A Pilot Study of whether or Not Vegetable and Fruit Juice Containing Lactobacillus paracasei Lowers Blood Lipid Levels and Oxidative Stress Markers in Thai Patients with Dyslipidemia: A Randomized Controlled Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Probiotics Lactobacillus paracasei
2.3. Preparation of Vegetable and Fruit Juice (VFJ) with and without Probiotic Lactobacillus paracasei
2.4. Determination of Bioactive Compounds and Nutritions in Vegetable and Fruit Juice (VFJ)
2.5. Ethical Considerations
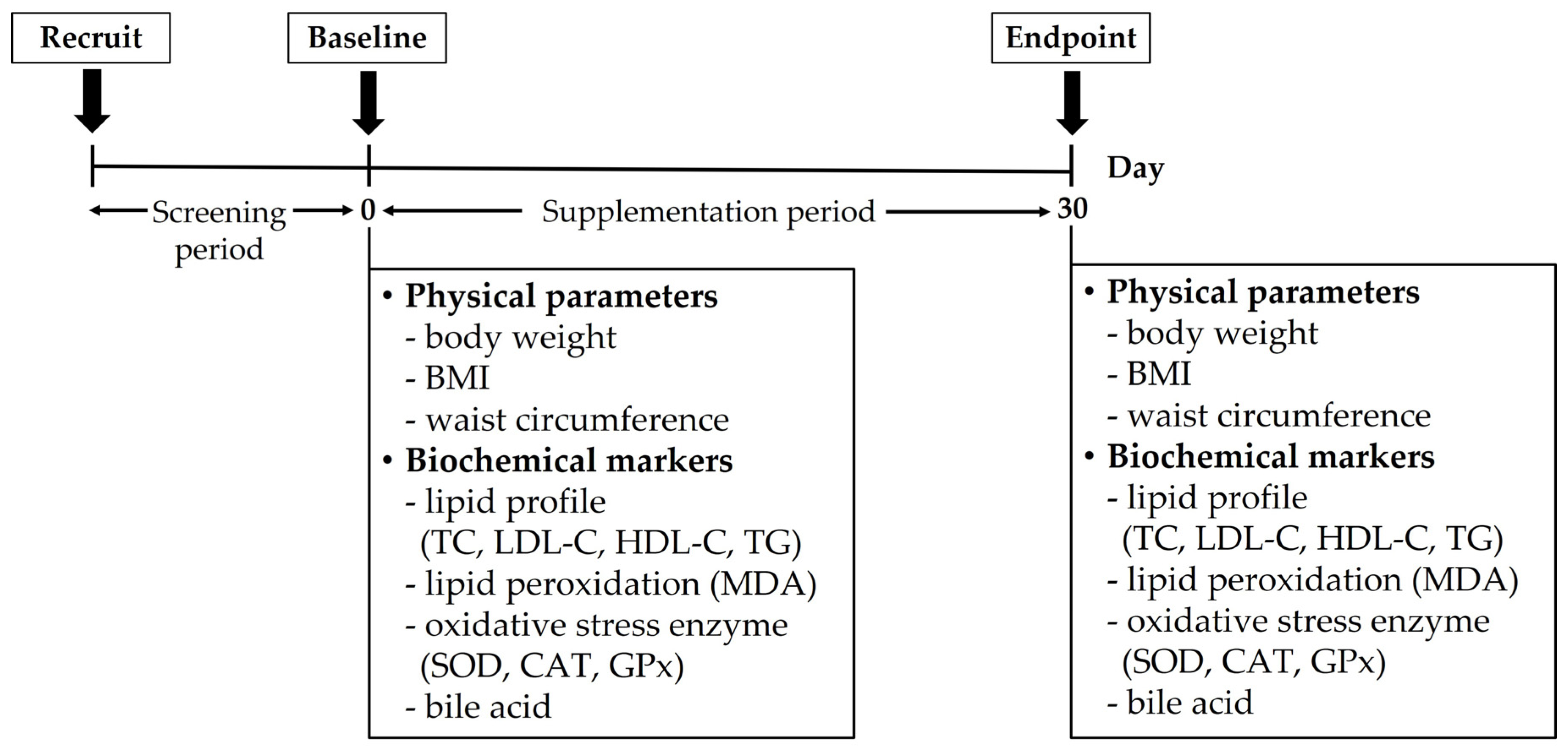
2.6. Participants and Study Design
2.7. Outcome Assessment
2.7.1. Physical Examination
2.7.2. Lipid Profile
2.7.3. Lipid Peroxidation
2.7.4. Oxidative Stress Enzymes
2.7.5. Bile Acid
2.8. Statistical Analysis
3. Results
3.1. Nutrition Information of the Vegetable and Fruit Juice (VFJ)
3.2. Participant Characteristics
3.3. Effect of the Vegetable and Fruit Juice (VFJ) with and without Probiotic L. paracasei on Physical Parameters
3.4. Effect of the Vegetable and Fruit Juice (VFJ) with and without Probiotic L. paracasei on Lipid Profile
3.5. Effect of the Vegetable and Fruit Juice (VFJ) with and without Probiotic L. paracasei on Lipid Peroxidation
3.6. Effect of the Vegetable and Fruit Juice (VFJ) with and without Probiotic L. paracasei on Oxidative Stress Enzyme
3.7. Effect of the Vegetable and Fruit Juice (VFJ) with and without Probiotic L. paracasei on Bile Acids Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shenoy, C.; Shenoy, M.M.; Rao, G.K. Dyslipidemia in dermatological disorders. N. Am. J. Med. Sci. 2015, 7, 421. [Google Scholar] [CrossRef] [Green Version]
- Narindrarangkura, P.; Bosl, W.; Rangsin, R.; Hatthachote, P. Prevalence of dyslipidemia associated with complications in diabetic patients: A nationwide study in Thailand. Lipids Health Dis. 2019, 18, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lertwanichwattana, T.; Rangsin, R.; Sakboonyarat, B. Prevalence and associated factors of uncontrolled hyperlipidemia among Thai patients with diabetes and clinical atherosclerotic cardiovascular diseases: A cross-sectional study. BMC Res. Notes 2021, 14, 118. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diet, Nutrition, and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation (Vol. 916); World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Reiner, Z.; Catapano, A.L.; De Backer, G.; Graham, I.; Taskinen, M.R.; Wiklund, O.; Agewall, S.; Alegria, E.; Chapman, M.J.; Durrington, P.; et al. ESC/EAS Guidelines for the management of dyslipidaemias: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur. Heart J. 2011, 32, 1769–1818. [Google Scholar] [PubMed] [Green Version]
