A Review on Contaminants of Emerging Concern in the Environment: A Focus on Active Chemicals in Sub-Saharan Africa
Abstract
1. Introduction
2. Materials and Methods
3. Sources of Active Chemicals
4. Environmental Effects of Active Chemicals
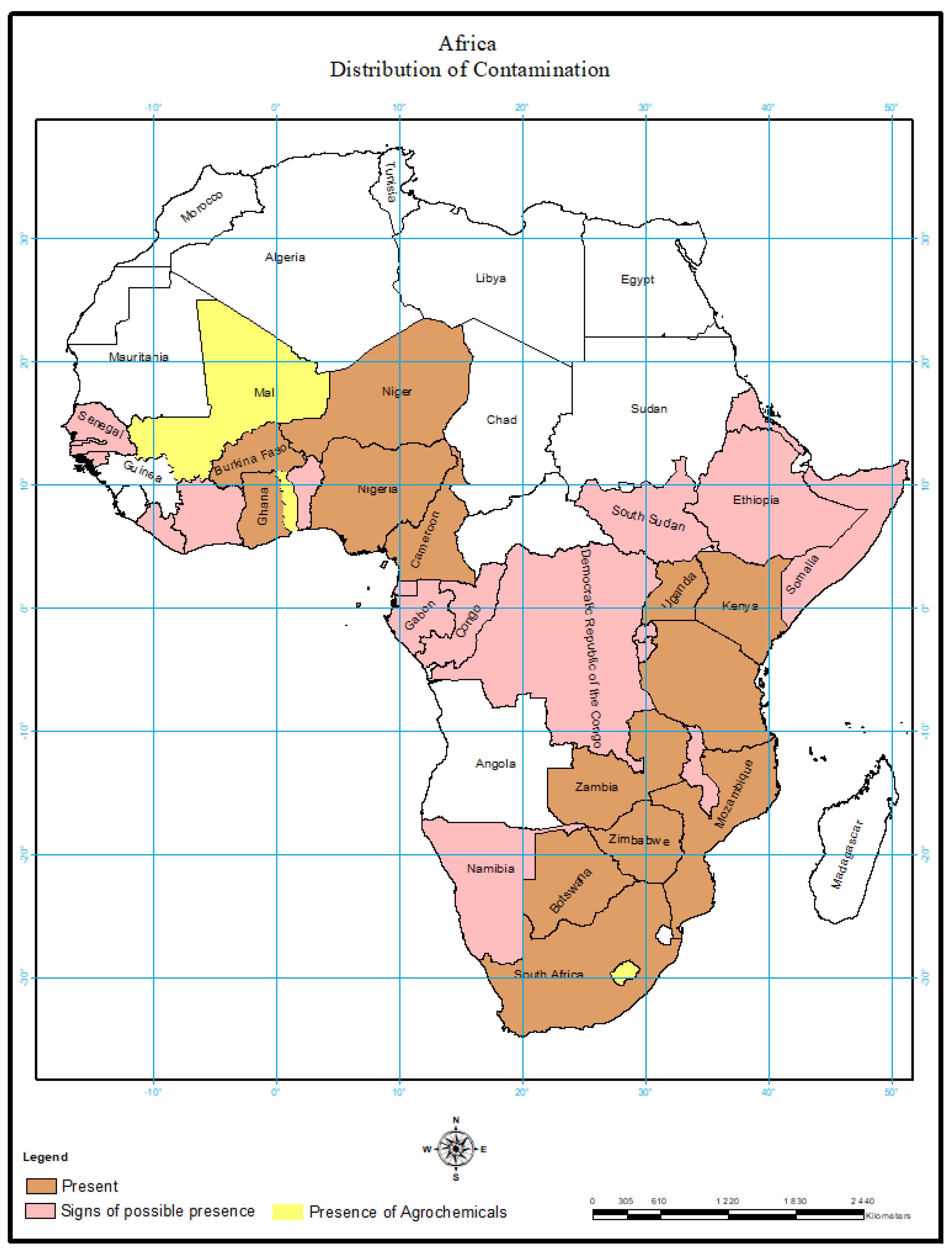
5. Environmental Load of Active Chemicals
Occurrences of Active Chemicals in Other Parts of Africa
6. Active Chemicals in the Sub-Saharan African Context
6.1. Kenya
| Substances | Effluents | Surface Water (ng/L) | Sediments (ng/Kg) | References |
|---|---|---|---|---|
| Norfloxacin | 4.2 | 1.6 to 4.9 | 248 to 776 | [44] |
| Trimethoprim | 15.8 | 3.8 to 4.4 | 11 to 90 | |
| Ciprofloxacin | 5.3 | 2.5 to 2.8 | 4125 to 1225 | |
| Sulfamethoxazole | 956.4 | 96.9 to 142.6 | 542 to 896 | |
| Lamivudine | 847.1 | 219.6 to 228.3 | 107 to 491 | |
| Zidovudine | 1.4 | 1.1 to 21 | 118 to 510 | |
| Clavulanic acid | 10–110 | [34] | ||
| Erythromycin | 100–150 | |||
| Sulfadimidine | 0–5 | |||
| Sulfamerazine | 2–20 | |||
| Minocycline | 0 | |||
| Tetracycline | 10–180 | |||
| Trimethoprim | 5–100 | |||
| Lincomycin | 5–80 |
6.2. Uganda
| Matrix | Substance | Concentration (ng/ L) | References |
|---|---|---|---|
| Lake Victoria water | Sulfamethoxazole | 1–5600 | [46] |
| Trimethoprim | 1–89 L | ||
| Tetracycline | 3–70 | ||
| Sulfacetamide | 1–13 | ||
| Erythromycin | 10–66 | ||
| Sulfamethazine | 2–50 | ||
| Carbamazepine | 5–72 | ||
| Ibuprofen | 6–780 | ||
| Diclofenac | 2–160 | ||
| Sulfamethoxazole | 1–5600 |
6.3. Tanzania
| Matrix | Substance | Concentration | References |
|---|---|---|---|
| Wastewater | Metronidazole | 0.065–0.104 ppm | [51] |
| Msimbazi River waters | Metronidazole | 0.0024 ppm | |
| Paracetamol | 0.0060 ppm | [53] | |
| Cetirizine | 0.0073 ppm | ||
| Ibuprofen | 0.0016 ppm | ||
| Wastewater effluents | Ampicillin | bdl to 0.367 ppm | [48] |
| Ciprofloxacin | bdl to 0.037 ppm | ||
| AMR Lake Victoria water | Sulfamethoxazole/trimethoprim | 100% | [52] |
| Ampicillin/cloxacillin | 100% | ||
| Erythromycin | 72.7% | ||
| Tetracycline | 90.9% | ||
| Nalidixic acid | 63.6% |
6.4. Zambia
| Matrix | Substance | Concentration | References |
|---|---|---|---|
| Urine | Sulfamethoxazole | 7740 µg/L | [56] |
| Trimethoprim | 12,800 µg/L | ||
| Lamivudine | 10,010 µg/L | ||
| Surface water | Antibiotics | 11,800 ng/L | |
| Antivirals | 49,700 ng/L | ||
| Effluents | Antibiotics | 100–300,400 ng/L | |
| Antivirals | 680–55,760 ng/L |
6.5. Zimbabwe
| Activity in (ng/L) | Effluents from | References | |||
|---|---|---|---|---|---|
| STPs | Ugumza | Matsheumhlope | Kihami | ||
| 17β-estradiol equivalent (EEq) | 33 | 237 | 9 | 2 | [62] |
| Dihydrotestosterone equivalent (TEq) | 55 | - | - | - | |
| Androgenic | 93 | - | - | - | |
6.6. Mozambique
| Matrix | Substance | Concentration (ng/L) | References |
|---|---|---|---|
| Surface water | Azithromycin | 8 | [34] |
| Clavulanic acid | 5–20 | ||
| Erythromycin | 20–1000 | ||
| Sulfapyridine | 800–1300 | ||
| Sulfamethoxazole | 12 | ||
| Oxytetracycline | 1000 | ||
| Trimethoprim | 800 |
6.7. Ethiopia
| Matrix | Substance | Concentration (ng/L) | References |
|---|---|---|---|
| Wastewater | Trimethoprim | 500 | [67] |
| Ciprofloxacin | 10–300 | ||
| Water | Trimethoprim | 7800 | |
| Caffeine | 3200 | ||
| Albendazole | 2100 | ||
| Hospital water | Caffeine | 320 | |
| Trimethoprim | 780 | ||
| Albendazole | 210 |
6.8. Malawi
6.9. Eritrea
6.10. Mauritius
6.11. Madagascar
6.12. Rwanda
6.13. South Sudan
6.14. Burundi
6.15. Seychelles
| Matrix | Substance (s) | Concentration (pg g−1 ww) | References |
|---|---|---|---|
| Muscle | Organochlorine pesticides (OCPs) | 5637 | [97] |
| Polychlorinated biphenyls (PCBs) | 491 | ||
| Perfluoroalkyl substances (PFASs) | 331 |
6.16. Somalia
6.17. Nigeria
| Matrix | Substance | Concentration | References |
|---|---|---|---|
| River water | Phenazone | <to 0.01 µ/L | [103] |
| Trimethoprim | <to 0.01 µ/L | ||
| Estrone | <to 0.01 µ/L | ||
| Estriol | <to 0.01 µ/L | ||
| Acetylsalicylic acid | <to 0.02 µ/L | ||
| Carbamazepine | <to 0.02 µ/L | ||
| Diclofenac | <to 0.02 µ/L | ||
| Roxithromycin | <to 0.02 µ/L | ||
| Indomethacin | <to 0.02 µ/L | ||
| Erythromycin | <to 0.06 µ/L | ||
| Clofibric acid | <to 0.02 µ/L | ||
| Borehole | Diclofenac | 0.39 mg/L | [101] |
| Artemether | 0.62 mg/L | ||
| Treated tape | Diclofenac | 0.17 mg/L | |
| Artemether | 0.04 mg/L | ||
| Well water | Diclofenac | 8.84–1100 µg/L | |
| Ofloxacin | 0.73, 0.24 and 0.08 ng/L | ||
| Sewage | Chemical load | 10–10,000 ng/L | [104] |
| Chemical effluent | Chemical load | 2–15,000 ng/L | |
| Urban river | Chemical load | 10–10,000 ng/L | |
| Hospital wastewater and landfill leachate | Bisphenol A | 2.3–59.2 ng/L | [106] |
| Acetamidophenol | Bdl–30.1 ng/L | ||
| Oxybenzone | 1.0–1.1 ng/L | ||
| Triclocarban | 39.3–47.2 ng/L | ||
| Triclosan | 55–63.6 ng/L |
6.18. Ghana
| Matrix | Substance | Concentration (ng/L) | References |
|---|---|---|---|
| Surface water | Oxytetracycline | 0–350 | [34] |
| Sulfadimidine | 0–50 | ||
| Minocycline | 2–14 | ||
| Chlortetracycline | 2–14 | ||
| Streptozotocin | 500 | ||
| Lincomycin | 2–10 | ||
| Tetracycline | 10–300 | ||
| Trimethoprim | 10–200 | ||
| Clavulanic acid | 5–14 | ||
| Azithromycin | 2–12 | ||
| Erythromycin | 10–110 | ||
| Hospital wastewater effluent/influent, river water, and in vegetables | Metronidazole | 247–420 | [109] |
| Ciprofloxacin | 11,352–15,733 | ||
| Erythromycin | 7944–10,613 | ||
| Trimethoprim | 94–4826 | ||
| Tetracycline | 58–116 | ||
| Oxytetracycline | 75–252 | ||
| Chlortetracycline | 16–24 | ||
| Amoxicillin | 2–6 | ||
| Ampicillin | 107–324 | ||
| Cephalexin | 1052–1557 | ||
| Sulfasalazine | 2315–3590 |
6.19. Togo
| Substances | Cowpea | Maize | River water | References |
|---|---|---|---|---|
| Gamma-hexachlorocyclohexane | 4.95 | 6.38 | 0.03 to 0.05 | [112] |
| 2,4-Dichlorodiphenyldichloroethane | 14.57 | 2.18 | 0.00 to 0.02 | |
| 4,4-Dichlorodiphenyldichloroethane | 15.76 | 5.29 | 0.00 | |
| 2,4-Dichlorodiphenyldichloroethylene | 11.66 | - | 0.03 to 0.15 | |
| 4,4-Dichlorodiphenyldichloroethylene | 4.71 | - | 0.07 | |
| 4,4-Dichlorodiphenyltrichloroethane | 12.49 | 21.79 | 0.11 | |
| Aldrin | 6 | 0.52 | - | |
| Dieldrin | 39.50 | 18.09 | 0.04 | |
| Heptachlor | 3.92 | 1.72 | 0.24 | |
| α-Endosulfan | 88.51 | 34.74 | 0.32 | |
| β-Endosulfan | 98.80 | 65.71 | 0.25 | |
| Heptachlor epoxide | 44.88 | 17.65 | 0.17 |
6.20. Burkina Faso
| Amount of Substances in (µg/L) | Ground Water | Surface Water | References |
|---|---|---|---|
| Endosulfan | 0.05 to 0.16 | 0.05 to 0.25 | [113] |
| Aldrin | 0.01 to 0.07 | 0.01 to 0.15 |
6.21. Liberia
6.22. Benin
6.23. Ivory Coast
6.24. Mali
| Matrix | Substance (s) | Concentration (Pg/m3) | References |
|---|---|---|---|
| Air | trans-Chlordane | 44 | [124] |
| Sum chlordanes | 48 | ||
| Chlorpyrifos | 9960 | ||
| Dacthal | 1.1 | ||
