Abstract
COVID-19 is a respiratory infectious disease that spreads readily between people, and an urgent issue of passengers’ exposure risk assessment in commercial aircraft has been raised because an aircraft cabin as a confined space may carry and transmit the disease worldwide. In this study, the droplets transmission process under different ventilation systems in a twin-aisle wide-body aircraft was studied using CFD simulations and the infection risk of passengers was assessed by the improved Wells–Riley model. Numerical results found that the transmission mechanism of droplets in the aircraft cabin was different depending on the type of ventilation systems and the location of the infectious source. Annular airflow could effectively enhance the ability of droplets transmission, while direct airflow, represented by displacement ventilation, could significantly inhibit droplets transmission. Accordingly, a new type of ventilation system was proposed based on the concept that the overall space is organized by annular airflow and the local area is direct airflow. Compared with sidewall mixing ventilation system, the infection risk of the new ventilation system presented in this study is reduced by 27%.
1. Introduction
In 1897, Flugge [1] discovered that the droplets produced by human respiratory behavior carried bacteria, which explained the possibility that respiratory infectious diseases could spread through the air. In recent years, various respiratory infectious diseases, such as severe acute respiratory syndrome (SARS), influenza A (H1N1) and tuberculosis (TB), have been threatening public health. Novel coronavirus (COVID-19) has spread rapidly all over the world, and the virus is highly contagious. Compared with previous infectious diseases, it affects a wider area and has more infected people, which has attracted the attention of WHO [2]. The aircraft cabin environment with confined enclosure and high occupant densities increases the risk of infectious diseases and enhances the ability of spreading the epidemic around the world. There is a typical case. On an international aircraft from Britain to Vietnam, there was a super disseminator infecting fourteen passengers and one crew member on the same aircraft. Therefore, it is essential to study the droplets transmission mechanism in the aircraft cabin.
Air distribution is the most important influencing factor of droplets transmission mechanism. At present, the influence of different ventilations on droplets transmission has been fully studied by researchers under indoor environment [3,4,5], and some important conclusions have been drawn. As for transportation tools, many scholars have carried out related research on droplets transmission in coach buses [6] and high-speed rails [7]. However, studies on droplets transmission mechanism in commercial aircraft cabin are relatively rare. Some contributions are focused on the phenomenon of droplets transmission under the existing ventilation system. Mangili et al. [8] point out that researchers could gain a deeper understanding of disease transmission in the aircraft cabin through a mathematical model of infection risk. Yin et al. [9] performed retrospective analysis on a tuberculosis epidemic on a commercial aircraft and the infection risks of the passengers were assessed using the dose–response model. Gupta [10] computed the transmission of the droplets exhaled by the infectious source seated in the middle of a seven-row twin-aisle cabin and found that the airflow pattern in the cabin played the most important role on the droplets transport. Sze-To [11] conducted some experiments to study the transmission of expiratory aerosols in an aircraft cabin mock-up. The study suggested that a higher supply airflow rate improved the dilution and reduced the infection risks of passengers seated close to the source, but the aerosol dispersion was enhanced. YAN et al. [12] studied the transmission characteristics of airborne droplets and the infection risks of passengers under the mixing ventilation system using a Boeing 737 cabin model. The study found that the spread of particles was related to the release locations. Then, Yan et al. [13,14] evaluated the effects of cough-jet on instantaneous cough contaminants transport characteristics in passenger’s local environments and found that cough flow could increase the residence times of contaminants. Additionally, the conclusion that the droplets released by passengers located in different positions have different effects on other passengers was re-emphasized. Liu et al. [15] found that the infection risks of passengers under displacement ventilation system was lower than that under mixing ventilation system and the infection risk could be reduced when passengers wore masks. In order to regulate the cabin environment on aircrafts, intervention measures [16] and innovative ventilation systems [17] were proposed. Unfortunately, Mboreha et al. did not consider the performance of the new ventilation system when the infectious source was located in different locations. In addition, the transmission of droplets of different sizes generated by talking, coughing and breathing activities in a cabin was also studied [18].
At present, there has been little research on reducing the infection risk of passengers by improving the ventilation system. Additionally, the air flow organization optimization aimed at improving the removal efficiency of pollutant and reducing pollutants in passengers’ breathing areas was mostly carried out by using gaseous pollutants representing droplets [19,20,21,22]. However, the density of droplets is usually several orders of magnitude higher than that of air. Therefore, gaseous pollutants cannot represent the transmission characteristics of droplets in the cabin.
Based on Lagrange method, the present study aims to obtain the dispersion process of coughed droplets in a commercial wide-body aircraft cabin and to understand the transport mechanism of the droplets. Three traditional ventilation systems and a personalized ventilation system are compared with the infection risk of passengers by the improved Wells–Riley model. Finally, the general airflow organization characteristics to reduce infection risk are given.