- Kelly, R.B. Diet and exercise in the management of hyperlipidemia. Am. Fam. Physician 2010, 81, 1097–1102. [Google Scholar] [PubMed]
- Suwimol, S.; Pimpanit, L.; Aporn, M.; Pichita, S.; Ratiyaporn, S.; Wiroj, J. Impact of fruit and vegetables on oxidative status and lipid profiles in healthy individuals. Food Public Health 2012, 2, 113–118. [Google Scholar] [CrossRef] [Green Version]
- Jideani, A.I.; Silungwe, H.; Takalani, T.; Omolola, A.O.; Udeh, H.O.; Anyasi, T.A. Antioxidant-rich natural fruit and vegetable products and human health. Int. J. Food Prop. 2021, 24, 41–67. [Google Scholar] [CrossRef]
- Van Duyn, M.A.S.; Pivonka, E. Overview of the health benefits of fruit and vegetable consumption for the dietetics professional: Selected literature. J. Am. Diet. Assoc. 2000, 100, 1511–1521. [Google Scholar] [CrossRef]
- Maron, D.J. Flavonoids for reduction of atherosclerotic risk. Curr. Atheroscler. Rep. 2004, 6, 73–78. [Google Scholar] [CrossRef]
- Liu, R.H. Health-promoting components of fruits and vegetables in the diet. Adv. Nutr. 2013, 4, 384S–392S. [Google Scholar] [CrossRef]
- Guan, Q.; Xiong, T.; Xie, M. Influence of probiotic fermented fruit and vegetables on human health and the related industrial development trend. Engineering 2021, 7, 212–218. [Google Scholar] [CrossRef]
- Tsuda, H.; Miyamoto, T. Production of Exopolysaccharide by Lactobacillus plantarum and the Prebiotic Activity of the Exopolysaccharide. Food Sci. Technol. Res. 2010, 16, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Markowiak, P.; Śliżewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.H.; Lu, T.Y.; Tseng, Y.Y.; Pan, T.M. The effects of Lactobacillus-fermented milk on lipid metabolism in hamsters fed on high-cholesterol diet. Appl. Microbiol. Biotechnol. 2006, 71, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Dehkohneh, A.; Jafari, P.; Fahimi, H. Effects of probiotic Lactobacillus paracasei TD3 on moderation of cholesterol biosynthesis pathway in rats. Iran. J. Basic Med. Sci. 2019, 22, 1004. [Google Scholar]
- Saenjum, C.; Chaiyasut, C.; Chansakaow, S.; Suttajit, M.; Sirithunyalug, B. Antioxidant and anti-inflammatory activities of gamma-oryzanol rich extracts from Thai purple rice bran. J. Med. Plant Res. 2012, 6, 1070–1077. [Google Scholar]
- Zeb, A.; Ullah, F. A simple spectrophotometric method for the determination of thiobarbituric acid reactive substances in fried fast foods. J. Anal. Methods Chem. 2016, 2016, 9412767. [Google Scholar] [CrossRef] [Green Version]
- Atasayar, S.; Orhan, H.; Özgüneş, H. Malondialdehyde quantification in blood plasma of tobacco smokers and non-smokers. FABAD J. Pharm. Sci. 2004, 29, 15–19. [Google Scholar]
