Influence of Carbon Dioxide Curing on the Corrosion Resistance of Reinforced Cement Mortar under the External Erosion of NaCl Freeze–Thaw Cycle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
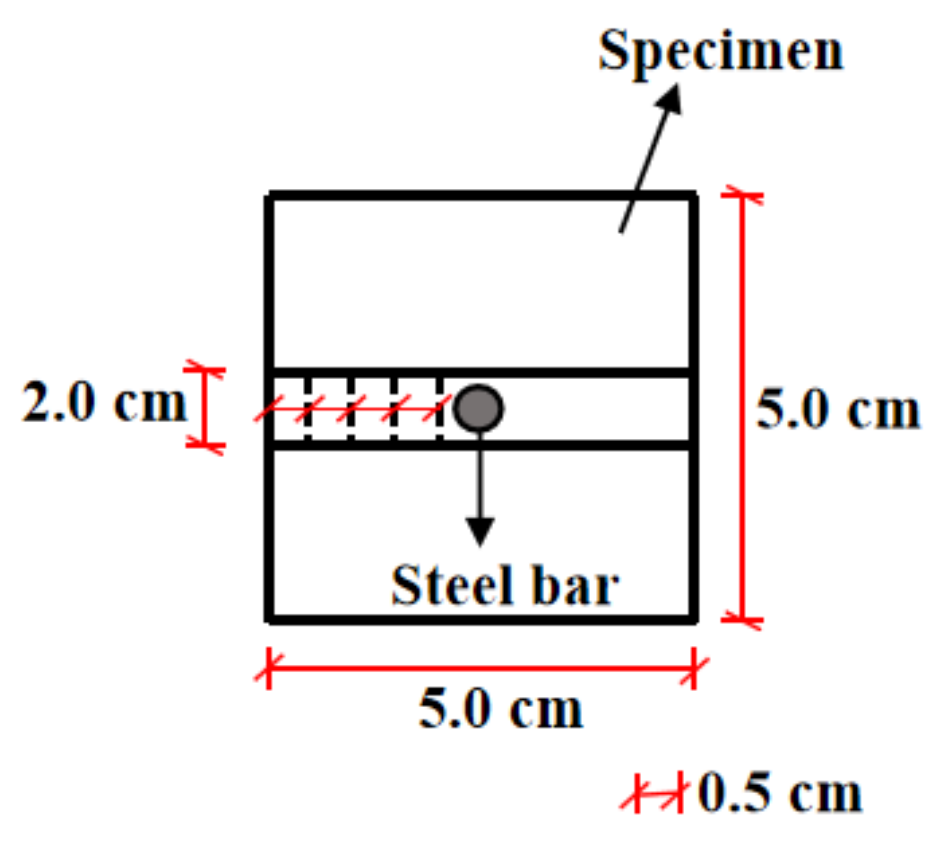
2.2. The Preparation of Samples
2.3. Measurement Methods
2.3.1. The Measurement of pH
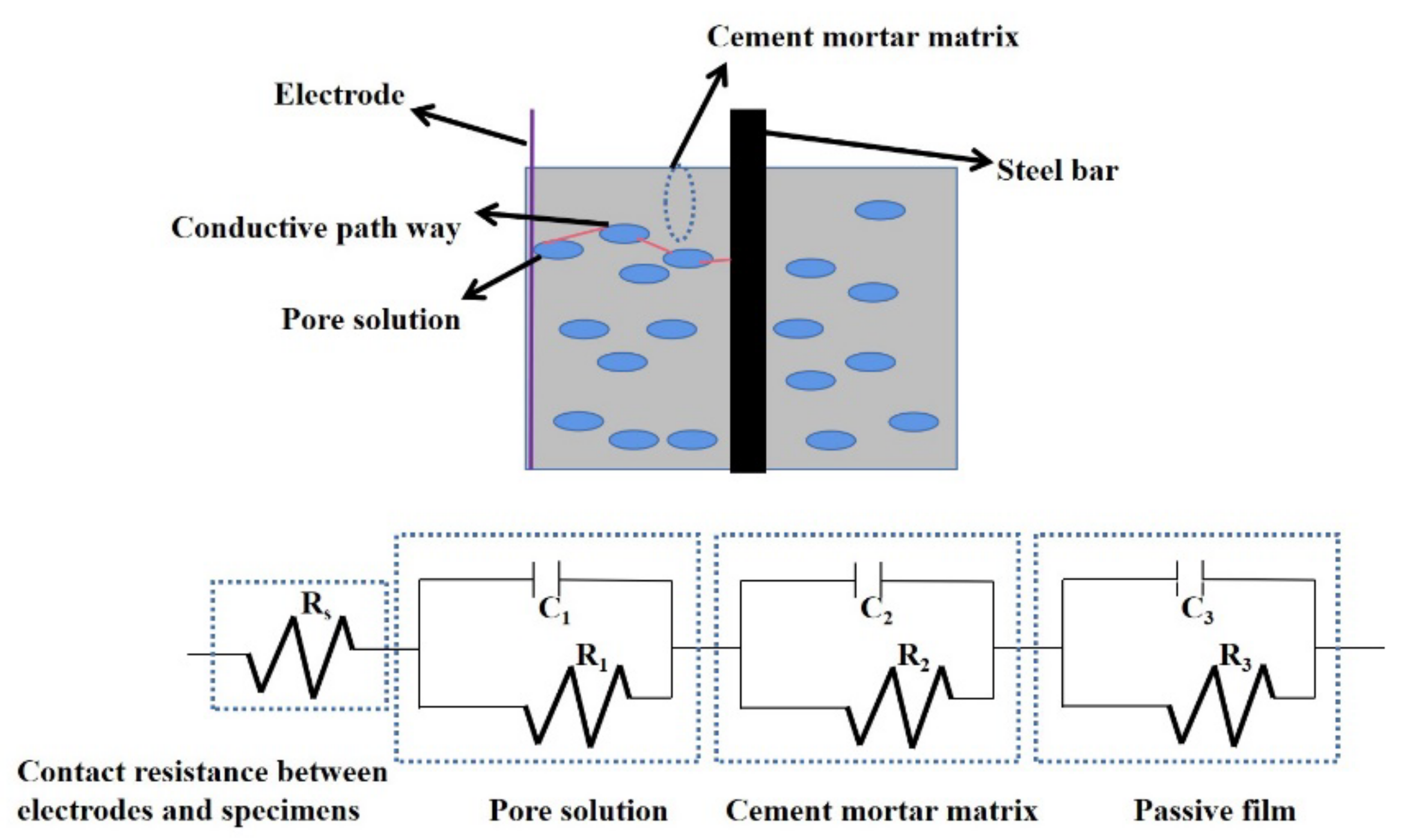
2.3.2. Electrical Parameters
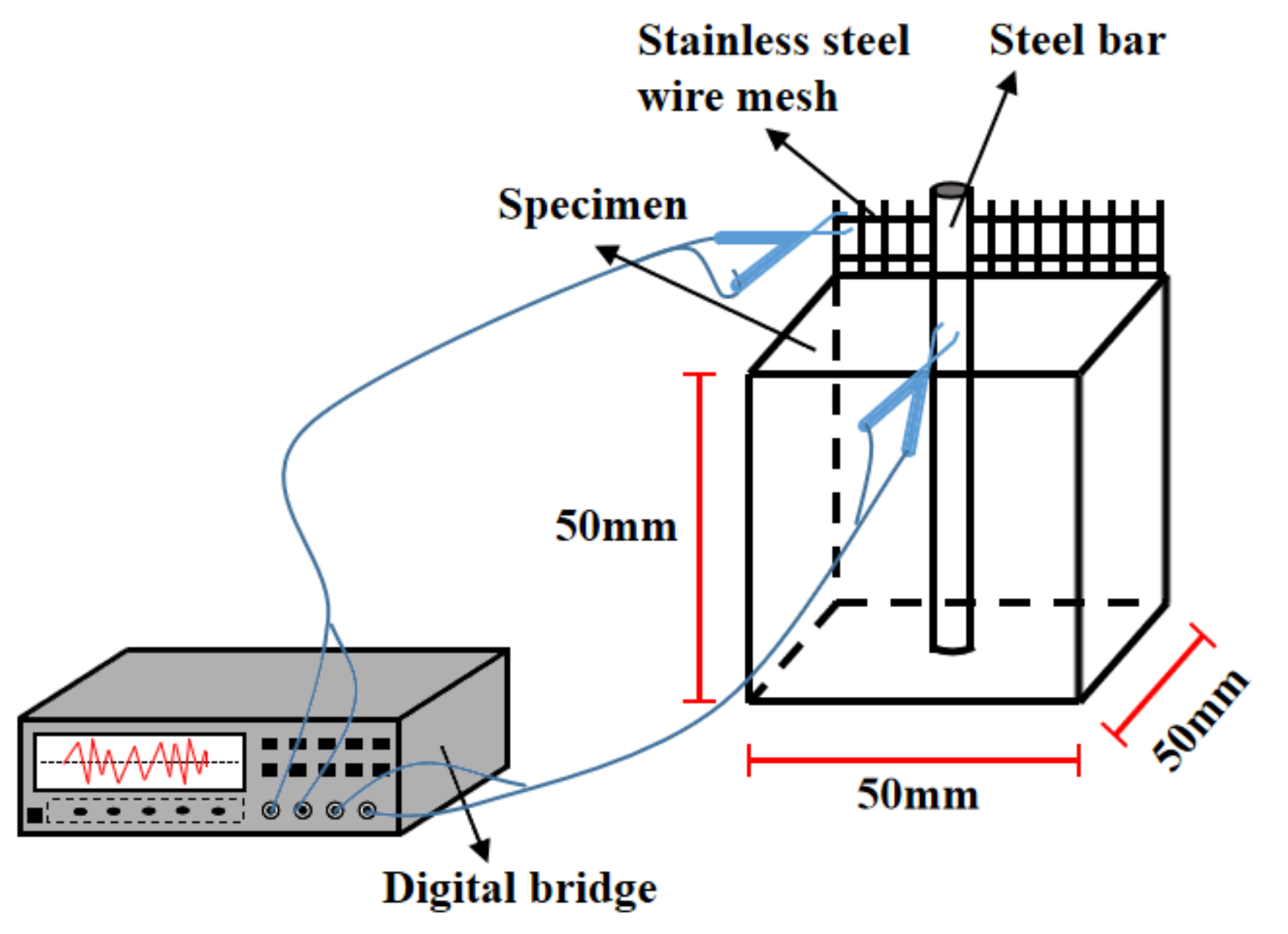
2.3.3. Ultrasonic Velocity
2.3.4. Percentage of Mass Loss
2.3.5. Microscopic Tests
3. Results and Discussions
3.1. The pH of the Cement Mortar
3.2. Macroscopic Properties before Sodium Chloride Action
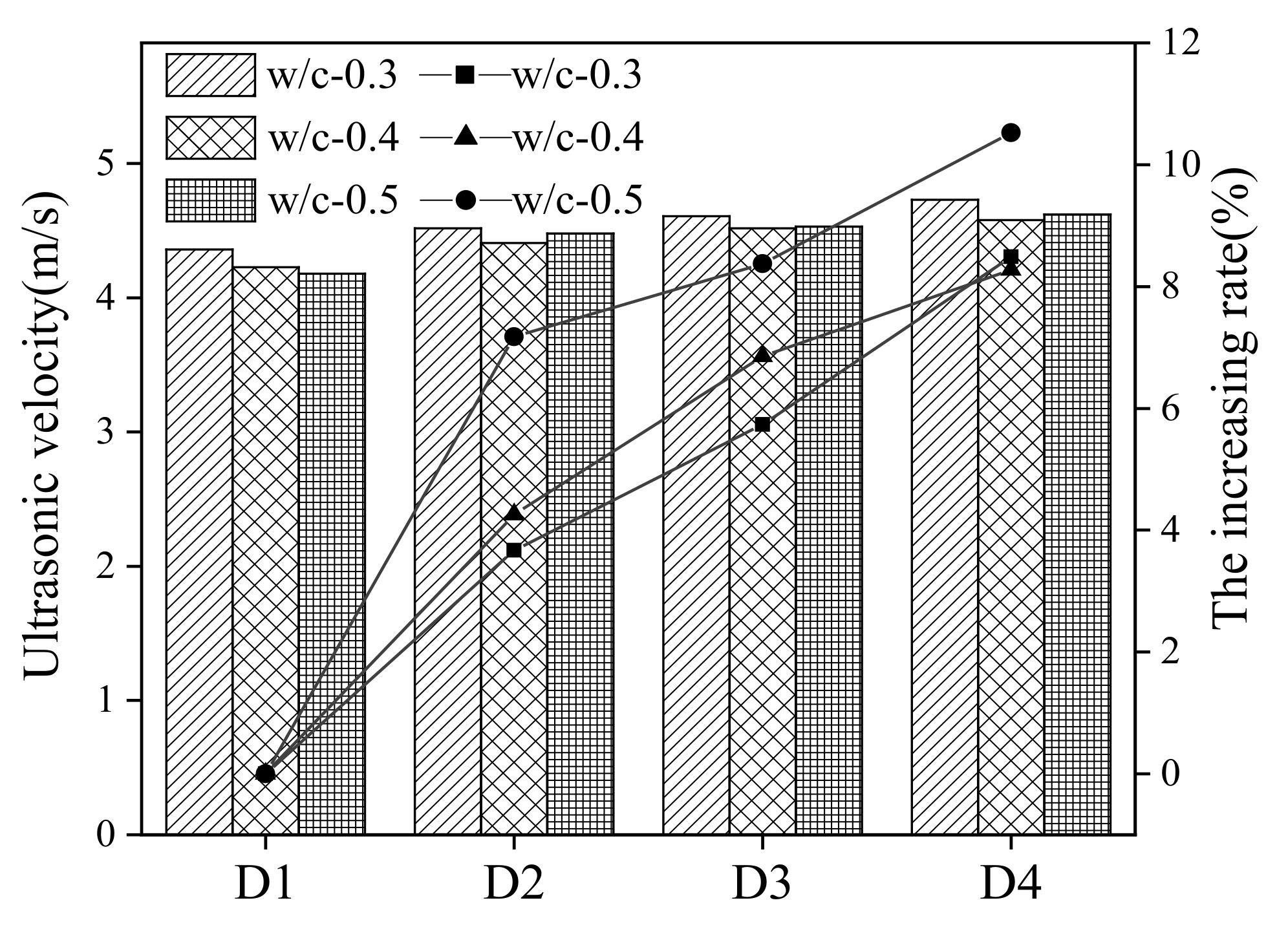
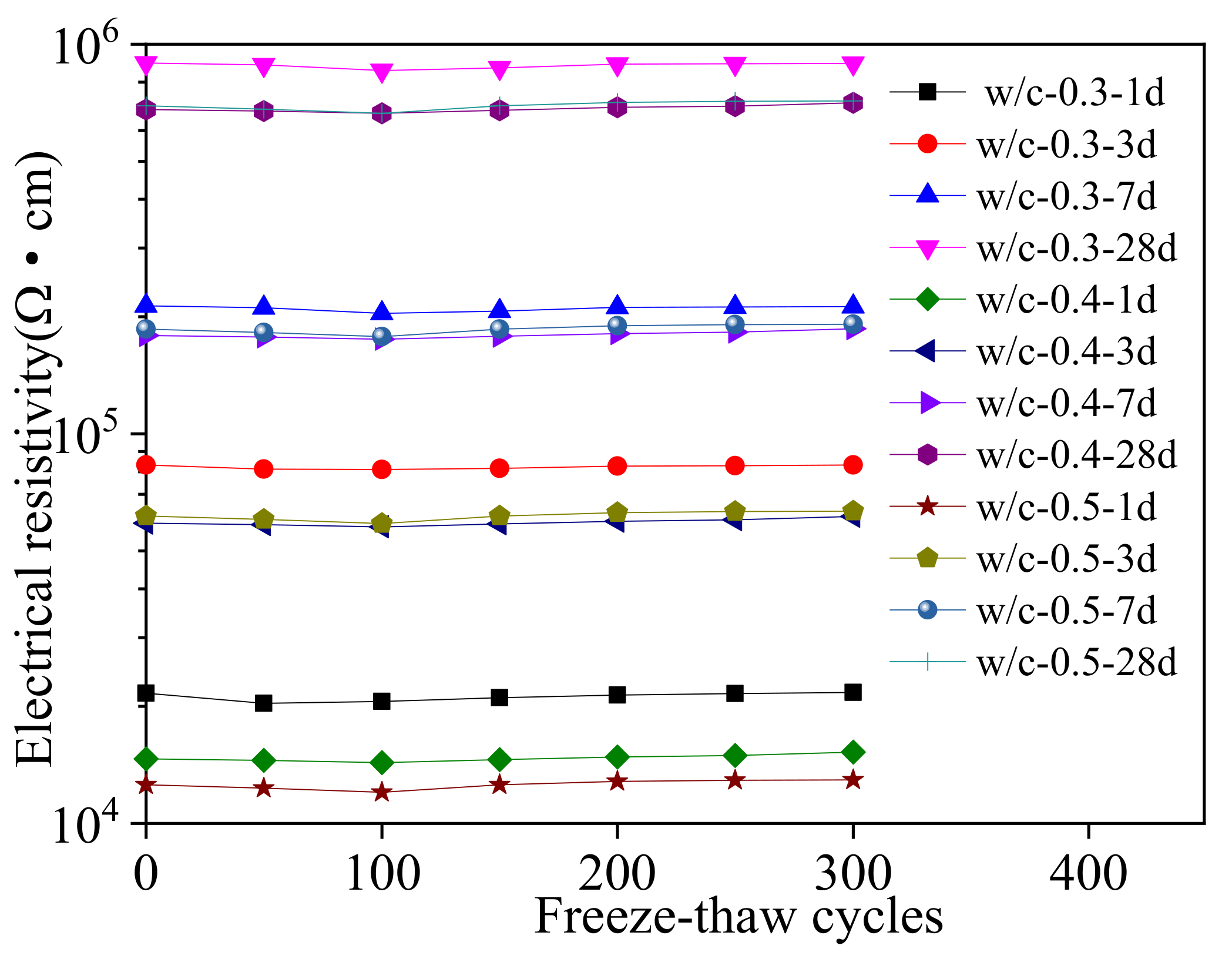
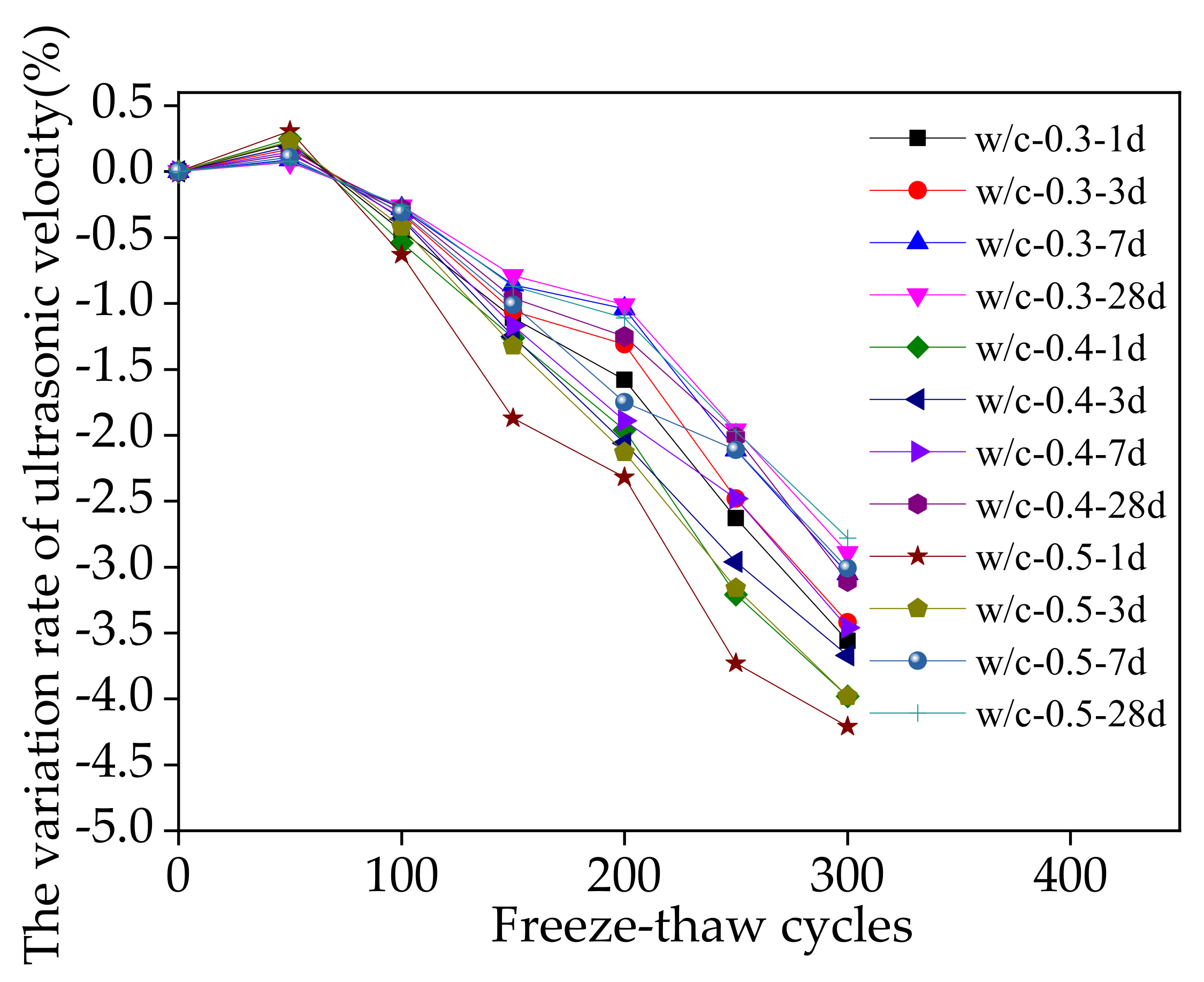
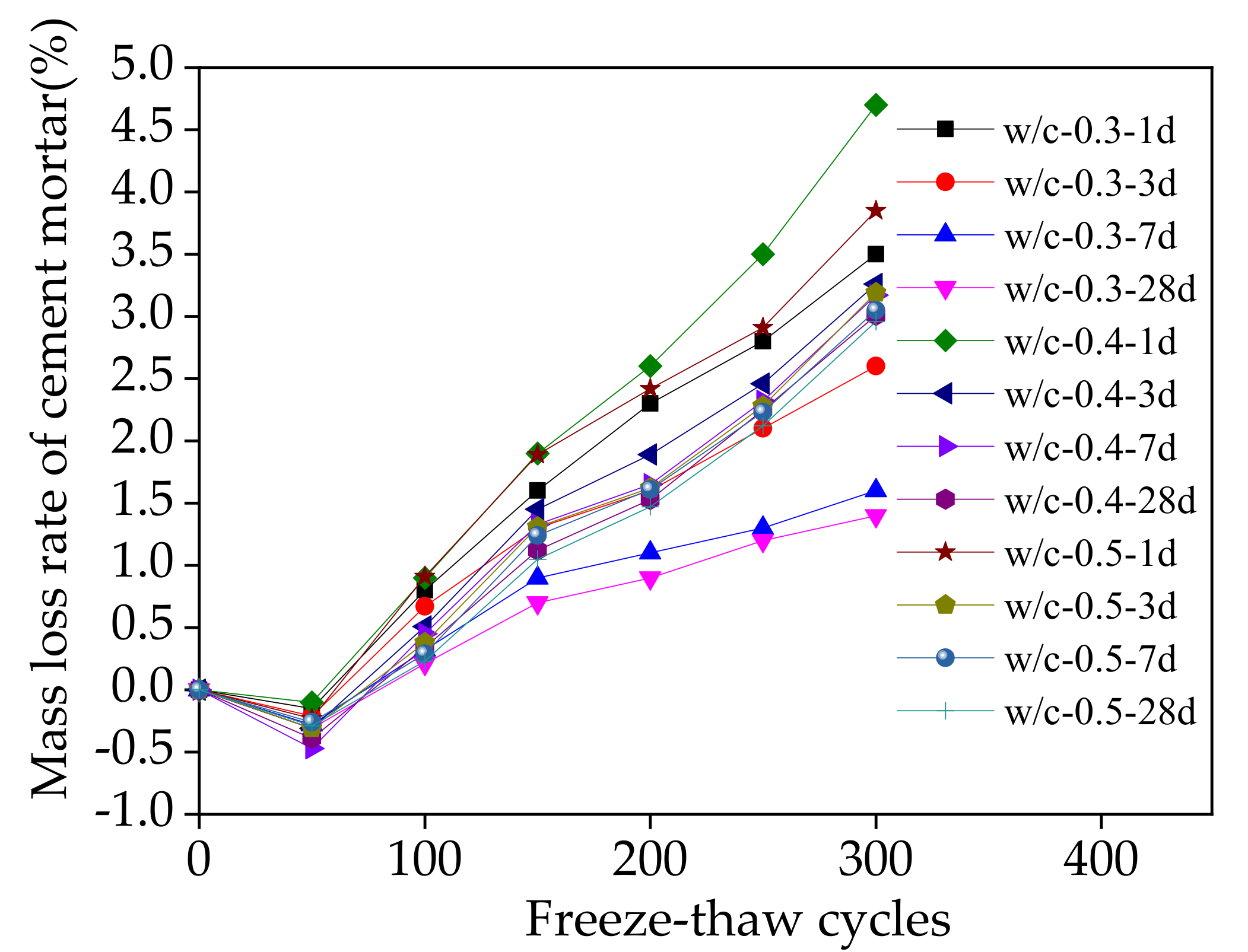
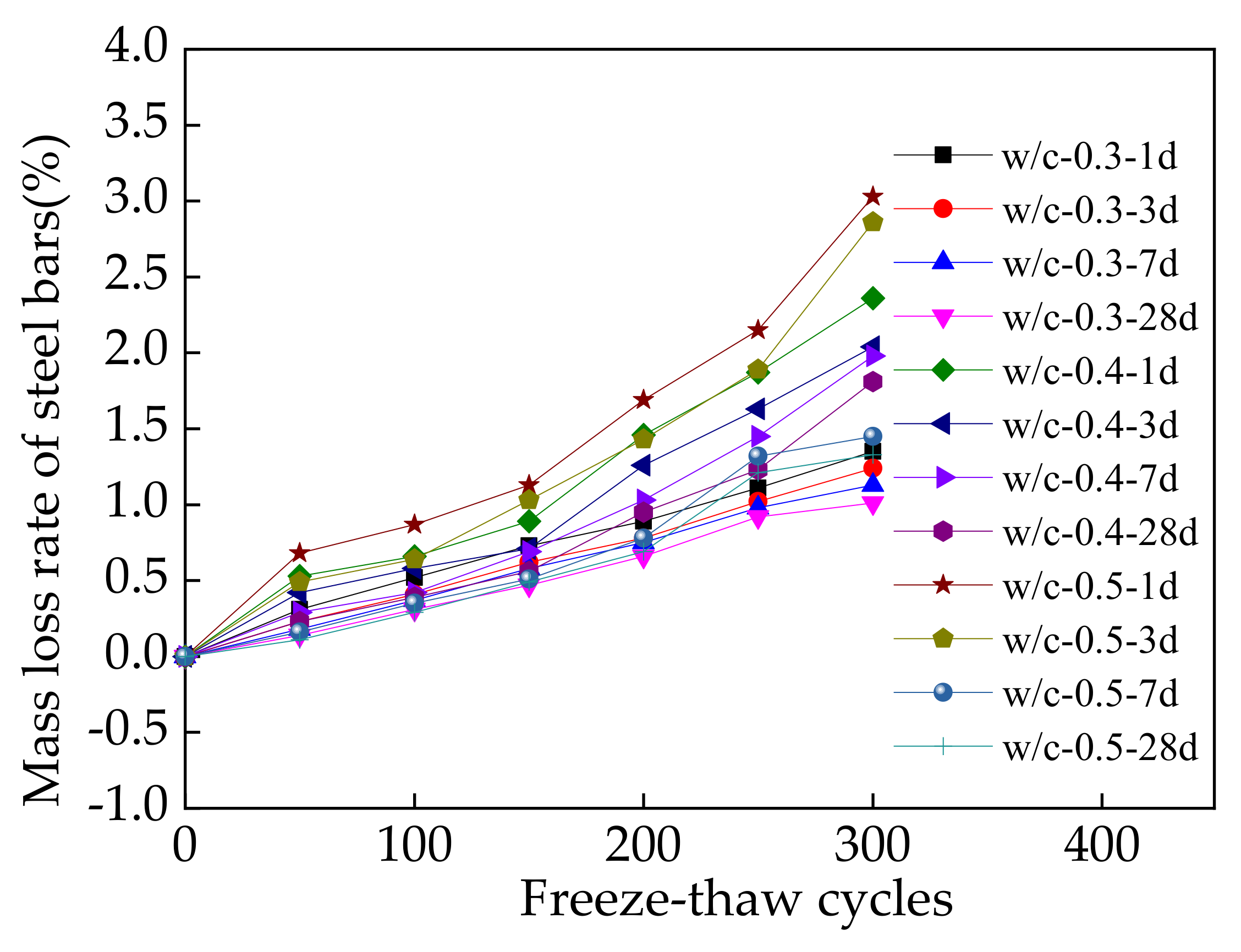
3.3. Macroscopic Properties during Sodium Chloride Action
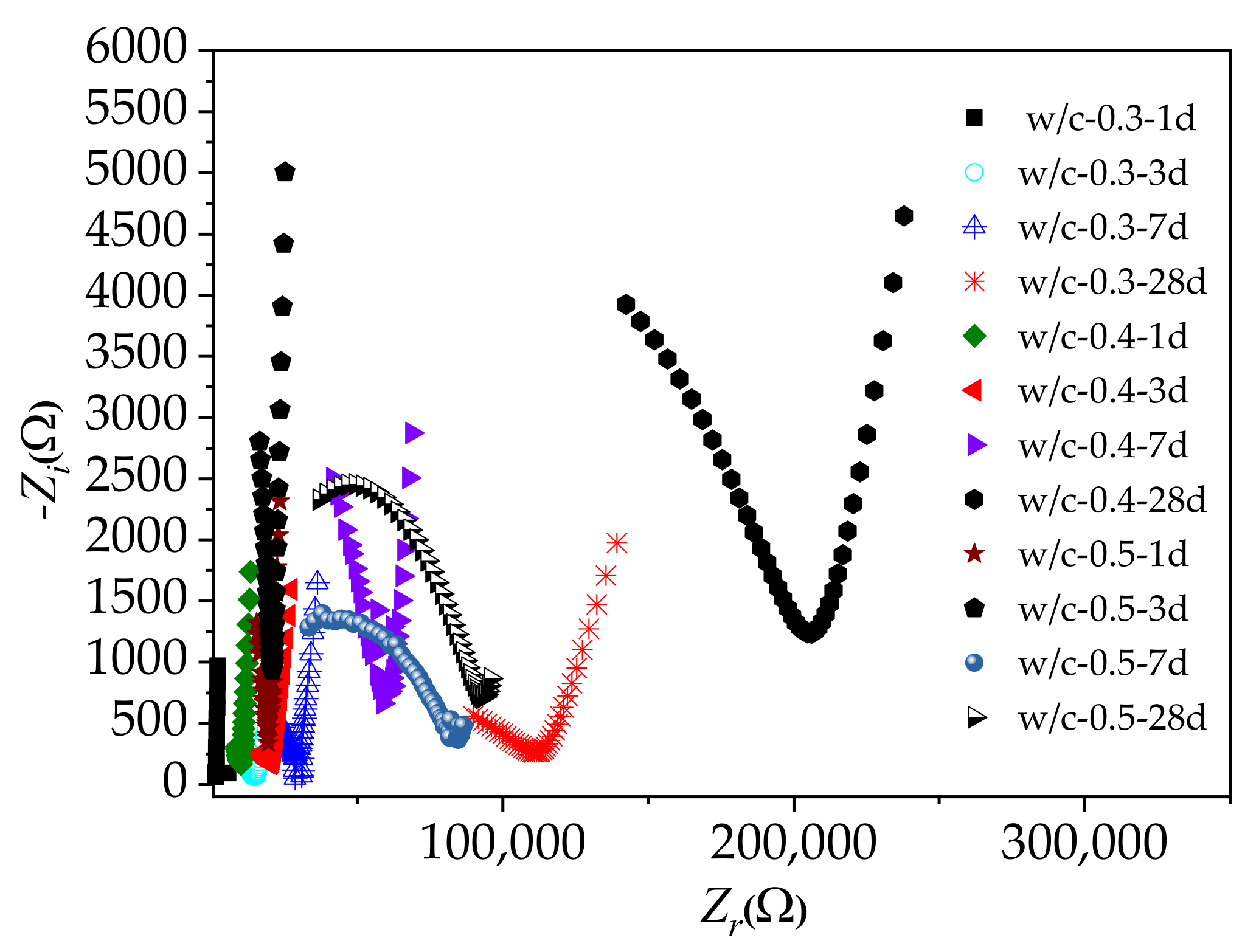
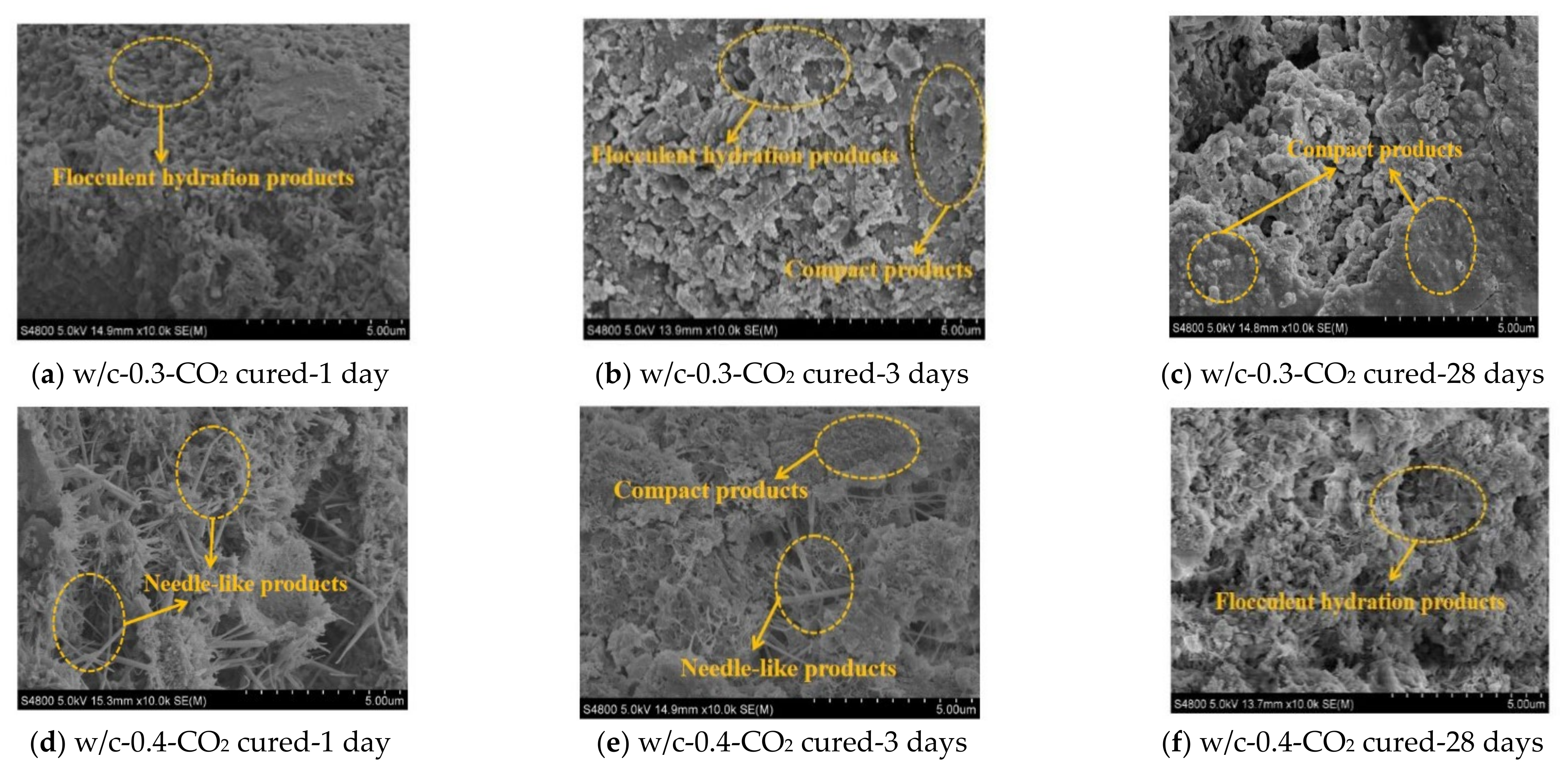
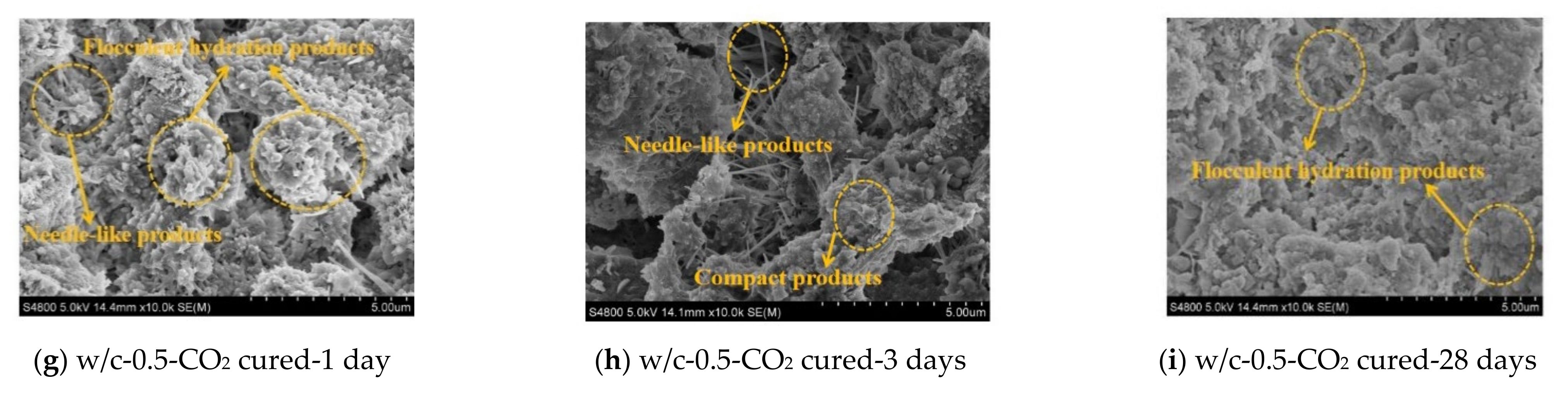
3.4. Microscopic Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mostafaeipour, A.; Bidokhti, A.; Fakhrzad, M.; Sadegheih, A.; Mehrjerdi, Y. A new model for the use of renewable electricity to reduce carbon dioxide emissions. Energy 2022, 238, 121602. [Google Scholar] [CrossRef]
- Kongboon, R.; Gheewala, S.H.; Sampattagulad, S. Greenhouse gas emissions inventory data acquisition and analytics for low carbon cities. J. Clean. Prod. 2022, 343, 130711. [Google Scholar] [CrossRef]
- Zhu, J.; Qu, Z.; Liang, S.; Li, B.; Du, T.; Wang, H. The macro-scopic and microscopic properties of cement paste with carbon dioxide curing. Materials 2022, 15, 1578. [Google Scholar] [CrossRef] [PubMed]
- Adesina, A. Recent advances in the concrete industry to reduce its carbon dioxide emissions. Environ. Chall. 2020, 1, 100004. [Google Scholar] [CrossRef]
- Xian, X.; Zhang, D.; Lin, H.; Shao, Y. Ambient pressure carbonation curing of reinforced concrete for CO2 utilization and corrosion resistance. J. CO2 Util. 2022, 56, 101861. [Google Scholar] [CrossRef]
