Antibacterial and Anti-Inflammatory Potential of Mouthwash Composition Based on Natural Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material and Extraction Process
2.2. Strain Culture
2.3. pH Value
2.4. Measurement of Antibacterial Activity
2.5. Macrophage Cell Culture
2.6. Measurement of Cell Viability
2.7. Measurement of Nitric Oxide Production
2.8. Statistical Analysis
3. Results
3.1. pH Measurement
3.2. The Antibacterial Effect
3.3. Cell Survival
3.4. Anti-Inflammatory Effect
4. Discussion
5. Conclusions
- Each natural extract and their mixture series were observed to affect some of the eleven types of oral pathogenic bacteria. Among them, PG, SG, M2, and M4 were observed to have no effect on the eleven types of oral pathogenic bacteria. All samples were observed to have no effect on the nonpathogenic bacteria. However, it was observed that M1, GA, and LIS had a high effect of about 77–90% compared to the other samples.
- The natural extract mixtures, M1–M5, showed a level of cell viability similar to the control group, which was RAW 264.7 cells, but those of the natural extracts (PG, CSK, SG) were similar to those of the chemical mixture mouthwash (GA and LIS). This suggests that natural extract mixtures offered more cell stability than natural extracts.
- The anti-inflammatory effects of PG, CSK, SG, M4, M5, GA and LIS were confirmed through NO assay.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, E.-J.; Lee, M.-O. Analysis on the Effect of the Dental Health Characteristics of Adult on the Status of Recognition and Practical Application of Dental Hygiene Devices. J. Dent. Hyg. Sci. 2010, 10, 241–250. [Google Scholar]
- Oliveira, L.M.; Pazinatto, J.; Zanatta, F.B. Are oral hygiene instructions with aid of plaque-disclosing methods effective in improving self-performed dental plaque control? A systematic review of randomized controlled trials. Int. J. Dent. Hyg. 2021, 1–16. [Google Scholar] [CrossRef]
- Lee, Y.H.; Moon, H.S.; Paik, D.I.; Kim, J.B. A Survey on Family Dental Health Behavior in Seoul Capital City. J. Korean Acad. Oral Health 2000, 24, 239–254. [Google Scholar]
- Stanley, A.; Wilson, M.; Newman, H.N. The in Vitro Effects of Chlorhexidine on Subgingival Plaque Bacteria. J. Clin. Periodontol. 1989, 16, 259–264. [Google Scholar] [CrossRef]
- Pratten, J.; Smith, A.W.; Wilson, M. Response of Single Species Biofilms and Microcosm Dental Plaques to Pulsing with Chlorhexidine. J. Antimicrob. Chemoth. 1998, 42, 453–459. [Google Scholar] [CrossRef]
- Holbeche, J.D.; Ruljancich, M.K.; Reade, P.C. A Clinical Trial of the Efficacy of a Cetylpyridinium Chloride-based Mouth Wash*: 1. Effect on Plaque Accumulation and Gingival Condition. Aust. Dent. J. 1975, 20, 397–404. [Google Scholar] [CrossRef]
- McCullough, M.; Farah, C. The Role of Alcohol in Oral Carcinogenesis with Particular Reference to Alcohol-containing Mouthwashes. Aust. Dent. J. 2008, 53, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Wynder, E.L.; Kabat, G.; Rosenberg, S.; Levenstein, M. Oral Cancer and Mouthwash Use. JNCI J. Natl. Cancer Inst. 1983. [Google Scholar] [CrossRef]
