The Combination of Low-Temperature Plasma and Tripterygium wilfordii Hook F on Ameliorating Imiquimod-Induced Psoriasiform Dermatitis in Mice
Abstract
:1. Introduction
2. Experiment Setup and Methods
2.1. Reagents
2.2. IMQ-Induced Psoriatic-Like Mouse Model
2.3. Measurement of Skin Inflammation Severity, Immunohistochemistry and Cytokine Detection via Quantitative Real-Time PCR
2.4. Statistical Analyses
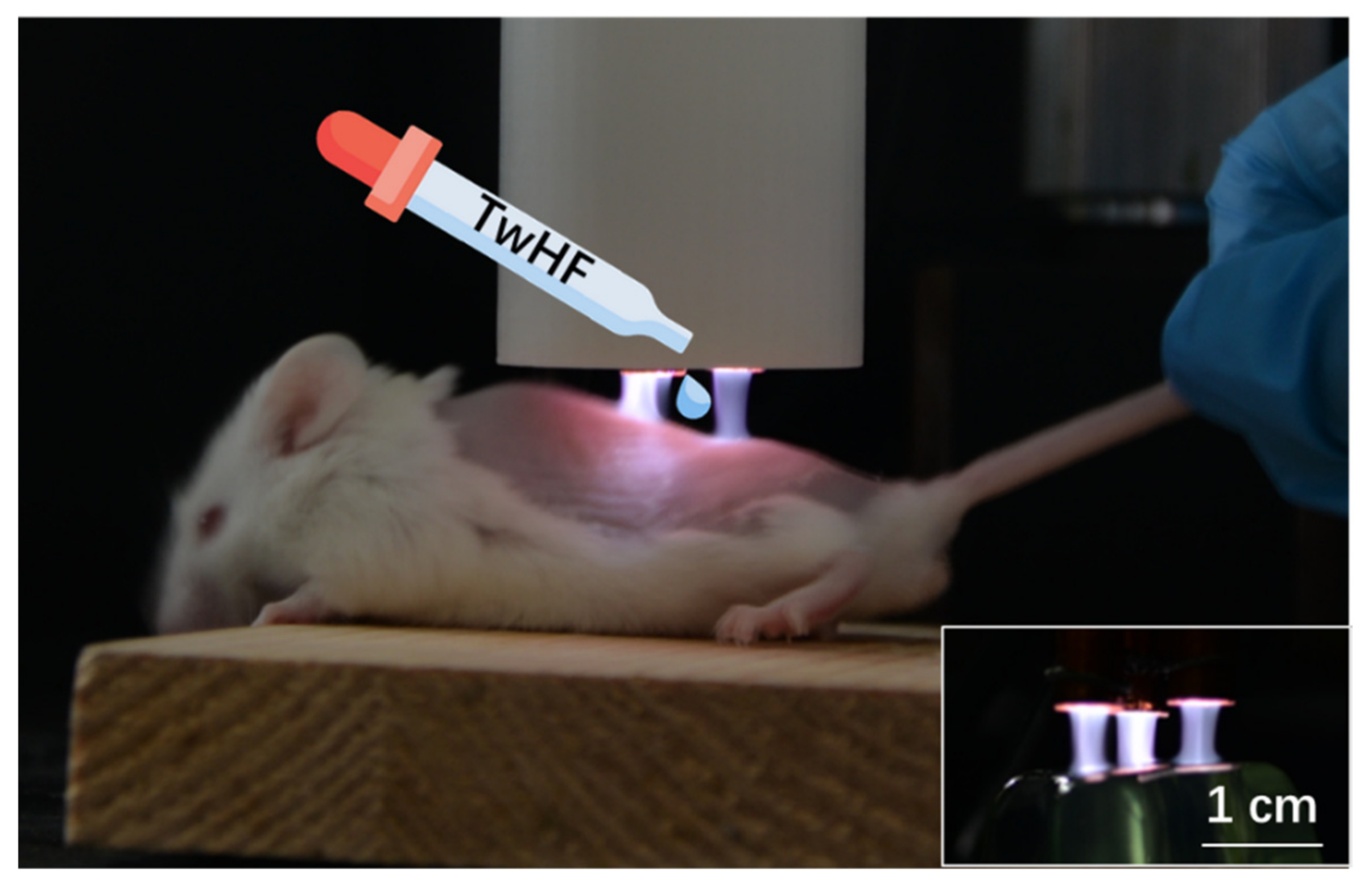
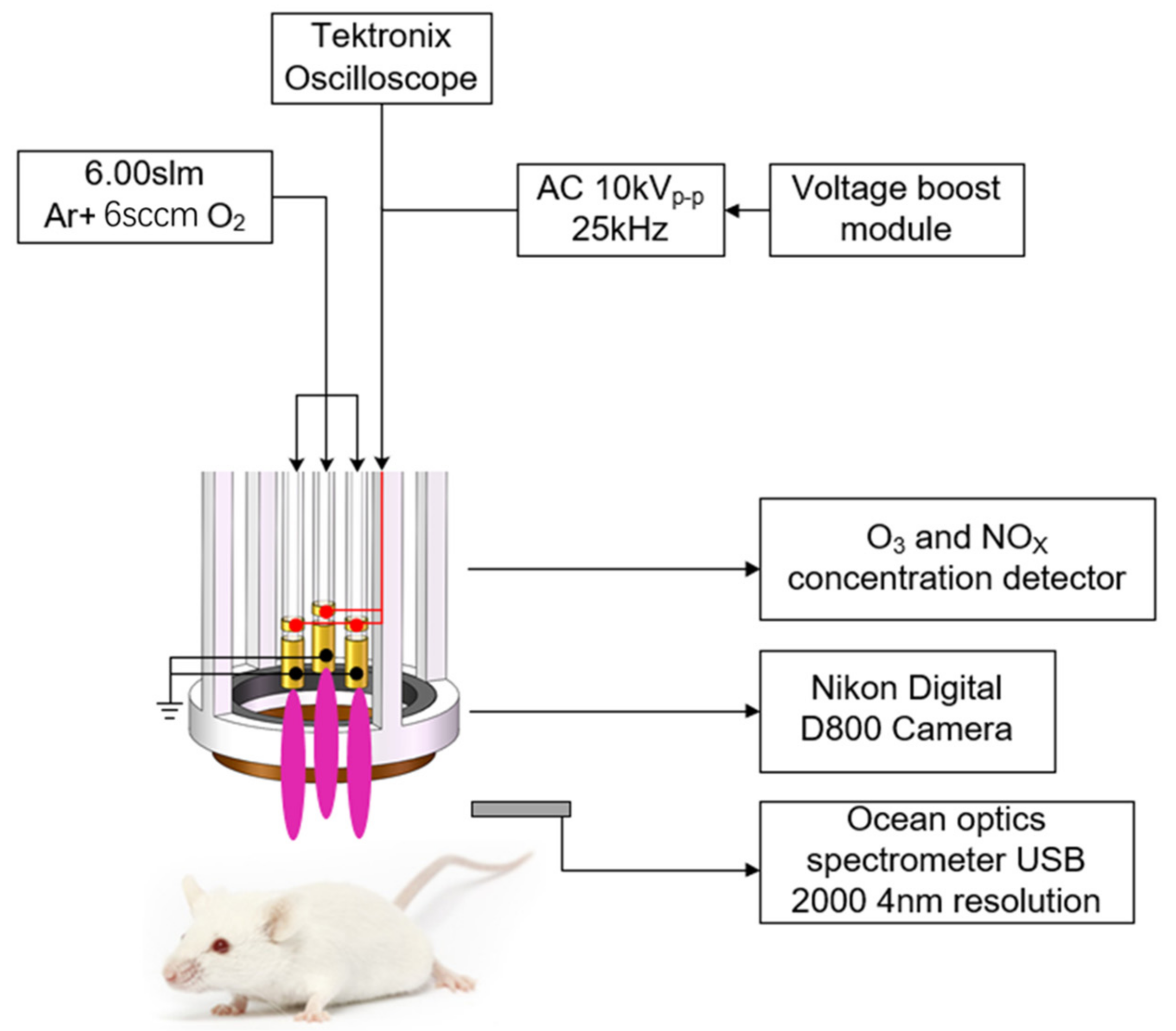
2.5. LTP Jet Array System
3. Results and Analysis
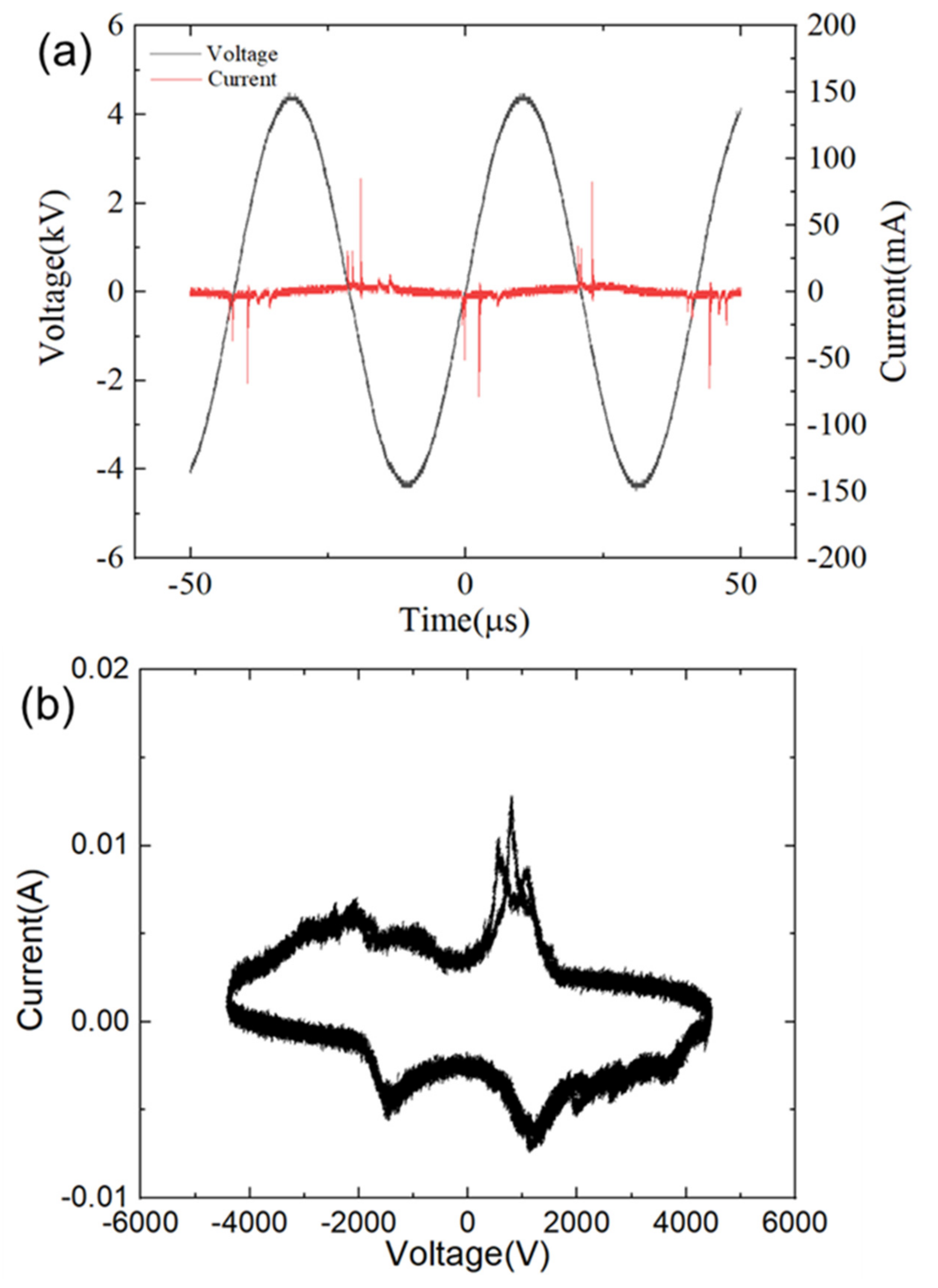
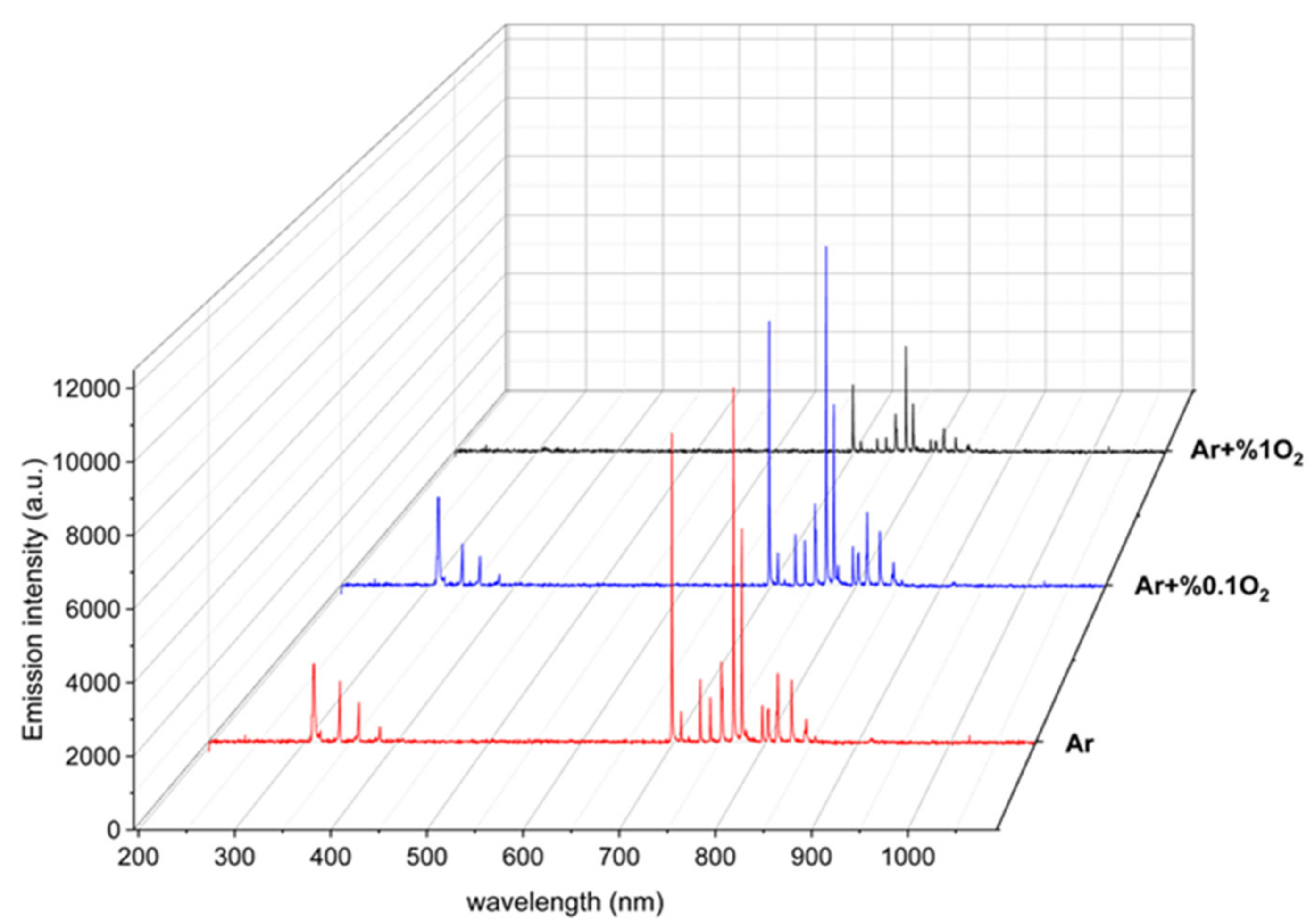
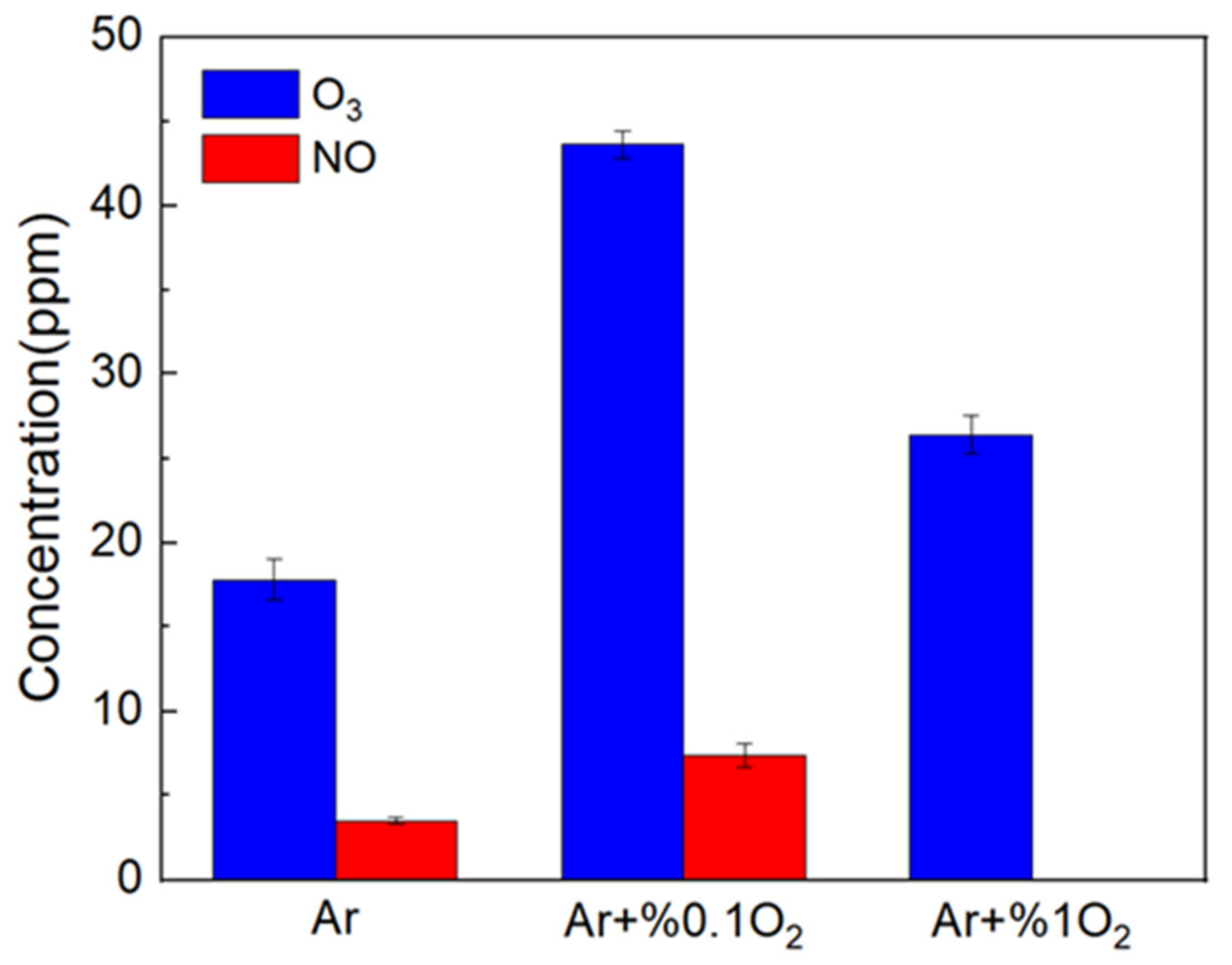
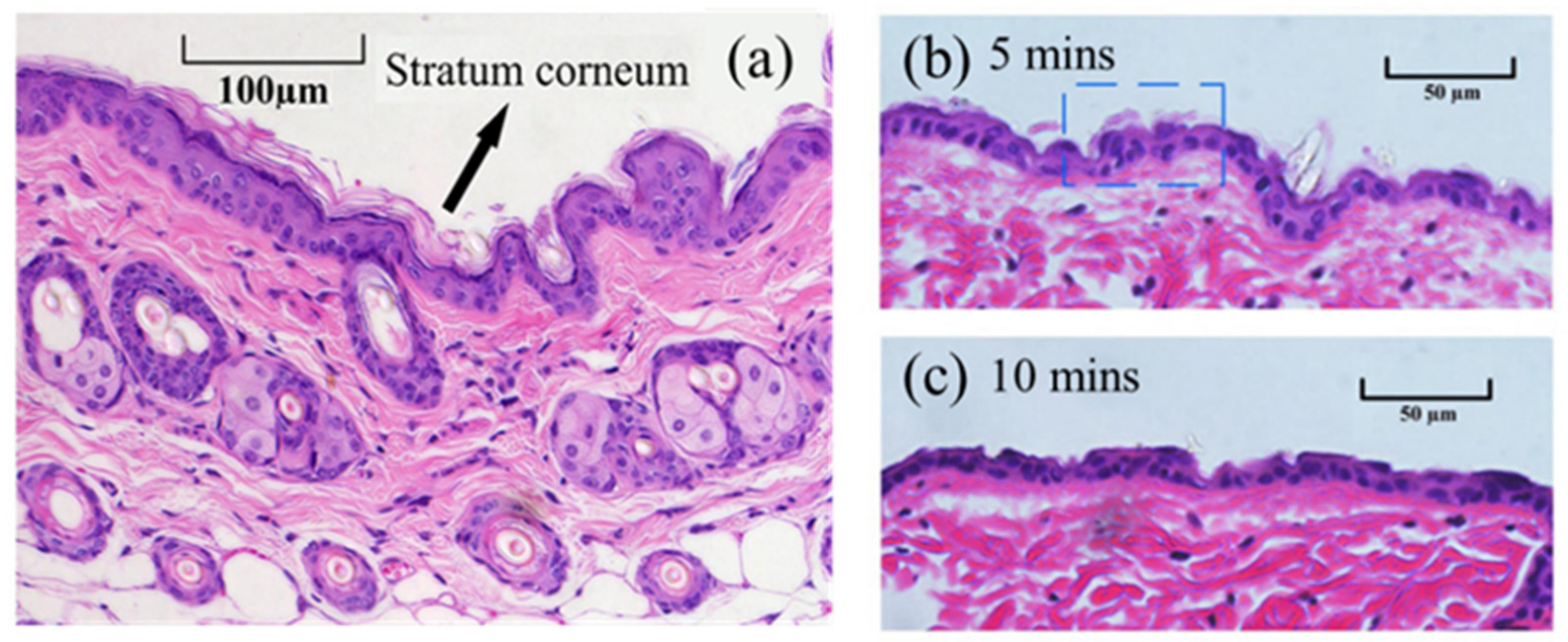
3.1. Characteristics of LTP Jet Array
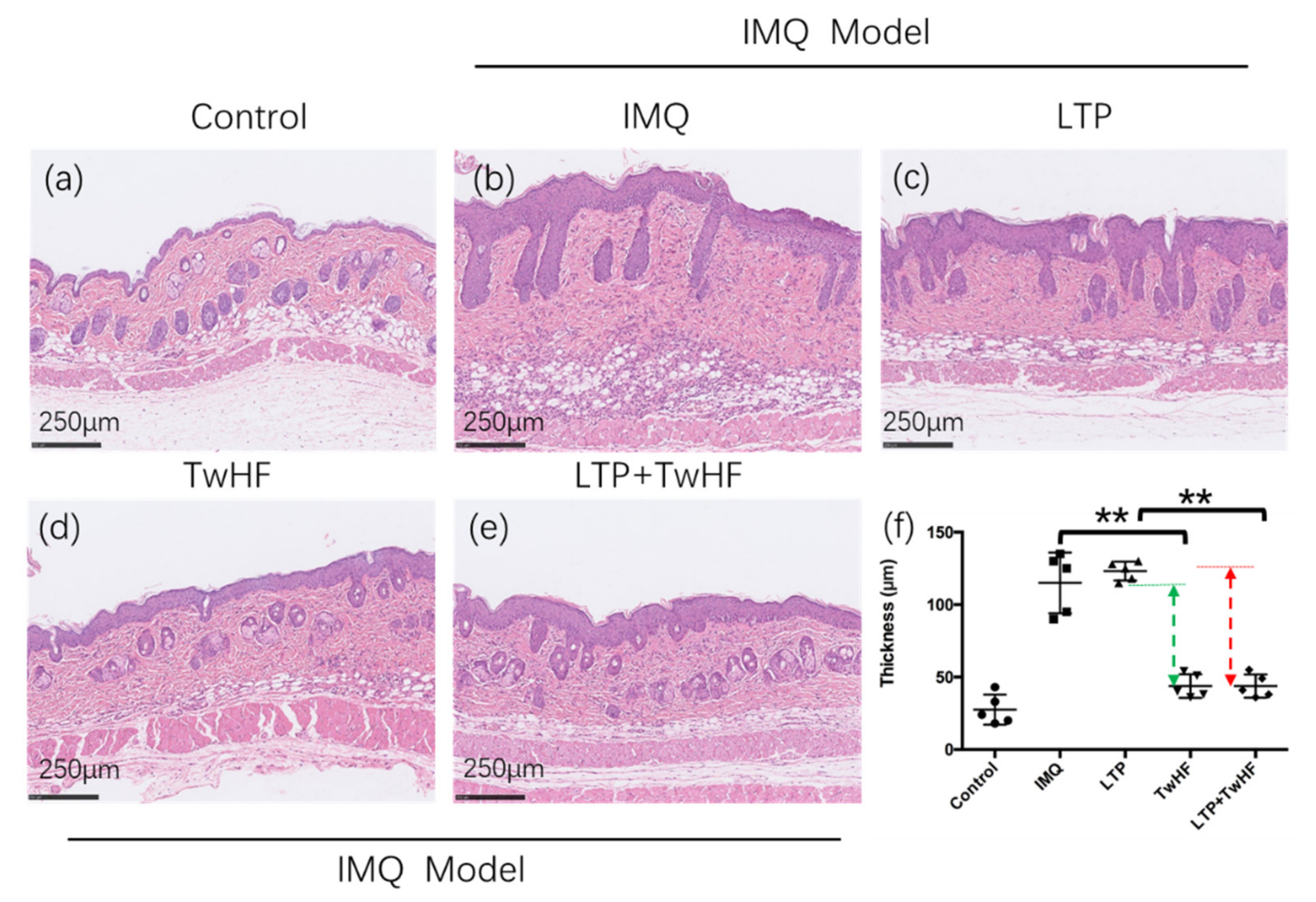
3.2. Plasma + TwHF Treatment Ameliorated IMQ-Induced Psoriatic Skin Lesion
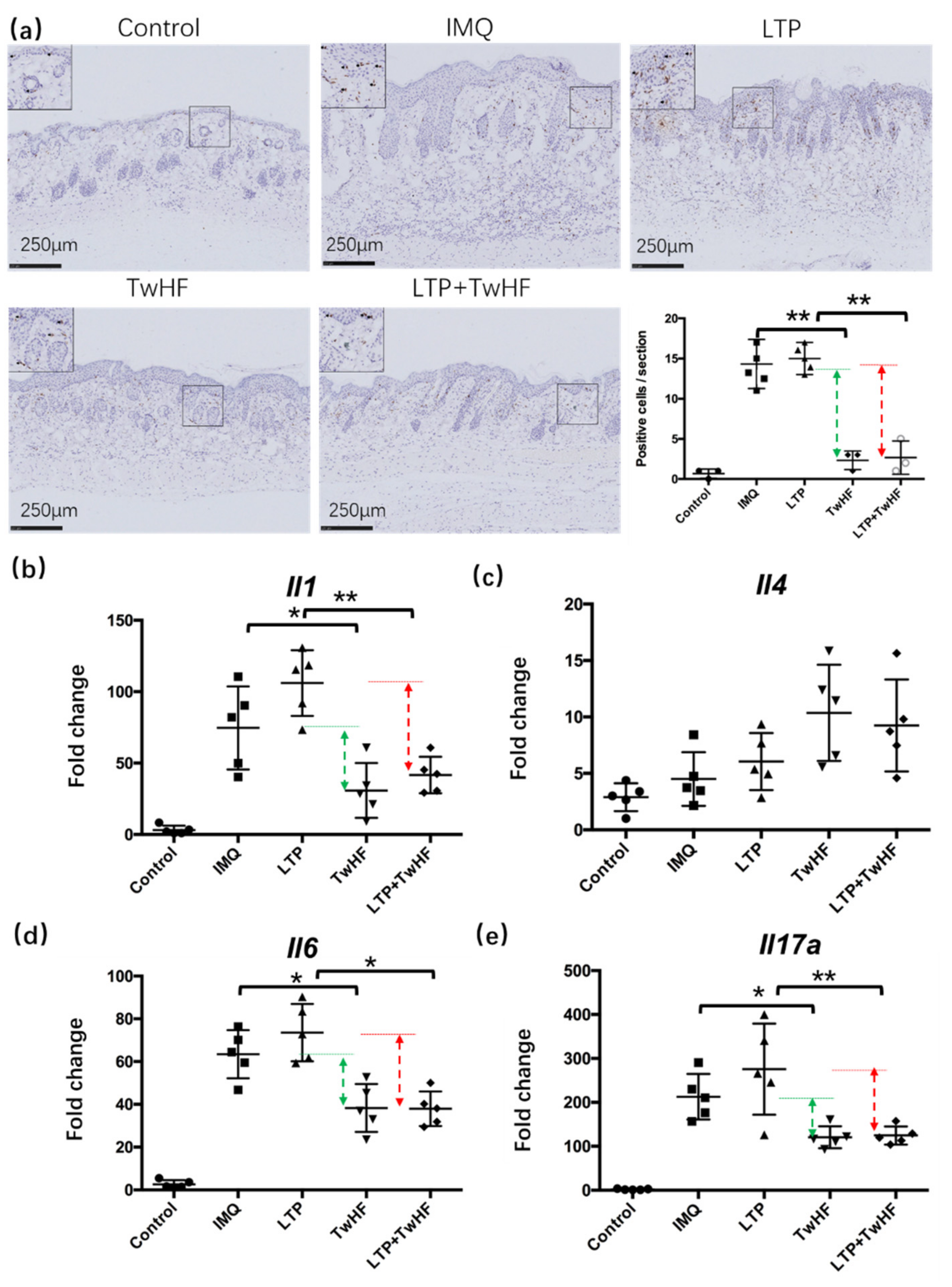
3.3. Plasma + TwHF Treatment Inhibited Inflammatory Cell Infiltration and Inflammatory Cytokines Released in the Skin in the IMQ-Induced Mouse Model
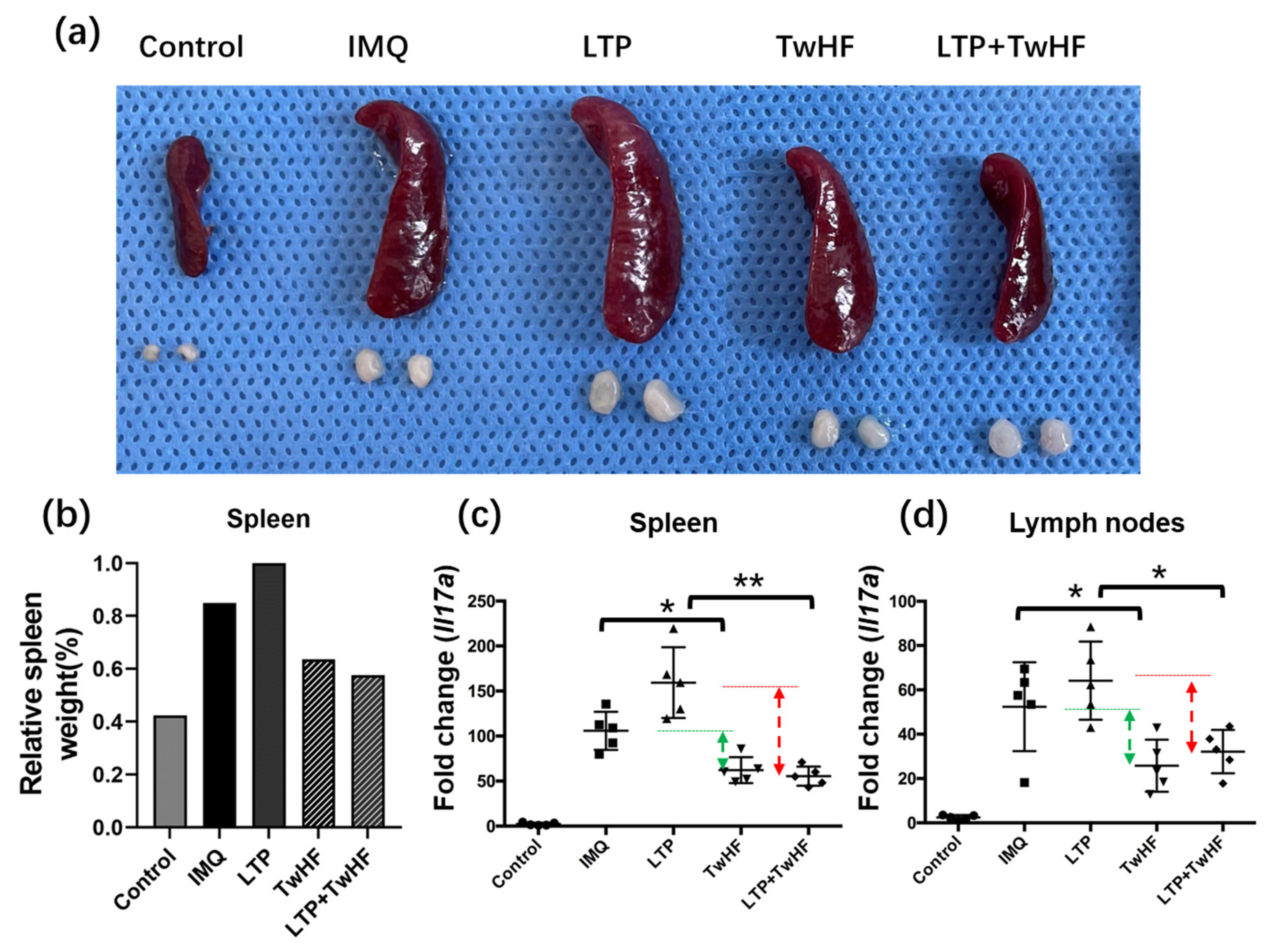
3.4. Plasma + TwHF Treatment Suppressed IMQ-Induced Systemic Inflammation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gan, L.; Duan, J.; Zhang, S.; Liu, X.; Poorun, D.; Liu, X.; Lu, X.; Duan, X.; Liu, D.; Chen, H. Cold Atmospheric Plasma Ameliorates Imiquimod-Induced Psoriasiform Dermatitis in Mice by Mediating Antiproliferative Effects. Free Radic. Res. 2019, 53, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Zhang, S.; Poorun, D.; Liu, D.; Lu, X.; He, M.; Duan, X.; Chen, H. Medical Applications of Nonthermal Atmospheric Pressure Plasma in Dermatology. J. Dtsch. Dermatol. Ges. 2018, 16, 7–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Zhang, Y.; Xu, M.; Chen, H.; Lu, X.; Ostrikov, K. Cold Atmospheric Pressure Plasmas in Dermatology: Sources, Reactive Agents, and Therapeutic Effects. Plasma Process. Polym. 2020, 17, 1900218. [Google Scholar] [CrossRef]
- Griffiths, C.E.; Barker, J.N. Pathogenesis and Clinical Features of Psoriasis. Lancet 2007, 370, 263–271. [Google Scholar] [CrossRef]
- Michalek, I.M.; Loring, B.; John, S.M. A Systematic Review of Worldwide Epidemiology of Psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 205–212. [Google Scholar] [CrossRef]
- Ogawa, E.; Sato, Y.; Minagawa, A.; Okuyama, R. Pathogenesis of Psoriasis and Development of Treatment. J. Dermatol. 2018, 45, 264–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandra, A.; Ray, A.; Senapati, S.; Chatterjee, R. Genetic and Epigenetic Basis of Psoriasis Pathogenesis. Mol. Immunol. 2015, 64, 313–323. [Google Scholar] [CrossRef]
- Sala, M.; Elaissari, A.; Fessi, H. Advances in Psoriasis Physiopathology and Treatments: Up to Date of Mechanistic Insights and Perspectives of Novel Therapies Based on Innovative Skin Drug Delivery Systems (ISDDS). J. Control. Release 2016, 239, 182–202. [Google Scholar] [CrossRef]