| O; P-Dichlorodiphenyldichloroethane | 152 | ||
| P; P-Dichlorodiphenyldichloroethane | 314 | ||
| O; P-Dichlorodiphenyldichloroethylene | 81 | ||
| P; P-Dichlorodiphenyldichloroethylene | 990 | ||
| O; P-Dichlorodiphenyltrichloroethane | 537 | ||
| P; P-Dichlorodiphenyltrichloroethane | 810 | ||
| Diazinon | 810 | ||
| Dieldrin | 1100 | ||
| Endosulfan I | 10,500 | ||
| Endosulfan II | 2510 |
6.25. Cape Verde
| Matrix | Substance (s) | Amount in pg/m3 | References |
|---|---|---|---|
| Air | trans-Chlordane | 0.1 | [124] |
| trans-Nonachlor | 0.2 | ||
| Chlorpyrifos | 1.0 | ||
| Dacthal | 0.1 | ||
| O, P-Dichlorodiphenyldichloroethane | 0.4 | ||
| P, P-Dichlorodiphenyldichloroethane | 0.8 | ||
| O, P-Dichlorodiphenyldichloroethylene | 0.3 | ||
| P, P-Dichlorodiphenyldichloroethylene | 0.2 | ||
| O, P-Dichlorodiphenyltrichloroethane | 0.6 | ||
| P, P-Dichlorodiphenyltrichloroethane | 0.5 | ||
| Diazinon | 0.7 | ||
| Dieldrin | 0.8 | ||
| Endosulfan I | 4.4 | ||
| Endosulfan II | 0.4 |
6.26. Guinea-Bissau
6.27. Gambia
6.28. Senegal
6.29. Equatorial Guinea
6.30. Cameroon
| Matrix | Substances (s) | Concentration (μg/L) | References |
|---|---|---|---|
| Hospital wastewater | Paracetamol | 211.93 | [137] |
| Ibuprofen | 141 | ||
| Tramadol | 76 | ||
| O-desmethyltramadol | 141 | ||
| Erythromycin anhydrate | 7 | ||
| Ciprofloxacin | 24 | ||
| Clarithromycin | 0.088 | ||
| Propranolol | 0.3 | ||
| Cimetidine | 34 | ||
| Hydroxy omeprazole | 5 | ||
| Diphenhydramine | 0.38 | ||
| Metformin | 154 | ||
| Sucralose | 13.07 | ||
| Azithromycin | 0.39 | ||
| Sulfamethoxazole | 0.16 | ||
| Trimethoprim | 0.27 | ||
| Caffeine | 5.8 | ||
| Carbamazepine | 0.94 | ||
| Atenolol | 0.43 | ||
| Acetochlor | 4.1 | [133] | |
| Atrazine | 2.2 | ||
| Chlortoluron | 6.2 | ||
| Diuron | 1.5 | ||
| Linuron | 01 | ||
| Flazasulfuron | 1.0 |
6.31. Angola
6.32. Gabon
6.33. Congo-Brazzaville
6.34. Democratic Republic of Congo (DRC)
6.35. Namibia
6.36. Lesotho
| Matrix | Substance (s) | Concentration (ng/g) | References |
|---|---|---|---|
| Lake water, sediments | Hexachlorobenzene | 0.1–0.3 | [153] |
| Total polycyclic aromatic hydrocarbons | 50–250 | ||
| Total 16 USEPA polycyclic aromatic hydrocarbons | 0–150 | ||
| High-molecular-weight polycyclic aromatic hydrocarbons (4–6-rings) | 0–10 | ||
| Benzo(a)pyrene | 0.5–1 | ||
| Indeno (1, 2, 3, c, d)pyrene | 1–3 | ||
| Benzo (g, h, 2)pyrene | 1–3 | ||
| Total polychlorinated biphenyls | 0.1–3 | ||
| Total 7 polychlorinated biphenyls | 0.1–2 |
6.37. Botswana
| Matrix | Antimicrobial Resistant (AMR) | Percentage | References |
|---|---|---|---|
| Wastewater influent, effluent, and downstream environment | Ampicillin | 54 | [154] |
| Penicillin | 85 | ||
| Erythromycin | 76 | ||
| Cephalosporin | 69 | ||
| Sulfamethoxazole | 54 | ||
| Trimethoprim | 85 |
7. Lack of AC Reports for Some Sub-Saharan African Countries
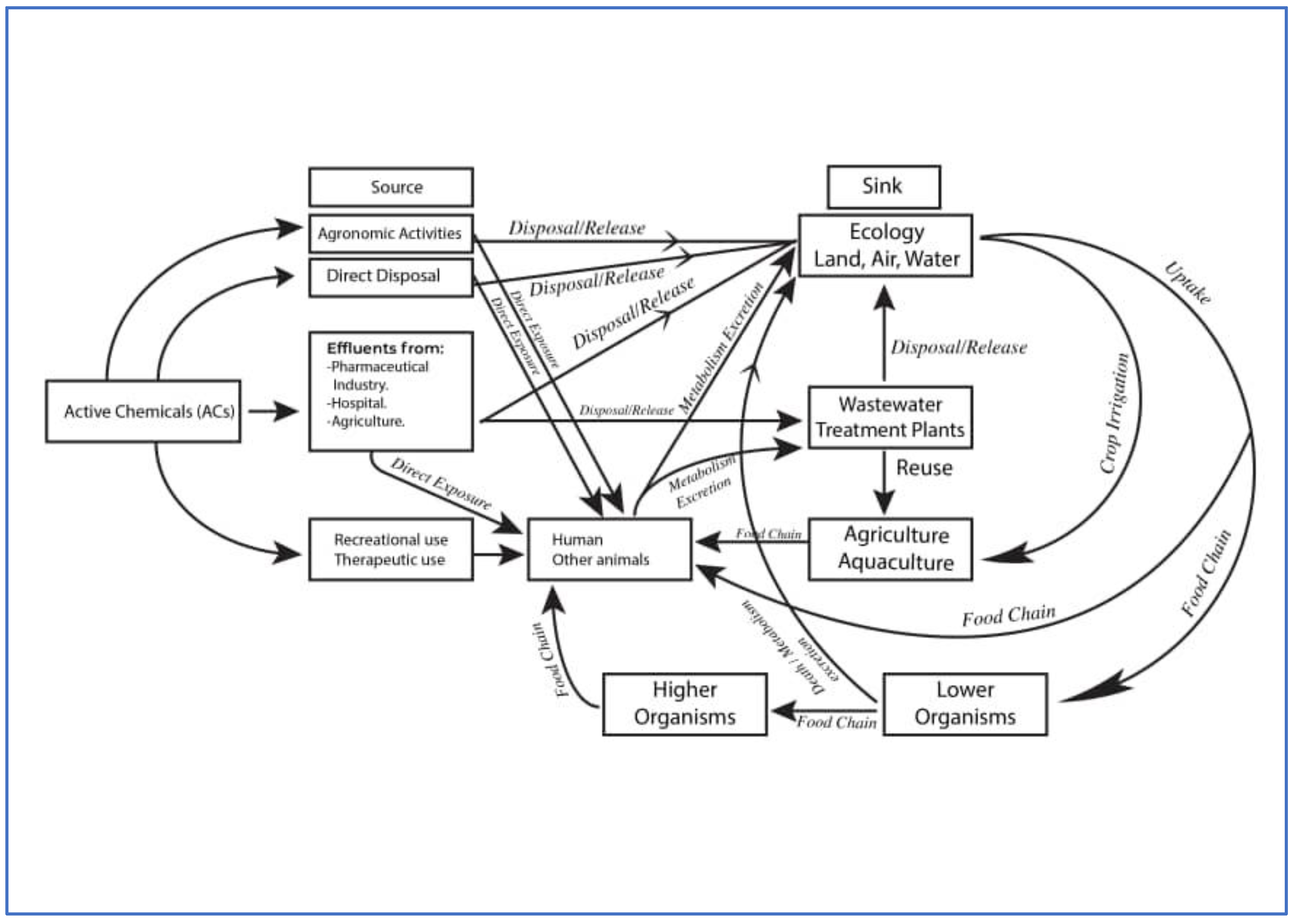
8. Flow and Fate of Active Chemicals
8.1. Environmental and Human Health Effects
8.2. Challenges Associated with ACs in the Environment
8.3. Limitations of Wastewater Treatment
8.4. Overcoming the Challenges Relating to ACs
9. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; Mao, K.; Du, W.; Cai, M.; Zhang, Z.; Li, X. Diluted concentrations of methamphetamine in surface water induce behavior disorder, transgenerational toxicity, and ecosystem-level consequences of fish. Water Res. 2020, 184, 116164. [Google Scholar] [CrossRef]
- Mohan, H.; Rajput, S.S.; Jadhav, E.B.; Sankhla, M.S.; Sonone, S.S.; Jadhav, S.; Kumar, R. Ecotoxicity, Occurrence, and Removal of Pharmaceuticals and Illicit Drugs from Aquatic Systems. Biointerface Res. Appl. Chem. 2021, 1, 12530–12546. [Google Scholar] [CrossRef]
- Bonnefille, B.; Gomez, E.; Courant, F.; Escande, A.; Fenet, H. Diclofenac in the marine environment: A review of its occurrence and effects. Mar. Pollut. Bull. 2018, 131, 496–506. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 108, 309–318. [Google Scholar] [CrossRef]
- Michael, C.A.; Dominey-Howes, D.; Labbate, M. The antimicrobial resistance crisis: Causes, consequences, and management. Front. Public Health 2014, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Moo, C.-L.; Yang, S.-K.; Yusoff, K.; Ajat, M.; Thomas, W.; Abushelaibi, A.; Lim, S.-H.-E.; Lai, K.-S. Mechanisms of antimicrobial resistance (AMR) and alternative approaches to overcome AMR. Curr. Drug Discov. Technol. 2020, 17, 430–447. [Google Scholar] [CrossRef]
- Jansen, K.U.; Anderson, A.S. The role of vaccines in fighting antimicrobial resistance (AMR). Hum. Vaccines Immunother. 2018, 14, 2142–2149. [Google Scholar] [CrossRef]
- Hu, X.-Y.; Logue, M.; Robinson, N. Antimicrobial resistance is a global problem–a UK perspective. Eur. J. Integr. Med. 2020, 36, 101136. [Google Scholar] [CrossRef]
- Dadgostar, P. Antimicrobial resistance: Implications and costs. J. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, N.; Vlăduț, V.; Dincă, M.; Zăbavă, B.-Ș. Reuse of wastewater for irrigation, a sustainable practice in arid and semi-arid regions. In Proceedings of the 7th International Conference on Thermal Equipment, Renewable Energy and Rural Development (TE-RE-RD), Drobeta-Turnu Severin, Romania, 31 May–2 June 2018; pp. 379–384. [Google Scholar]
- Akponikpè, P.I.; Wima, K.; Yacouba, H.; Mermoud, A. Reuse of domestic wastewater treated in macrophyte ponds to irrigate tomato and eggplant in semi-arid West-Africa: Benefits and risks. J. Agric. Water Manag. 2011, 98, 834–840. [Google Scholar] [CrossRef]
- Miraji, H.; Othman, O.C.; Ngassapa, F.; Mureithi, E.J.S. Research trends in emerging contaminants on the aquatic environments of Tanzania. Scientifica 2016, 2016, 3769690. [Google Scholar] [CrossRef]
- Sorensen, J.; Lapworth, D.; Nkhuwa, D.; Stuart, M.; Bell, R.; Chirwa, M.; Kabika, J. Emerging organic contaminants in urban and peri-urban groundwater sources in sub-Saharan Africa: A case study from Kabwe, Zambia. In Proceedings of the International Association of Hydrogeologists IAH, the Moroccan Chapter—41st IAH International Congress “Groundwater: Challenges and Strategies” Marrakech, Marrakech, Morocco, 15–19 September 2014; pp. 15–19. [Google Scholar]
- Adegoke, A.A.; Amoah, I.D.; Stenström, T.A.; Verbyla, M.E.; Mihelcic, J.R. Epidemiological evidence and health risks associated with agricultural reuse of partially treated and untreated wastewater: A review. Front. Public Health 2018, 6, 337. [Google Scholar] [CrossRef]
- Abelkop, A.D.; Graham, J.D.; Royer, T.V. Persistent, Bioaccumulative, and Toxic (PBT) Chemicals: Technical Aspects, Policies, and Practices, 1st ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar] [CrossRef]
- Zhang, W.; Asiri, A.M.; Liu, D.; Du, D.; Lin, Y. Nanomaterial-based biosensors for environmental and biological monitoring of organophosphorus pesticides and nerve agents. TrAC Trends Anal. Chem. 2014, 54, 1–10. [Google Scholar] [CrossRef]
