Properties of Emulsion Paint with Modified Natural Rubber Latex/Polyvinyl Acetate Blend Binder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Methods
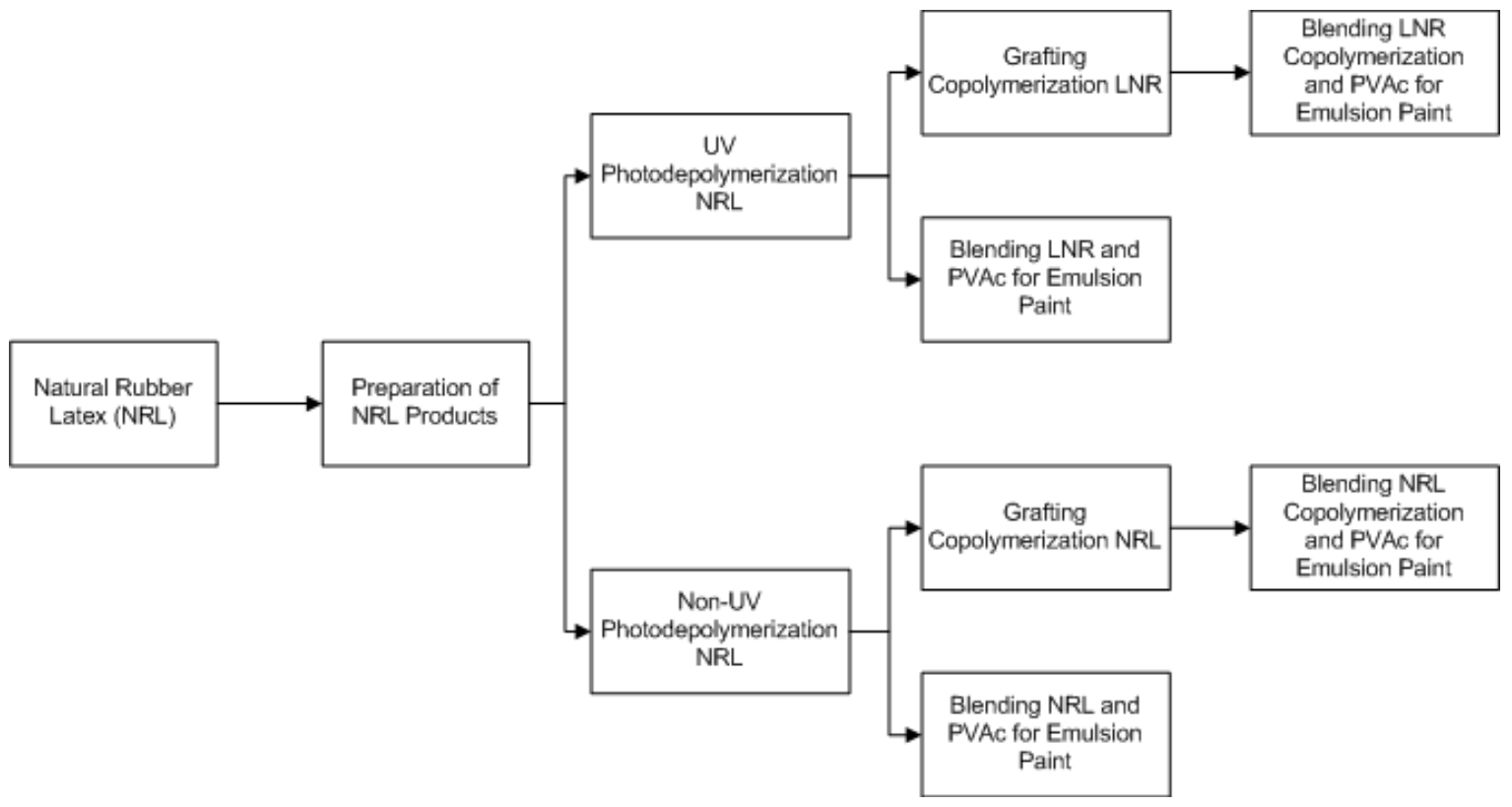
2.2.1. Preparation of Natural Rubber Latex (NRL) Products
2.2.2. Modification of NRL by UV Photodepolymerization
2.2.3. Modification of NRL and LNR with Grafting Copolymerization
2.2.4. Latex Binder Preparation for Emulsion Paints
2.3. Emulsion Paint Sample Preparation
2.4. Characterization of the Emulsion Paint
3. Results and Discussion
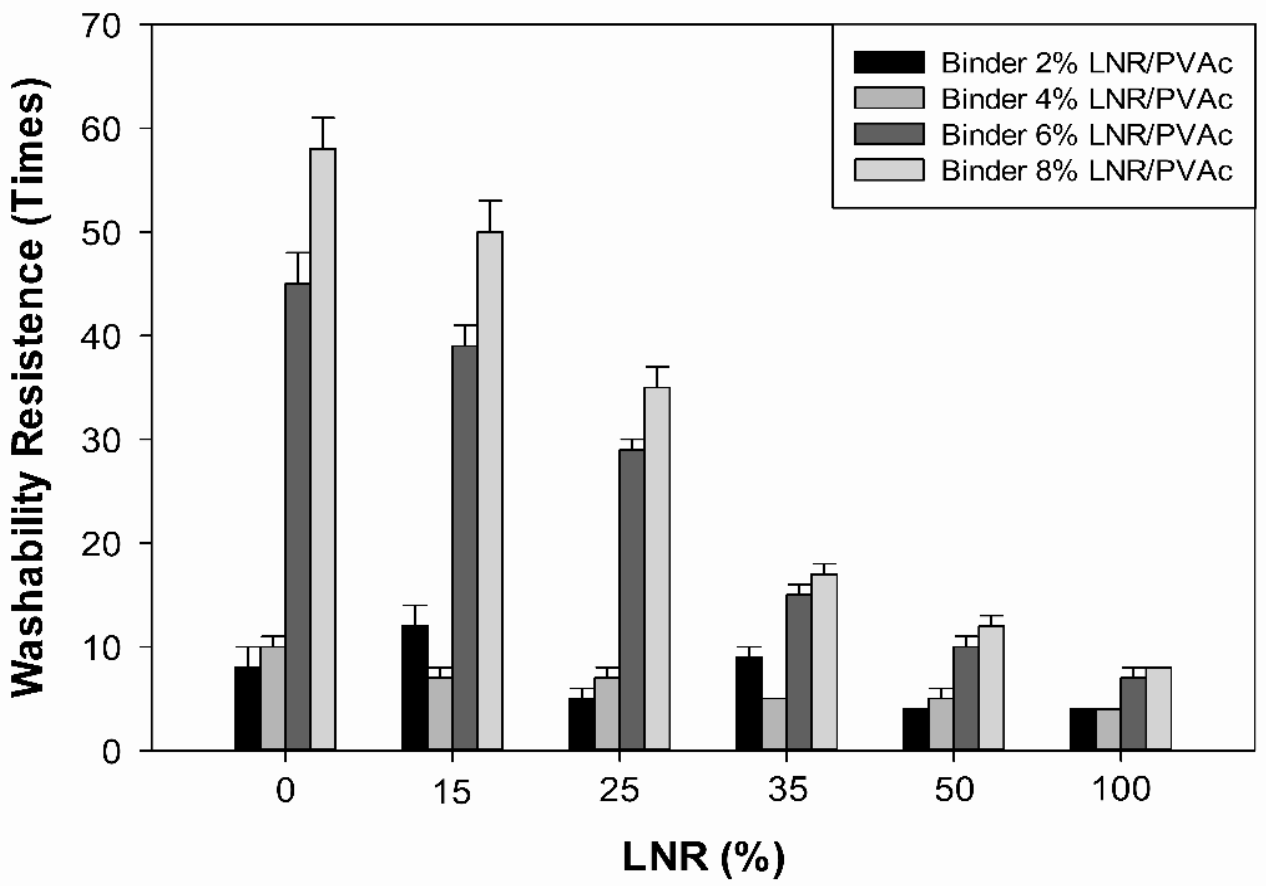
3.1. Washability Resistance Properties of Emulsion Paints
3.2. Opacity Properties of Emulsion Paints
3.3. Properties of Set Touch Drying Time of Emulsion Paints
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vijetha, P.; Prasanna Kumar, Y.; Kumaraswamy, K.; Kumari, A.; Singham, P.; Satyasree, N. Comparative studies of natural and synthetic rubber. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 851–857. [Google Scholar]
- Thongseenuch, S.; Taweepreda, W.; Suchiva, K. Effect of low molecular weight natural rubber on mixing and vulcanized properties of low energy processing natural rubber. Sains Malays. 2017, 46, 1379–1384. [Google Scholar] [CrossRef]
- Ramli, R.; Bao, C.A.; Rasdi, F.R.M.; Kamaruddin, S.; Aziz, A.A.; Song, T.K.; Lang, M.K.; Hou, H.J. Preliminary study on epoxidized natural rubber latex composites intended for shoe soles applic. Int. J. Emerg. Trends Eng. Res. 2020, 8, 122–131. [Google Scholar]
- Azevedo Borges, F.; Cesar Bolognesi, L.F.; Trecco, A.; de Camargo Drago, B.; Baldo de Arruda, L.; Noronha Lisboa Filho, P.; Gonçalves Pierri, E.; de Oliveira Graeff, C.F.; Gonzaga dos Santos, A.; Romeiro Miranda, M.C.; et al. Natural Rubber Latex: Study of a Novel Carrier for Casearia sylvestris Swartz Delivery. ISRN Polym. Sci. 2014, 11, 125. [Google Scholar] [CrossRef]
- Nawamawat, K.; Sakdapipanich, J.T.; Ho, C.C.; Ma, Y.; Song, J.; Vancso, J.G. Surface nanostructure of Hevea brasiliensis natural rubber latex particles. Colloids Surf. A Physicochem. Eng. Asp. 2011, 390, 157–166. [Google Scholar] [CrossRef]