- Low Temperature Plasma Technology: Methods and Applications, 1st ed.; Chu, P.K.; Lu, X. (Eds.) CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Lu, X.; Reuter, S.; Laroussi, M.; Liu, D.; Reuter, S.; Laroussi, M.; Liu, D. Nonequilibrium Atmospheric Pressure Plasma Jets Fundamentals, Diagnostics, and Medical Applications; CRC Press: Boca Raton, FL, USA, 2019; ISBN 978-0-429-05366-5. [Google Scholar]
- Laroussi, M.; Kong, M.G.; Morfill, G.; Stolz, W. Plasma Medicine: Applications of Low-Temperature Gas Plasmas in Medicine and Biology; Cambridge University Press: Cambridge, UK, 2012; ISBN 978-1-107-00643-0. [Google Scholar]
- Kong, M.G.; Kroesen, G.; Morfill, G.; Nosenko, T.; Shimizu, T.; van Dijk, J.; Zimmermann, J.L. Plasma Medicine: An Introductory Review. New J. Phys. 2009, 11, 115012. [Google Scholar] [CrossRef]
- Heinlin, J.; Morfill, G.; Landthaler, M.; Stolz, W.; Isbary, G.; Zimmermann, J.L.; Shimizu, T.; Karrer, S. Plasma Medicine: Possible Applications in Dermatology. J. Dtsch. Derm. Ges. 2010, 8, 968–976. [Google Scholar] [CrossRef]
- Graves, D.B. Low Temperature Plasma Biomedicine: A Tutorial Review. Phys. Plasmas 2014, 21, 080901. [Google Scholar] [CrossRef] [Green Version]
- Lv, M.; Deng, J.; Tang, N.; Zeng, Y.; Lu, C. Efficacy and Safety of Tripterygium Wilfordii Hook F on Psoriasis Vulgaris: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Evid. Based Complement. Altern. Med. 2018, 2018, e2623085. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Jin, H.-Z.; Shu, D.; Li, F.; He, C.-X.; Qiao, J.; Yu, X.-L.; Zhang, Y.; He, Y.-B.; Liu, T.-J. Efficacy and Safety of Tripterygium Wilfordii Hook F Versus Acitretin in Moderate to Severe Psoriasis Vulgaris: A Randomized Clinical Trial. Chin. Med. J. 2015, 128, 443–449. [Google Scholar] [CrossRef]