- Kuyama, K.; Yamamoto, H. A Study of Effects of Mouthwash on the Human Oral Mucosae: With Special References to Sites, Sex Differences and Smoking. J. Nihon Univ. Sch. Dent. 1997, 39, 202–210. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jahangir, G.Z.; Ashraf, D.S.; Nasir, I.A.; Sadiq, M.; Shahzad, S.; Naz, F.; Iqbal, M.; Saeed, A. The Myth of Oral Hygiene Using Synthetic Mouthwash Products. Springerplus 2016, 5, 1481. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saleem, H.G.M.; Seers, C.A.; Sabri, A.N.; Reynolds, E.C. Dental Plaque Bacteria with Reduced Susceptibility to Chlorhexidine Are Multidrug Resistant. BMC Microbiol. 2016, 16, 214. [Google Scholar] [CrossRef]
- Carnovale, C.E.; Ronco, M.T. Role of Nitric Oxide in Liver Regeneration. Ann. Hepatol. 2012, 11, 636–647. [Google Scholar] [CrossRef]
- Blot, S. Antiseptic Mouthwash, the Nitrate–Nitrite–Nitric Oxide Pathway, and Hospital Mortality: A Hypothesis Generating Review. Intensive Care Med. 2021, 47, 28–38. [Google Scholar] [CrossRef]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the Normal Bacterial Flora of the Oral Cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef]
- Colombo, A.P.V.; Teles, R.P.; Torres, M.C.; Souto, R.; Rosalém, W.; Mendes, M.C.S.; Uzeda, M. Subgingival Microbiota of Brazilian Subjects with Untreated Chronic Periodontitis. J. Periodontol. 2002, 73, 360–369. [Google Scholar] [CrossRef]
- Lee, J.-H.; Jeong, W.-S.; Seo, S.-J.; Kim, H.-W.; Kim, K.-N.; Choi, E.-H.; Kim, K.-M. Non-Thermal Atmospheric Pressure Plasma Functionalized Dental Implant for Enhancement of Bacterial Resistance and Osseointegration. Dent. Mater. 2017, 33, 257–270. [Google Scholar] [CrossRef]
- Pita, P.P.C.; Rodrigues, J.A.; Ota-Tsuzuki, C.; Miato, T.F.; Zenobio, E.G.; Giro, G.; Figueiredo, L.C.; Gonçalves, C.; Gehrke, S.A.; Cassoni, A.; et al. Oral Streptococci Biofilm Formation on Different Implant Surface Topographies. Biomed. Res. Int. 2015, 2015, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.M.; Knight, E.T.; Al-Harthi, L.; Leichter, J.W. Chronic Periodontitis and Implant Dentistry. Periodontology 2000 2017, 74, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Gross, E.L.; Beall, C.J.; Kutsch, S.R.; Firestone, N.D.; Leys, E.J.; Griffen, A.L. Beyond Streptococcus Mutans: Dental Caries Onset Linked to Multiple Species by 16S RRNA Community Analysis. PLoS ONE 2012, 7, e47722. [Google Scholar] [CrossRef]
- Lemos, J.A.; Palmer, S.R.; Zeng, L.; Wen, Z.T.; Kajfasz, J.K.; Freires, I.A.; Abranches, J.; Brady, L.J. The Biology of Streptococcus Mutans. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Conrads, G.; de Soet, J.J.; Song, L.; Henne, K.; Sztajer, H.; Wagner-Döbler, I.; Zeng, A.-P. Comparing the Cariogenic Species Streptococcus Sobrinus and S. Mutans on Whole Genome Level. J. Oral Microbiol. 2014, 6, 26189. [Google Scholar] [CrossRef]