- Witoon, T.; Lapkeatseree, V.; Numpilai, T.; Cheng, C.; Limtrakul, J. CO2 hydrogenation to light olefins over mixed Fe-Co-K-Al oxides catalysts prepared via precipitation and reduction methods. Chem. Eng. J. 2022, 428, 131389. [Google Scholar] [CrossRef]
- Witoon, T.; Numpilai, T.; Nijpanich, S.; Chanlek, N.; Kidkhunthod, P.; Cheng, C.K.; Ng, K.H.; Vo, D.-V.N.; Ittisanronnachai, S.; Wattanakit, C.; et al. Enhanced CO2 hydrogenation to higher alcohols over K-Co promoted In2O3 catalysts. Chem. Eng. J. 2022, 431, 133211. [Google Scholar] [CrossRef]
- AL-Ameeri, A.S.; ImranRafiq, M.; Tsioulou, O.; Rybdylova, O. Impact of climate change on the carbonation in concrete due to carbon dioxide ingress: Experimental investigation and modelling. J. Build. Eng. 2021, 44, 102594. [Google Scholar] [CrossRef]
- Peng, Y.; Meng, X.; Song, F.; Xu, G. Experimental study on the corrosion characteristics of concrete exposed to acid water containing aggressive carbon dioxide and sodium sulfate. Constr. Build. Mater. 2022, 321, 126397. [Google Scholar] [CrossRef]
- Smirnov, V.G.; Manakov, A.Y.; Dyrdin, V.V.; Ismagilov, Z.R.; Mikhailova, E.S.; Rodionova, T.V. The formation of carbon dioxide hydrate from water sorbed by coals. Fuel 2018, 228, 123–131. [Google Scholar] [CrossRef]
- Atsbha, T.G.; Zhutovsky, S. The effect of external curing methods on the development of mechanical and durability-related properties of normal-strength concrete. Constr. Build. Mater. 2022, 324, 126706. [Google Scholar] [CrossRef]
- Kamal, N.L.M.; Itam, Z.; Sivaganese, Y.; Beddu, S. Carbon dioxide sequestration in concrete and its effects on concrete compressive strength. Mater. Today Proc. 2020, 31, A18–A21. [Google Scholar] [CrossRef]
- Glass, G.; Buenfeld, N. The influence of chloride binding on the chloride induced corrosion risk in reinforced concrete. Corros. Sci. 2000, 42, 329–344. [Google Scholar] [CrossRef]
- Qin, L.; Gao, X.; Chen, T. Influence of mineral admixtures on carbonation curing of cement paste. Constr. Build. Mater. 2019, 212, 653–662. [Google Scholar] [CrossRef]