- Ru, Y.; Li, H.; Zhang, R.; Luo, Y.; Song, J.; Kuai, L.; Xing, M.; Hong, S.; Sun, X.; Ding, X.; et al. Role of Keratinocytes and Immune Cells in the Anti-Inflammatory Effects of Tripterygium Wilfordii Hook. f. in a Murine Model of Psoriasis. Phytomedicine 2020, 77, 153299. [Google Scholar] [CrossRef]
- Chen, B.; Huang, Z.; Liu, D. Deposition of Charged Aerosols by E × B Enhanced Low-Temperature Plasma Jet Array. Plasma Process. Polym. 2021, 18, e2100085. [Google Scholar] [CrossRef]
- Pei, X.; Liu, J.; Xian, Y.; Lu, X. A Battery-Operated Atmospheric-Pressure Plasma Wand for Biomedical Applications. J. Phys. D Appl. Phys. 2014, 47, 145204. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, J.; Yu, J.; Chen, X.; Zhang, F.; Wei, W.; Zhang, L.; Chen, W.; Lin, N.; Wu, Y. Hyperforin Ameliorates Imiquimod-Induced Psoriasis-Like Murine Skin Inflammation by Modulating IL-17A–Producing Γδ T Cells. Front. Immunol. 2021, 12, 635076. [Google Scholar] [CrossRef]
- Liu, Z.; Tian, Y.; Niu, G.; Wang, X.; Duan, Y. Direct Oxidative Nitrogen Fixation from Air and H2O by a Water Falling Film Dielectric Barrier Discharge Reactor at Ambient Pressure and Temperature. ChemSusChem 2021, 14, 1507–1511. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wang, G.; Chen, B.; Zhang, Y.; Liu, D.; Lu, X.; He, G.; Ostrikov, K. Atmospheric-Pressure Non-Equilibrium Plasmas for Effective Abatement of Pathogenic Biological Aerosols. Plasma Sour. Sci. Technol. 2021, 30, 1507–1511. [Google Scholar] [CrossRef]