- Listgrarten, M.A.; Johnson, D.; Nowotny, A.; Tanner, A.C.R.; Socransky, S.S. Histopathology of Periodontal Disease in Gnotobiotic Rats Monoinfected with Eikenella Corrodens. J. Periodontal Res. 1978, 13, 134–148. [Google Scholar] [CrossRef]
- Arana, E.; Vallcanera, A.; Santamaría, J.A.; Sanguesa, C.; Cortina, H. Eikenella Corrodens Skull Infection: A Case Report with Review of the Literature. Surg. Neurol. 1997, 47, 389–391. [Google Scholar] [CrossRef]
- Olopoenia, L.A.; Mody, V.; Reynolds, M. Eikenella Corrodens Endocarditis in an Intravenous Drug User: Case Report and Literature Review. J. Natl. Med. Assoc. 1994, 86, 313–315. [Google Scholar] [PubMed]
- Baqui, A.A.; Meiller, T.F.; Chon, J.J.; Turng, B.F.; Falkler, W.A. Granulocyte-Macrophage Colony-Stimulating Factor Amplification of Interleukin-1beta and Tumor Necrosis Factor Alpha Production in THP-1 Human Monocytic Cells Stimulated with Lipopolysaccharide of Oral Microorganisms. Clin. Diagn. Lab. Immun. 1998, 5, 341–347. [Google Scholar] [CrossRef]
- Henderson, B.; Ward, J.M.; Ready, D. Aggregatibacter (Actinobacillus) Actinomycetemcomitans: A Triple A* Periodontopathogen? Periodontology 2000 2010, 54, 78–105. [Google Scholar] [CrossRef] [PubMed]
- Mysak, J.; Podzimek, S.; Sommerova, P.; Lyuya-Mi, Y.; Bartova, J.; Janatova, T.; Prochazkova, J.; Duskova, J. Porphyromonas Gingivalis: Major Periodontopathic Pathogen Overview. J. Immunol. Res. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Kamma, J.J.; Nakou, M.; Gmür, R.; Baehni, P.C. Microbiological Profile of Early Onset/Aggressive Periodontitis Patients. Oral Microbiol. Immun. 2004, 19, 314–321. [Google Scholar] [CrossRef]
- Teanpaisan, R.; Douglas, C.W.I.; Walsh, T.F. Characterisation of Black-pigmented Anaerobes Isolated from Diseased and Healthy Periodontal Sites. J. Periodontal Res. 1995, 30, 245–251. [Google Scholar] [CrossRef]
- Tanzer, J.M.; Kurasz, A.B.; Clive, J. Competitive Displacement of Mutans Streptococci and Inhibition of Tooth Decay by Streptococcus Salivarius TOVE-R. Infect. Immun. 1985, 48, 44–50. [Google Scholar] [CrossRef]
- Hoogmoed, C.G.V.; Geertsema-doornbusch, G.I.; Teughels, W.; Quirynen, M.; Busscher, H.J.; Mei, H.C.V. der Reduction of Periodontal Pathogens Adhesion by Antagonistic Strains. Oral Microbiol. Immun. 2008, 23, 43–48. [Google Scholar] [CrossRef]
- Koo, H.; Falsetta, M.L.; Klein, M.I. The Exopolysaccharide Matrix. J. Dent. Res. 2013, 92, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- del Carmen Ahumada Ostengo, M.; Wiese, B.; Nader-Macias, M.E. Inhibitory Effect of Sodium Fluoride and Chlorhexidine on the Growth of Oral Lactobacilli. Can. J. Microbiol. 2005, 51, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Kõll-Klais, P.; Mändar, R.; Leibur, E.; Marcotte, H.; Hammarström, L.; Mikelsaar, M. Oral Lactobacilli in Chronic Periodontitis and Periodontal Health: Species Composition and Antimicrobial Activity. Oral Microbiol. Immun. 