Histological Change in Cucumber Tissue and Cellulase Activity of Plectosphaerella melonis Strain 502
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain
2.2. Artificial Infectious Background (AIB)
2.3. Plant Material
2.4. Histological Processing of Samples and Stain Preparation
2.5. pH Growth
2.6. Physiological Phases of Growth
2.7. Cellulase Assay
2.8. Statistical Methods
3. Results and Discussion
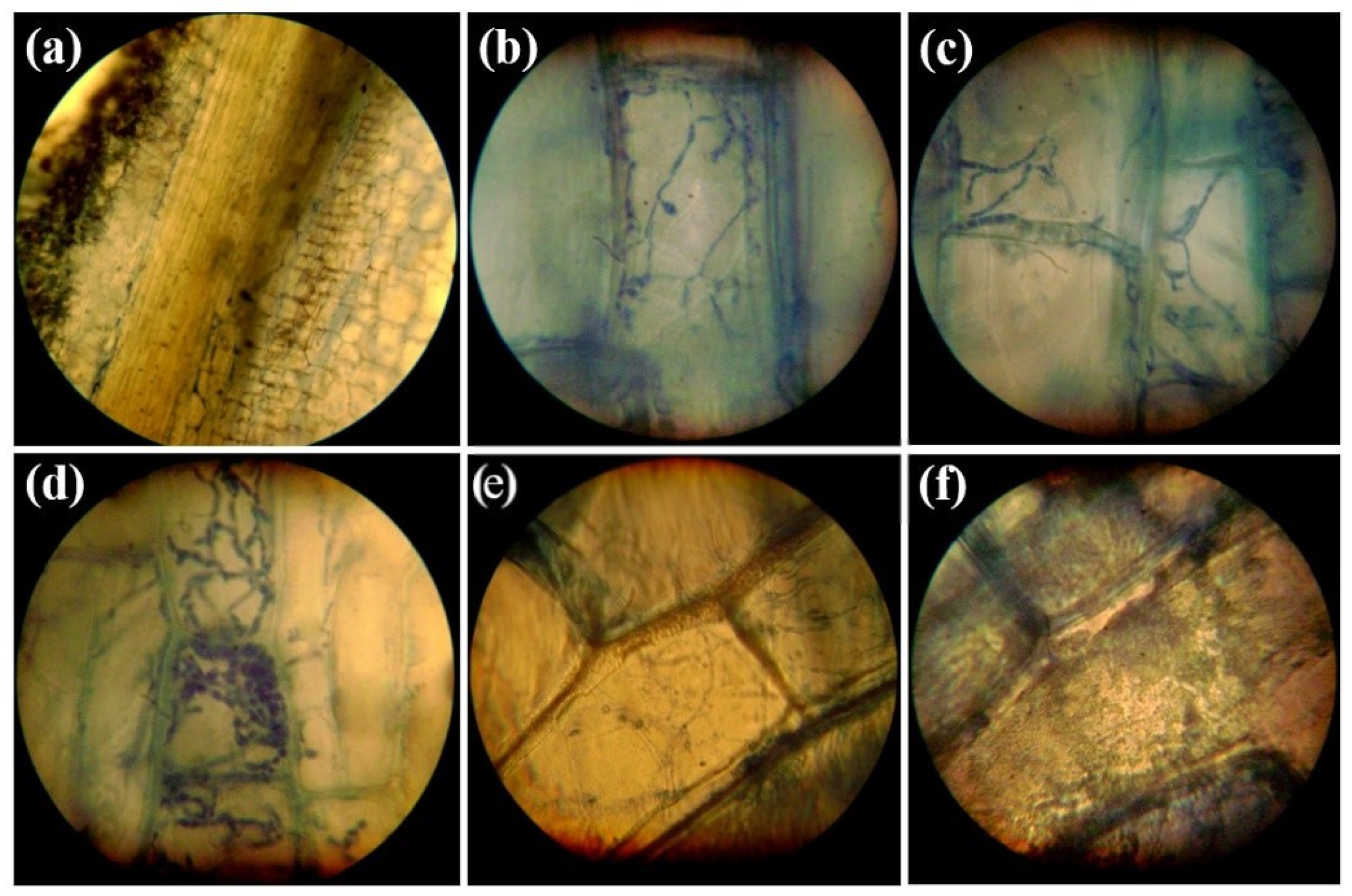
3.1. Histopathology of Infections Caused by P. melonis 502 on the Root Tissues of Cucumber
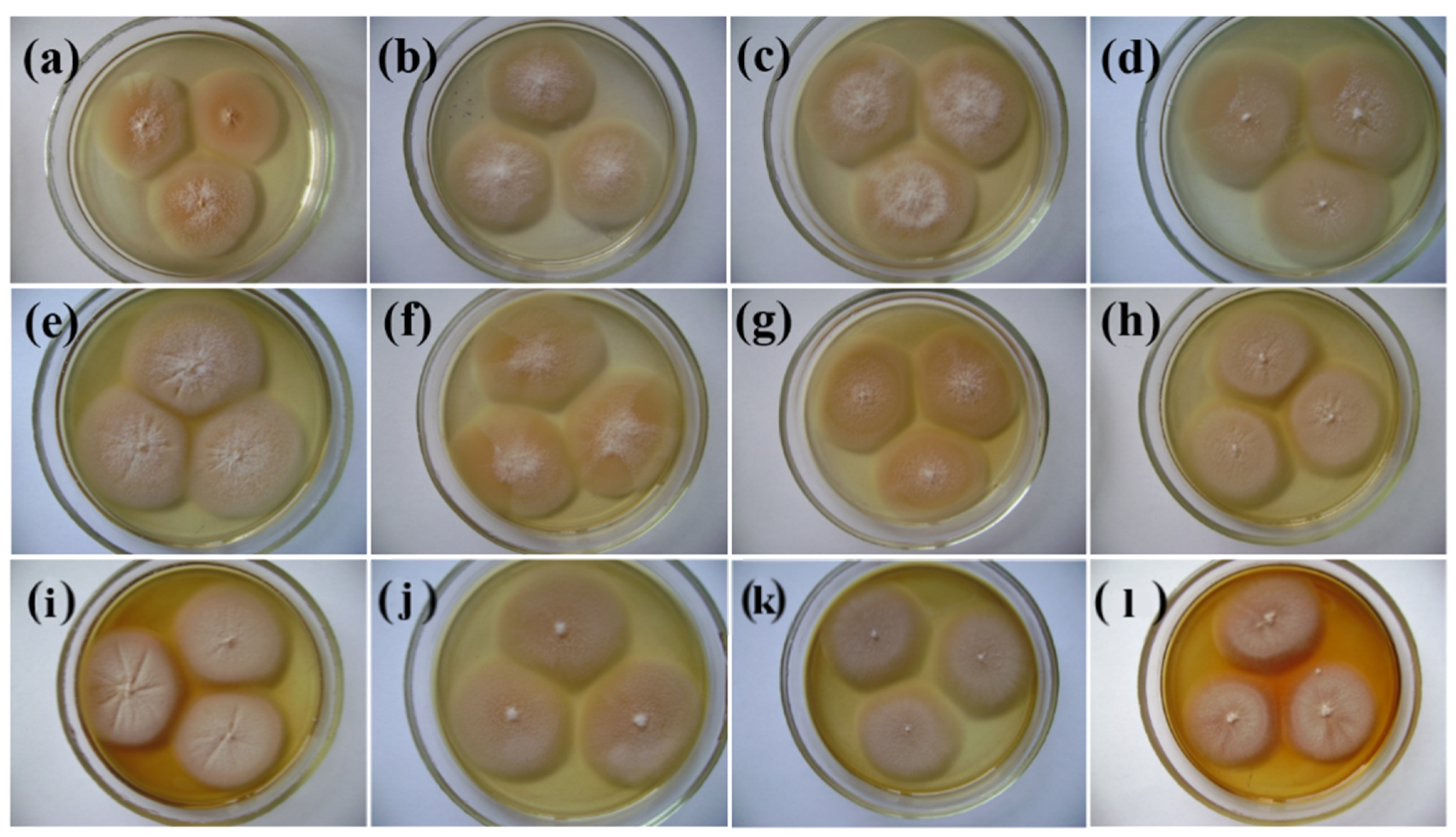
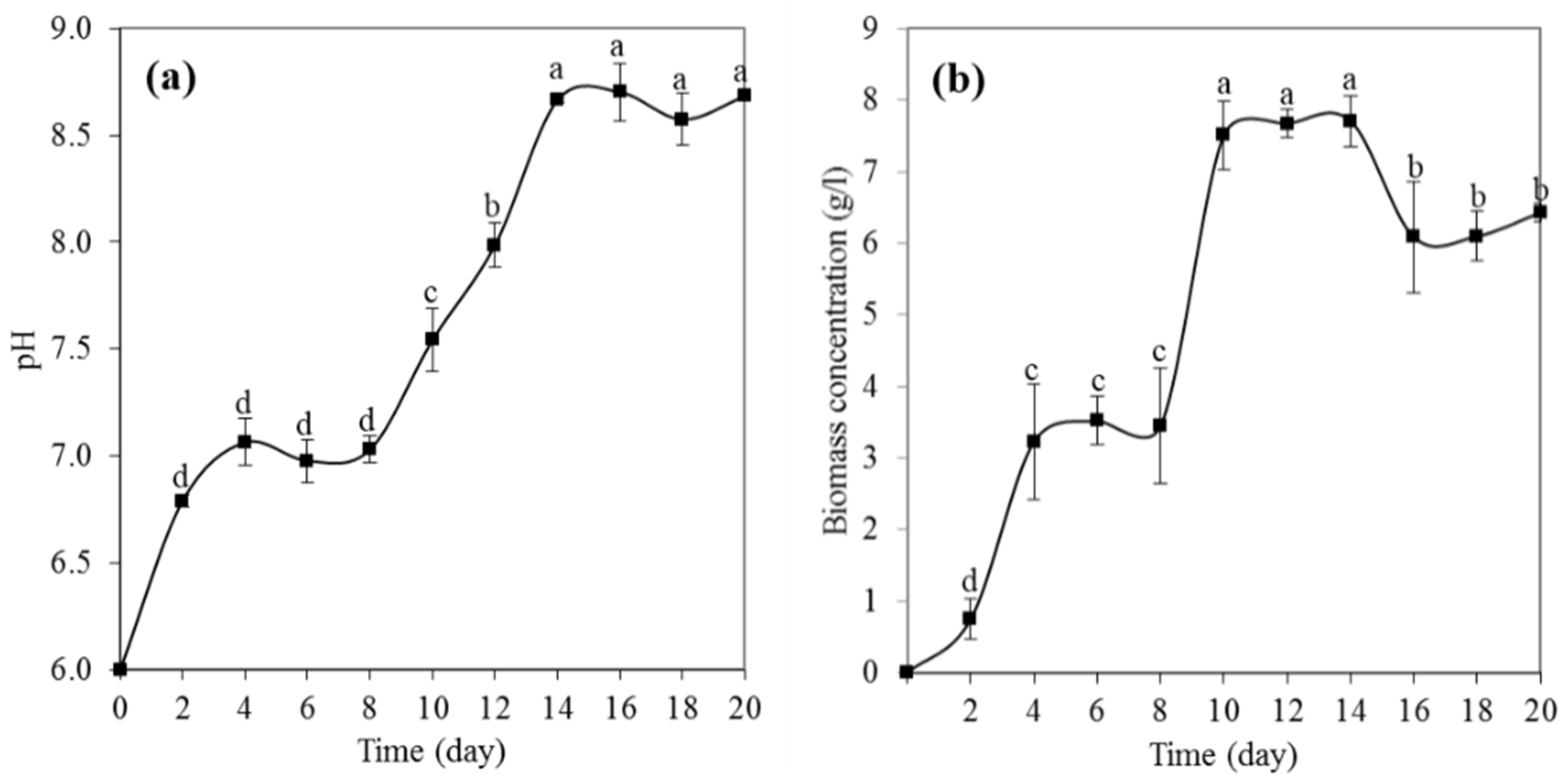
3.2. pH Growth of P. melonis 502
3.3. Physiological Growth Phases of P. melonis 502
3.4. Cellulase Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bruton, B.D. Soilborne diseases in cucurbitaceae: Pathogen virulence and host resistance. In Cucurbitaceae 98: Evaluation and Enhancement of Cucurbit Germplasm; McCreight, J.D., Ed.; ASHS Press: Alexandria, VA, USA, 1998; pp. 143–166. ISBN 978-0961502799. [Google Scholar]