- Pandey, S.; Goswami, G.K.; Okoro, H.K.; Fosso-Kankeu, E. Carbon nanotubes in the 21st Century: An Advancement in real time monitoring and control of environmental water. In Nano and Bio-Based Technologies for Wastewater; Scrivener Publishing LLC: Beverly, UK, 2019; pp. 265–301. [Google Scholar] [CrossRef]
- Miraji, H.; Othman, O.C.; Ngassapa, F.; Eunice, M. Analytical perspectives on emerging organic contaminants in the aquatic ecosystem. In Effects of Emerging Chemical Contaminants on Water Resources and Environmental Health; IGI Global: Hershey, PA, USA, 2020; pp. 68–80. [Google Scholar]
- Jarque, S.; Quirós, L.; Grimalt, J.O.; Gallego, E.; Catalan, J.; Lackner, R.; Piña, B. Background fish feminization effects in European remote sites. Sci. Rep. 2015, 5, 11292. [Google Scholar] [CrossRef] [PubMed]
- García-Berthou, E.; Bae, M.-J.; Benejam, L.; Alcaraz, C.; Casals, F.; de Sostoa, A.; Solà, C.; Munné, A. Fish-based indices in Catalan rivers: Intercalibration and comparison of approaches. In Experiences from Surface Water Quality Monitoring; Springer: Berlin/Heidelberg, Germany, 2015; pp. 125–147. [Google Scholar]
- Hamilton, P.B.; Nicol, E.; De-Bastos, E.S.R.; Williams, R.J.; Sumpter, J.P.; Jobling, S.; Stevens, J.R.; Tyler, C.R. Populations of a cyprinid fish are self-sustaining despite widespread feminization of males. BMC Biol. 2014, 12, 1. [Google Scholar] [CrossRef]
- Pérez, M.R.; Fernandino, J.I.; Carriquiriborde, P.; Somoza, G.M. Feminization and altered gonadal gene expression profile by ethinylestradiol exposure to pejerrey, Odontesthes bonariensis, a South American teleost fish. Environ. Toxicol. Chem. Lat. Am. 2012, 31, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, W.; Sremski, W.; Piccini, B.; Palluel, O.; Maillot-Marechal, E.; Betoulle, S.; Jaffal, A.; Aït-Aïssa, S.; Brion, F.; Thybaud, E.; et al. Adverse effects in wild fish living downstream from pharmaceutical manufacture discharges. Environ. Int. 2011, 37, 1342–1348. [Google Scholar] [CrossRef] [PubMed]
- Tyler, C.R.; Jobling, S. Roach, Sex, and Gender-Bending Chemicals: The Feminization of Wild Fish in English Rivers. BioScience 2008, 58, 1051–1059. [Google Scholar] [CrossRef]
- Richards, S.M.; Wilson, C.J.; Johnson, D.J.; Castle, D.M.; Lam, M.; Mabury, S.A.; Sibley, P.K.; Solomon, K.R. Effects of pharmaceutical mixtures in aquatic microsms. Environ. Toxicol. Chem. 2004, 23, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, M.I.; Lambrianides, A.; Schneider, M.; Kümmerer, K.; Fatta-Kassinos, D. Environmental side effects of pharmaceutical cocktails: What we know and what we should know. J. Hazard. Mater. 2014, 279, 169–189. [Google Scholar] [CrossRef]
- Michael, I.; Vasquez, M.I.; Hapeshi, E.; Haddad, T.; Baginska, E.; Kümmerer, K.; Fatta-Kassinos, D. Metabolites and Transformation Products of Pharmaceuticals in the Aquatic Environment as Contaminants of Emerging Concern; Wiley, John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 413–459. [Google Scholar]
- Kamba, P.F.; Kaggwa, B.; Munanura, E.I.; Okurut, T.; Kitutu, F.E. Why regulatory indifference towards pharmaceutical pollution of the environment could be a missed opportunity in public health protection. a holistic view. Pan Afr. Med. J. 2017, 27, 4. [Google Scholar] [CrossRef]
- Madikizela, L.M.; Tavengwa, N.T.; Chimuka, L. Luke Chimuka Status of pharmaceuticals in African water bodies: Occurrence, removal and analytical methods. Environ. Manag. 2017, 193, 211–230. [Google Scholar] [CrossRef]
- Atnafie, S.A.; Muluneh, N.Y.; Getahun, K.A.; Tsegaw Woredekal, A.; Kahaliw, W. Pesticide Residue Analysis of Khat Leaves and Health Risks among Khat Chewers in the Amhara Region, Northwestern Ethiopia. J. Environ. Public Health 2021, 2021, 4680573. [Google Scholar] [CrossRef]
- Ngubane, N.P.; Naicker, D.; Ncube, S.; Chimuka, L.; Madikizela, L.M. Determination of naproxen, diclofenac and ibuprofen in Umgeni estuary and seawater: A case of northern Durban in KwaZulu-Natal Province of South Africa. Reg. Stud. Mar. Sci. 2019, 29, 100675. [Google Scholar] [CrossRef]
- Oyedemi, S.; Oyedemi, B.; Coopoosamy, R.; Prieto, J.; Stapleton, P.; Gibbons, S. Antibacterial and norfloxacin potentiation activities of Ocimum americanum L. against methicillin resistant Staphylococcus aureus. S. Afr. J. Bot. 2017, 109, 308–314. [Google Scholar] [CrossRef]
- Lorenzo, M.; Campo, J.; Suárez-Varela, M.M.; Picó, Y. Occurrence, distribution and behavior of emerging persistent organic pollutants (POPs) in a Mediterranean wetland protected area. Sci. Total Environ. 2019, 646, 1009–1020. [Google Scholar] [CrossRef]
- Segura, P.A.; Takada, H.; Correa, J.A.; El Saadi, K.; Koike, T.; Onwona-Agyeman, S.; Ofosu-Anim, J.; Sabif, E.B.; Wasongag, O.V.; Mghalu, J.M.; et al. Global occurrence of anti-infectives in contaminated surface waters: Impact of income inequality between countries. Environ. Int. 2015, 80, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Ngqwala, N.P.; Muchesa, P. Occurrence of pharmaceuticals in aquatic environments: A review and potential impacts in South Africa. S. Afr. J. Sci. 2020, 116, 1–7. [Google Scholar] [CrossRef]
- Schoeman, C.; Mashiane, M.; Dlamini, M.; Okonkwo, O. Quantification of selected antiretroviral drugs in a wastewater treatment works in South Africa using GC-TOFMS. J. Chromatogr. Sep. Tech. 2015, 6, 1–7. [Google Scholar]
- Matongo, S.; Birungi, G.; Moodley, B.; Ndungu, P. Pharmaceutical residues in water and sediment of Msunduzi River, kwazulu-natal, South Africa. Chemosphere 2015, 134, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Rimayi, C.; Chimuka, L.; Gravell, A.; Fones, G.R.; Mills, G.A. Use of the Chemcatcher® passive sampler and time-of-flightmass spectrometry to screen for emerging pollutants in rivers in Gauteng Province of South Africa. Environ. Monit. Assess 2019, 2019, 388. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.P.; Duvenage, C.S.; Rohwer, E. The occurrence of anti-retroviral compounds used for HIV treatment in South African surface water. J. Environ. Pollut. 2015, 199, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Madikizela, L.M.; Ncube, S.; Chimuka, L. Luke Chimuka Analysis, occurrence and removal of pharmaceuticals in African water resources: A current status. Environ. Manag. 2020, 253, 109741. [Google Scholar] [CrossRef]
- Lapworth, D.J.; Nkhuwa, D.C.W.; Okotto-Okotto, J.; Pedley, S.; Stuart, M.E.; Tijani, M.N.; Wright, J.J.H.J. Urban groundwater quality in sub-Saharan Africa: Current status and implications for water security and public health. Hydrogeol. J. 2017, 25, 1093–1116. [Google Scholar] [CrossRef] [PubMed]
- Muriuki, C.W.; Home, P.G.; Raude, J.M.; Ngumba, E.K.; Munala, G.K.; Kairigo, P.K.; Gachanja, A.N.; Tuhkanen, T.A. Occurrence, distribution, and risk assessment of pharmerciuticals in wastewater and open surface drains of peri-urban areas: Case study of Juja town, Kenya. Environ. Pollut. 2020, 267, 115503. [Google Scholar] [CrossRef]