- Ma, S.; Cheng, H.; Li, J.; Xu, M.; Liu, D.; Ostrikov, K. Large-Scale Ion Generation for Precipitation of Atmospheric Aerosols. Atmos. Chem. Phys. 2020, 20, 11717–11727. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, D. Enhanced Transmembrane Transport of Reactive Oxygen Species by Electroporation Effect of Plasma. Plasma Process. Polym. 2021, 18, e2100054. [Google Scholar] [CrossRef]
- Chen, B.; Liu, D. Mass Spectrometry Study on Ions Generated by Low-Temperature Plasma Jet. IEEE Trans. Plasma Sci. 2021, 49, 1190–1194. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, H.; Gao, H.; Liu, D.; Lu, X. On the Charged Aerosols Generated by Atmospheric Pressure Non-Equilibrium Plasma. High Volt. 2021, 6, 408–425. [Google Scholar] [CrossRef]
- Liu, D.W.; Iza, F.; Kong, M.G. Electron Heating in Radio-Frequency Capacitively Coupled Atmospheric-Pressure Plasmas. Appl. Phys. Lett. 2008, 93, 261503. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.W.; Iza, F.; Kong, M.G. Electron Avalanches and Diffused Gamma-Mode in Radio-Frequency Capacitively Coupled Atmospheric-Pressure Microplasmas. Appl. Phys. Lett. 2009, 95, 031501. [Google Scholar] [CrossRef] [Green Version]
- Reuter, S.; Winter, J.; Schmidt-Bleker, A.; Schroeder, D.; Lange, H.; Knake, N.; der Gathen, V.S.; Weltmann, K.-D. Atomic Oxygen in a Cold Argon Plasma Jet: TALIF Spectroscopy in Ambient Air with Modelling and Measurements of Ambient Species Diffusion. Plasma Sour. Sci. Technol. 2012, 21, 024005. [Google Scholar] [CrossRef]