2. Methods
2.1. Model Description and Numerical Case Design
2.1.1. Cabin Ventilation Systems
The geometrical model used in this study is a twin-aisle wide-body aircraft cabin with seat distribution of 3-3-3. There are 6 rows with 54 seats in the cabin. The study involves three traditional ventilation systems and a personalized ventilation system. In the personalized ventilation system, a series of personalized exhaust diffusers are added on the back of passenger seats based on the sidewall mixing ventilation system. The schematic diagram of the specific ventilation system is shown in Figure 1.
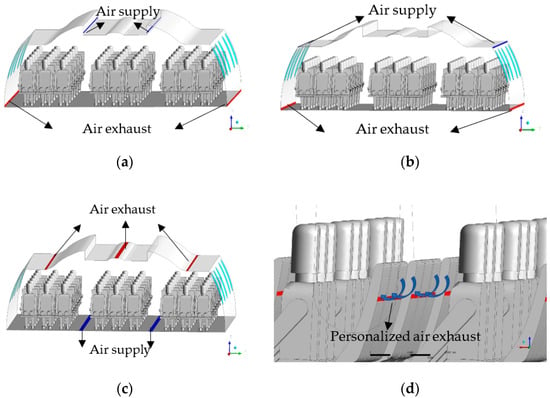
Figure 1.
Schematic of ventilation systems. (a) Ceiling mixing ventilation system (CMVS). (b) Sidewall mixing ventilation system (SMVS). (c) Displacement ventilation system (DVS). (d) Personalized ventilation system (PVS).
The parameters of air supply and exhaust diffusers of various ventilation systems are shown in Table 1.

Table 1.
Parameters of ventilation systems.
According to Reference [23], the passenger’s mouth as the inlet is 1.2 cm2 when breathing, and the inlet is simplified as a circle with a diameter of 1.24 cm, as shown in Figure 2. In order to assess the infection risk of passengers, a spherical breathing area with a radius of 20 cm is established around the face. Droplets inside the breathing area are regarded as particles that threaten passengers. In addition, the seat arrangement is marked as follows.
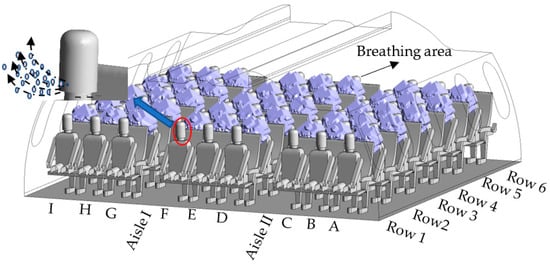
Figure 2.
Schematic of mouth and breathing area of passengers.
2.1.2. Boundary Conditions
Software ICEM 18.0 is used to build the model and the unstructured mesh is generated. A total of 14 million elements is chosen after the grid independence test. The boundary conditions of numerical simulation are depicted in Table 2. The air supply flow rate, air supply temperature and walls temperature are obtained through literature research [24,25,26,27,28]. It is worth noting that the recirculated air is filtered by absolutely perfect HEPA filters, which means that the air supply diffusers provide 100% fresh air. The size of droplets generated by respiration is in the submicron level [29,30,31,32,33]. In this paper, the size of droplets is set to 1 μm, and the concentration of droplets is 1000/L. It is assumed that the droplets exhaled by the infectious source are composed of water and sodium chloride, and the density of droplets are 1010 kg/m3. The droplets evaporate in the cabin and finally form the droplets nuclei.

Table 2.
Boundary conditions of numerical simulation.
2.2. Numerical Simulation Method
2.2.1. Turbulence Model
The RNG k-ε turbulence model is used to simulate the airflow organization inside the aircraft cabin in this paper. The specific expressions of the RNG k-ε turbulence model are depicted as follows.
where αk and αε are the inverse effective Prandtl numbers for k and ε, respectively, Gk represents the generation of turbulence kinetic energy due to the mean velocity gradients, Gb represents the generation of turbulence kinetic energy due to buoyancy, YM represents the contribution of the fluctuating dilatation in compressible turbulence to the overall dissipation rate, C1ε, C2ε and C3ε are constant, Sk and Sε are user-defined source terms, Rε represents the additional term of the RNG k-ε Model to Standard RNG Model and can be expressed as Equation (3).
where η = Sk/ε, η0 = 4.38 and β = 0.012.
In order to make the model better handle low-Reynolds and near-wall flows, Equation (4) is integrated to obtain an accurate description of how the effective turbulent transport varies with the effective Reynolds number.
where μt = μeff/μ, Cv ≈ 100.