- Aegerter, B.J.; Gordon, T.R.; Davis, R.M. Occurrence and pathogenicity of fungi associated with melon root rot and vine decline in California. Plant Dis. 2000, 84, 224–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfaro-Fernández, A.; García-Luis, A. Colonisation and histological changes in muskmelon and autumn squash tissues infected by Acremonium cucurbitacearum or Monosporascus cannonballus. Eur. J. Plant Pathol. 2009, 125, 73–85. [Google Scholar] [CrossRef]
- Alfaro-García, A.; Armengol, J.; Bruton, B.D.; Gams, W.; García-Jiménez, J.; Martínez-Ferrer, G. The taxonomic position of the causal agent of Acremonium collapse. Mycologia 1996, 88, 804–808. [Google Scholar] [CrossRef]
- Forti, J. Aspectos Patológicos, Epidemiológicos y Culturales de Acremonium cucurbitacearum Alfaro-Garcia, w. Gams et j. Garcia-Jiménez. Ph.D. Thesis. Universidad Politecnica de Valencia, Valencia, Spain, 1997. Available online: https://dialnet.unirioja.es/servlet/tesis?codigo=245756 (accessed on 7 April 2022).
- Armengol, J.; Sanz, E.; Martínez-Ferrer, G.; Sales, R.; Bruton, B.D.; García-Jiménez, J. Host range of acremonium cucurbitacearum, cause of acremonium collapse of muskmelon. Plant Pathol. 1998, 47, 29–35. [Google Scholar] [CrossRef]
- Bruton, B.D.; Davis, R.M.; Gordon, T.R. Occurrence of Acremonium sp. and Monosporascus cannonballus in the major cantaloupe and watermelon growing areas of California. Plant Dis. 1995, 79, 754. [Google Scholar] [CrossRef]
- Bruton, B.D.; Garcia-Jimenez, J.; Armengol, J. Analysis of the relationship between temperature and vine declines caused by Acremonium cucurbitacearum and Monosporascus cannonballus on Muskmelon. Subtrop. Plant Sci. 1999, 51, 23–28. Available online: http://www.subplantsci.org/wp-content/uploads/2016/03/SPSJ-51-23-28-Bruton-et-al.pdf (accessed on 7 April 2022).