- K’oreje, K.O.; Kandie, F.J.; Vergeynst, L.; Abira, M.A.; Van Langenhove, H.; Okoth, M.; Demeestere, K. Occurrence, fate and removal of pharmaceuticals, personal care products and pesticides in wastewater stabilization ponds and receiving rivers in the Nzoia Basin, Kenya. Sci. Total Environ. 2018, 637, 336–348. [Google Scholar] [CrossRef]
- Kairigo, P.; Ngumba, E.; Sundberg, L.-R.; Gachanja, A.; Tuhkanen, T. Contamination of Surface Water and River Sediments by Antibiotic and Antiretroviral Drug Cocktails in Low and Middle-Income Countries: Occurrence, Risk and Mitigation Strategies. Water 2020, 12, 1376. [Google Scholar] [CrossRef]
- Ekane, N.; Mertz, C.K.; Slovic, P.; Kjellén, M.; Westlund, H. Risk and benefit judgment of excreta as fertilizer in agriculture: An exploratory investigation in Rwanda and Uganda. Hum. Ecol. Risk Assess. 2015, 2015, 639–666. [Google Scholar] [CrossRef]
- Nantaba, F.; Wasswa, J.; Kylin, H.; Palm, W.-U.; Bouwman, H.; Kümmerer, K. Occurrence, distribution, and ecotoxicological risk assessment of selected pharmaceutical compounds in water from Lake Victoria, Uganda. Chemosphere 2020, 239, 124642. [Google Scholar] [CrossRef]
- Dalahmeh, S.; Tirgani, S.; Komakech, A.J.; Niwagaba, C.B.; Ahrens, L. Per- and polyfluoroalkyl substances (PFASs) in water, soil and plants in wetlands and agricultural areas in Kampala, Uganda. Sci. Total Environ. 2018, 631, 660–667. [Google Scholar] [CrossRef]
- Kihampa, C. β-lactams and Fluoroquinolone Antibiotics in influents and effluents of Wastewater treatment plants, Dar es Salaam, Tanzania. Res. J. Chem. Sci. 2014, 2231, 606. [Google Scholar]
- Kaseva, M.E.; Mwegoha, W.J.S.; Kihampa, C.; Matiko, S. Performance of a waste stabilization pond system treating domestic and hospital wastewater and its implications to the aquatic environment-a case study in Dar es Salaam, Tanzania. J. Build. Land Dev. 2008, 15, 14. [Google Scholar]
- Mhongole, O.J.; Mdegela, R.H.; Kusiluka, L.J.M.; Forslund, A.; Dalsgaard, A. Characterization of Salmonella spp. from wastewater used for food production in Morogoro, Tanzania. World J. Microbiol. Biotechnol. 2017, 33, 42. [Google Scholar] [CrossRef]
- Makokola, S.K.; Ripanda, A.; Miraji, H. Quantitative Investigation of Potential Contaminants of Emerging Concern in Dodoma City: A Focus at Swaswa Wastewater Stabilization Ponds. Egypt. J. Chem. 2019, 63, 427–436. [Google Scholar]
- Baniga, Z.; Hounmanou, Y.M.G.; Kudirkiene, E.; Kusiluka, L.J.M.; Mdegela, R.H.; Dalsgaard, A. Genome-Based Analysis of Extended-Spectrum β-Lactamase-Producing Escherichia coli in the Aquatic Environment and Nile Perch (Lates niloticus) of Lake Victoria, Tanzania. Front. Microbiol. 2020, 11, 108. [Google Scholar] [CrossRef]
- Hossein, M.; Chande, O.; Faustin, N.; Erwin, M. Spatial Occurrence and Fate Assessment of Potential Emerging Contaminants in the Flowing Surface Waters. Chem. Sci. Int. J. 2018, 24, 1–11. [Google Scholar] [CrossRef]
- Outwater, A.H.; Pamba, S.; Outwater, A.B. Water-Related Diseases of People Using Municipal Wastewater: Risks, Exposure, Effects on Health and Control Approaches in Tanzania; VLIR-UOS: Da res Salaam, Tanzania, 2013; Volume 132, p. 133. [Google Scholar]
- Ettler, V.; Mihaljevic, M.; Kříbek, B.; Majer, V.; Šebek, O. Tracing the spatial distribution and mobility of metal/metalloid contaminants in Oxisols in the vicinity of the Nkana copper smelter, Copperbelt province, Zambia. Geoderma 2011, 164, 73–84. [Google Scholar] [CrossRef]
- Ngumba, E.; Gachanja, A.; Nyirenda, J.; Maldonado, J.; Tuhkanen, T. Occurrence of antibiotics and antiretroviral drugs in source-separated urine, groundwater, surface water and wastewater in the peri-urban area of Chunga in Lusaka, Zambia. Water SA 2020, 46, 278–284. [Google Scholar]
- Mapanda, F.; Mangwayana, E.; Nyamangara, J.; Giller, K. Uptake of heavy metals by vegetables irrigated using wastewater and the subsequent risks in Harare, Zimbabwe. Phys. Chem. Earth Parts A/B/C 2007, 32, 1399–1405. [Google Scholar] [CrossRef]
- Muchuweti, M.; Birkett, J.; Chinyanga, E.; Zvauya, R.; Scrimshaw, M.; Lester, J. Heavy metal content of vegetables irrigated with mixtures of wastewater and sewage sludge in Zimbabwe: Implications for human health. Agric. Ecosyst. Environ. 2006, 112, 41–48. [Google Scholar] [CrossRef]
- Meck, M.; Mudimbu, D.; Davies, T. Accumulation of potentially harmful elements in edible parts of vegetables grown on two different geological substrates in Zimbabwe. J. Geochem. Explor. 2020, 208, 106392. [Google Scholar] [CrossRef]
- Gwenzi, W. Autopsy, thanatopraxy, cemeteries and crematoria as hotspots of toxic organic contaminants in the funeral industry continuum. Sci. Total Environ. 2021, 753, 141819. [Google Scholar] [CrossRef] [PubMed]
- Kearns, J. The role of chemical exposures in reducing the effectiveness of water–sanitation–hygiene interventions in Bangladesh, Kenya, and Zimbabwe. Wiley Interdiscip. Rev. Water 2020, 7, 1478. [Google Scholar] [CrossRef]
- Teta, C.; Holbech, B.F.; Norrgren, L.; Naik, Y.S. Occurrence of oestrogenic pollutants and widespread feminisation of male tilapia in peri-urban dams in Bulawayo, Zimbabwe. Afr. J. Aquat. Sci. 2018, 43, 17–26. [Google Scholar] [CrossRef]
- Remili, A.; Gallego, P.; Pinzone, M.; Castro, C.; Jauniaux, T.; Garigliany, M.-M.; Malarvannan, G.; Covaci, A.; Das, K. Humpback whales (Megaptera novaeangliae) breeding off Mozambique and Ecuador show geographic variation of persistent organic pollutants and isotopic niches. Environ. Pollut. 2020, 267, 115575. [Google Scholar] [CrossRef] [PubMed]
- Ayele, Y.; Mamu, M. Assessment of knowledge, attitude and practice towards disposal of unused and expired pharmaceuticals among community in Harar city, Eastern Ethiopia. J. Pharm. Policy Pract. 2018, 11, 27. [Google Scholar] [CrossRef]
- Kahsay, H.; Ahmedin, M.; Kebede, B.; Gebrezihar, K.; Araya, H.; Tesfay, D. Assessment of Knowledge, Attitude, and Disposal Practice of Unused and Expired Pharmaceuticals in Community of Adigrat City, Northern Ethiopia. J. Environ. Public Health 2020, 2020, 6725423. [Google Scholar] [CrossRef]
- Gudeta, T.; Assefa, D. Assessment of Pharmaceuticals Waste Practices Among Private Drug Retail Outlets in Ethiopia. J. Prim. Care Community Health 2020, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tegegne, B.; Chandravanshi, B.S.; Zewge, F.; Chimuka, L. Solid-phase optimisation for simultaneous determination of thirteen pharmaceuticals in Ethiopian water samples with HPLC-DAD detection: An initial assessment. Environ. Monit. Assess. 2021, 193, 310. [Google Scholar] [CrossRef]
- Kosamu, I.; Kaonga, C.; Utembe, W. A Critical Review of the Status of Pesticide Exposure Management in Malawi. Int. J. Environ. Res. Public Health 2020, 17, 6727. [Google Scholar] [CrossRef] [PubMed]