- Liu, X.Y.; Hu, J.T.; Liu, J.H.; Xiong, Z.L.; Liu, D.W.; Lu, X.P.; Shi, J.J. The Discharge Mode Transition and O(5p1) Production Mechanism of Pulsed Radio Frequency Capacitively Coupled Plasma. Appl. Phys. Lett. 2012, 101, 043705. [Google Scholar] [CrossRef]
- Lu, X.; Laroussi, M.; Puech, V. On Atmospheric-Pressure Non-Equilibrium Plasma Jets and Plasma Bullets. Plasma Sour. Sci. Technol. 2012, 21, 034005. [Google Scholar] [CrossRef]
- Marschewski, M.; Hirschberg, J.; Omairi, T.; Höfft, O.; Viöl, W.; Emmert, S.; Maus-Friedrichs, W. Electron Spectroscopic Analysis of the Human Lipid Skin Barrier: Cold Atmospheric Plasma-Induced Changes in Lipid Composition. Exp. Dermatol. 2012, 21, 921–925. [Google Scholar] [CrossRef]
- Maiti, S.; Sen, K.K. Advanced Technology for Delivering Therapeutics; BoD–Books: Norderstedt, Germany, 2017; ISBN 978-953-51-3122-9. [Google Scholar]
- Liu, X.; Gan, L.; Ma, M.; Zhang, S.; Liu, J.; Chen, H.; Liu, D.; Lu, X. A Comparative Study on the Transdermal Penetration Effect of Gaseous and Aqueous Plasma Reactive Species. J. Phys. D Appl. Phys. 2018, 51, 075401. [Google Scholar] [CrossRef]
- Duan, J.; Ma, M.; Yusupov, M.; Cordeiro, R.M.; Lu, X.; Bogaerts, A. The Penetration of Reactive Oxygen and Nitrogen Species across the Stratum Corneum. Plasma Process. Polym. 2020, 17, 2000005. [Google Scholar] [CrossRef]