- Kaya, S.; Sütçü, R.; Cetin, E.S.; Arıdogan, B.C.; Delibaş, N.; Demirci, M. Lipid peroxidation level and antioxidant enzyme activities in the blood of patients with acute and chronic fascioliasis. Int. J. Infect. Dis. 2007, 11, 251–255. [Google Scholar] [CrossRef] [Green Version]
- Rush, J.W.; Sandiford, S.D. Plasma glutathione peroxidase in healthy young adults: Influence of gender and physical activity. Clin. Biochem. 2003, 36, 345–351. [Google Scholar] [CrossRef]
- Vijayvargiya, P.; Camilleri, M.; Shin, A.; Saenger, A. Methods for diagnosis of bile acid malabsorption in clinical practice. Clin. Gastroenterol. Hepatol. 2013, 11, 1232–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khutami, C.; Sumiwi, S.A.; Khairul Ikram, N.K.; Muchtaridi, M. The Effects of Antioxidants from Natural Products on Obesity, Dyslipidemia, Diabetes and Their Molecular Signaling Mechanism. Int. J. Mol. Sci. 2022, 23, 2056. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, L.; Rosner, B.; Willett, W.W.; Sacks, F.M. Cholesterol-lowering effects of dietary fiber: A meta-analysis. Am. J. Clin. Nutr. 1999, 69, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Kleniewska, P.; Hoffmann, A.; Pniewska, E.; Pawliczak, R. The influence of probiotic Lactobacillus casei in combination with prebiotic inulin on the antioxidant capacity of human plasma. Oxid. Med. Cell. Longev. 2016, 2016, 1340903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surampudi, P.; Enkhmaa, B.; Anuurad, E.; Berglund, L. Lipid lowering with soluble dietary fiber. Curr. Atheroscler. Rep. 2016, 18, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, M.C.; Lajo, T.; Carrión, J.M.; Cuné, J. Cholesterol-lowering efficacy of Lactobacillus plantarum CECT 7527, 7528 and 7529 in hypercholesterolaemic adults. Br. J. Nutr. 2013, 109, 1866–1872. [Google Scholar] [CrossRef] [Green Version]
- Ahn, H.Y.; Kim, M.; Chae, J.S.; Ahn, Y.T.; Sim, J.H.; Choi, I.D.; Lee, S.H.; Lee, J.H. Supplementation with two probiotic strains, Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032, reduces fasting triglycerides and enhances apolipoprotein AV levels in non-diabetic subjects with hypertriglyceridemia. Atherosclerosis 2015, 241, 649–656. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, T.; Xu, C.; Yang, H.; Zhang, T.; Liu, Y. Probiotics can further reduce waist circumference in adults with morbid obesity after bariatric surgery: A systematic review and meta-analysis of randomized controlled trials. Evid. Based Complement. Altern. Med. 2021, 2021. [Google Scholar] [CrossRef]