- Mmanga, M.; Singini, W.; Di Bella, V.; Flaherty, M.G.; Holm, R.H. Unpacking healthcare waste management at rural village health clinics in the Ntcheu District (Malawi). Environ. Monit. Assess. 2019, 191, 175. [Google Scholar] [CrossRef]
- Srikanth, R.; Naik, D. Prevalence of Giardiasis due to wastewater reuse for agriculture in the suburbs of Asmara City, Eritrea. Int. J. Environ. Health Res. 2004, 14, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Shahid, M.; Tahir, N.; Bibi, I.; Sarwar, T.; Shah, A.H.; Niazi, N.K. A Review of Environmental Contamination and Health Risk Assessment of Wastewater Use for Crop Irrigation with a Focus on Low and High-Income Countries. Int. J. Environ. Res. Public Health 2018, 15, 895. [Google Scholar] [CrossRef]
- Tesfalema, N.; Tesfamariam, A.; Okbaslasiec, A.; Tesfayd, K. Physico-chemical Analysis of Groundwater Around MaiBela, Asmara, Eritrea. Am. Sci. Res. J. Eng. Technol. Sci. 2019, 57, 161–186. [Google Scholar]
- Subratty, A.; Nathire, M.H. A survey on home generated medical waste in Mauritius. Int. J. Environ. Health Res. 2005, 15, 45–52. [Google Scholar] [CrossRef]
- Saulick, B.; Bhoyroo, V.; Nazurally, N.; Lalljee, B. Heavy metal bioaccumulation in commercial Lethrinidae fish species in Mauritius. Ital. J. Food Saf. 2017, 6, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Beeharry, Y.; Bekaroo, G.; Bussoopun, D.; Bokhoree, C.; Phillips, M.R. Perspectives of leisure operators and tourists on the environmental impacts of coastal tourism activities: A case study of Mauritius. Environ. Dev. Sustain. 2021, 23, 10702–10726. [Google Scholar] [CrossRef]
- Bastaraud, A.; Rakotondramanga, J.M.; Mahazosaotra, J.; Ravaonindrina, N.; Jambou, R. Environmental Factors and the Microbial Quality of Urban Drinking Water in a Low-Income Country: The Case of Madagascar. Water 2018, 10, 1450. [Google Scholar] [CrossRef]
- van der Schyff, V.; Yive, N.S.C.K.; Polder, A.; Cole, N.C.; Tatayah, V.; Kylin, H.; Bouwman, H. Persistent organic pollutants in sea bird eggs from the Indian Ocean’s Mascarene Basin. Sci. Total Environ. 2021, 771, 145348. [Google Scholar] [CrossRef]
- Miraji, H.; Ripanda, A.; Moto, E. A review on the occurrences of persistent organic pollutants in corals, sediments, fish and waters of the Western Indian Ocean. Egypt. J. Aquat. Res. 2021, 1–7. [Google Scholar] [CrossRef]
- Elisephane, I.; Ishigaki, Y. The Effect Assessment of Industrial Activities on Air Pollution at Cimerwa and its Surrounding Areas, Rusizi-District-Rwanda. Int. J. Sustain. Energy Environ. Res. 2020, 9, 87–97. [Google Scholar] [CrossRef]
- Theoneste, S.; Vincent, N.M.; Xavier, N.F. The Effluent Quality Discharged and Its Impacts on the Receiving Environment Case of Kacyiru Sewerage Treatment Plant, Kigali, Rwanda. Int. J. Environ. Agric. Res. 2020, 6, 11. [Google Scholar] [CrossRef]
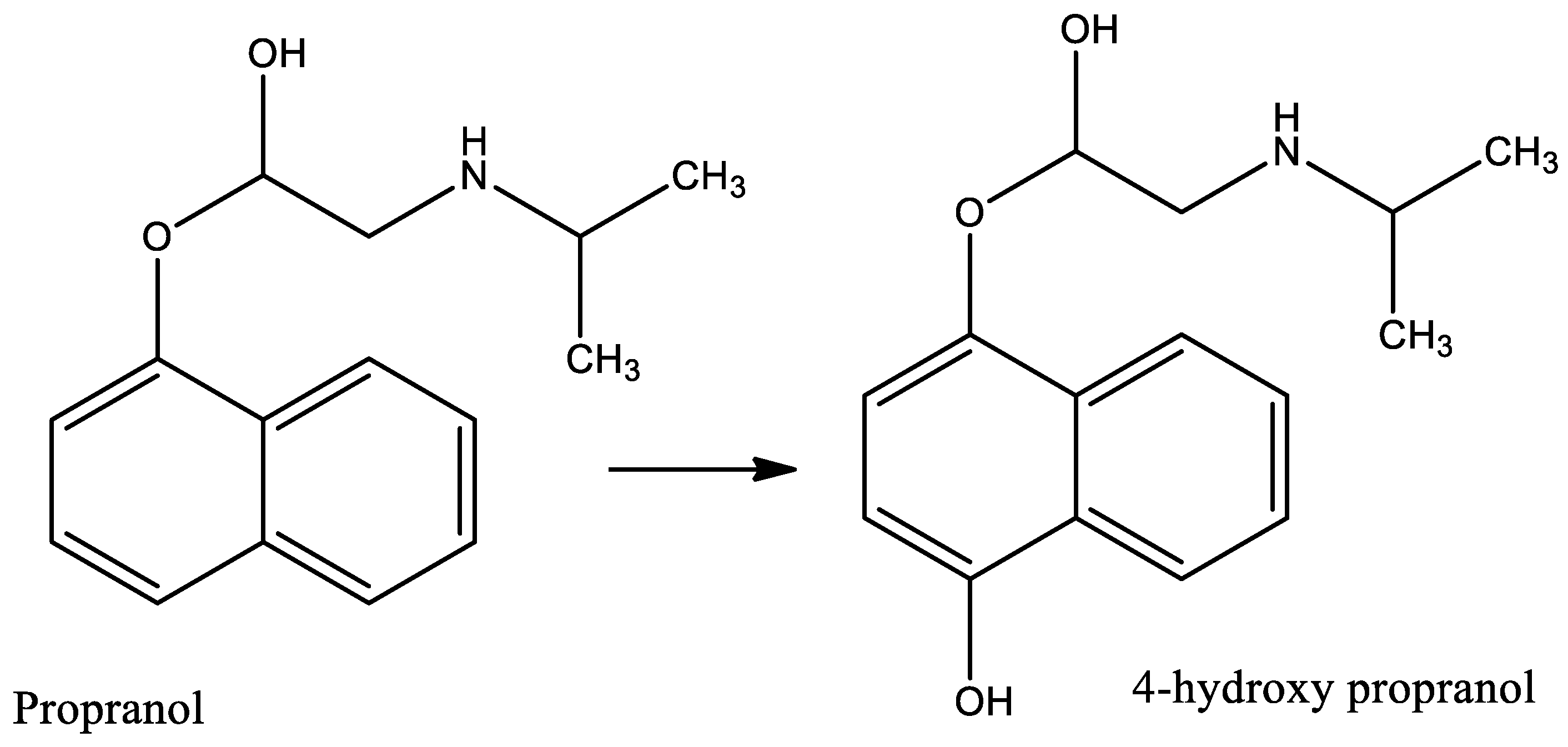
- Rastogi, T.; Leder, C.; Kümmerer, K. Re-Designing of Existing Pharmaceuticals for Environmental Biodegradability: A Tiered Approach with β-Blocker Propranolol as an Example. Environ. Sci. Technol. 2015, 49, 11756–11763. [Google Scholar] [CrossRef] [PubMed]
- Harelimana, V.; Gao, Z.J.; Nyiranteziryayo, E.; Nwankwegu, A.S. Identification of weaknesses in the implementation of environmental impact assessment regulations in industrial sector: A case study of some industries in Rwanda, Africa. J. Clean. Prod. 2020, 258, 120677. [Google Scholar] [CrossRef]
- Moya, B.; Parker, A.; Sakrabani, R.; Mesa, B. Evaluating the Efficacy of Fertilisers Derived from Human Excreta in Agriculture and Their Perception in Antananarivo, Madagascar. Waste Biomass Valorization 2017, 10, 941–952. [Google Scholar] [CrossRef]
- Pragst, F.; Stieglitz, K.; Runge, H.; Runow, K.-D.; Quig, D.; Osborne, R.; Runge, C.; Ariki, J. High concentrations of lead and barium in hair of the rural population caused by water pollution in the Thar Jath oilfields in South Sudan. Forensic Sci. Int. 2017, 274, 99–106. [Google Scholar] [CrossRef]
- Ruley, J.A.; Tumuhairwe, J.B.; Amoding, A.; Opolot, E.; Oryem-Origa, H.; Basamba, T. Assessment of plants for phytoremediation of hydrocarbon-contaminated soils in the Sudd Wetland of South Sudan. Plant Soil Environ. 2019, 65, 463–469. [Google Scholar] [CrossRef]
- Loboka, M.K.; Shihua, Q.; Celestino, J.L.; Hassan, S.O.; Wani, S. Municipal solid waste management practices and fecal coliform water contamination in the cities of the developing countries: The case of Juba, South Sudan. Int. J. Environ. Sci. 2013, 3, 1614–1624. [Google Scholar]
- Kuch, S.G.; Bavumiragira, J.P. Impacts of crude oil exploration and production on environment and its implications on human health: South Sudan Review. Int. J. Sci. Res. Publ. 2019, 9, 29322. [Google Scholar] [CrossRef]
- Boying, H.H.; Ping, F. Non-point source pollution and its impact on drinking water quality in River Nile- A case study of Juba South Sudan. Int. J. Sci. Res. Publ. 2020, 10, 483–496. [Google Scholar] [CrossRef]
- Mier, I.A.M.; Zhuo, L. Current Status of Municipal Solid Waste Management in Juba City, South Sudan. Int. J. Sci. Res. Publ. 2020, 10, 671–684. [Google Scholar] [CrossRef]
- Saad, S.A.G. Management of hospitals solid waste in Khartoum State. Environ. Monit. Assess. 2013, 185, 8567–8582. [Google Scholar] [CrossRef]
- Manirakiza, N.; Ndikumana, T.; Jung, C.G. Municipal Solid Waste Sorting in Burundi, Inventory and Perspectives: Case of Bujumbura City. Technium Conf. 2020, 5, 1148–1155. [Google Scholar]
- Quansah, A.; Ntaryamira, T.; Rwemera, J. Sludge Wastewater Management by Conventional Treatment Process: Case Study-Bujumbura Municipal Sewage. Int. J. Sci. 2018, 4, 52–65. [Google Scholar] [CrossRef]
- Niyongaboa, E.; Jang, Y.C.; Kang, D.; Sung, K. Generation, management practices and rapid risk assessment of solid medical wastes: A case study in Burundi. J. Mater. Cycles Waste Manag. 2019, 21, 950–961. [Google Scholar] [CrossRef]
- Mizero, M.; Ndikumana, T.; Jung, C.G. Briquettes From Solid Waste: A substitute For Charcoal in Burundi. Ann. Assoc. Am. Geogr. 1985, 75, 163–184. [Google Scholar]
- Gwimbi, P.; Kotelo, T.; Selimo, M.J. Heavy metal concentrations in sediments and Cyprinus carpio from Maqalika Reservoir –Maseru, Lesotho: An analysis of potential health risks to Fish consumers. Toxicol. Rep. 2020, 7, 475–479. [Google Scholar] [CrossRef]
- Chouvelon, T.; Brach-Papa, C.; Auger, D.; Bodin, N.; Bruzac, S.; Crochet, S.; Degroote, M.; Hollanda, S.J.; Hubert, C.; Knoery, J.; et al. Chemical contaminants (trace metals, persistent organic pollutants) in albacore tuna from western Indian and south-eastern Atlantic Oceans: Trophic influence and potential as tracers of populations. Sci. Total Environ. 2017, 596, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Munschy, C.; Bely, N.; Héas-Moisan, K.; Olivier, N.; Pollono, C.; Hollanda, S.; Bodin, N. Tissue-specific bioaccumulation of a wide range of legacy and emerging persistent organic contaminants in swordfish (Xiphias gladius) from Seychelles, Western Indian Ocean. Mar. Pollut. Bull. 2020, 158, 111436. [Google Scholar] [CrossRef]