- Helmke, A.; Hoffmeister, D.; Mertens, N.; Emmert, S.; Schuette, J.; Vioel, W. The Acidification of Lipid Film Surfaces by Non-Thermal DBD at Atmospheric Pressure in Air. New J. Phys. 2009, 11, 115025. [Google Scholar] [CrossRef]
- Shimizu, K. Biological Effects and Enhancement of Percutaneous Absorption on Skin by Atmospheric Microplasma Irradiation. PMED 2015, 5, 205–221. [Google Scholar] [CrossRef]
- Duan, J.; Gan, L.; Nie, L.; Sun, F.; Lu, X.; He, G. On the Penetration of Reactive Oxygen and Nitrogen Species Generated by a Plasma Jet into and through Mice Skin with/without Stratum Corneum. Phys. Plasmas 2019, 26, 43504. [Google Scholar] [CrossRef]
- Wen, X.; Xin, Y.; Hamblin, M.R.; Jiang, X. Applications of Cold Atmospheric Plasma for Transdermal Drug Delivery: A Review. Drug Deliv. Transl. Res. 2021, 11, 741–747. [Google Scholar] [CrossRef]
- Shimizu, K.; Tran, N.A.; Hayashida, K.; Blajan, M. Comparison of Atmospheric Microplasma and Plasma Jet Irradiation for Increasing of Skin Permeability. J. Phys. D Appl. Phys. 2016, 49, 315201. [Google Scholar] [CrossRef]
- Lee, S.; Choi, J.; Kim, J.; Jang, Y.; Lim, T.H. Atmospheric Pressure Plasma Irradiation Facilitates Transdermal Permeability of Aniline Blue on Porcine Skin and the Cellular Permeability of Keratinocytes with the Production of Nitric Oxide. Appl. Sci. 2021, 11, 2390. [Google Scholar] [CrossRef]