2005, 20, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Shinjo, T.; Ishikado, A.; Hasturk, H.; Pober, D.M.; Paniagua, S.M.; Shah, H.; Wu, I.; Tinsley, L.J.; Matsumoto, M.; Keenan, H.A.; et al. Characterization of Periodontitis in People with Type 1 Diabetes of 50 Years or Longer Duration. J. Periodontol. 2019, 90, 565–575. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A.; Alibrandi, A.; Williams, R.C.; Leonardi, R. Independent Impact of Periodontitis and Cardiovascular Disease on Elevated Soluble Urokinase-type Plasminogen Activator Receptor (SuPAR) Levels. J. Periodontol. 2020. [Google Scholar] [CrossRef]
- Aoyama, N.; Kobayashi, N.; Hanatani, T.; Ashigaki, N.; Yoshida, A.; Shiheido, Y.; Sato, H.; Takamura, C.; Yoshikawa, S.; Matsuo, K.; et al. Periodontal Condition in Japanese Coronary Heart Disease Patients: A Comparison between Coronary and Non-coronary Heart Diseases. J. Periodontal Res. 2019, 54, 259–265. [Google Scholar] [CrossRef]
- Bhattarai, G.; Min, C.; Jeon, Y.; Bashyal, R.; Poudel, S.B.; Kook, S.; Lee, J. Oral Supplementation with P-coumaric Acid Protects Mice against Diabetes-associated Spontaneous Destruction of Periodontal Tissue. J. Periodontal Res. 2019, 54, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Lv, H.; Shi, B.-H.; Hou, X.; Xu, X. Inhibition of IL-6 and IL-8 Production in LPS-Stimulated Human Gingival Fibroblasts by Glycyrrhizin via Activating LXRα. Microb. Pathogenesis. 2017, 110, 135–139. [Google Scholar] [CrossRef]
- Liu, F.; Rabinovich, G.A. Galectins: Regulators of Acute and Chronic Inflammation. Ann. N. Y. Acad. Sci. 2010, 1183, 158–182. [Google Scholar] [CrossRef] [PubMed]
- Loimaranta, V.; Hepojoki, J.; Laaksoaho, O.; Pulliainen, A.T. Galectin-3-binding Protein: A Multitask Glycoprotein with Innate Immunity Functions in Viral and Bacterial Infections. J. Leukoc. Biol. 2018, 104, 777–786. [Google Scholar] [CrossRef]
- Agnello, L.; Bivona, G.; Sasso, B.L.; Scazzone, C.; Bazan, V.; Bellia, C.; Ciaccio, M. Galectin-3 in Acute Coronary Syndrome. Clin. Biochem. 2017, 50, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Taşdemir, İ.; Yılmaz, H.E.; Narin, F.; Sağlam, M. Assessment of Saliva and Gingival Crevicular Fluid Soluble Urokinase Plasminogen Activator Receptor (SuPAR), Galectin-1, and TNF-α Levels in Periodontal Health and Disease. J. Periodontal Res. 2020, 55, 622–630. [Google Scholar] [CrossRef]
- Obeid, S.; Yousif, N.; Davies, A.; Loretz, R.; Saleh, L.; Niederseer, D.; Noor, H.A.; Amin, H.; Mach, F.; Gencer, B.; et al. Prognostic Role of Plasma Galectin-3 Levels in Acute Coronary Syndrome. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Joshipura, K.; Muñoz-Torres, F.; Fernández-Santiago, J.; Patel, R.P.; Lopez-Candales, A. Over-the-Counter Mouthwash Use, Nitric Oxide and Hypertension Risk. Blood Press. 2019, 29, 1–10. [Google Scholar] [CrossRef]