- Cao, H.; Liang, Z.; Peng, X.; Cai, X.; Wang, K.; Wang, H.; Lyu, Z. Research of Carbon Dioxide Curing on the Properties of Reactive Powder Concrete with Assembly Unit of Sulphoaluminate Cement and Ordinary Portland Cement. Coatings 2022, 12, 209. [Google Scholar] [CrossRef]
- Siddique, S.; Naqi, A.; Jang, J. Influence of water to cement ratio on CO2 uptake capacity of belite-rich cement upon exposure to carbonation curing. Cem. Concr. Compos. 2020, 111, 103616. [Google Scholar] [CrossRef]
- El-Hassan, H.; Shao, Y. Early carbonation curing of concrete masonry units with Portland limestone cement. Cem. Concr. Compos. 2015, 62, 168–177. [Google Scholar] [CrossRef]
- Shi, W.; Wang, T.-Z.; Dong, Z.-H.; Guo, X.-P. Application of wire beam electrode technique to investigate themigrating behavior of corrosion inhibitors in mortar. Constr. Build. Mater. 2017, 134, 167–175. [Google Scholar] [CrossRef]
- Liu, J.; Geng, J.; Wang, H.; Ba, M.; He, Z. Influence of nitrite on chemical composition of passivation film of steel bars under the coupling effects of carbonization and chloride. Anti-Corros. Mater. Methods 2019, 6, 230–235. [Google Scholar] [CrossRef]
- Goyal, A.; Ganjian, E.; Pouya, H.S.; Tyrerd, M. Inhibitor efficiency of migratory corrosion inhibitors to reduce corrosion in reinforced concrete exposed to high chloride environment. Constr. Build. Mater. 2021, 303, 124461. [Google Scholar] [CrossRef]
- Li, Z.; Jin, Z.; Wang, P.; Zhao, T. Corrosion mechanism of reinforced bars inside concrete and relevant monitoring or detection apparatus: A review. Constr. Build. Mater. 2021, 279, 122432. [Google Scholar] [CrossRef]
- Wang, H.; Gao, X.; Liu, J. Effects of salt freeze-thaw cycles and cyclic loading on the piezoresistive properties of carbon nanofibers mortar. Constr. Build. Mater. 2018, 177, 192–201. [Google Scholar] [CrossRef]
- Rinaldi, Z.; Carlo, F.D.; Spagnuolo, S.; Meda, A. Influence of localised corrosion on the cyclic response of reinforced concrete columns. Eng. Struct. 2022, 256, 114037. [Google Scholar] [CrossRef]
- Ting, M.; Wong, K.; Rahman, M.; Joo, M. Mechanical and durability performance of marine sand and seawater concrete incorporating silicomanganese slag as coarse aggregate. Constr. Build. Mater. 2020, 254, 119195. [Google Scholar] [CrossRef]