2.2.2. Droplets Model
The temperature of droplets is determined by the heat balance in the evaporation process. When the influence of radiation is ignored, the heat balance equation of droplets is given as follows.
where mp is the mass of droplets, cp is the heat capacity of droplets, Tp is the temperature of droplets, hp is the convection heat transfer coefficient between droplets and air, Ap is the surface area of droplets, T is the temperature of local airflow and hfg is the latent heat.
The influence of convection cannot be ignored in the evaporation process since the evaporation rate of droplets entering the aircraft cabin is fast. Therefore, the Convection/Diffusion Controlled Model is selected as the mass transfer model, and the evaporation rate can be expressed as:
where kc is the mass transfer coefficient, ρ is the density of gas and Bm is given by:
where Cs is the mass fraction of water vapor on the particles surface and C∞ is the water vapor mass fraction in the mainstream.
The effects of gravity, Saffman force, drag force, buoyancy and thermophoresis force are considered in this paper. According to the force of droplets and Newton’s second law, the motion equation of the particle orbit can be established:
where mp is the mass of droplet, up is the motion velocity of droplet, FG is the gravity and buoyancy, FD is the drag force, FT is the thermophoresis force and FS is the Saffman force.
2.2.3. Algorithm Verification
The transmission characteristics of the droplets from coughing are studied in this paper and compared with the study by Liu et al. [35]. The comparison between the results in this paper and those in the literature is shown in Figure 3.
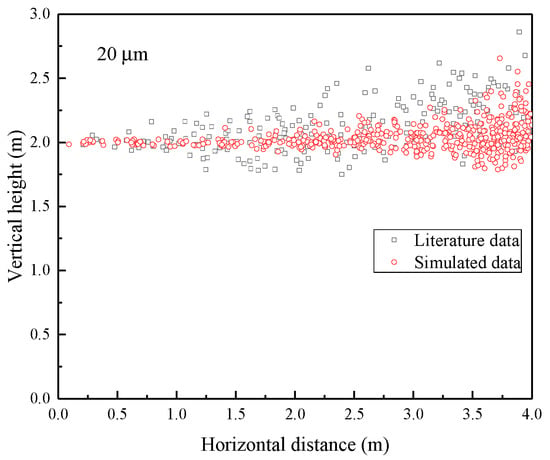
Figure 3.
Droplets dispersion profile of 20 μm droplets in a steady turbulent cough jet.
It can be seen from Figure 3 that the simulation results in this paper are in great agreement with the theoretical analysis results in the literature. When the particle size is 20 μm, the droplets always float in the air without settling. Additionally, the particles have obvious characteristics of diffusion along the streamlines.
2.3. Risk Assessment Model
In the field of epidemiology, disease transmission can be effectively predicted based on inhalation of infection risk models. We use the improved Wells–Riley model proposed by Zhai [36] to predict the infection risk in order to quantitatively analyze the probability of infection risk of passengers in an aircraft cabin. The total quanta inhaled per person during the exposure time te, N, is defined.
where n represents the transient inhaled number of quanta, C represents the mass concentration of pathogen, qs represents the inhalation rate of one susceptible person, qe represents the exhalation rate of the infectious source, σ represents the exhaled number of quanta per second, (x, y, z) represents any location in space and (x0, y0, z0) represents the spatial location of the infectious source.
The probability of infection (PI) can be expressed as follows.
3. Results and Discussion
3.1. Flow Fields of Different Ventilation Systems
The flow fields of different ventilation systems were analyzed and presented as shown in Figure 4.
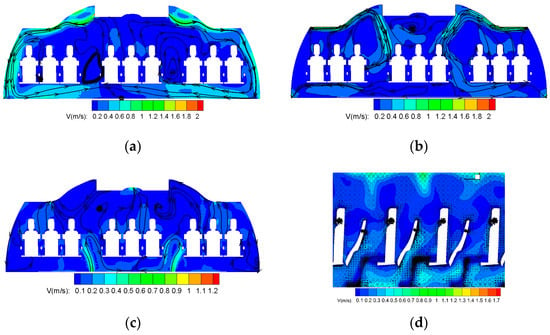
Figure 4.
Flow fields of different ventilation systems. (a) Flow field of CMVS. (b) Flow field of SMVS. (c) Flow field of DVS. (d) Flow field of PVS.