- Ochigbo, S.S.; Suleiman, M.A.T. Formulation and Characterization of Waterborne Paints from the Blends of Natural Rubber (NR) Latex and Polyvinyl acetate (PVAc) Emulsion. Int. J. Sci. 2014, 3, 145. [Google Scholar]
- Moonprasith, N.; Poonsrisawat, A.; Champreda, V.; Kongkaew, C.; Loykulnant, S.; Suchiva, K. Deproteinization of Nonammonia and Ammonia Natural Rubber Latices by Ethylenediaminetetraacetic Acid. Adv. Mater. Sci. Eng. 2017, 17, 1516945. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, M.; Mendonça, R.J.; Coutinho-Netto, J.; Mulato, M. Angiogenic properties of natural rubber latex biomembranes and the serum fraction of Hevea brasiliensis. Braz. J. Phys. 2009, 39, 564–569. [Google Scholar] [CrossRef]
- Chaikumpollert, O.; Yamamoto, Y.; Suchiva, K.; Nghia, P.T.; Kawahara, S. Preparation and characterization of protein-free natural rubber. Polym. Adv. Technol. 2012, 23, 825–828. [Google Scholar] [CrossRef]
- Klinklai, W.; Saito, T.; Kawahara, S.; Tashiro, K.; Suzuki, Y.; Sakdapipanich, J.T.; Isono, Y. Hyperdeproteinized natural rubber prepared with urea. J. Appl. Polym. Sci. 2004, 93, 555–559. [Google Scholar] [CrossRef]
- Anderson, C.D.; Daniels, E.S. Emulsion Polymerisation and Latex Applications; iSmithers Rapra Publishing: Shropshire, UK, 2003; Volume 14. [Google Scholar]
- Ndibe, H.C.; Iyasele, J.U.; Imanah, E.O.; Okpara, G.E.; Eriamiatoe, I. Utilization of Binary Blends of Liquid Natural Rubber and Polyvinyl Acetate in Emulsion Paint. J. Chem. Soc. Niger. 2021, 46, 72–78. [Google Scholar] [CrossRef]
- Fadhillah, I.; Warinata, A.; Zahrina, I. Molecular Weight of Liquid Natural Rubber (LNR) Product from the Chemical Depolymerization Process of High Molecular Weight Narutal Rubber Latex. J. Phys. Conf. Ser. 2020, 1655, 012091. [Google Scholar]
- Clark, M.D. X-Polymers-D-Paints and Pigments. Paint. Res. Assoc. Teddingt. 2000, 11, 19. [Google Scholar]