- Li, L.; Hou, B.; Lu, Z.; Liu, F. Fatigue behaviour of sea sand concrete beams reinforced with basalt fibre-reinforced polymer bars. Constr. Build. Mater. 2018, 179, 160–171. [Google Scholar] [CrossRef]
- Xu, Q.; Ji, T.; Yang, Z.; Ye, Y. Steel rebar corrosion in artificial reef concrete with sulphoaluminate cement, sea water and marine sand. Constr. Build. Mater. 2019, 227, 116685. [Google Scholar] [CrossRef]
- Song, J.; Li, Y.; Xu, W.; Liu, H.; Xu, Y. Inexpensive and non-fluorinated superhydrophobic concrete coating for anti-icing and anti-corrosion. J. Colloid Interface Sci. 2019, 514, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Harb, M.B.; Abubshait, S.; Etteyeb, N.; Kamoun, M.; Dhouib, A. Olive leaf extract as a green corrosion inhibitor of reinforced concrete contaminated with seawater. Arab. J. Chem. 2020, 13, 4846–4856. [Google Scholar] [CrossRef]
- Jiang, C.; Wub, Y.; Dai, M. Degradation of steel-to-concrete bond due to corrosion. Constr. Build. Mater. 2018, 158, 1073. [Google Scholar] [CrossRef]
- Lei, L.; Wang, Q.; Xu, S.; Wang, N.; Zheng, X. Fabrication of superhydrophobic concrete used in marine environment with anti-corrosion and stable mechanical properties. Constr. Build. Mater. 2020, 251, 118946. [Google Scholar] [CrossRef]
- Liang, J.; Zhu, H.; Chen, L.; Qinglin, X.; Ying, G. Rebar corrosion investigation in rubberaggregate concrete via the chloride electro-accelerated test. Materials 2019, 12, 862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.; Wang, Q.; Xu, S.; Zheng, X. Eco-Friendly and safe method of fabricatingsuperhydrophobic surfaces on stainless steel substrates. J. Phys. Chem. C 2019, 123, 25738–25746. [Google Scholar] [CrossRef]
- Cui, L.; Wang, H. Research on the Mechanical Strengths and the Following Corrosion Resistance of Inner Steel Bars of RPC with Rice Husk Ash and Waste Fly Ash. Coatings 2021, 11, 1480. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, A.; Zhang, L.; Wang, Q.; Han, Y.; Liu, J.; Gao, X.; Shi, F.; Lin, X.; Feng, L. Hydration process of rice husk ash cement paste and the following corrosion resistance of embedded steel bar. J. Cent. South Univ. 2020, 11, 3464–3476. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, A.; Zhang, L.; Liu, J.; Han, Y.; Wang, J. Research on the Influence of Carbonation on the Content and State of Chloride Ions and the Following Corrosion Resistance of Steel Bars in Cement Paste. Coatings 2020, 10, 1071. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, A.; Zhang, L.; Liu, J.; Han, Y.; Shu, H.; Wang, J. Study on the influence of compound rust inhibitor on corrosion of steel bars in chloride concrete by electrical parameters. Constr. Build. Mater. 2020, 262, 120763. [Google Scholar] [CrossRef]
- Mahoutian, M.; Ghouleh, Z.; Shao, Y. Carbon dioxide activated ladle slag binder. Constr. Build. Mater. 2014, 66, 214–221. [Google Scholar] [CrossRef]
- Vogler, N.; Lindemann, M.; Drabetzki, P.; Kühne, H. Alternative pH-indicators for determination of carbonation depth on cement-based concretes. Cem. Concr. Compos. 2020, 109, 103565. [Google Scholar] [CrossRef]
- Villain, G.; Thiery, M.; Platret, G. Measurement methods of carbonation profiles in concrete: Thermogravimetry, chemical analysis and gammadensimetry. Cem. Concr. Res. 2007, 37, 1182–1192. [Google Scholar] [CrossRef]
- Dong, S.; Han, B.; Ou, J.; Li, Z.; Han, L.; Yu, X. Electrically conductive behaviors and mechanisms of short-cut super-fine stainless wire reinforced reactive powder concrete. Cem. Concr. Compos. 2016, 72, 48–65. [Google Scholar] [CrossRef] [Green Version]
- Bertos, M.F.; Simons, S.J.R.; Hills, C.D.; Careyet, P.J. A review of accelerated carbonation technology in the treatment of cement-based materials and sequestration of CO2. J. Hazard. Mater. 2004, 112, 193–205. [Google Scholar]
- Qin, L.; Gao, X.; Su, A.; Li, Q. Effect of carbonation curing on sulfate resistance of cement-coal gangue paste. J. Clean. Prod. 2021, 278, 123897. [Google Scholar] [CrossRef]
- Shi, X.; Fay, L.; Peterson, M.; Yang, Z. Freeze-thaw damage and chemical change of a Portland cement concrete in the presence of diluted deicers. Mater. Struct. 2010, 43, 933–946. [Google Scholar] [CrossRef]