Figure 4a shows the cross-sectional velocity field of CMVS. There are two streams of strong airflow along the sidewalls from air supply diffusers in air distribution of CMVS. The two streams are influenced by the thermal plumes of the middle passenger on the way, converging in the middle of the cabin and rising. Thus, the two large scale symmetrical vortex formed on either side of the cabin. However, the two vortices generated in SMVS are opposite to those generated in CMVS. As shown in Figure 4b, the cold air comes out from the air supply diffusers and forms an attached jet flowing along the ceiling. Due to the obstruction of the luggage rack, the supply air loses a lot of kinetic energy and drops down. Then, because of the negative pressure in the exhaust, the airflow flows to the bottom of the cabin. In the process, one part of the airflow directly flows to the side exhaust diffusers and the other part of the airflow moves upward with the human thermal plume. Two clockwise vortices are generated in SMVS. Figure 4c shows the cross-sectional velocity field of DVS. It can be seen that the cold air supplied by the exhaust diffuser firstly rises to the height of the handrail, and then gathers in the passengers’ leg area due to its relatively large density. Driven by the thermal plume of passengers, the air rises again and finally exhausts by the outlets at the ceiling. Unlike CMVS and SMVS, there is no large-scale air vortex, and the air distribution mainly presents a straight flow. The air velocity in the whole aircraft cabin is extremely slow. In Figure 4d, since PVS is based on CMVS, the air distribution is almost similar to that of CMVS. The influence area of seat exhaust diffusers is concentrated in the microenvironment of the passengers. The negative pressure formed at the exhaust diffusers on the seat is conducive to more fresh air entering the microenvironment around the passengers and promoting the air ventilation.
3.2. Droplets Transmission of CMVS
Because the diffusion mechanism of droplet in different ventilation systems has some similarity, CMVS is taken as the representative to analyze its diffusion performance of droplets. Figure 5 indicates the droplets transmission with time in case of the patient appearing in a different location. In Figure 5a, the droplets in CMVS case A move forward with the respiratory flow within 1 s, and then move downward at the right side of the cabin with fresh air. A part of droplets is exhausted through the air exhaust diffusers and other part of droplets move to the interior of the cabin at the height of the passengers’ legs. Then, under the combined mechanism of the thermal plume of the middle passengers and the local airflow in the cabin, the droplets diffuse to the ceiling. The droplets transmission is mainly concentrated at the right side of the cabin due to two large scale vortexes. As shown in Figure 5b, the droplets exhaled by patient B float up at the beginning because patient B is located in the vortex center. As shown in Figure 5c, the distribution of droplets exhaled by patient C is the same as that of case A at the initial time. As shown in Figure 5d, the distribution of droplets exhaled by patient D are the same as that of the droplets exhaled patient B at the initial time. However, the ability of the droplets exhaled by patient D move upward is stronger than that of case B. The main reason for the above phenomenon is that the droplets motion in case B is significantly affected by the interaction of the thermal plume and the air circulation in the cabin. In Figure 5e, the distribution of droplets exhaled by patient E are the same as that of case D at the initial time. However, since patient E is at the intersection of the two large vortex, the droplets do not immediately flow to the sidewalls of the cabin until Row 6.
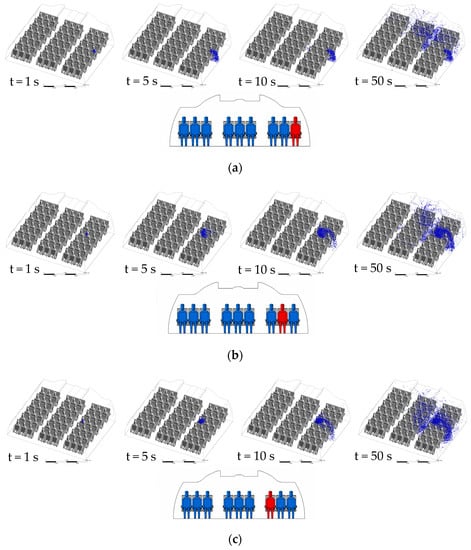
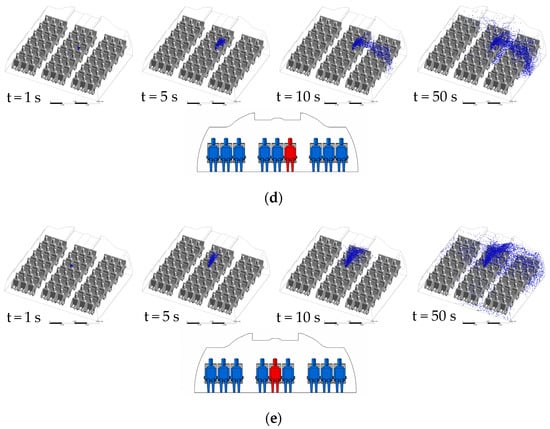
Figure 5.
Droplets transient transmission of CMVS: (a) case A—patient A in Row 4; (b) case B—patient B in Row 4; (c) case C—patient C in Row 4; (d) case D—patient D in Row 4; (e) case E—patient E in Row 4.
3.3. Droplets Infection Risk Assessment
3.3.1. CMVS
Figure 6 and Table 3 display the infection risks and intensity of CMVS in case of different infectious sources, respectively.