- Michael, D.R.; Jack, A.A.; Masetti, G.; Davies, T.S.; Loxley, K.E.; Kerry-Smith, J.; Plummer, J.F.; Marchesi, J.R.; Mullish, B.H.; McDonald, J.A.K.; et al. A randomised controlled study shows supplementation of overweight and obese adults with lactobacilli and bifidobacteria reduces bodyweight and improves well-being. Sci. Rep. 2020, 10, 4183. [Google Scholar] [CrossRef]
- Ooi, L.G.; Liong, M.T. Cholesterol-lowering effects of probiotics and prebiotics: A review of in vivo and in vitro findings. Int. J. Mol. Sci. 2010, 11, 2499–2522. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Nagpal, R.; Kumar, R.; Hemalatha, R.; Verma, V.; Kumar, A.; Chakraborty, C.; Singh, B.; Marotta, F.; Jain, S.; et al. Cholesterol-lowering probiotics as potential biotherapeutics for metabolic diseases. Exp. Diabetes Res. 2012, 2012, 902917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamadshahi, M.; Veissi, M.; Haidari, F.; Javid, A.Z.; Mohammadi, F.; Shirbeigi, E. Effects of probiotic yogurt consumption on lipid profile in type 2 diabetic patients: A randomized controlled clinical trial. J. Res. Med. Sci. 2014, 19, 531. [Google Scholar] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Du, R.; Wang, L.; Zhang, H. The antioxidative effects of probiotic Lactobacillus casei Zhang on the hyperlipidemic rats. Eur. Food Res. Technol. 2010, 231, 151–158. [Google Scholar] [CrossRef]
- Chamari, M.; Djazayery, A.; Jalali, M.; Sadrzadeh yeganeh, H.; Hosseini, S.; Heshmat, R.; Behbahani Haeri, B. The effect of daily consumption of probiotic and conventional yogurt on some oxidative stress factors in plasma of young healthy women. ARYA Atheroscler. 2008, 4, 175–179. [Google Scholar]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant properties of probiotic bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human nutrition, the gut microbiome, and immune system: Envisioning the future. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Chiang, J.Y. Bile acid metabolism and signaling. Compr Physiol. 2013, 3, 1191–1212. [Google Scholar]
- Di Ciaula, A.; Garruti, G.; Lunardi, B.R.; Molina-Molina, E.; Bonfrate, L.; Wang, D.Q.H. Bile Acid Physiology. Ann. Hepatol. 2017, 16, S4–S14. [Google Scholar] [CrossRef]
- Bourgin, M.; Kriaa, A.; Mkaouar, H.; Mariaule, V.; Jablaoui, A.; Maguin, E.; Rhimi, M. Bile Salt Hydrolases: At the Crossroads of Microbiota and Human Health. Microorganisms 2021, 9, 1122. [Google Scholar] [CrossRef] [PubMed]
- Luz, P.L.D.; Favarato, D.; Faria-Neto Junior, J.R.; Lemos, P.; Chagas, A.C.P. High ratio of triglycerides to HDL-cholesterol predicts extensive coronary disease. Clinics 2008, 63, 427–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Ingredients | Content (% w/w) |