- Zhang, D.; Shao, Y. Effect of early carbonation curing on chloride penetration and weathering carbonation in concrete. Constr. Build. Mater. 2016, 123, 516–526. [Google Scholar] [CrossRef]
- Hong, X.; Wang, H.; Shi, F. Influence of NaCl freeze thaw cycles and cyclic loading on the mechanical performance and permeability of sulphoaluminate cement reactive powder concrete. Coatings 2020, 10, 1227. [Google Scholar] [CrossRef]
- Sun, L.; Jiang, K.; Zhu, X.; Xu, L. An alternating experimental study on the combined effect of freeze-thaw and chloride penetration in concrete. Constr. Build. Mater. 2020, 252, 119025. [Google Scholar] [CrossRef]
- Wang, H.; Gao, X.; Liu, J. Coupling effect of salt freeze-thaw cycles and cyclic loading on performance degradation of carbon nanofiber mortar. Cold Reg. Sci. Technol. 2018, 154, 95–102. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, H. Influence of NaCl Freeze–thaw Cycles on the Mechanical Strength of Reactive Powder Concrete with the Assembly Unit of Sulphoaluminate Cement and Ordinary Portland Cement. Coatings 2021, 11, 1238. [Google Scholar] [CrossRef]
- Huang, G.; Wang, H.; Shi, F. Coupling Effect of Salt Freeze-thaw Cycles and Carbonation on the Mechanical Performance of Quick Hardening Sulphoalu-minate Cement-based Reactive Powder Concrete with Basalt Fibers. Coatings 2021, 11, 1142. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, A.; Li, K.; Wang, Q.; Liu, J.; Wang, H. Inflfluence of Rust Inhibitor on the Corrosion Resistance of Reinforcement in Cement Paste with Chloride. Coatings 2021, 11, 11050606. [Google Scholar]
- Wang, H.; Shi, F.; Shen, J.; Zhang, A.; Zhang, L.; Huang, H.; Liu, J.; Jin, K.; Feng, L.; Tang, Z. Research on the self-sensing and mechanical properties of aligned stainless steel fiber reinforced reactive powder concrete. Cem. Concr. Compos. 2021, 119, 104001. [Google Scholar] [CrossRef]
- Castellote, M.; Andrade, C.; Turrillas, X.; Campo, J.; Cuello, G.J. Accelerated carbonation of cement pastes in situ monitored by neutron diffraction. Cem. Concr. Res. 2008, 38, 1365–1373. [Google Scholar] [CrossRef]