- Jae, M.-H.; Chang, K.-W.; Ma, D.-S. The Effects of Origanum Oil, Red Ginseng Extract, and Green Tea Extract on Oral Microorganisms and Volatile Sulfur Compounds. J. Korean Acad. Oral Health 2011, 35, 396–404. [Google Scholar]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Heo, N.S.; Choi, H.J.; Hwang, S.M.; Choi, Y.W.; Lee, Y.G.; Joo, W.H. Antimicrobial and Anti-Oral Malodor Efficacy of Schizandra Chinensis Extracts against Oral Pathogens. J. Life Sci. 2013, 23, 443–447. [Google Scholar] [CrossRef]
- Lee, S.-J.; Bang, W.-S.; Hong, J.-Y.; Kwon, O.-J.; Shin, S.-R.; Yoon, K.-Y. Antioxidant and Antimicrobial Activities of Black Doraji (Platycodon Grandiflorum). Korean J. Food Preserv. 2013, 20, 510–517. [Google Scholar] [CrossRef]
- Sawai, R.; Kuroda, K.; Shibata, T.; Gomyou, R.; Osawa, K.; Shimizu, K. Anti-Influenza Virus Activity of Chaenomeles Sinensis. J. Ethnopharmacol. 2008, 118, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Lussi, A.; Portmann, P.; Burhop, B. Erosion on Abraded Dental Hard Tissues by Acid Lozenges: An in Situ Study. Clin. Oral Investig. 1998, 1, 191–194. [Google Scholar] [CrossRef]
- Grishagin, I.V. Automatic Cell Counting with ImageJ. Anal. Biochem. 2015, 473, 63–65. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Q.; Sun, Y.; Jin, Y.; Zhang, J.; Wu, J. Melatonin Induces Anti-Inflammatory Effects via Endoplasmic Reticulum Stress in RAW264.7 Macrophages. Mol. Med. Rep. 2018, 17, 6122–6129. [Google Scholar] [CrossRef] [PubMed]
- Choo, G.-S.; Lim, D.-P.; Kim, S.-M.; Yoo, E.-S.; Kim, S.-H.; Kim, C.-H.; Woo, J.-S.; Kim, H.-J.; Jung, J.-Y. Anti-Inflammatory Effects of Dendropanax Morbifera in Lipopolysaccharide-Stimulated RAW264.7 Macrophages and in an Animal Model of Atopic Dermatitis. Mol. Med. Rep. 2019, 19, 2087–2096. [Google Scholar] [CrossRef]
- WEISZ, A.; CICATIELLO, L.; ESUMI, H. Regulation of the Mouse Inducible-Type Nitric Oxide Synthase Gene Promoter by Interferon-γ, Bacterial Lipopolysaccharide and NG-Monomethyl-l-Arginine. Biochem. J. 1996, 316, 209–215. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, B.; Zhang, X.; Wang, X.; Wu, K.; Guan, Q. Effects of Cathelicidin-Derived Peptide from Reptiles on Lipopolysaccharide-Induced Intestinal Inflammation in Weaned Piglets. Vet. Immunol. Immunop. 2017, 192, 41–53. [Google Scholar] [CrossRef]
- Choi, H.-J.; Lee, H.-J.; Jeong, S.-S.; Choi, C.-H.; Hong, S.-J. Effect of Mouthrinse with Low PH on the Surface Microhardness of Artificial Carious Enamel. J. Korean Acad. Oral Health 2012, 36, 161–166. [Google Scholar]
- Peter, H. Plaque Control and Oral Hygiene Methods. J. Irish Dent. Assoc. 2017, 63, 151–156. [Google Scholar]
- Pontefract, H.; Hughes, J.; Kemp, K.; Yates, R.; Newcombe, R.G.; Addy, M. The Erosive Effects of Some Mouthrinses on Enamel. J. Clin. Periodontol. 2001, 28, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, K.; Stahl, V. Cannabinoids Infused Mouthwash Products Are as Effective as Chlorhexidine on Inhibition of Total-Culturable Bacterial Content in Dental Plaque Samples. J. Cannabis. Res. 2020, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Kałduńska, J.; Jakubczak, K.; Gutowska, I.; Dalewski, B.; Janda, K. Fluoride content in dietary supplements of spirulina (Arthrospira spp.) from conventional and organic cultivation. Fluoride 2020, 5, 469–476. [Google Scholar]