- Zainudin, Z.; Baharulrazi, N.; Hajjar, S.; Man, C. Natural rubber derivatives for adhesives applications: A review. Chem. Eng. Trans. 2021, 83, 493–498. [Google Scholar]
- Radabutra, S.; Saengsuwan, S.; Jitchati, R.; Kalapat, M. Preparation and characterization of modified telechelic natural rubber-based pressure-sensitive adhesive. J. Adhes. Sci. Technol. 2017, 31, 2682–2696. [Google Scholar] [CrossRef]
- Zhang, K.; Huang, C.; Shen, H.; Chen, H. Grafting of methyl methacrylate and styrene onto polychloroprene latex for compatibilization of polychloroprene latex/styrene-acrylate emulsion blends. J. Adhes. 2015, 91, 419–433. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Duy, H.N.; Anh, D.T.; Thi, T.N.; Nguyen, T.H.; Van, N.N.; Quang, T.T.; Huy, T.N.; Thi, T.T. Improvement of Thermal and Mechanical Properties of Vietnam Deproteinized Natural Rubber via Graft Copolymerization with Methyl Methacrylate. Int. J. Polym. Sci. 2020, 2020, 6–8. [Google Scholar] [CrossRef]
- Moolsin, S.; Saksayamkul, N.; Wichien, A.N. Natural rubber grafted poly(methyl methacrylate) as compatibilizer in 50/50 natural rubber/nitrile rubber blend. J. Elastomers Plast. 2017, 49, 422–439. [Google Scholar] [CrossRef]
- Sari, T.I.; Saputra, A.H.; Bismo, S.; Maspanger, D.R.; Cifriadi, A. The effect of styrene monomer in the graft copolymerization of acrylonitrile onto deproteinized natural rubber. Int. J. Technol. 2015, 6, 1164–1173. [Google Scholar] [CrossRef]
- Akinterinwa, A.; Osemeahon, S.A.; Nkafamiya, I.I.; Dass, P.M. Formulation of Emulsion Paint from a Copolymer Composite of Dimethylol Urea/Polystyrene. Chem. Mater. Res. 2015, 7, 2225–2956. [Google Scholar]
- Aiza Jaafar, C.N.; Zainol, I.; Ishak, N.S.; Ilyas, R.A.; Sapuan, S.M. Effects of the liquid natural rubber (LNR) on mechanical properties and microstructure of epoxy/silica/kenaf hybrid composite for potential automotive applications. J. Mater. Res. Technol. 2021, 12, 1026–1038. [Google Scholar] [CrossRef]


| Components | Utility |
|---|---|
| Water | Dispersion medium |
| Hydroxyethylcellulose | Thickening agent |