- Hassan, D.A.T. Management of Abattoir Waste in Somalia: A Case Study of Mogadishu Slaughterhouse; Kesmonds International University: Bamenda, Cameroon, 2020. [Google Scholar]
- Massimo, C. An investigation of constraints and opportunities in setting up hygiene standards in Somalia meat export industry. In Veterinary Public Health; University of Nairobi: Nairobi, Kenya, 2015. [Google Scholar]
- Said, A.A.; Yurtal, R.; Cetin, M.; Gölpinar, M.S. Evaluation of some groundwater quality parameters using geostatistics in the urban coastal aquifer of Bosaso plain, Somalia. Tarım Bilim. Derg. 2021, 27, 88–97. [Google Scholar] [CrossRef]
- Ogah, C.; Adetifa, I.; Basheeru, K. Pharmaceuticals in the Environment: Levels of Selected Drugs in Water in Lagos, Nigeria. Niger. J. Pharm. Appl. Sci. Res. 2020, 9, 13–18. [Google Scholar]
- Wesström, T.; Andersson, J. Pharmaceutical Pollution in Irrigation Water: A Minor Field Study in Chirapatre Estates in Kumasi, Ghana, in Department of Earth Sciences; Uppsala University, Disciplinary Domain of Science and Technology, Earth Sciences: Uppsala, Sweden, 2014; p. 39. [Google Scholar]
- Oluwatosin, O.; Adekunle, B.; Obih, U.; Arne, H.; Olarinmoye, O.; Bakare, A.; Ugwumba, O.; Hein, A. Quantification of pharmaceutical residues in wastewater impacted surface waters and sewage sludge from Lagos, Nigeria. J. Environ. Chem. Ecotoxicol. 2016, 8, 14–24. [Google Scholar] [CrossRef]
- Ogunbanwo, O.M.; Kay, P.; Boxall, A.B.; Wilkinson, J.; Sinclair, C.J.; Shabi, R.A.; Fasasi, A.E.; Lewis, G.A.; Amoda, O.A.; Brown, L.E. High Concentrations of Pharmaceuticals in a Nigerian River Catchment. Environ. Toxicol. Chem. 2020, 1–8. [Google Scholar] [CrossRef]
- Idris, M.A.; Kolo, B.G.; Sani, A. Organic pollution indicator and anion concentration of pharmaceutical effluent and surface water In Minna, Niger State, Nigeria. Int. J. Eng. Sci. 2013, 2, 27–32. [Google Scholar]
- Inam, E.; Offiong, N.-A.; Kang, S.; Yang, P.; Essien, J. Assessment of the Occurrence and Risks of Emerging Organic Pollutants (EOPs) in Ikpa River Basin Freshwater Ecosystem, Niger Delta-Nigeria. Bull. Environ. Contam. Toxicol. 2015, 95, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Ite, A.E.; Harry, T.A.; Obadimu, C.O.; Asuaiko, E.R.; Inim, I.J. Petroleum Hydrocarbons Contamination of Surface Water and Groundwater in the Niger Delta Region of Nigeria. J. Environ. Pollut. Hum. Health 2018, 6, 51–61. [Google Scholar] [CrossRef]
- Germer, J.; Sinar, E. Pharmaceutical consumption and residuals potentially relevant to nutrient cycling in Greater Accra, Ghana. J. Agric. Rural. Dev. Trop. Subtrop. 2010, 111, 41–53. [Google Scholar]
- Azanu, D.; Styrishave, B.; Darko, G.; Weisser, J.J.; Abaidoo, R.C. Occurrence and risk assessment of antibiotics in water and lettuce in Ghana. Sci. Total Environ. 2018, 622-623, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Gnandi, K.; Tchangbedji, G.; Killi, K.; Baba, G.; Abbe, K. The Impact of Phosphate Mine Tailings on the Bioaccumulation of Heavy Metals in Marine Fish and Crustaceans from the Coastal Zone of Togo. Mine Water Environ. 2006, 25, 56–62. [Google Scholar] [CrossRef]
- Diallo, A.; Zotchi, K.; Lawson-Evi, P.; Bakoma, B.; Badjabaissi, E.; Kwashie, E.-G. Pesticides Use Practice by Market Gardeners in Lome (Togo). J. Toxicol. 2020, 2020, 1–5. [Google Scholar] [CrossRef]
- Mawussi, G.; Sanda, K.; Merlina, G.; Pinelli, E. Assessment of average exposure to organochlorine pesticides in southern Togo from water, maize (Zea mays) and cowpea (Vigna unguiculata). Food Addit. Contam. Part A 2009, 26, 348–354. [Google Scholar] [CrossRef]
- Tapsoba, K.; Bonzi-Coulibaly, Y. Production cotonnière et pollution des eaux par les pesticides au Burkina Faso. J.-Soc. Ouest Afr. Chim. 2006, 21, 87. [Google Scholar]
- Enaruvbe, G.; Keculah, K.; Atedhor, G.; Osewole, A. Armed conflict and mining induced land-use transition in northern Nimba County, Liberia. Glob. Ecol. Conserv. 2019, 17, e00597. [Google Scholar] [CrossRef]
- Wilson, S.T.K.; Wang, H.; Kabenge, M.; Qi, X. The mining sector of Liberia: Current practices and environmental challenges. Environ. Sci. Pollut. Res. 2017, 24, 18711–18720. [Google Scholar] [CrossRef]
- Daouda, M.M.A.; Hounkpè, S.P.; Djihouessi, M.B.; Akowanou, A.V.O.; Aïna, M.P.; Drogui, P. Physicochemical assessment of urban wastewater of Cotonou (Benin). Water Sci. Technol. 2021, 83, 1499–1510. [Google Scholar] [CrossRef]
- Yao, K.A.F.; Yao, B.K.; Belcourt, O.; Salze, D.; Lasm, T.; Lopez-Ferber, M.; Junqua, G. Mining Impacts Assessment Using the LCA Methodology: Case Study of Afema Gold Mine in Ivory Coast. Integr. Environ. Assess. Manag. 2021, 17, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Giannakis, S.; Rtimi, S.; Pulgarin, C. Light-Assisted Advanced Oxidation Processes for the Elimination of Chemical and Microbiological Pollution of Wastewaters in Developed and Developing Countries. Molecules 2017, 22, 1070. [Google Scholar] [CrossRef] [PubMed]
- Salomon, K.Y.; Brahima, S.; Maxime, G.F.; Martial, K.N.; Audrey, K.N. Wastewater Management of Korhogo City. Asian J. Environ. Ecol. 2019, 11, 1–11. [Google Scholar] [CrossRef]
- Toure, A.; Wenbiao, D.; Keita, Z.; Dembele, A.; Elzaki, E.E.A. Drinking water quality and risk for human health in Pelengana commune, Segou, Mali. J. Water Health 2019, 17, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Toure, A.; Wenbiao, D.; Keita, Z. An investigation of some water quality properties from different sources in Pelengana commune, Segou, Mali. J. Water Sanit. Hyg. Dev. 2018, 8, 449–458. [Google Scholar] [CrossRef]
- Keraita, B.; Drechsel, P.; Klutse, A.; Cofie, O. On-farm Treatment Options for Wastewater, Greywater and Fecal Sludge with Special Reference to West Africa; CGIAR Research Program on Water, Land and Ecosystems, International Water ManagementInstitute (IWMI): Colombo, Sri Lanka, 2014; Volume 1. [Google Scholar]
- Martínez-Santos, P.; Martín-Loeches, M.; García-Castro, N.; Solera, D.; Díaz-Alcaide, S.; Montero, E.; García-Rincón, J. A survey of domestic wells and pit latrines in rural settlements of Mali: Implications of on-site sanitation on the quality of water supplies. Int. J. Hyg. Environ. Health 2017, 220, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Garrison, V.; Majewski, M.; Foreman, W.; Genualdi, S.; Mohammed, A.; Simonich, S.M. Persistent organic contaminants in Saharan dust air masses in West Africa, Cape Verde and the eastern Caribbean. Sci. Total Environ. 2014, 468, 530–543. [Google Scholar] [CrossRef]
- Shah, K. Air Pollution in Small Island Developing States: A Growing but Avoidable Challenge Requiring Multilevel Interventions. Available online: https://ssrn.com/abstract=3776750 (accessed on 31 January 2021).