- Bruton, B.D.; Garcia-Jimenez, J.; Armengol, J.; Popham, T.W. Assessment of virulence of Acremonium cucurbitacearum and Monosporascus cannonballus on Cucumis melo. Plant Dis. 2000, 84, 907–913. [Google Scholar] [CrossRef] [Green Version]
- Bruton, B.D.; Popham, T.W.; García-Jiménez, J.; Armengol, J.; Miller, M.E. Disease reaction among selected Cucurbitaceae to an Acremonium cucurbitacearum isolate from Texas. Hortscience 2000, 35, 677–680. [Google Scholar] [CrossRef] [Green Version]
- Carlucci, A.; Raimondo, M.L.; Santos, J.; Phillips, A.J.L. Plectosphaerella species associated with root and collar rots of horticultural crops in southern Italy. Pers. Mol. Phylogeny Evol. Fungi 2012, 28, 34–48. [Google Scholar] [CrossRef] [Green Version]
- Chilosi, G.; Reda, R.; Aleandri, M.P.; Camele, I.; Altieri, L.; Montuschi, C.; Languasco, L.; Rossi, V.; Agosteo, G.E.; Macrì, C.; et al. Fungi associated with root rot and collapse of melon in Italy. OEPP/EPPO Bull. 2008, 38, 147–154. [Google Scholar] [CrossRef]
- Garsia-Jimenez, J.; Velazquez, M.T.; Jorda, C.; Alfaro-Garcia, A. Acremonium species as the causal agent of muskmelon collapse in Spain. Plant Dis. 1994, 78, 416–419. [Google Scholar] [CrossRef]
- Kopilov, E.; Tsekhmister, H.; Nadkernychna, O.; Kyslynska, A. Identification of Plectosphaerella melonis from cucumber plants in Ukraine. Phytopathol. Mediterr. 2021, 60, 259–263. [Google Scholar] [CrossRef]
- Martinez-Culebras, P.V.; Abad-Campos, P.; Garcia-Jimenez, J. Molecular characterization and PCR detection of the melon pathogen Acremonium cucurbitacearum. Eur. J. Plant Pathol. 2004, 110, 801–809. [Google Scholar] [CrossRef]
- Mostafa, M.A.; Attia, M.F.; Merghany, M.M.; Salama, R.M. Association of Plectosphaerella melonis with cantaloupe decline for the first time in Egypt. Plant Arch. 2019, 19, 2565–2573. Available online: http://plantarchives.org/19-2/2565-2573%20(5261).pdf (accessed on 7 April 2022).
- Raimondo, M.L.; Carlucci, A. Characterization and pathogenicity assessment of Plectosphaerella species associated with stunting disease on tomato and pepper crops in Italy. Plant Pathol. 2018, 67, 626–641. [Google Scholar] [CrossRef]
- Raimondo, M.L.; Carlucci, A. Characterization and pathogenicity of Plectosphaerella spp. collected from basil and parsley in Italy. Phytopathol. Mediterr. 2018, 57, 284–295. [Google Scholar] [CrossRef]
- Tsekhmister, H.V.; Kyslynska, A.S.; Kopilov, E.P.; Nadkernychna, O.V. Plant growth regulatory activity in the phytopathogenic fungus Plectosphaerella melonis strain 502. Agric. Sci. Pract. 2021, 8, 13–24. [Google Scholar] [CrossRef]
- Schulz, B.; Boyle, C. The endophytic continuum. Mycol. Res. 2006, 109, 661–686. [Google Scholar] [CrossRef] [Green Version]
- Selim, K.A.; El-Beih, A.A.; AbdEl-Rahman, T.M.; El-Diwany, A.I. Biology of endophytic Fungi. Curr. Res. Environ. Appl. Mycol. J. Fungal Biol. 2012, 2, 31–82. [Google Scholar] [CrossRef]
- Blagoveshchenskaya, E.Y. Endophyte-plant as a complex dynamic system. Mycol. Today 2011, 2, 126–134. Available online: https://istina.msu.ru/media/publications/article/d02/ec8/1497555/Blagoveshchenskaya2011_endoph_system.pdf (accessed on 7 April 2022). (In Russian).