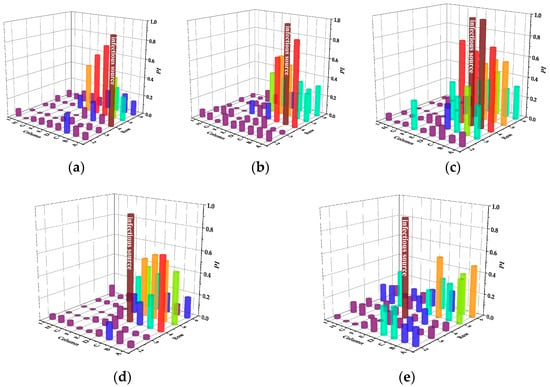
Figure 6.
Infection risks of CMVS in case of different infectious sources. (a) Case A. (b) Case B. (c) Case C. (d) Case D. (e) Case E.

Table 3.
Infection intensity of CMVS in case of different infectious sources.
As shown in Figure 6, the infection risk of the next two rows of passengers is relatively high in case A. The infection risk of passengers on Aisle II in the fifth row is greater than 0.5, which is higher than that of the passengers directly behind the infectious source. In case B, the two rows behind the infectious source also have a relatively high risk of infection, which is the same in case A. However, the two passengers in the same row of the infectious source have the highest risk in the cabin. In particular, the passenger near the window has the highest infection risk of 0.9. In case C, the infection risk of passengers in the same row is higher than that in case B and the infection risk of passenger B even reached 1.0. In case D, the infection risk between the same row is reduced because the exhaled droplets moved in the leg areas of passengers B and C in the same row. In case E, the infection risk in the same row is further reduced. In addition, different from the previous four cases, the passengers on both sides of the cabin behind the infectious source have the risk of infection. As shown in Table 3, case C is the least desirable condition of all, which is prone to infection throughout the cabin. It is worth noting that the number of these bullets is deduced from Figure 6 in the following way: 0 bullet/person (PI ≤ 0.3), 1 bullet/person (0.3 < PI ≤ 0.6), 2 bullets/person (0.6 < PI ≤ 0.8), 3 bullets/person (0.8 < PI ≤ 1), and then the sum of all the bullets for all people is the corresponding infection intensity.
3.3.2. SMVS
Figure 7 and Table 4 display the infection risks and intensity of SMVS in case of different infectious sources, respectively.
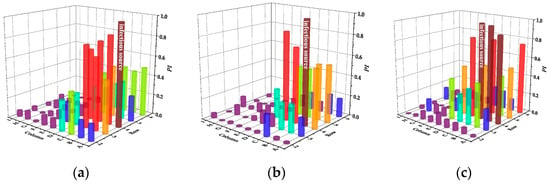
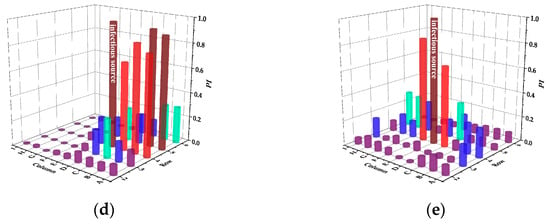
Figure 7.
Infection risks of SMVS in case of different infectious sources. (a) Case A. (b) Case B. (c) Case C. (d) Case D. (e) Case E.

Table 4.
Infection intensity of SMVS in case of different infectious sources.
In case A, passengers in the same row have a greater risk of infection and Passenger B in the same row is at the highest risk of 0.86. In addition, the droplets exhaled by passenger A not only affect the back-row passengers, but also spread to the front row, causing a large number of infected people. In case B, the infection intensity of the same row and the back row is reduced. However, compared with CMVS, the affected area of the same row increases. In case C, the infection range between the same rows is larger. In case D, the most susceptible person is passenger B in the five-row. Different from CMVS, only the two passengers in the adjacent seat of the infectious passenger are mainly at higher risk of infection in case E.
3.3.3. DVS
Figure 8 and Table 5 display the infection risks and intensity of DVS in case of different infectious sources, respectively.
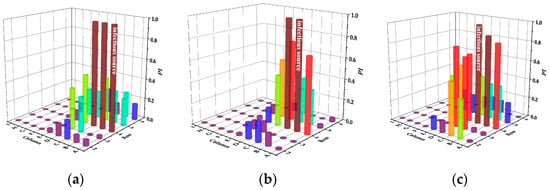
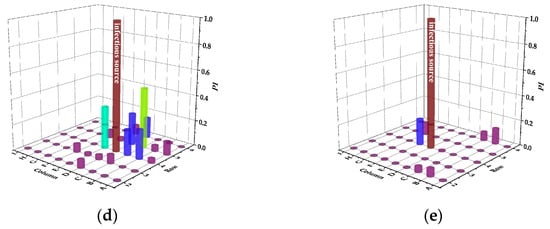
Figure 8.