- Maniyar, R.; Umashankar, G.K. Effectiveness of Spirulina Mouthwash on Reduction of Dental Plaque and Gingivitis: A Clinical Study. Int. J. Pharm. Pharm. Sci. 2017, 9, 136–139. [Google Scholar] [CrossRef]
- Isola, G.; Giudice, A.L.; Polizzi, A.; Alibrandi, A.; Murabito, P.; Indelicato, F. Identification of the Different Salivary Interleukin-6 Profiles in Patients with Periodontitis: A Cross-Sectional Study. Arch. Oral Biol. 2021, 122, 104997. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A.; Patini, R.; Ferlito, S.; Alibrandi, A.; Palazzo, G. Association among Serum and Salivary A. Actinomycetemcomitans Specific Immunoglobulin Antibodies and Periodontitis. BMC Oral Health 2020, 20, 283. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Polizzi, A.; Alibrandi, A.; Williams, R.C.; Giudice, A.L. Analysis of Galectin-3 Levels as a Source of Coronary Heart Disease Risk during Periodontitis. J. Periodontal Res. 2021. [Google Scholar] [CrossRef]
- Skottrup, P.D.; Dahlén, G.; Baelum, V.; Lopez, R. Soluble Urokinase-type Plasminogen Activator Receptor Is Associated with Signs of Periodontitis in Adolescents. Eur. J. Oral Sci. 2018, 126, 292–299. [Google Scholar] [CrossRef]
- Shoji, M.; Nakayama, K. Glycobiology of the Oral Pathogen Porphyromonas Gingivalis and Related Species. Microb. Pathog. 2016, 94, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, M.; Yamaguchi, Y.; Yoshitake, K.; Saeki, Y. Effects of TNFα and Prostaglandin E2 on the Expression of MMPs in Human Periodontal Ligament Fibroblasts. J. Periodontal Res. 2002, 37, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Hyun, J.-W.; Kim, Y.-G. Anticancer Effects of Natural Medicinal Plant Extracts on Oral Carcinoma Cells. J. Appl. Pharm. 1999, 7, 153–157. [Google Scholar]
- Park, H.-R.; Hong, S.-J. Research on Natural Medicine for Wellness and Oral Health. J. Digit. Converg. 2015, 13, 357–363. [Google Scholar] [CrossRef]
- Shim, K.-M.; Kim, S.-E.; Choi, J.-Y.; Choi, J.-C.; Jeong, S.-J.; Lee, J.-Y.; Bae, C.-S.; Park, D.-H.; Kim, D.-M.; Jeong, M.-J.; et al. Effects of Aucuba Japonica Extract on Oral Wound Healing. Korean Soc. Vet. Clin. 2006, 23, 55–60. [Google Scholar]
- Kim, J.-H.; Ji, C.-S.; Jung, B.-M.; Kim, B.-O.; Yu, S.-J. Effect of Mouthwash Containing Sun-Dried Salt on Gingivitis and Halitosis. Oral Biol. Res. 2015, 39, 120. [Google Scholar] [CrossRef]
- Brahmi, F.; Nury, T.; Debbabi, M.; Hadj-Ahmed, S.; Zarrouk, A.; Prost, M.; Madani, K.; Boulekbache-Makhlouf, L.; Lizard, G. Evaluation of Antioxidant, Anti-Inflammatory and Cytoprotective Properties of Ethanolic Mint Extracts from Algeria on 7-Ketocholesterol-Treated Murine RAW 264.7 Macrophages. Antioxidants 2018, 7, 184. [Google Scholar] [CrossRef] [PubMed]

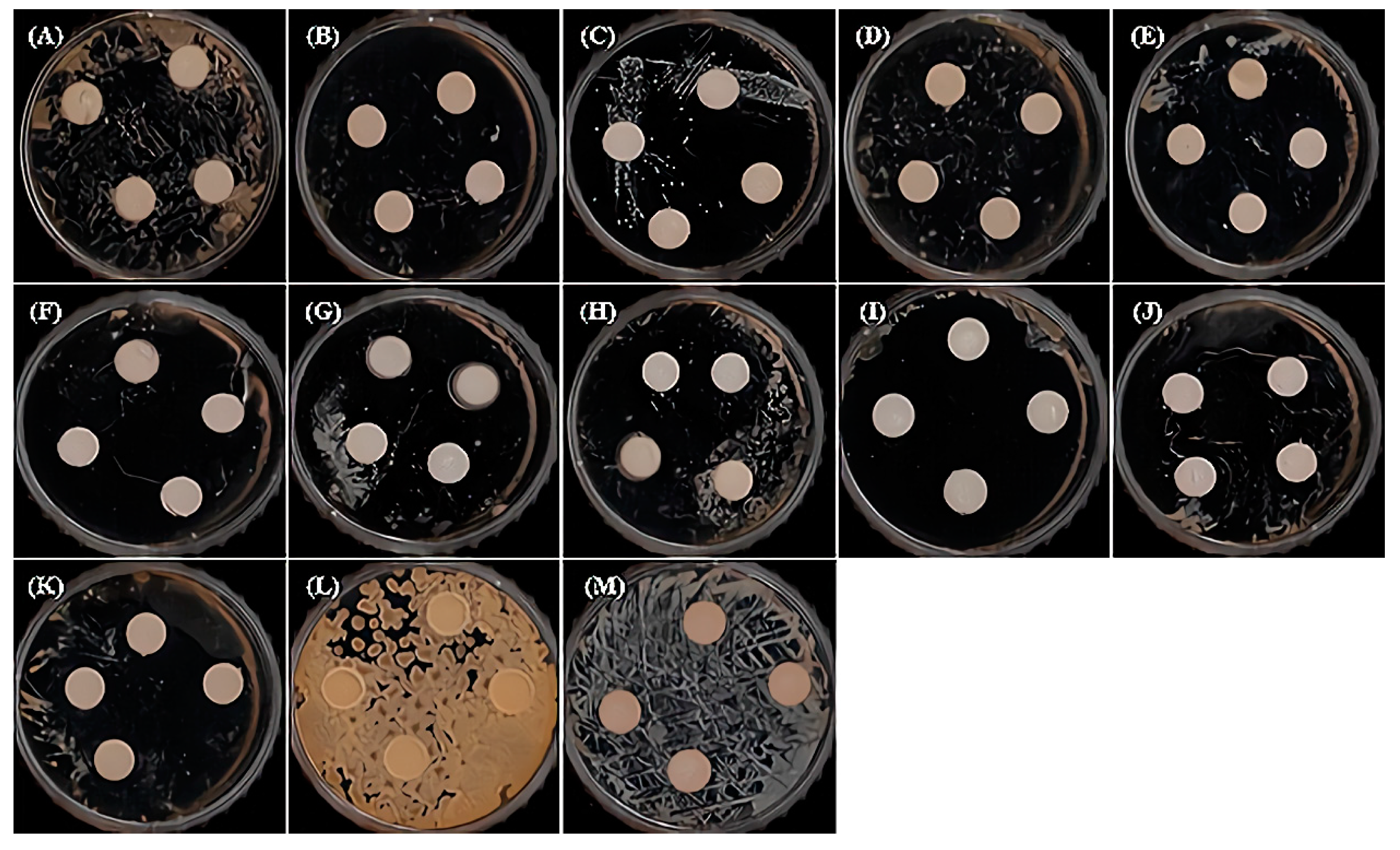
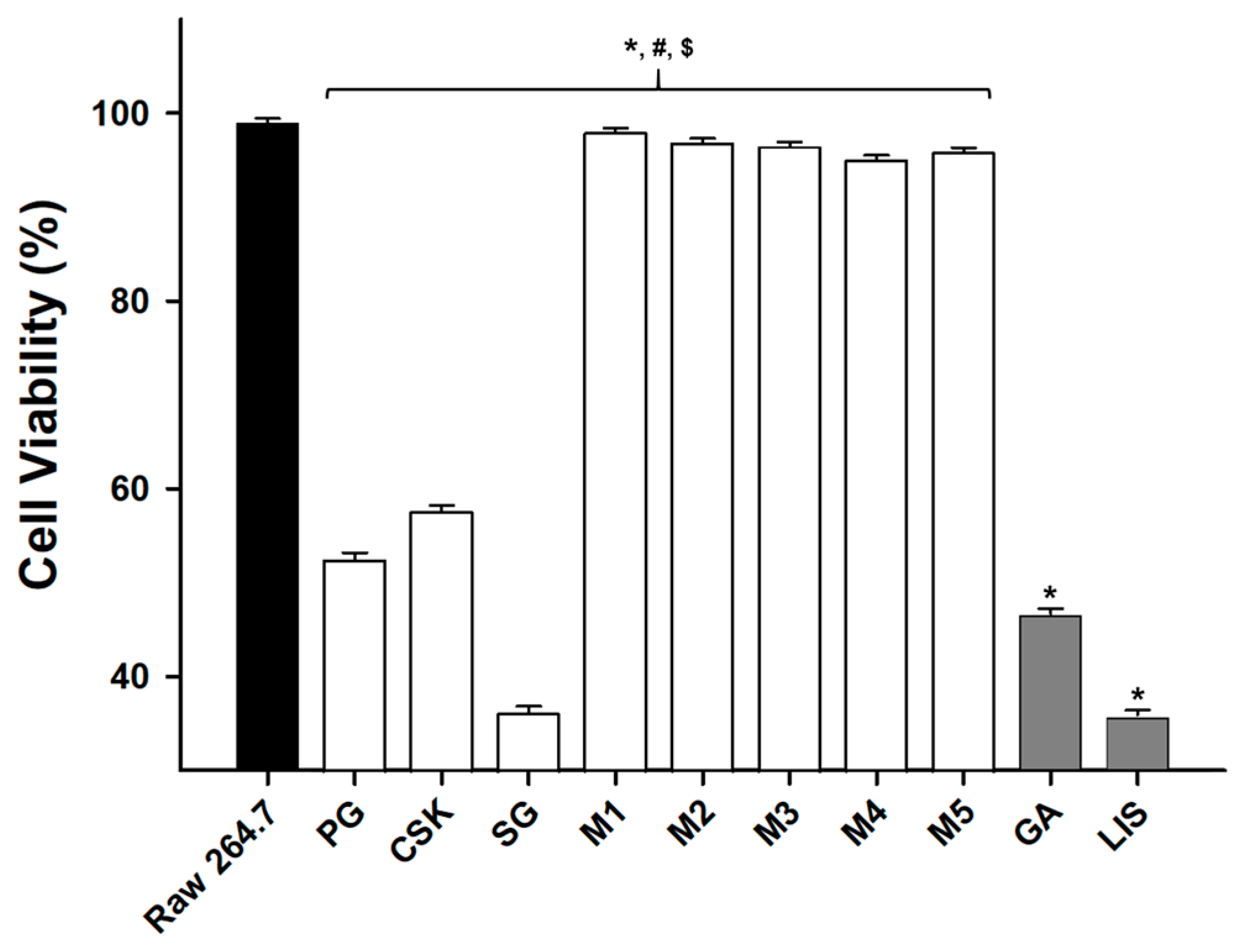
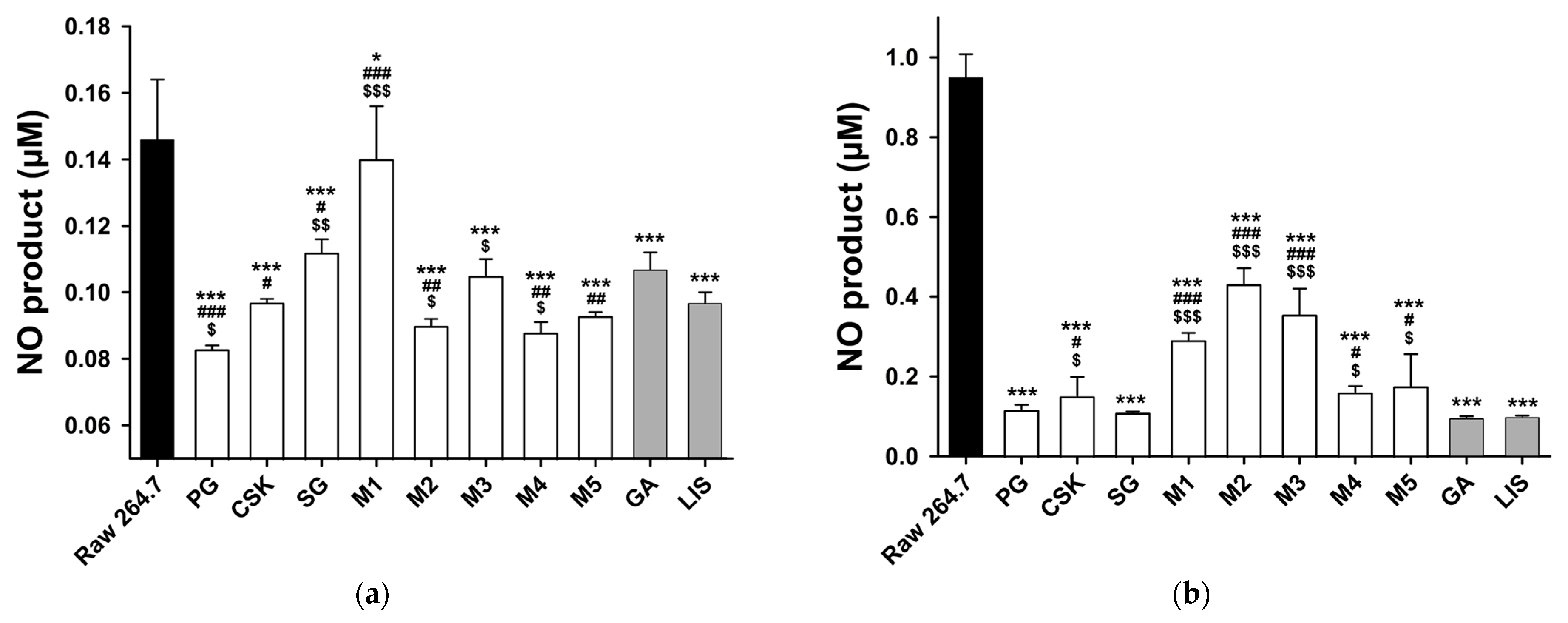