| Caustic soda | pH control |
| Ultramarine blue | Blue pigment |
| Alkylphenol ethoxylate | Surfactant |
| TiO2 | Opacity agent/White agent |
| CaO | Hiding power agent |
| CaCO3 | Extender |
| Polypropylene glycol | Anti-settling agent |
| Eastman (pentaerythritol esters) | Additive agent |
| Dodecylbenzene sulfonat | Wetting agent |
| LNR, NRL-g-(MMA-co-St) or LNR-g-(MMA-co-St)/PVAc Blends | Binder |
| Sample | Opacity (% Absorbance) with PVAc Ratio | |||||
|---|---|---|---|---|---|---|
| 0:100 | 15:80 | 25:75 | 35:65 | 50:50 | 100:0 | |
| NRL/PVAc Binder 4% | 1.6346 | 1.6359 | 1.6192 | 1.5387 | 1.5644 | 1.593 |
| NRL/PVAc Binder 6% | 1.6193 | 1.6309 | 1.6216 | 1.5611 | 1.5476 | 1.5923 |
| NRL/PVAc Binder 8% | 1.6169 | 1.6426 | 1.5957 | 1.649 | 1.5802 | 1.5355 |
| LNR/PVAc Binder 2% | 1.6093 | 1.6989 | 1.5754 | 1.6342 | 1.4943 | 1.6535 |
| LNR/PVAc Binder 4% | 1.6346 | 1.6292 | 1.6315 | 1.6098 | 1.5823 | 1.6313 |
| LNR/PVAc Binder 6% | 1.6193 | 1.6013 | 1.721 | 1.653 | 1.6909 | 1.5788 |
| LNR/PVAc Binder 8% | 1.6169 | 1.6144 | 1.6142 | 1.6417 | 1.6055 | 1.7298 |
| NRL-g-(MMA-co-St) 60:40 | 1.6346 | 1.6961 | 1.693 | 1.6698 | 1.6589 | 1.5267 |
| NRL-g-(MMA-co-St) 65:35 | 1.6346 | 1.5205 | 1.3726 | 1.4448 | 1.3532 | 1.4177 |
| NRL-g-(MMA-co-St) 70:30 | 1.6346 | 1.637 | 1.5653 | 1.4196 | 1.5645 | 1.4096 |
| NRL-g-(MMA-co-St) 75:25 | 1.6346 | 1.3897 | 1.42 | 1.2402 | 1.1751 | 1.3511 |
| NRL-g-(MMA-co-St) 80:20 | 1.6346 | 1.7782 | 1.7673 | 1.7655 | 1.7533 | 1.7159 |
| LNR-g-(MMA-co-St) 60:40 | 1.6346 | 1.6708 | 1.6383 | 1.6451 | 1.6338 | 1.5689 |
| LNR-g-(MMA-co-St) 65:35 | 1.6346 | 1.5036 | 1.6143 | 1.659 | 1.5743 | 1.5485 |
| LNR-g-(MMA-co-St) 75:25 | 1.6346 | 1.5624 | 1.4107 | 1.5309 | 1.3856 | 1.5281 |
| LNR-g-(MMA-co-St) 70:30 | 1.6346 | 1.4728 | 1.5118 | 1.3575 | 1.2358 | 1.6171 |
| LNR-g-(MMA-co-St) 80:20 | 1.6346 | 1.5844 | 1.4826 | 1.3422 | 1.1793 | 0.9267 |
| Sample | Set Touch Drying Times (Minutes) | |||||
|---|---|---|---|---|---|---|
| 0:100 | 15:80 | 25:75 | 35:65 | 50:50 | 100:0 | |