- Thines, E.; Aguirre, J.; Foster, A.J.; Deising, H.B. Genetics of phytopathology: Secondary metabolites as virulence determinants of fungal plant pathogens. Prog. Bot. 2006, 67, 134–161. [Google Scholar] [CrossRef]
- Asoufi, H.; Hameed, K.M.; Mahasneh, A. The cellulase and pectinase activities associated with the virulence of indigenous Sclerotinia sclerotiorum isolates in Jordan Valley. Plant Pathol. J. 2007, 23, 233–238. [Google Scholar] [CrossRef] [Green Version]
- Hubballi, M.; Sornakili, A.; Nakkeeran, S.; Anand, T.; Raguchander, T. Virulence of Alternaria alternata infecting noni associated with production of cell wall degrading enzymes. J. Plant Prot. Res. 2011, 51, 87–92. [Google Scholar] [CrossRef]
- Jain, V.; Dhawan, K. Major cell wall degrading enzymes in two contrasting cultivars of Brassica juncea infected with Alternaria brassicae. Crucif. Newslett. 2008, 27, 20–21. Available online: https://www.brassica.info/info/publications/cn/CruciferaeNewsletter_vol27_2008.pdf#page=20 (accessed on 7 April 2022).
- Moussa, T.A.A.; Tharwat, N.A. Optimization of cellulase and -glucosidase induction by sugarbeet pathogen Sclerotium rolfsii. Afr. J. Biotechnol. 2007, 6, 1048–1054. Available online: https://academicjournals.org/journal/AJB/article-full-text-pdf/1F42A327000 (accessed on 7 April 2022).
- Hassan, A.G. Formulations as biocontrol and role of pectinase and cellulose in pathogenicity. J. Agric. Food Environ. 2020, 1, 1–5. [Google Scholar] [CrossRef]
- Ziedan, E.S.H.; Saad, M.M.; Hemida, K.A.; El-Naggar, M.A.E.A.; Mostafa, M.H.; El-Samman, M.G.E.R. In vitro: Stem cutting a simple technique for determining aggressive potential of fungal isolates causing root rot disease of grapevine. GSC Biol. Pharm. Sci. 2020, 11, 141–147. [Google Scholar] [CrossRef]
- Lalaoui, F.; Halama, P.; Dumortier, V.; Paul, B. Cell wall-degrading enzymes produced in vitro by isolates of Phaeosphaeria nodorum differing in aggressiveness. Plant Pathol. 2000, 49, 727–733. [Google Scholar] [CrossRef]
- Ogórek, R. Enzymatic activity of potential fungal plant pathogens and the effect of their culture filtrate on seed germination and seedling growth of garden cress (Lepidium sativum L.). Eur J. Plant. Pathol 2016, 145, 469–481. [Google Scholar] [CrossRef]
- Methods of Experimental Mycology. Moscow, ISBN 978-5-458-27224-7. 2014. Available online: https://www.bookvoed.ru/files/3515/10/62/35.pdf (accessed on 7 April 2022). (In Russian).
- Pirt, J. Principles of Microbe and Cell Cultivation; Blackwell Scientific: Oxford, UK, 1975; ISBN 0632081503. [Google Scholar]
- Kurchenko, I.M.; Pavlichenko, A.K.; Yurieva, E.M. Growth characteristics of strains of Fusarium poae (Peck) Wollenw and Penicillium funiculosum Thom. Mikrobiolohichnyi Zhurnal 2013, 75, 47–51. (In Russian) [Google Scholar]
- Somogyi, M. Notes on sugar determination. J. Biol. Chem. 1952, 195, 19–23. [Google Scholar] [CrossRef]
- Nelson, N. A photometric adaptation of the Somogyi method for the determination of glucose. J. Biochem. 1944, 153, 375–380. [Google Scholar] [CrossRef]
- Del Rio, J.A.; Gonzalez, A.; Fuster, M.D.; Botia, J.M.; Gomez, P.; Frias, V.; Ortuño, A. Tylose formation and changes in phenolic compounds of grape roots infected with Phaeomoniella chlamydospora and Phaeoacremonium species. Phytopathol. Mediterr. 2001, 40, 394–399. Available online: http://www.jstor.org/stable/44981647 (accessed on 7 April 2022).