- James, A.; Bazarchi, E.; Chiniforush, A.; Aghdam, P.P.; Hosseini, M.R.; Akbarnezhad, A.; Martek, I.; Ghodoosi, F. Rebar corrosion detection, protection, and rehabilitation of reinforced concrete structures in coastal environments: A review. Constr. Build. Mater. 2019, 224, 1026–1039. [Google Scholar] [CrossRef]
- Huijgen, W.J.; Witkamp, G.J.; Comans, R.N. Mechanisms of aqueous wollastonite carbonation as a possible CO2 sequestration process. Chem. Eng. Sci. 2006, 61, 4242–4251. [Google Scholar] [CrossRef]
- Velazco, G.; Almanza, J.M.; Cortés, D.A.; Escobedo, J.C.; Escalante-Garcia, J.I. Effect of citric acid and the hemihydrate amount on the properties of a calcium sulphoaluminate cement. Mater. Constr. 2014, 64, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.; Li, M.; He, X.; Su, Y.; Zhang, J.; Pan, H.; Yang, J.; Wang, Y. Preparation for micro-lithium slag via wet grinding and its application as accelerator in Portland cement. J. Clean. Prod. 2020, 250, 119528. [Google Scholar] [CrossRef]
- Gong, Y.; Wu, X.; Dong, Y.; Liu, Q.; Li, Z.; Wang, G. Cathodic photoelectrochemical immunoassay based on glucose-oxidase mediated biocatalysis to inhibit the exciton trapping of cupric ions for PbS quantum dots. Sens. Actuators B. Chem. 2018, 266, 408–415. [Google Scholar] [CrossRef]
- Zhang, L.; Han, B.; Ouyang, J.; Yu, X.; Sun, S.; Ou, J. Multifunctionality of cement based composite with electrostatic self-assembled CNT/NCB composite filler. Arch. Civ. Mech. Eng. 2017, 17, 354–364. [Google Scholar] [CrossRef]
- Zhang, D.; Shao, Y. Surface scaling of CO2-cured concrete exposed to freeze-thaw cycles. J. CO2 Util. 2018, 27, 137–144. [Google Scholar] [CrossRef]
- Ben Mansour, H.; Dhouibi, L.; Idrissi, H. Effffect of phosphate-based inhibitor on prestressing tendons corrosion in simulated concrete pore solution contaminated by chloride ions. Constr. Build. Mater. 2018, 171, 250–260. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, A.; Zhang, L.; Wang, Q.; Yang, X.-H.; Gao, X.; Shi, F. Electrical and piezoresistive properties of carbon nanofifiber cement mortar under difffferent temperatures and water contents. Constr. Build. Mater. 2020, 265, 120740. [Google Scholar] [CrossRef]