|---|---|
| Green lettuce | 7 |
| Chinese celery | 0.5 |
| Cherry tomato | 15 |
| Onion | 3 |
| Apple | 20 |
| Lime juice | 3 |
| Honey | 10 |
| Fresh water | 41.5 |
| Nutrition | Amount (Per 100 mL) |
|---|---|
| Energy | 43.08 kcal |
| Moisture | 91.92 g |
| Protein | 0.43 g |
| Total Carbohydrate | 10.34 g |
| Total dietary fiber | 1.35 g |
| Soluble dietary fiber | 0.71 g |
| Insoluble dietary fiber | 0.65 g |
| Vitamin C | 3.06 mg |
| Calcium | 8.99 mg |
| Phosphorus | 27.97 mg |
| Sodium | 14.73 mg |
| Potassium | 64.94 mg |
| Magnesium | 3.97 mg |
| Iron | 0.51 mg |
| Zinc | 0.06 mg |
| Copper | 0.02 mg |
| Chloride | 9.39 mg |
| Pantothenic acid | 0.04 mg |
| Total polyphenol | 41.99 mg eq GA 1 |
| Naringenin | 636.82 µg |
| Quercetin | 1088.34 µg |
| Lutein | 48.76 µg |
| Lycopene | 239.10 µg |
| Parameters | Placebo Group | Probiotic Group | p-Value |
|---|---|---|---|
| Male, n (%) | 1 (10) | 2 (20) | 1.000 |
| Female, n (%) | 9 (90) | 8 (80) | |
| Age (years) | 44.40 ± 2.40 | 43.80 ± 2.07 | 0.852 |
| Weight (kg) | 62.62 ± 3.20 | 65.01 ± 2.53 | 0.566 |
| Height (cm) | 157.40 ± 1.69 | 157.50 ± 1.87 | 0.969 |
| BMI (kg/m2) | 25.36 ± 1.44 | 26.25 ± 1.06 | 0.625 |
| WC (cm) | 81.50 ± 2.46 | 86.00 ± 3.03 | 0.264 |
| LDL-C (mg/dL) | 145.20 ± 3.93 | 146.60 ± 3.54 | 0.794 |
| Parameters | Placebo Group | Probiotic Group | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Endpoint | % Change | p-Value | Baseline | Endpoint | % Change | p-Value | |
| BW (kg) | 62.62 ± 3.20 | 62.08 ± 3.15 | −0.86 | 0.255 | 65.01 ± 2.53 | 64.57 ± 2.57 | −0.68 | 0.192 |
| BMI (kg/m2) | 25.36 ± 1.43 | 25.16 ± 1.43 | −0.79 | 0.281 | 26.25 ± 1.06 | 26.06 ± 1.04 | −0.72 | 0.163 |
| WC (cm) | 81.50 ± 2.46 | 81.10 ± 2.26 | −0.49 | 0.343 | 86.00 ± 3.02 | 85.20 ± 3.02 | −0.93 | 0.087 * |
| TC (mg/dL) | 202.60 ± 9.46 | 203.00 ± 6.40 | 0.20 | 0.955 | 203.80 ± 5.11 | 192.40 ± 4.53 | −5.59 | 0.041 ** |
| LDL-C (mg/dL) | 145.20 ± 3.93 | 145.80 ± 4.97 | 0.41 | 0.818 | 146.60 ± 3.54 | 137.80 ± 4.04 | −6.00 | 0.003 *** |
| HDL-C (mg/dL) | 51.40 ± 2.87 | 50.80 ± 2.56 | −1.17 | 0.712 | 49.20 ± 2.53 | 53.30 ± 2.36 | 8.33 | 0.009 *** |
| TG (mg/dL) | 122.40 ± 12.84 | 101.80 ± 8.39 | −16.83 | 0.111 | 121.80 ± 11.59 | 92.40 ± 9.83 | −24.14 | 0.029 ** |
| TG/HDL-C ratio | 2.48 ± 0.32 | 2.06 ± 0.21 | −16.93 | 0.160 | 2.53 ± 0.27 | 1.75 ± 0.18 | −30.83 | 0.016 ** |
| MDA (µM) | 0.18 ± 0.07 | 0.07 ± 0.01 | −61.11 | 0.147 | 0.19 ± 0.07 | 0.06 ± 0.01 | −68.42 | 0.067 * |
| CAT (unit/mL) | 159.08 ± 3.97 | 163.39 ± 5.43 | 2.71 | 0.542 | 153.60 ± 2.89 | 166.00 ± 4.36 | 8.07 | 0.005 *** |