- Ferrante, M.; Signorelli, S.S.; Ferlito, S.L.; Grasso, A.; Dimartino, A.; Copat, C. Groundwater-based water wells characterization from Guinea Bissau (Western Africa): A risk evaluation for the local population. Sci. Total Environ. 2018, 619–620, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Mathiarasan, S.; Hüls, A. Impact of Environmental Injustice on Children’s Health—Interaction between Air Pollution and Socioeconomic Status. Int. J. Environ. Res. Public Health 2021, 18, 795. [Google Scholar] [CrossRef] [PubMed]
- Chatkin, J.; Correa, L.; Santos, U. External Environmental Pollution as a Risk Factor for Asthma. Clin. Rev. Allergy Immunol. 2021, 1–18. [Google Scholar] [CrossRef]
- Sly, P.D. Adverse Environmental Exposure and Respiratory Health in Children. Pediatr. Clin. N. Am. 2021, 68, 277–291. [Google Scholar] [CrossRef]
- Kavegue, A.; Eguavoen, I. The Experience and Impact of Urban Floods and Pollution in Ebo Town, Greater Banjul Area, in The Gambia; ZEF Working Paper Series; University of Bonn, Center for Development Research (ZEF): Bonnin, Gambia, 2016; p. 37. [Google Scholar]
- Dieng, C.; Mberu, B.; Dimbuene, Z.T.; Faye, C.; Amugsi, D.; Aboderin, I. Biomedical waste management in Dakar, Senegal: Legal framework, health and environment issues; policy and program options. Cities Health 2020, 2020, 1–15. [Google Scholar] [CrossRef]
- Appel, H. The Licit Life of Capitalism: US Oil in Equatorial Guinea; Duke University Press: Durham, UK; London, UK, 2019. [Google Scholar] [CrossRef]
- Branchet, P.; Cadot, E.; Fenet, H.; Sebag, D.; Ngatcha, B.N.; Borrell-Estupina, V.; Ngoupayou, J.R.N.; Kengne, I.; Braun, J.; Gonzalez, C. Polar pesticide contamination of an urban and peri-urban tropical watershed affected by agricultural activities (Yaoundé, Center Region, Cameroon). Environ. Sci. Pollut. Res. 2018, 25, 17690–17715. [Google Scholar] [CrossRef]
- Fantong, W.Y.; Satake, H.; Aka, F.T.; Ayonghe, S.N.; Asai, K.; Mandal, A.K.; Ako, A.A. Hydrochemical and isotopic evidence of recharge, apparent age, and flow direction of groundwater in Mayo Tsanaga River Basin, Cameroon: Bearings on contamination. Environ. Earth Sci. 2010, 60, 107–120. [Google Scholar] [CrossRef]
- Asongwe, G.A.; Yerima, B.P.; Tening, A.S. Vegetable production and the livelihood of farmers in Bamenda Municipality, Cameroon. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 682–700. [Google Scholar]
- Forton, O.T.; Manga, V.E.; Tening, A.S.; Asaah, A.V. Land contamination risk management in Cameroon: A critical review of the existing policy framework. Land Use Policy 2012, 29, 750–760. [Google Scholar] [CrossRef]
- Mayoudom, E.V.T.; Nguidjoe, E.; Mballa, R.N.; Tankoua, O.F.; Fokunang, C.; Anyakora, C.; Blackett, K.N. Identification and quantification of 19 pharmaceutical active compounds and metabolites in hospital wastewater in Cameroon using LC/QQQ and LC/Q-TOF. Environ. Monit. Assess. 2018, 190, 723. [Google Scholar] [CrossRef]
- Pouokam, G.B.; Foudjo, B.U.S.; Samuel, C.; Yamgai, P.F.; Silapeux, A.K.; Sando, J.T.; Atonde, G.F.; Frazzoli, C. Contaminants in Foods of Animal Origin in Cameroon: A One Health Vision for Risk Management “from Farm to Fork”. Front. Public Health 2017, 5, 197. [Google Scholar] [CrossRef] [PubMed]
- Matos, R.; Ferreira, F.; Alves, L.; Ramos, E.; Costa, L.; Matos, J. Multi-Criteria Framework for Selection of City-Wide Sanitation Solutions in Coastal Towns in Northern Angola. Sustainability 2021, 13, 5627. [Google Scholar] [CrossRef]
- Reksten, A.M.; Victor, A.M.J.C.; Neves, E.B.N.; Christiansen, S.M.; Ahern, M.; Uzomah, A.; Lundebye, A.-K.; Kolding, J.; Kjellevold, M. Nutrient and Chemical Contaminant Levels in Five Marine Fish Species from Angola—The EAF-Nansen Programme. Foods 2020, 9, 629. [Google Scholar] [CrossRef]
- Paca, J.M.; Santos, F.M.; Pires, J.C.; Leitão, A.A.; Boaventura, R.A. Quality assessment of water intended for human consumption from Kwanza, Dande and Bengo rivers (Angola). Environ. Pollut. 2019, 254, 113037. [Google Scholar] [CrossRef]
- Yala, J.F.; Moundounga, H.K.; Pambo-Pambo, A.B.; Mabika, R.M.; Minko, E.S.; Lepengué, A.N.; Souza, E.A. Evaluation of physicochemical and bacteriological pollution of some water bodies of Franceville (Gabon). J. Biodivers. Environ. Sci. 2017, 11, 365–375. [Google Scholar]
- Mbehang Nguema, P.P.; Onanga, R.; Ndong Atome, G.R.; Obague Mbeang, J.C.; Mabika, A.; Yaro, M.; Lounnas, M.; Dumont, Y.; Zohra, Z.F.; Godreuil, S.; et al. Characterization of ESBL-producing enterobacteria from fruit bats in an unprotected area of Makokou, Gabon. Microorganisms 2020, 8, 138. [Google Scholar] [CrossRef]
- Muliele, T.M.; Manzenza, C.M.; Ekuke, L.W.; Diaka, C.P.; Ndikubwayo, D.M.; Kapalay, O.M.; Mundele, A.N. Utilisation et gestion des pesticides en cultures maraîchères: Cas de la zone de Nkolo dans la province du Kongo Central, République démocratique du Congo. J. Appl. Biosci. 2017, 11, 11954–11972. [Google Scholar] [CrossRef]
- Litébé, A.C.; Ngakegni-Limbili, C.A.; Mvouezolo, R.F.L.; Loupangou, C.N.; Nzobadila, D.; Ouamba, J.M. Impact of Reject of Dairy Wastewater into the Aquatic Environment: Case of the Bayo Dairy Company (Brazzaville-Congo). Int. J. Environ. Clim. Chang. 2020, 10, 1–12. [Google Scholar] [CrossRef][Green Version]
- Ngoulou, T.B.; Moyen, R.; Nguimb, E.; Ahombo, G.; Matini, L. Study of the Physico-Chemical and Microbiological Parameters of Household Wastewater in Brazzaville. Int. J. Environ. Sci. 2019, 4, 80–90. [Google Scholar]
- Moyen, R.; Ngoulou, T.B.; Nguimbi, E.; Ahombo, G. Antibiotic Resistance Phenotypes of Enterobacteriaceae Isolated from Household Wastewater in Brazzaville, Republic of Congo. Adv. Microbiol. 2021, 11, 27–36. [Google Scholar] [CrossRef]
- Kindzierski, W.B.; Gabos, S. Health effects associated with wastewater treatment, disposal, and reuse. Water Environ. Res. 1995, 67, 749–755. [Google Scholar] [CrossRef]
- Kindzierski, W.B.; Gabos, S. Health effects associated with wastewater treatment, disposal, and reuse. Water Environ. Res. 1994, 66, 651–657. [Google Scholar] [CrossRef]
- Moisès, D.J.; Kgabi, N.; Lewis, E. Developing a contamination susceptibility index for the Goreangab Dam in Namibia. Phys. Chem. Earth Parts A/B/C 2020, 124, 102916. [Google Scholar] [CrossRef]
- Julies, E.; Alema, A.; Smith, B.; Gebrekidanasgedom, A.; Zenebe, G.; Jampani, M.; Ito, M.; Hettiarachchi, H. Application of Bioaugmentation for Sustainable Treatment of Used Water in Namibia and Ethiopia. In Proceedings of the Dresden Nexus Conference, Dresden, Germany, 25 March 2015. [Google Scholar]
- Bu, Q.; Shi, X.; Yu, G.; Huang, J.; Wang, B. Assessing the persistence of pharmaceuticals in the aquatic environment: Challenges and needs. Emerg. Contam. 2016, 2, 145–147. [Google Scholar] [CrossRef]
- Rose, N.L.; Milner, A.M.; Fitchett, J.; Langerman, K.E.; Yang, H.; Turner, S.; Jourdan, A.-L.; Shilland, J.; Martins, C.C.; de Souza, A.C.; et al. Natural archives of long-range transported contamination at the remote lake Letšeng-la Letsie, Maloti Mountains, Lesotho. Sci. Total Environ. 2020, 737, 139642. [Google Scholar] [CrossRef]
- Tapela, K.; Rahube, T. Isolation and antibiotic resistance profiles of bacteria from influent, effluent and downstream: A study in Botswana. Afr. J. Microbiol. Res. 2019, 13, 279–289. [Google Scholar]
- Spray, J.; Wolf, S. Industries without Smokestacks in Uganda and Rwanda; Oxford University Press: Oxford, UK, 2018; pp. 341–363. [Google Scholar] [CrossRef]
- Luzardo, O.P.; Boada, L.D.; Carranza, C.; Ruiz-Suárez, N.; Henríquez-Hernández, L.A.; Valerón, P.F.; Zumbado, M.; Camacho, M.; Arellano, J.L.P. Socioeconomic development as a determinant of the levels of organochlorine pesticides and PCBs in the inhabitants of Western and Central African countries. Sci. Total Environ. 2014, 497–498, 97–105. [Google Scholar] [CrossRef]
- Biošić, M.; Dabić, D.; Škorić, I.; Babić, S. Effects of environmental factors on nitrofurantoin photolysis in water and its acute toxicity assessment. Environ. Sci. Process. Impacts 2021, 23, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Bu, Q.; Li, Q.; Zhang, H.; Wu, D.; Yu, G. Using a fugacity model to determine the degradation rate of typical polycyclic musks in the field: A case study in the North Canal River watershed of Beijing, China. J. Environ. Manag. 2021, 302, 114096. [Google Scholar] [CrossRef] [PubMed]
- Aftab, N.F.; Ahmad, K.S.; Gul, M.M. Sorptive and degradative assessments of environmentally pestilential Benzimidazole fungicide Fuberidazole in pedosphere. Int. J. Environ. Anal. Chem. 2021, 2021, 1–18. [Google Scholar] [CrossRef]
- Munschy, C.; Vigneau, E.; Bely, N.; Héas-Moisan, K.; Olivier, N.; Pollono, C.; Hollanda, S.; Bodin, N. Legacy and emerging organic contaminants: Levels and profiles in top predator fish from the western Indian Ocean in relation to their trophic ecology. Environ. Res. 2020, 188, 109761. [Google Scholar] [CrossRef] [PubMed]
- Hossein, M. Toxicological Aspects of Emerging Contaminants, in Emerging and Eco-Friendly Approaches for Waste Management; Springer Nature Singapore Pte Ltd.: Singapore, 2019; pp. 33–58. [Google Scholar] [CrossRef]
- aus der Beek, T.; Weber, F.A.; Bergmann, A.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A. Pharmaceuticals in the environment: Global occurrence and potential cooperative action under the Strategic Approach to International Chemicals Management. Ger. Fed. Environ. Agency 2016, 35, 823–835. [Google Scholar]
- Al Aukidy, M.; Al Chalabi, S.; Verlicchi, P. Hospital Wastewater Treatments Adopted in Asia, Africa, and Australia. In The Handbook of Environmental Chemistry; Springer: Cham, Switzerland, 2017; Volume 60, pp. 171–188. [Google Scholar] [CrossRef]
- Trautwein, C.; Berset, J.-D.; Wolschke, H.; Kümmerer, K. Occurrence of the antidiabetic drug Metformin and its ultimate transformation product Guanylurea in several compartments of the aquatic cycle. Environ. Int. 2014, 70, 203–212. [Google Scholar] [CrossRef]
- Kidd, K.A.; Blanchfield, P.; Mills, K.; Palace, V.P.; Evans, R.E.; Lazorchak, J.; Flick, R.W. Collapse of a fish population after exposure to a synthetic estrogen. Proc. Natl. Acad. Sci. USA 2007, 104, 8897–8901. [Google Scholar] [CrossRef]