Infection risks of DVS in case of different infectious sources. (a) Case A. (b) Case B. (c) Case C. (d) Case D. (e) Case E.

Table 5.
Infection intensity of DVS in case of different infectious sources.
In case A, the infection risk of passengers B and C are all 1.0, indicating that the adjacent passengers of the infectious passenger will be infected if they do not take additional protective measures. Although DVS increases the infection risk of passenger A to the other passengers in the same row, the risk of the passengers in the front and back rows is significantly reduced compared with the mixing ventilation system. In case B, the infection risk of adjacent passenger C is still 1.0, but the infection risk of passenger A is only 0.7. In case C, the infection risk of the passengers in the same row is still very high. Because the settlement effect of exhaled particles from passenger C is more significant than that from passenger B, more particles are dispersed to the right side of the cabin due to the obstruction of the seat. In case D and E, the number of passengers at risk of infection is greatly reduced, and the susceptible passengers is the safest in case E. Due to the direct airflow effect, the infection risk of passengers on the left side of the cabin is close to zero.
3.3.4. PVS
Figure 9 and Table 6 display the infection risks and intensity of PVS in case of different infectious sources, respectively.
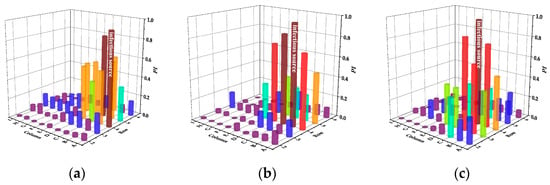
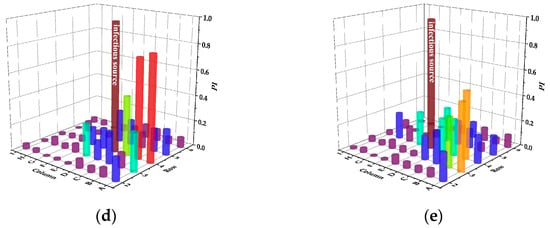
Figure 9.
Infection risks of PVS in case of different infectious sources. (a) Case A. (b) Case B. (c) Case C. (d) Case D. (e) Case E.

Table 6.
Infection intensity of PVS in case of different infectious sources.
In case A, the infection risk of passengers in the fourth row is basically the same as that in the fifth row and Passenger C in the fifth row is at the highest risk of 0.84. In case A and B, compared with SMVS, the infection risk of the front row is reduced. In case C, D and E, although the infection risk of the back row is reduced, the infection risk of the front row is increased. Among them, case C has the greatest influence on the front row, and Passenger C in the third row is at the highest risk of 0.87. Different from SMVS, the passengers in the same row have nearly the same risk of infection in case E.
3.3.5. Performance Comparison of Different Ventilation Systems
Figure 10 shows the overall cabin infection risk under different ventilation systems based on the average infection risk of 45 passengers from row two to row six.
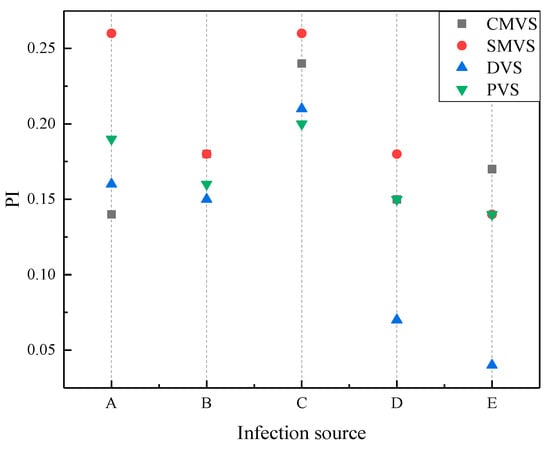
Figure 10.
Overall cabin infection risk of different the infectious sources under different ventilation systems.
As shown in Figure 10, displacement ventilation has the lowest infection risk among the four ventilation systems when the passengers B, D and E are the sources of infection. The infection risk of sidewall mixing ventilation was the highest when the passengers A, B, C and D are the infectious sources. Overall, the infection risk of mixing ventilation is greater than that of displacement ventilation, which is related to air distribution. The annular flow of mixing ventilation leads to a larger dispersion range of droplets so that more passengers are affected, while the direct flow of displacement ventilation can quickly take away the droplets and inhibit their dispersion. Personalized ventilation system makes use of the advantages of displacement ventilation to reduce the infection risk of passengers under mixing ventilation. Compared with sidewall mixing ventilation system, the infection risk of passengers is reduced by up to 27%. In addition, under the same ventilation system, when passenger C is the infectious source, the cabin environment is at the highest risk.