| GPx (unit/mL) | 0.61 ± 0.11 | 0.78 ± 0.08 | 27.87 | 0.196 | 0.63 ± 0.06 | 0.83 ± 0.13 | 31.75 | 0.060 * |
| SOD (unit/mL) | 588.84 ± 62.54 | 643.14 ±40.22 | 9.22 | 0.350 | 562.41 ± 73.44 | 678.92 ± 51.68 | 20.71 | 0.115 |
| BA (µmol/L) | 30.06 ± 3.65 | 29.29 ± 2.16 | −2.56 | 0.834 | 31.60 ± 2.90 | 37.73 ± 2.48 | 19.40 | 0.054 * |
| Parameters | Coefficient | 95% CI | p-Value |
|---|---|---|---|
| BW (kg) | 2.49 | −1.24 to 1.31 | 0.953 |
| BMI (kg/m2) | 0.90 | −0.52 to 0.51 | 0.985 |
| WC (cm) | 4.10 | −1.40 to 1.24 | 0.898 |
| TC (mg/dL) | −10.60 | −23.05 to 2.48 | 0.106 |
| LDL-C (mg/dL) | −8.00 | −23.42 to 6.78 | 0.259 |
| HDL-C (mg/dL) | 2.50 | −0.02 to 7.37 | 0.051 * |
| TG (mg/dL) | −9.40 | −35.10 to 15.44 | 0.420 |
| TG/HDL-C ratio | −0.31 | −0.86 to 0.26 | 0.270 |
| MDA (µM) | −0.01 | −0.04 to 0.03 | 0.825 |
| CAT (unit/mL) | 2.61 | −10.82 to 22.17 | 0.475 |
| GPx (unit/mL) | 0.05 | −0.29 to 0.30 | 0.980 |
| SOD (unit/mL) | 35.78 | −101.65 to 160.33 | 0.640 |
| BA (µmol/L) | 8.44 | 1.52 to 15.15 | 0.020 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siripun, P.; Chaiyasut, C.; Lailerd, N.; Makhamrueang, N.; Kaewarsar, E.; Sirilun, S. A Pilot Study of whether or Not Vegetable and Fruit Juice Containing Lactobacillus paracasei Lowers Blood Lipid Levels and Oxidative Stress Markers in Thai Patients with Dyslipidemia: A Randomized Controlled Clinical Trial. Appl. Sci. 2022, 12, 4913. https://doi.org/10.3390/app12104913
Siripun P, Chaiyasut C, Lailerd N, Makhamrueang N, Kaewarsar E, Sirilun S. A Pilot Study of whether or Not Vegetable and Fruit Juice Containing Lactobacillus paracasei Lowers Blood Lipid Levels and Oxidative Stress Markers in Thai Patients with Dyslipidemia: A Randomized Controlled Clinical Trial. Applied Sciences. 2022; 12(10):4913. https://doi.org/10.3390/app12104913
Chicago/Turabian StyleSiripun, Pattharaparn, Chaiyavat Chaiyasut, Narissara Lailerd, Netnapa Makhamrueang, Ekkachai Kaewarsar, and Sasithorn Sirilun. 2022. "A Pilot Study of whether or Not Vegetable and Fruit Juice Containing Lactobacillus paracasei Lowers Blood Lipid Levels and Oxidative Stress Markers in Thai Patients with Dyslipidemia: A Randomized Controlled Clinical Trial" Applied Sciences 12, no. 10: 4913. https://doi.org/10.3390/app12104913
APA StyleSiripun, P., Chaiyasut, C., Lailerd, N., Makhamrueang, N., Kaewarsar, E., & Sirilun, S. (2022). A Pilot Study of whether or Not Vegetable and Fruit Juice Containing Lactobacillus paracasei Lowers Blood Lipid Levels and Oxidative Stress Markers in Thai Patients with Dyslipidemia: A Randomized Controlled Clinical Trial. Applied Sciences, 12(10), 4913. https://doi.org/10.3390/app12104913