- Hassanisaadi, M.; Bonjar, G.; Rahdar, A.; Pandey, S.; Hosseinipour, A.; Abdolshahi, R. Environmentally Safe Biosynthesis of Gold Nanoparticles Using Plant Water Extracts. Nanomaterials 2021, 11, 2033. [Google Scholar] [CrossRef]
- Alaba, P.A.; Sani, Y.M.; Olupinla, S.F.; Daud, W.M.W.; Mohammed, I.Y.; Enweremadu, C.C.; Ayodele, O.O. Toward N-nitrosamines free water: Formation, prevention, and removal. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2448–2489. [Google Scholar] [CrossRef]
- Margot, J.; Kienle, C.; Magnet, A.; Weil, M.; Rossi, L.; de Alencastro, L.F.; Abegglen, C.; Thonney, D.; Chèvre, N.; Schärer, M.; et al. Treatment of micropollutants in municipal wastewater: Ozone or powdered activated carbon? Sci. Total Environ. 2013, 461, 480–498. [Google Scholar] [CrossRef]
- NDMA. Background Technical Information for N-Nitrosodimethylamine (NDMA). In Water Research Foundation Advancing the Science of Water; Water Research Foundation: Alexandria, VA, USA, 2015. [Google Scholar]
- Haddad, T.; Kümmerer, K. Characterization of photo-transformation products of the antibiotic drug Ciprofloxacin with liquid chromatography–tandem mass spectrometry in combination with accurate mass determination using an LTQ-Orbitrap. Chemosphere 2014, 115, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Teta, C.; Naik, Y.S. Vitellogenin induction and reduced fecundity in zebrafish exposed to effluents from the City of Bulawayo, Zimbabwe. Chemosphere 2017, 167, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.K.; Rehman, M.Y.A.; Malik, R.N. Fate and toxicity of pharmaceuticals in water environment: An insight on their occurrence in South Asia. J. Environ. Manag. 2020, 271, 111030. [Google Scholar] [CrossRef]
- Shen, R.; Andrews, S.A. Demonstration of 20 pharmaceuticals and personal care products (PPCPs) as nitrosamine precursors during chloramine disinfection. Water Res. 2011, 45, 944–952. [Google Scholar] [CrossRef]
- Li, C.-M. Performances and influential factors of adsorption of chlorpheniramine and N-nitrosamine formation potentials by graphene oxide-iron oxide composites. In Environmental Engineering; Institute of Environmental Engineering, Natinal Sun Yet-sen University: Guangzhou, China, 2016; p. 147. [Google Scholar]
- Sakai, H.; Takamatsu, T.; Oguma, K.; Murakami, M.; Kosaka, K.; Asami, M.; Takizawa, S. Effects of natural organic matter and nitrate on the behavior of nitrosodimethylamine during ultraviolet irradiation and chloramination. J. Water Supply Res. Technol. 2014, 63, 260–267. [Google Scholar] [CrossRef]
- Lampard, J.; Leusch, F.D.L.; Roiko, A.; Chapman, H.F. Contaminants of concern in recycled water. Water 2010, 37, 54–60. [Google Scholar]
- Lewis, G.A.; Mathieu, D.; Phan-Tan-Luu, R. Pharmaceutical Experimental Design; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Kümmerer, K. The presence of pharmaceuticals in the environment due to human use – present knowledge and future challenges. J. Environ. Manag. 2009, 90, 2354–2366. [Google Scholar] [CrossRef]
- Demadis, K.D.; Paspalaki, M.; Theodorou, J. Controlled Release of Bis(phosphonate) Pharmaceuticals from Cationic Biodegradable Polymeric Matrices. Ind. Eng. Chem. Res. 2011, 50, 5873–5876. [Google Scholar] [CrossRef]
- Koller, M. Biodegradable and Biocompatible Polyhydroxy-alkanoates (PHA): Auspicious Microbial Macromolecules for Pharmaceutical and Therapeutic Applications. Molecules 2018, 23, 362. [Google Scholar] [CrossRef]
- Tscharntke, T.; Grass, I.; Wanger, T.C.; Westphal, C.; Batáry, P. Beyond organic farming – harnessing biodiversity-friendly landscapes. Trends Ecol. Evol. 2021, 36, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.-S.; Wang, S.; Zhang, F.; Desneux, N. Biological Control with Trichogramma in China: History, Present Status, and Perspectives. Annu. Rev. Èntomol. 2021, 66, 463–484. [Google Scholar] [CrossRef]
- Farhan, S.A.; Dadoosh, R.M.; Jassim, A. Evaluation of Phytochemical, Total phenolic and Antioxidant Activity of Carica Papaya Seed for Its Use in Biosynthesis of Gold Nanoparticles. Egypt. J. Chem. 2021, 64, 4301–4310. [Google Scholar] [CrossRef]
- Botteon, C.E.A.; Silva, L.B.; Ccana-Ccapatinta, G.V.; Silva, T.S.; Ambrosio, S.R.; Veneziani, R.C.S.; Bastos, J.K.; Marcato, P.D. Biosynthesis and characterization of gold nanoparticles using Brazilian red propolis and evaluation of its antimicrobial and anticancer activities. Sci. Rep. 2021, 11, 1974. [Google Scholar] [CrossRef] [PubMed]
- Hosny, M.; Fawzy, M.; Abdelfatah, A.M.; Fawzy, E.E.; Eltaweil, A.S. Comparative study on the potentialities of two halophytic species in the green synthesis of gold nanoparticles and their anticancer, antioxidant and catalytic efficiencies. Adv. Powder Technol. 2021, 32, 3220–3233. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Umayavalli, M.; Kokila, P.; Jeyalalitha, T.; Vijayapreetha, T.S.; Otchadevan, S. Biosynthesis of Gold Nanoparticles Using Solanum Lycopersicum. Synthesis 2021, 9, 2078–2080. [Google Scholar]
- Amiri, M.; Mohammadzadeh, V.; Yazdi, M.; Barani, M.; Rahdar, A.; Kyzas, G. Plant-Based Gums and Mucilages Applications in Pharmacology and Nanomedicine: A Review. Molecules 2021, 26, 1770. [Google Scholar] [CrossRef]
- Mahmud, S.; Biswas, S.; Paul, G.K.; Mita, M.A.; Promi, M.M.; Afrose, S.; Hasan, R.; Zaman, S.; Uddin, S.; Dhama, K.; et al. Plant-based phytochemical screening by targeting main protease of SARS-CoV-2 to design effective potent inhibitors. Biology 2021, 10, 589. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hao, Y.; Liu, Y.; Li, R.; Huang, D.; Pan, Y. Nanomedicine in lung cancer: Current states of overcoming drug resistance and improving cancer immunotherapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1654. [Google Scholar] [CrossRef]
- Cho, C.; Yu, C.; Wu, C.; Ho, J.; Yang, C.; Yu, D. Decreased drug resistance of bladder cancer using phytochemicals treatment. Kaohsiung J. Med. Sci. 2021, 37, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Mishra, S.B. Chemical Nanosensors for Monitoring Environmental Pollution. Appl. Nanotechnol. Water Res. 2014, 309–332. [Google Scholar] [CrossRef]
- Brahmkhatri, V.; Pandit, P.; Rananaware, P.; D’Souza, A.; Kurkuri, M.D. Recent progress in detection of chemical and biological toxins in Water using plasmonic nanosensors. Trends Environ. Anal. Chem. 2021, 30, e00117. [Google Scholar] [CrossRef]
- Sharma, P.; Pandey, V.; Sharma, M.M.M.; Patra, A.; Singh, B.; Mehta, S.; Husen, A. A Review on Biosensors and Nanosensors Application in Agroecosystems. Nanoscale Res. Lett. 2021, 16, 136. [Google Scholar] [CrossRef]
- Mahmoud, A.E.D.; Fawzy, M. Nanosensors and Nanobiosensors for Monitoring the Environmental Pollutants. Funct. Graded Mater. 2021, 229–246. [Google Scholar] [CrossRef]
- Johnson, M.S.; Sajeev, S.; Nair, R.S. Role of Nanosensors in agriculture. In Proceedings of the 2021 International Conference on Computational Intelligence and Knowledge Economy (ICCIKE), Dubai, United Arab Emirates, 17–18 March 2021; pp. 58–63. [Google Scholar]
- Sharma, P.; Kumar, S. Bioremediation of heavy metals from industrial effluents by endophytes and their metabolic activity: Recent advances. Bioresour. Technol. 2021, 339, 125589. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.P.; Almeida, C.M.R.; Salgado, M.A.; Carvalho, M.F.; Mucha, A.P. Pharmaceutical Compounds in Aquatic Environments—Occurrence, Fate and Bioremediation Prospective. Toxics 2021, 9, 257. [Google Scholar] [CrossRef]
- Kaczorek, E.; Smułek, W. Special Issue “Study of Biodegradation and Bioremediation”. Processes 2021, 9, 1130. [Google Scholar] [CrossRef]
- Mojiri, A.; Baharlooeian, M.; Zahed, M. The Potential of Chaetoceros muelleri in Bioremediation of Antibiotics: Performance and Optimization. Int. J. Environ. Res. Public Health 2021, 18, 977. [Google Scholar] [CrossRef]
- Jaswal, R.; Bedi, A.; Bedi, I.; Jaiswar, A.; Jasrotia, R.S. Phytoremediation of soil and water. In Phytoremediation; Elservier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2022; pp. 239–262. [Google Scholar]
| Matrix | Substance (s) | Concentration (ng/L) | References |
|---|---|---|---|
| Wastewater | Clarithromycin | 5–30 | [34] |
| Erythromycin | 10–100 | ||
| Sulfadimidine | 0–10 | ||
| Sulfamethoxazole | 5–1000 | ||
| Sulfapyridine | 5–110 | ||
| Chlortetracycline | 90 | ||
| Oxytetracycline | 100 | ||
| Trimethoprim | 5–10,000 | ||
| Seawater | Ibuprofen | 160 | [31] |
| Naproxen | 160 | ||
| Wastewater | Nevirapine and efavirenz | 2100 ng/L 17,400 ng/L | [36] |
| Wastewater | Ibuprofen | 117,000 | |
| Surface water | 84,600 | [37] | |
| Sediments | 65,900 | ||
| Water | Concentrations were efavirenz > nevirapine > carbamazepine > methocarbamol > bromacil > venlafaxine. | 164–593 | [38] |
| Surface water | Antiretrovirals (ARVs) | 26.5–430 | [39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ripanda, A.S.; Rwiza, M.J.; Nyanza, E.C.; Njau, K.N.; Vuai, S.A.H.; Machunda, R.L. A Review on Contaminants of Emerging Concern in the Environment: A Focus on Active Chemicals in Sub-Saharan Africa. Appl. Sci. 2022, 12, 56. https://doi.org/10.3390/app12010056
Ripanda AS, Rwiza MJ, Nyanza EC, Njau KN, Vuai SAH, Machunda RL. A Review on Contaminants of Emerging Concern in the Environment: A Focus on Active Chemicals in Sub-Saharan Africa. Applied Sciences. 2022; 12(1):56. https://doi.org/10.3390/app12010056
Chicago/Turabian StyleRipanda, Asha S., Mwemezi Johaiven Rwiza, Elias C. Nyanza, Karoli N. Njau, Said A. H. Vuai, and Revocatus L. Machunda. 2022. "A Review on Contaminants of Emerging Concern in the Environment: A Focus on Active Chemicals in Sub-Saharan Africa" Applied Sciences 12, no. 1: 56. https://doi.org/10.3390/app12010056
APA StyleRipanda, A. S., Rwiza, M. J., Nyanza, E. C., Njau, K. N., Vuai, S. A. H., & Machunda, R. L. (2022). A Review on Contaminants of Emerging Concern in the Environment: A Focus on Active Chemicals in Sub-Saharan Africa. Applied Sciences, 12(1), 56. https://doi.org/10.3390/app12010056