- Zheng, J.R.; Fang, J.L.; Gu, K.X.; Xu, L.F.; Gao, J.W.; Guo, H.Z.; Yu, Y.H.; Sun, H.Z. Screening of active anti-inflammatory-immunosuppressive and antifertile compositions from Tripterygium wilfordii. I. Screening of 8 components from total glucosides of Tripterygium wilfordii (TII). Zhongguo Yi Xue Ke Xue Yuan Xue Bao 1987, 9, 317–322. [Google Scholar]
- Han, R.; Rostami-Yazdi, M.; Gerdes, S.; Mrowietz, U. Triptolide in the Treatment of Psoriasis and Other Immune-Mediated Inflammatory Diseases. Br. J. Clin. Pharmacol. 2012, 74, 424–436. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Chen, B.; Liu, D.; Chen, H. The Combination of Low-Temperature Plasma and Tripterygium wilfordii Hook F on Ameliorating Imiquimod-Induced Psoriasiform Dermatitis in Mice. Appl. Sci. 2022, 12, 356. https://doi.org/10.3390/app12010356
Zhang S, Chen B, Liu D, Chen H. The Combination of Low-Temperature Plasma and Tripterygium wilfordii Hook F on Ameliorating Imiquimod-Induced Psoriasiform Dermatitis in Mice. Applied Sciences. 2022; 12(1):356. https://doi.org/10.3390/app12010356
Chicago/Turabian StyleZhang, Song, Baihan Chen, Dawei Liu, and Hongxiang Chen. 2022. "The Combination of Low-Temperature Plasma and Tripterygium wilfordii Hook F on Ameliorating Imiquimod-Induced Psoriasiform Dermatitis in Mice" Applied Sciences 12, no. 1: 356. https://doi.org/10.3390/app12010356
APA StyleZhang, S., Chen, B., Liu, D., & Chen, H. (2022). The Combination of Low-Temperature Plasma and Tripterygium wilfordii Hook F on Ameliorating Imiquimod-Induced Psoriasiform Dermatitis in Mice. Applied Sciences, 12(1), 356. https://doi.org/10.3390/app12010356