- Pivonia, S.; Cohen, R.; Kafkafi, U.; Ben Ze’ev, I.S.; Katan, J. Sudden wilt of melons in Southern Israel: Fungal agents and relationship with plant development. Plant Dis. 1997, 81, 1264–1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jumpponen, A.; Trappe, J.M. Dark septate endophytes: A review of facultative biotrophic root-colonizing fungi. New Phytol. 1998, 140, 295–310. [Google Scholar] [CrossRef]
- Kazan, K.; Lyons, R. Intervention of phytohormone pathways by pathogen effectors. Plant Cell 2014, 26, 2285–2309. [Google Scholar] [CrossRef] [Green Version]
- Mattoo, A.K.; Handa, A.K. Ethylene signaling in plant cell death. In Plant Cell Death Processes; Nooden, L., Ed.; Academic Press: New York, NY, USA, 2004; pp. 125–142. [Google Scholar]
- Lutova, L.A.; Provorov, N.A. Plant Development Genetics; SPb. Nauka: Saint Petersburg, Russia, 2000; 539p., Available online: https://www.twirpx.com/file/327348/ (accessed on 15 February 2022). (In Russian)
- Wang, S.; Park, Y.S.; Yang, Y.; Borrego, E.J.; Isakeit, T.; Gao, X.; Kolomiets, M.V. Seed-derived ethylene facilitates colonization but not aflatoxin production by Aspergillus flavus in maize. Front. Plant Sci. 2017, 8, 415. [Google Scholar] [CrossRef] [Green Version]
- Grum-Grzhimaylo, A.A.; Debets, A.J.M.; van Diepeningen, A.D.; Georgieva, M.L.; Bilanenko, E.N. Sodiomyces alkalinus, a new alkaliphilic ascomycete within the Plectosphaerellaceae. Pers. Mol. Phylogeny Evol. Fungi 2013, 31, 147–158. [Google Scholar] [CrossRef] [Green Version]
- Bondarenko, S.A.; Georgieva, M.L.; Bilanenko, E.N. Alkalitolerant micromycetes in acidic and neutral soils of the temperate zone. Microbiology 2016, 85, 737–744. [Google Scholar] [CrossRef]
- Idnurm, A.; Howlett, B.J. Pathogenicity genes of phytopathogenic fungi. Mol. Plant Pathol. 2001, 2, 241–255. [Google Scholar] [CrossRef]
- Prusky, D.; Yakoby, N. Pathogenic fungi: Leading or led by ambient pH? Mol. Plant Pathol. 2003, 4, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Jennings, D.H. Some perspectives on nitrogen and phosphorus metabolism in fungi. In Nitrogen, Phosphorus and Sulphur Utilization by Fungi; Boddy, L., Machant, R., Read, D.J., Eds.; Cambridge University Press: Cambridge, UK, 1989; pp. 1–31. ISBN 9780521106245. [Google Scholar]
- Vieira, G.R.T.; Liebl, M.; Tavares, L.B.; Paulert, R.; Smania, A.J. Submerged culture conditions for the production of mycelial biomass and antimicrobial metabolites by polyporus tricholoma mont. Braz. J. Microbiol. 2008, 39, 561–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Bernardis, F.; Muhlschlegel, F.A.; Cassone, A. The pH of the host niche controls gene expression in and virulence of Candida albicans. Infect. Immun. 1998, 66, 3317–3325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- St Leger, R.J.; Joshi, L.; Roberts, D. Ambient pH is a major determinant in the expression of cuticle-degrading enzymes and hydrophobin by Metarhizium anisopliae. Appl. Environ. Microbiol. 1998, 64, 709–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eshel, D.; Miyara, I.; Tong, A.; Dinoor, A.; Prusky, D. pH Regulates endoglucanase expression and virulence of Alternaria alternata in persimmon fruit. Mol. Plant-Microbe Interact. (MPMI) 2002, 15, 774–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prusky, D.; McEvoy, J.L.; Leverentz, B.; Conway, W.S. Local modulation of host pH by Colletotrichum species as a mechanism to increase virulence. Mol. Plant-Microbe Interact. 2001, 14, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Maccheroni, W., Jr.; Araújo, W.L.; Azevedo, J.L. Ambient pH-regulated enzime secretion in endophytic and pathogenic isolates of the fungal genus Colletotrichum. Sci. Agric. 2004, 61, 298–302. [Google Scholar] [CrossRef] [Green Version]
- Ramanathan, G.; Banupriya, S.; Abirami, D. Production and optimization of cellulase from Fusarium oxysporum by submerged fermentation. J. Sci. Ind. Res. 2010, 69, 454–459. Available online: http://nopr.niscair.res.in/bitstream/123456789/9685/1/JSIR%2069(6)%20454-459.pdf (accessed on 7 April 2022).
- Acosta-Rodríguez, I.; Piñón-Escobedo, C.; Zavala-Páramo, M.G.; López-Romero, E.; Cano-Camacho, H. Degradation of cellulose by the bean-pathogenic fungus Colletotrichum lindemuthianum. Production of extracellular cellulolytic enzymes by cellulose induction. Antonie Van Leeuwenhoek 2005, 87, 301–310. [Google Scholar] [CrossRef]
- Ramezani, Y.; Taheri, P.; Mamarabadi, M. Identification of Alternaria spp. associated with tomato early blight in Iran and investigating some of their virulence factors. J. Plant Pathol. 2019, 101, 647–659. [Google Scholar] [CrossRef]
- Yasmin, A.; Hameed, A.; Ghaffar, A. Enzymatic activity of fungal pathogens in corn. Pak. J. Bot. 2006, 38, 1305–1316. Available online: http://www.pakbs.org/pjbot/PDFs/38(4)/PJB38(4)1305.pdf (accessed on 7 April 2022).
