosengren, A.; Reddy, S.K.; Sjöberg, J.S.; Aurelius, O.; Logan, D.T.; Kolenová, K.; Stålbrand, H. An Aspergillus nidulans β-mannanase with high transglycosylation capacity revealed through comparative studies within glycosidase family 5. Appl. Microbiol. Biotechnol. 2014, 98, 10091–10104. [Google Scholar] [CrossRef] [Green Version]
- Malbert, Y.; Pizzut-Serin, S.; Massou, S.; Cambon, E.; Laguerre, S.; Monsan, P.; Lefoulon, F.; Morel, S.; André, I.; Remaud-Simeon, M. Extending the structural diversity of α-flavonoid glycosides with engineered glucansucrases. ChemCatChem 2014, 6, 2282–2291. [Google Scholar] [CrossRef]
- Wu, S.; Snajdrova, R.; Moore, J.C.; Baldenius, K.; Bornscheuer, U.T. Biocatalysis: Enzymatic Synthesis for Industrial Applications. Angew. Chem. Int. Ed. 2021, 60, 88–119. [Google Scholar] [CrossRef]
- Valladares-Diestra, K.K.; de Souza Vandenberghe, L.P.; Soccol, C.R. A biorefinery approach for enzymatic complex production for the synthesis of xylooligosaccharides from sugarcane bagasse. Bioresour. Technol. 2021, 333, 125174. [Google Scholar] [CrossRef] [PubMed]
- de Jong, E.; Jungmeier, G. Chapter 1—Biorefinery Concepts in Comparison to Petrochemical Refineries. In Industrial Biorefineries & White Biotechnology; Pandey, A., Höfer, R., Taherzadeh, M., Nampoothiri, K.M., Laroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3–33. [Google Scholar] [CrossRef]

| Acceptor | BoGal36A | Aga27A | Product |
|---|---|---|---|
| pNP-Gal | N.D. | + | pNP-Gal-Gal |
| 10% Methanol | + | + | Methyl-Gal |
| 5% Propanol | + | + | Propyl-Gal |
| 10% Glycerol | + | + | Glyceryl-Gal |
| 5% Allyl-OH | + | + | Allyl-Gal |
| 2.5% Propargyl-OH | + | + | Propargyl-Gal |
| Acceptor | BoGal36A | Aga27A | Product |
|---|---|---|---|
| Raffinose | + | + | Raffinose-Gal |
| 10% Methanol | + | + | Methyl-Gal |
| 5% Propanol | + | + | Propyl-Gal |
| 10% Glycerol | + | + | Glycerol-Gal |
| 5% Allyl-OH | N.D. | + | Allyl-Gal |
| 2.5% Propargyl-OH | N.D. | + | Propargyl-Gal |
| Acceptor | BoGal36A | Aga27A | Product |
|---|---|---|---|
| 10% Methanol | + | + | Methyl-Gal |
| 5% Propanol | N.D. | + | Propyl-Gal |
| 10% Glycerol | N.D. | + | Glycerol-Gal |
| 5% Allyl-OH | N.D. | + | Allyl-Gal |
| 2.5% Propargyl-OH | N.D. | N.D. | Propargyl-Gal |
| Donor | Enzyme | [Me-Gal] (mM) | [Gal] (mM) | Product Yield (%) ([Me-Gal]/([Me-Gal]+[Gal])) | rS/rH ([Me-Gal]/[Gal]) |
|---|---|---|---|---|---|
| 0.4 w/v% LBG | BoGal36A (24 h) | 0.7 (±0.1) | 2.1 (±0.4) | 27 | 0.33 (±0.06) |
| Aga27A (24 h) | 1.0 (±0.1) | 2.4 (±0.1) | 30 | 0.42 (±0.05) | |
| 400 mM raffinose | BoGal36A (24 h) | 41 (±7) | 70 (±11) | 37 | 0.58 (±0.01) |
| Aga27A (24 h) | 35 (±11) | 55 (±13) | 39 | 0.63 (±0.05) | |
| 40 mM raffinose | BoGal36A (30 min) | 1.3 (±0.1) | 1.4 (±0.2) | 48 | 0.99 (±0.06) |
| BoGal36A (2h) | 3.1 (±0.1) | 4.2 (±0.3) | 42 | 0.74 (±0.07) | |
| Aga27A (30 min) | 2.3 (±0.3) | 3.3 (±0.4) | 41 | 0.71 (±0.03) | |
| Aga27A (3 h) | 4.0 (±0.6) | 6.4 (±1.0) | 38 | 0.63 (±0.04) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiemann, M.; Axell, E.; Stålbrand, H. A Comparison of the Transglycosylation Capacity between the Guar GH27 Aga27A and Bacteroides GH36 BoGal36A α-Galactosidases. Appl. Sci. 2022, 12, 5123. https://doi.org/10.3390/app12105123
Wiemann M, Axell E, Stålbrand H. A Comparison of the Transglycosylation Capacity between the Guar GH27 Aga27A and Bacteroides GH36 BoGal36A α-Galactosidases. Applied Sciences. 2022; 12(10):5123. https://doi.org/10.3390/app12105123
Chicago/Turabian StyleWiemann, Mathias, Emil Axell, and Henrik Stålbrand. 2022. "A Comparison of the Transglycosylation Capacity between the Guar GH27 Aga27A and Bacteroides GH36 BoGal36A α-Galactosidases" Applied Sciences 12, no. 10: 5123. https://doi.org/10.3390/app12105123
APA StyleWiemann, M., Axell, E., & Stålbrand, H. (2022). A Comparison of the Transglycosylation Capacity between the Guar GH27 Aga27A and Bacteroides GH36 BoGal36A α-Galactosidases. Applied Sciences, 12(10), 5123. https://doi.org/10.3390/app12105123