| Type | Resource Number | Oral Microorganisms |
|---|---|---|
| Pathogenic | * KCOM 1039 | Streptococcus constellatus (A) |
| KCOM 1070 | Streptococcus sanguinis | |
| KCOM 1128 | Streptococcus mutans | |
| * KCOM 1314 | Streptococcus constellatus (B) | |
| KCTC 15198 | Eikenella corrodens | |
| KCTC2640 | Fusobacterium nucleatum | |
| KCTC5272 | Streptococcus sobrinus | |
| KCTC2581 | Aggregatibacter actinomycetemcomitans | |
| KCTC5352 | Porphyromonas gingivalis | |
| KCTC5686 | Prevotella nigrescens | |
| KCTC5692 | Prevotella intermedia | |
| nonpathogenic | KCOM1429 | Streptococcus Salivarius |
| KCTC3600 | Lactobacillus Salivarius |
| Product Name | Major Components |
|---|---|
| PG | Platycodon grandiflorum |
| CSK | Chaenomeles sinensis Koehne |
| SG | Siraitia grosvenorii |
| M1 | Platycodon grandiflorum, Chaenomeles sinensis Koehne, Siraitia grosvenorii, enzyme salt, xylitol, mint, green tea, lemon, propolis, maltodextrin |
| M2 | Platycodon grandiflorum, Chaenomeles sinensis Koehne, Siraitia grosvenorii, enzyme salt, xylitol, mint, green tea, lemon, propolis, silicon dioxide, magnesium stearate |
| M3 | Platycodon grandiflorum, Chaenomeles sinensis Koehne, Siraitia grosvenorii, enzyme salt, xylitol, mint, green tea, lemon, propolis, silicon dioxide, magnesium stearate |
| M4 | Platycodon grandiflorum, Chaenomeles sinensis Koehne, Siraitia grosvenorii, enzyme salt, xylitol, mint, green tea, lemon, propolis, silicon dioxide, magnesium stearate |
| M5 | Platycodon grandiflorum, Chaenomeles sinensis Koehne, Siraitia grosvenorii, enzyme salt, xylitol, mint, green tea, lemon, propolis, silicon dioxide, magnesium stearate |
| GA | Eucalyptol, menthol, thymol, methyl salicylate, sodium fluoride, cetylpyridinium chloride, ethanol |
| LIS | Eucalyptol, thymol, methyl salicylate, menthol, benzoic acid, Green3, methyl salicylate, poloxamer 407, sodium benzoate, sodium saccharin, sorbitol, alcohol |
| Product Name | Ingredient Contents | Origin | Raw Material | Solvent |
|---|---|---|---|---|
| Xylitol | 40–70% | Finland | Ready-made | Hot water |
| Enzyme Salt | 3–10% | Korea | Sea slat + Fermented plant mixture liquid | Hot water |
| Mint extract powder | 10–15% | Korea | Leaf | Hot water |
| Platycodon grandiflorum | 0.5–12% | China | Root | Hot water |
| Chaenomeles sinensis Koehne | 0.1–11% | Korea | Fruit | Hot water |
| Green tea extract powder | 3–11% | Korea | Leaf | Hot water |
| Lemon extract powder | 2–10% | Korea | Fruit | Hot water |
| Propolis powder | 0.01–0.3% | Australia | Body | Alcohol extract |
| Siraitia grosvenorii | 0.01–0.5% | China | Fruit | Hot water |
| Exp. | Con. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacteria | PG1 | CSK1 | SG1 | M1 | M2 | M3 | M4 | M5 | GA | LIS | |
| P A T H O G E N | S. constellatus (A) | 82.5 ± 5.5 **,## | 83.5 ± 2.7 **,# | 89.7 ± 5.5 * | 99.9 ± 0.9 # | 90.8 ± 3.2 * | 84.6 ± 3.8 **,# | 81.5 ± 4.3 **,## | 81.4 ± 6.1 **,## | 96.9 ± 0.6 | 92.8 ± 3.6 |