- Chi, L.; Wang, Z.; Lu, S.; Zhao, D.; Yao, Y. Development of mathematical models for predicting the compressive strength and hydration process using the EIS impedance of cementitious materials. Constr. Build. Mater. 2019, 208, 659–668. [Google Scholar] [CrossRef]







| Types | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | SO3 | Loss on Ignition |
|---|---|---|---|---|---|---|---|
| P·O cement | 20.86 | 5.47 | 3.94 | 1.73 | 62.23 | 2.66 | 3.11 |
| Standard sand | 99.2 | 0 | 0.42 | 0 | 0 | 0 | 0.38 |
| Types | Particle Size/μm | ||||||
|---|---|---|---|---|---|---|---|
| 0.3 | 0.6 | 1 | 4 | 8 | 64 | 360 | |
| P·O cement | 0 | 0.33 | 2.66 | 15.01 | 28.77 | 93.59 | 100 |
| Water | Cement | Sand |
|---|---|---|
| 157.9 | 526.3 | 1315.8 |
| 210.5 | 526.3 | 1315.8 |
| 263.2 | 526.3 | 1315.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Liu, S.; Song, L.; Qu, Z.; Wang, H. Influence of Carbon Dioxide Curing on the Corrosion Resistance of Reinforced Cement Mortar under the External Erosion of NaCl Freeze–Thaw Cycle. Appl. Sci. 2022, 12, 5061. https://doi.org/10.3390/app12105061
Zhu J, Liu S, Song L, Qu Z, Wang H. Influence of Carbon Dioxide Curing on the Corrosion Resistance of Reinforced Cement Mortar under the External Erosion of NaCl Freeze–Thaw Cycle. Applied Sciences. 2022; 12(10):5061. https://doi.org/10.3390/app12105061
Chicago/Turabian StyleZhu, Jing, Shaotong Liu, Lizhuo Song, Zijian Qu, and Hui Wang. 2022. "Influence of Carbon Dioxide Curing on the Corrosion Resistance of Reinforced Cement Mortar under the External Erosion of NaCl Freeze–Thaw Cycle" Applied Sciences 12, no. 10: 5061. https://doi.org/10.3390/app12105061
APA StyleZhu, J., Liu, S., Song, L., Qu, Z., & Wang, H. (2022). Influence of Carbon Dioxide Curing on the Corrosion Resistance of Reinforced Cement Mortar under the External Erosion of NaCl Freeze–Thaw Cycle. Applied Sciences, 12(10), 5061. https://doi.org/10.3390/app12105061