- Kopilov, E.; Kyslynska, A.; Nadkernychna, O.; Tsekhmister, H. Formation and functioning of Chaetomium cochliodes/Fagopyrum esculentum endophytic association. J. Microbiol. Biotechnol. Food Sci. 2020, 10, 190–196. [Google Scholar] [CrossRef]
- Bilay, V.I.; Bilay, T.I.; Musich, E.G. Transformatsiya Tsellyulozyi Gribami; Naukova Dumka: Kyiv, Ukraine, 1982; 295p, Available online: https://koha.lib.tsu.ru/cgi-bin/koha/opac-detail.pl?biblionumber=232959 (accessed on 7 April 2022). (In Russian)
- Dankevych, L.; Leonova, N.; Dragovoz, I.; Patyka, V.; Kalinichenko, A.; Wlodarczyk, P.; Wlodarczyk, B. The synthesis of plant growth stimulators by phytopathogenic bacteria as factor of pathogenicity. Appl. Ecol. Environ. Res. 2019, 16, 1581–1593. [Google Scholar] [CrossRef]
- Kolomiiets, Y.V.; Grygoryuk, I.P.; Butsenko, L.M.; Kalinichenko, A.V. Biotechnological control methods against phytopathogenic bacteria in tomatoes. Appl. Ecol. Environ. Res. 2019, 17, 3215–3230. [Google Scholar] [CrossRef]
- Zhang, G.M.; Huang, J.; Huang, G.R.; Ma, L.X.; Zhang, X. Molecular cloning and heterologous expression of a new xylanase gene from Plectosphaerella cucumerina. Appl. Microbiol. Biotech. 2007, 74, 339–346. [Google Scholar] [CrossRef]
- Velmurugan, N.; Kalpana, D.; Han, J.H.; Cha, H.J.; Lee, Y.S. A novel low temperature chitinase from the marine fungus Plectosphaerella sp. strain MF-1. Bot. Mar. 2011, 54, 75–81. [Google Scholar] [CrossRef]

| pH | Colony Diameter, mm (10 days) | Radial Growth Rate, mm per h |
|---|---|---|
| 6.0 | 20.3 ± 0.2 e | 0.084 ± 0.000 e |
| 6.5 | 21.0 ± 0.0 e | 0.088 ± 0.000 e |
| 7.0 | 23.1 ± 0.1 c | 0.096 ± 0.000 c |
| 7.5 | 23.6 ± 0.2 b | 0.098 ± 0.001 b |
| 8.0 | 24.0 ± 0.0 b | 0.100 ± 0.000 b |
| 8.5 | 24.8 ± 0.1 a | 0.103 ± 0.001 a |
| 9.0 | 23.9 ± 0.1 b | 0.100 ± 0.000 b |
| 9.5 | 23.8 ± 0.2 b | 0.099 ± 0.001 b |
| 10.0 | 23.1 ± 0.1 c | 0.096 ± 0.001 c |
| 10.5 | 23.2 ± 0.1 bc | 0.097 ± 0.001 bc |
| 11.0 | 21.9 ± 0.1 d | 0.091 ± 0.000 d |
| 11.5 | 20.7 ± 0.2 e | 0.086 ± 0.001 e |
| 12.0 | 14.7 ± 0.3 f | 0.061 ± 0.001 f |
| Cultivation Period, Week | Activity (U mL−1) ± SD pH | ||
|---|---|---|---|
| 5.5 | 7.0 | 8.5 | |
| 1 | 0.031 ± 0.002 aa | 0.047 ± 0.002 aa | 0.077 ± 0.005 ab |
| 2 | 0.020 ± 0.001 aa | 0.025 ± 0.000 aa | 0.053 ± 0.005 ab |
| 3 | 0.046 ± 0.004 aa | 0.065 ± 0.006 ba | 0.118 ± 0.004 bb |
| 4 | 0.048 ± 0.002 aa | 0.071 ± 0.002 ba | 0.132 ± 0.006 bb |
| 5 | 0.066 ± 0.005 aa | 0.072 ± 0.003 ba | 0.163 ± 0.006 cb |
| 6 | 0.050 ± 0.005 aa | 0.068 ± 0.010 ba | 0.326 ± 0.019 db |
| 7 | 0.066 ± 0.023 aa | 0.039 ± 0.013 ca | 0.145 ± 0.011 eb |
| 8 | 0.021 ± 0.001 ba | 0.048 ± 0.006 cb | 0.149 ± 0.007 ec |
| Cultivation Period, Week | Activity (U mL−1) ± SD pH | ||
|---|---|---|---|
| 5.5 | 7.0 | 8.5 | |
| 1 | 0.121 ± 0.003 aa | 0.128 ± 0.002 aa | 0.135 ± 0.010 aa |