4. Conclusions
The COVID-19 pandemic has severely affected the air transport industry, forcing airlines to pay attention to the ventilation system in order to ensure the safety of passengers and crewmembers. In this study, the infection risk of passengers in a twin-aisle wide-body aircraft under three traditional ventilation systems was studied, and a new personalized exhaust system was proposed and investigated.
The conclusions arising from this study are the follows:
- (1)
- The droplets transmission mechanism and the infection risk of passengers are closely related to the type of ventilation system used in the aircraft cabin and the location of the infectious source.
- (2)
- Under the same ventilation system, when passenger C is the infectious source, the cabin environment is at greatest risk compared with other infectious sources.
- (3)
- The infection risk of mixing ventilation is greater than that of displacement ventilation. Therefore, from the perspective of epidemic prevention, the cabin air flow should be organized as direct as possible, and the passenger breathing area should avoid the air flow pathways between the passenger’s mouth and the air outlet as far as possible.
- (4)
- The personalized ventilation system proposed in this study can effectively reduce the infection risk of the cabin, the infection risk of passengers is reduced by up to 27%.
Author Contributions
Conceptualization, B.K., H.S. and Y.J.; methodology, B.K. and H.S.; software, B.K.; validation, B.K., H.S. and Y.J.; investigation, B.K.; resources, Y.Z. and M.C.; data curation, B.K.; writing—original draft preparation, B.K.; writing—review and editing, Y.Z., M.C., H.S. and Y.J.; visualization, B.K.; supervision, H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Flugge, C. Uber luftinfection. Z. Hyg. Infekt. 1897, 25, 179–224. [Google Scholar]
- World Health Organization. Novel Coronavirus (2019-nCoV): Situation Report; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Chao, C.Y.H.; Wan, M.P.; Sze To, G.N. Transport and removal of expiratory droplets in hospital ward environment. Aerosol Sci. Technol. 2008, 42, 377–394. [Google Scholar] [CrossRef]
- Qian, H.; Li, Y. Removal of exhaled particles by ventilation and deposition in a multibed airborne infection isolation room. Indoor Air 2010, 20, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhu, H.; Song, Y.; Cao, G. Quantitative distribution of human exhaled particles in a ventilation room. Build. Simul. 2022, 15, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ou, C.; Yang, H.; Liu, L.; Song, T.; Kang, M.; Lin, H.; Hang, J. Transmission of pathogen-laden expiratory droplets in a coach bus. J. Hazard. Mater. 2020, 397, 122609. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y. Dispersion of coughed droplets in a fully-occupied high-speed rail cabin. Build. Environ. 2012, 47, 58–66. [Google Scholar] [CrossRef]
- Mangili, A.; Gendreau, M.A. Transmission of infectious diseases during commercial air travel. Lancet 2005, 365, 989–996. [Google Scholar] [CrossRef]
- Yin, S.; Sze To, G.N.; Chao, C.Y.H. Retrospective analysis of multi-drug resistant tuberculosis outbreak during a flight using computational fluid dynamics and infection risk assessment. Build. Environ. 2012, 47, 50–57. [Google Scholar] [CrossRef]
- Gupta, J.K.; Lin, C.; Chen, Q. Transport of expiratory droplets in an aircraft cabin. Indoor Air 2011, 21, 3–11. [Google Scholar] [CrossRef]
- Sze To, G.N.; Wan, M.P.; Chao, C.Y.H.; Fang, L.; Melikov, A. Experimental study of dispersion and deposition of expiratory aerosols in aircraft cabins and impact on infectious disease transmission. Aerosol Sci. Technol. 2009, 43, 466–485. [Google Scholar] [CrossRef]
- Yan, Y.; Li, X.; Shang, Y.; Tu, J. Evaluation of airborne disease infection risks in an airliner cabin using the Lagrangian-based Wells-Riley approach. Build. Environ. 2017, 121, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Li, X.; Yang, L.; Yan, P.; Tu, J. Evaluation of cough-jet effects on the transport characteristics of respiratory-induced contaminants in airline passengers’ local environments. Build. Environ. 2020, 183, 107206. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Li, X.; Fang, X.; Yan, P.; Tu, J. Transmission of COVID-19 virus by cough-induced particles in an airliner cabin section. Eng. Appl. Comput. Fluid Mech. 2021, 15, 934–950. [Google Scholar] [CrossRef]