| NRL/PVAc Binder 4% | 84 | 91 | 99 | 109 | 110 | 110 |
| NRL/PVAc Binder 6% | 99 | 101 | 102 | 103 | 106 | 106 |
| NRL/PVAc Binder 8% | 115 | 105 | 110 | 116 | 99 | 112 |
| LNR/PVAc Binder 2% | 81 | 79 | 63 | 66 | 106 | 71 |
| LNR/PVAc Binder 4% | 84 | 40 | 51 | 61 | 194 | 59 |
| LNR/PVAc Binder 6% | 101 | 99 | 80 | 84 | 79 | 76 |
| LNR/PVAc Binder 8% | 115 | 97 | 103 | 61 | 97 | 121 |
| NRL-g-(MMA-co-St) 60:40 | 84 | 74 | 104 | 108 | 110 | 96 |
| NRL-g-(MMA-co-St) 65:35 | 84 | 104 | 95 | 103 | 108 | 115 |
| NRL-g-(MMA-co-St) 70:30 | 84 | 82 | 104 | 103 | 104 | 115 |
| NRL-g-(MMA-co-St) 75:25 | 84 | 110 | 116 | 118 | 120 | 112 |
| NRL-g-(MMA-co-St) 80:20 | 84 | 92 | 96 | 106 | 111 | 120 |
| LNR-g-(MMA-co-St) 60:40 | 84 | 104 | 85 | 99 | 99 | 95 |
| LNR-g-(MMA-co-St) 65:35 | 84 | 116 | 117 | 122 | 129 | 127 |
| LNR-g-(MMA-co-St) 75:25 | 84 | 125 | 104 | 105 | 110 | 119 |
| LNR-g-(MMA-co-St) 70:30 | 84 | 110 | 105 | 108 | 113 | 115 |
| LNR-g-(MMA-co-St) 80:20 | 84 | 95 | 98 | 105 | 113 | 115 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, B.; Helwani, Z.; Fadhillah, I.; Wiranata, A.; Miharyono, J. Properties of Emulsion Paint with Modified Natural Rubber Latex/Polyvinyl Acetate Blend Binder. Appl. Sci. 2022, 12, 296. https://doi.org/10.3390/app12010296
Ibrahim B, Helwani Z, Fadhillah I, Wiranata A, Miharyono J. Properties of Emulsion Paint with Modified Natural Rubber Latex/Polyvinyl Acetate Blend Binder. Applied Sciences. 2022; 12(1):296. https://doi.org/10.3390/app12010296
Chicago/Turabian StyleIbrahim, Bahruddin, Zuchra Helwani, Ivan Fadhillah, Arya Wiranata, and Joni Miharyono. 2022. "Properties of Emulsion Paint with Modified Natural Rubber Latex/Polyvinyl Acetate Blend Binder" Applied Sciences 12, no. 1: 296. https://doi.org/10.3390/app12010296
APA StyleIbrahim, B., Helwani, Z., Fadhillah, I., Wiranata, A., & Miharyono, J. (2022). Properties of Emulsion Paint with Modified Natural Rubber Latex/Polyvinyl Acetate Blend Binder. Applied Sciences, 12(1), 296. https://doi.org/10.3390/app12010296