| 2 | 0.080 ± 0.000 ba | 0.090 ± 0.002 ba | 0.141 ± 0.017 ab |
| 3 | 0.101 ± 0.013 ba | 0.161 ± 0.003 cb | 0.253 ± 0.002 bc |
| 4 | 0.068 ± 0.003 ca | 0.084 ± 0.001 da | 0.151 ± 0.001 cb |
| 5 | 0.115 ± 0.005 da | 0.142 ± 0.003 ea | 0.248 ± 0.010 db |
| 6 | 0.148 ± 0.015 ea | 0.225 ± 0.005 fa | 0.539 ± 0.019 ec |
| 7 | 0.136 ± 0.006 ea | 0.097 ± 0.012 gb | 0.156 ± 0.003 fd |
| 8 | 0.063 ± 0.004 fa | 0.095 ± 0.004 gb | 0.196 ± 0.010 gc |
| Cultivation Period, Week | Activity (U mL−1) ± SD pH | ||
|---|---|---|---|
| 5.5 | 7.0 | 8.5 | |
| 1 | 0.061 ± 0.001 aa | 0.059 ± 0.001 aa | 0.069 ± 0.002 aa |
| 2 | 0.037 ± 0.001 aa | 0.041 ± 0.001 aa | 0.067 ± 0.003 aa |
| 3 | 0.066 ± 0.000 aa | 0.139 ± 0.019 bb | 0.272 ± 0.016 bc |
| 4 | 0.084 ± 0.000 aa | 0.127 ± 0.022 ba | 0.337 ± 0.011 cb |
| 5 | 0.024 ± 0.001 aa | 0.090 ± 0.008 ba | 0.404 ± 0.005 db |
| 6 | 0.000 ± 0.000 aa | 0.016 ± 0.022 bb | 0.950 ± 0.071 eb |
| 7 | 0.000 ± 0.000 aa | 0.025 ± 0.009 ba | 0.233 ± 0.004 fb |
| 8 | 0.039 ± 0.002 aa | 0.049 ± 0.003 ba | 0.421 ± 0.043 gb |
| Cultivation Period, Week | Activity (U mL−1) ± SD pH | ||
|---|---|---|---|
| 5.5 | 7.0 | 8.0 | |
| 1 | 0.059 ± 0.001 aa | 0.078 ± 0.014 aa | 0.212 ± 0.006 ab |
| 2 | 0.036 ± 0.002 aa | 0.043 ± 0.005 aa | 0.174 ± 0.005 ab |
| 3 | 0.066 ± 0.000 aa | 0.161 ± 0.006 bb | 0.244 ± 0.016 bc |
| 4 | 0.090 ± 0.002 aa | 0.195 ± 0.010 bb | 0.293 ± 0.004 bc |
| 5 | 0.168 ± 0.007 ba | 0.245 ± 0.009 bb | 0.421 ± 0.024 cc |
| 6 | 0.363 ± 0.042 ca | 0.283 ± 0.006 bb | 0.795 ± 0.034 dc |
| 7 | 0.203 ± 0.019 da | 0.127 ± 0.018 cb | 0.398 ± 0.064 ec |
| 8 | 0.058 ± 0.013 ea | 0.106 ± 0.003 ca | 0.389 ± 0.012 eb |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patyka, V.; Tsekhmister, H.; Kopylov, Y.; Kyslynska, A.; Kalinichenko, A.; Sporek, M.; Stebila, J. Histological Change in Cucumber Tissue and Cellulase Activity of Plectosphaerella melonis Strain 502. Appl. Sci. 2022, 12, 5085. https://doi.org/10.3390/app12105085
Patyka V, Tsekhmister H, Kopylov Y, Kyslynska A, Kalinichenko A, Sporek M, Stebila J. Histological Change in Cucumber Tissue and Cellulase Activity of Plectosphaerella melonis Strain 502. Applied Sciences. 2022; 12(10):5085. https://doi.org/10.3390/app12105085
Chicago/Turabian StylePatyka, Volodymyr, Hanna Tsekhmister, Yevhenii Kopylov, Anna Kyslynska, Antonina Kalinichenko, Monika Sporek, and Jan Stebila. 2022. "Histological Change in Cucumber Tissue and Cellulase Activity of Plectosphaerella melonis Strain 502" Applied Sciences 12, no. 10: 5085. https://doi.org/10.3390/app12105085
APA StylePatyka, V., Tsekhmister, H., Kopylov, Y., Kyslynska, A., Kalinichenko, A., Sporek, M., & Stebila, J. (2022). Histological Change in Cucumber Tissue and Cellulase Activity of Plectosphaerella melonis Strain 502. Applied Sciences, 12(10), 5085. https://doi.org/10.3390/app12105085