- Liu, M.; Liu, J.; Cao, Q.; Li, X.; Liu, S.; Ji, S.; Lin, C.; Wei, D.; Shen, X.; Long, Z.; et al. Evaluation of different air distribution systems in a commercial airliner cabin in terms of comfort and COVID-19 infection risk. Build. Environ. 2022, 208, 108590. [Google Scholar] [CrossRef] [PubMed]
- Talaat, K.; Abuhegazy, M.; Mahfoze, O.; Anderoglu, O.; Poroseva, S. Simulation of aerosol transmission on a Boeing 737 airplane with intervention measures for COVID-19 mitigation. Phys. Fluids 2021, 33, 033312. [Google Scholar] [CrossRef] [PubMed]
- Mboreha, C.; Sun, J.; Wang, Y.; Sun, Z. Airflow and contaminant transport in innovative personalized ventilation systems for aircraft cabins: A numerical study. Sci. Technol. Built Environ. 2022, 28, 557–574. [Google Scholar] [CrossRef]
- Wang, W.; Wang, F.; Lai, D.; Chen, Q. Evaluation of SARS-CoV-2 transmission and infection in airliner cabins. Indoor Air 2022, 32, e12979. [Google Scholar] [CrossRef]
- Li, M.; Zhao, B.; Tu, J.; Yan, Y. Study on the carbon dioxide lockup phenomenon in aircraft cabin by computational fluid dynamics. Build. Simul. 2015, 8, 431–441. [Google Scholar] [CrossRef]
- Zhang, T.; Li, P.; Wang, S. A personal air distribution system with air terminals embedded in chair armrests on commercial airplanes. Build. Environ. 2012, 47, 89–99. [Google Scholar] [CrossRef]
- Dygert, R.K.; Dang, T.Q. Mitigation of cross-contamination in an aircraft cabin via localized exhaust. Build. Environ. 2010, 45, 2015–2026. [Google Scholar] [CrossRef]
- Dygert, R.K.; Dang, T.Q. Experimental validation of local exhaust strategies for improved IAQ in aircraft cabins. Build. Environ. 2012, 47, 76–88. [Google Scholar] [CrossRef]
- Gupta, J.K.; Lin, C.; Chen, Q. Characterizing exhaled airflow from breathing and talking. Indoor Air 2010, 20, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, Q. Novel air distribution systems for commercial aircraft cabins. Build. Environ. 2007, 42, 1675–1684. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, X.; Mazumdar, S.; Zhang, T.; Chen, Q. Experimental and numerical investigation of airflow and contaminant transport in an airliner cabin mockup. Build. Environ. 2009, 44, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Yin, S.; Wang, S. An under-aisle air distribution system facilitating humidification of commercial aircraft cabins. Build. Environ. 2010, 45, 907–915. [Google Scholar] [CrossRef]
- Zítek, P.; Vyhlídal, T.; Simeunovic, G.; Nováková, L.; Čížek, J. Novel personalized and humidified air supply for airliner passengers. Build. Environ. 2010, 45, 2345–2353. [Google Scholar] [CrossRef]
- Mboreha, C.A.; Tytelman, X.; Nwaokocha, C.; Layeni, A.; Okeze, R.C.; Amiri, A.S. Numerical simulations of the flow fields and temperature distribution in a section of a Boeing 767–300 aircraft cabin. Mater. Today Proc. 2021, 47, 4098–4106. [Google Scholar] [CrossRef]
- Almstrand, A.; Bake, B.; Ljungström, E.; Larsson, P.; Bredberg, A.; Mirgorodskaya, E.; Olin, A. Effect of airway opening on production of exhaled particles. J. Appl. Physiol. 2010, 108, 584–588. [Google Scholar] [CrossRef] [Green Version]
- Fabian, P.; Brain, J.; Houseman, E.; Gern, J.; Milton, D. Origin of exhaled breath particles from healthy and human rhinovirus-infected subjects. J. Aerosol Med. Pulm. Drug Deliv. 2011, 24, 137–147. [Google Scholar] [CrossRef] [Green Version]
- Haslbeck, K.; Schwarz, K.; Hohlfeld, J.M.; Seume, J.R.; Koch, W. Submicron droplet formation in the human lung. J. Aerosol Sci. 2010, 41, 429–438. [Google Scholar] [CrossRef]
- Johnson, G.; Morawska, L. The mechanism of breath aerosol formation. J. Aerosol Med. Pulm. Drug Deliv. 2009, 22, 229–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morawska, L.; Johnson, G.R.; Ristovski, Z.D.; Hargreaves, M.; Mengersen, K.; Corbett, S.; Chao, C.Y.H.; Li, Y.; Katoshevski, D. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J. Aerosol Sci. 2009, 40, 256–269. [Google Scholar] [CrossRef] [Green Version]
- Höppe, P. Temperatures of expired air under varying climatic conditions. Int. J. Biometeorol. 1981, 25, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wei, J.; LI, Y.; Ooi, A. Evaporation and dispersion of respiratory droplets from coughing. Indoor Air 2017, 27, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Li, H. Distributed probability of infection risk of airborne respiratory diseases. Indoor Built Environ. 2021, 1–10. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).