| S. sanguinis | 94.1 ± 1.0 # | 99.9 ± 2.6 **,## | 93.3 ± 0.5 # | 94.1 ± 0.8 # | 94.6 ± 1.9 # | 91.1 ± 3.8 | 96.8 ± 2.1 *,## | 94.0 ± 2.2 # | 92.3 ± 0.1 | 90.1 ± 1.7 | |
| S. mutans | 96.1 ± 3.1 | 96.4 ± 0.9 | 92.9 ± 1.4 # | 81.8 ± 6.1 **,## | 95.0 ± 6.3 | 92.7 ± 2.3 # | 94.0 ± 1.7 # | 92.4 ± 3.6 # | 93.8 ± 3.1 | 99.9 ± 1.8 | |
| S.constellatus (B) | 82.7 ± 7.6 **,## | 92.9 ± 0.9 | 94.9 ± 1.9 | 99.9 ± 0.3 * | 92.3 ± 2.1 | 84.7 ± 2.3 *,## | 93.6 ± 4.5 | 87.8 ± 0.5 # | 92.8 ± 5.2 | 95.7 ± 3.7 | |
| E. corrodens | 94.9 ± 5.1 ## | 95.9 ± 3.7 ## | 95.4 ± 1.5 ## | 97.0 ± 1.1 ## | 98.1 ± 4.5 ## | 99.9 ± 0.5 ## | 97.3 ± 1.1 ## | 92.4 ± 1.1 # | 95.1 ± 0.1 | 87.8 ± 3.2 | |
| F. nucleatum | 97.0 ± 2.4 # | 99.9 ± 0.2 *,## | 92.9 ± 2.0 | 94.0 ± 1.7 | 92.8 ± 0.4 | 88.9 ± 8.2 # | 90.9 ± 2.3 | 94.1 ± 0.3 | 94.6 ± 0.3 | 91.5 ± 0.5 | |
| S. sobrinus | 83.5 ± 6.4 **,## | 90.8 ± 1.8 *,# | 98.7 ± 1.9 | 91.6 ± 4.5 *,# | 85.4 ± 5.5 **,## | 94.7 ± 5.5 | 87.7 ± 3.5 **,## | 91.4 ± 6.0 *,# | 98.3 ± 2.8 | 99.9 ± 1.2 | |
| A. actinomycetemcomitans | 80.4 ± 6.9 **,## | 96.9 ± 7.6 | 88.7 ± 4.2 **,# | 98.0 ± 1.5 | 92.8 ± 2.4 * | 82.5 ± 2.0 **,## | 86.6 ± 5.8 **,# | 82.5 ± 0.1 **,## | 99.9 ± 1.2 | 94.9 ± 2.5 | |
| P. gingivalis | 71.9 ± 4.2 **,## | 91.7 ± 2.3 ** | 89.6 ± 3.5 **,# | 84.4 ± 3.9 **,## | 62.5 ± 6.1 **,## | 79.2 ± 1.7 **,## | 80.2 ± 1.6 **,## | 78.9 ± 5.6 **,## | 99.9 ± 2.7 | 94.6 ± 1.1 | |
| P. nigrescens | 91.7 ± 4.4 | 99.9 ± 3.2 | 96.8 ± 1.1 | 98.9 ± 3.7 | 96.1 ± 5.3 | 91.3 ± 0.7 | 92.7 ± 7.3 | 98.4 ± 4.1 | 97.8 ± 2.6 | 94.7 ± 1.6 | |
| P. intermedia | 93.9 ± 1.8 | 91.8 ± 2.9 | 91.1 ± 2.9 | 84.8 ± 5.1 * | 97.9 ± 1.6 # | 94.3 ± 4.9 | 92.8 ± 1.3 | 99.9 ± 3.5 *,## | 92.1 ± 2.2 | 88.0 ± 8.2 | |
| N O N | S. salivarius | 11.0 ± 11.0 **,# | 27.0 ± 0.8 ** | 28.0 ± 0.1 ** | 10.0 ± 2.9 **,# | 22.0 ± 1.1 ** | 22.0 ± 2.4 ** | 22.0 ± 11.0 ** | 27.0 ± 3.1 ** | 90.0 ± 2.6 | 18.0 ± 7.3 |
| L. salivarius | 44.0 ± 8.1 **,## | 40.0 ± 0.9 **,## | 35.0 ± 1.5 **,## | 86.0 ± 7.4 # | 39.0 ± 0.3 **,## | 38.0 ± 0.7 **,## | 39.0 ± 1.3 **,## | 44.0 ± 4.1 **,## | 86.0 ± 8.1 | 77.0 ± 2.7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-H.; Kim, W.-H.; Ju, K.-W.; Lee, M.-S.; Kim, H.-S.; Lee, J.-H.; Jung, Y.-J.; Kim, B.-J. Antibacterial and Anti-Inflammatory Potential of Mouthwash Composition Based on Natural Extracts. Appl. Sci. 2021, 11, 4227. https://doi.org/10.3390/app11094227
Lee S-H, Kim W-H, Ju K-W, Lee M-S, Kim H-S, Lee J-H, Jung Y-J, Kim B-J. Antibacterial and Anti-Inflammatory Potential of Mouthwash Composition Based on Natural Extracts. Applied Sciences. 2021; 11(9):4227. https://doi.org/10.3390/app11094227
Chicago/Turabian StyleLee, Sung-Ho, Won-Hyeon Kim, Kyung-Won Ju, Min-Sun Lee, Han-Soo Kim, Jong-Ho Lee, Yu-Jin Jung, and Bong-Ju Kim. 2021. "Antibacterial and Anti-Inflammatory Potential of Mouthwash Composition Based on Natural Extracts" Applied Sciences 11, no. 9: 4227. https://doi.org/10.3390/app11094227
APA StyleLee, S.-H., Kim, W.-H., Ju, K.-W., Lee, M.-S., Kim, H.-S., Lee, J.-H., Jung, Y.-J., & Kim, B.-J. (2021). Antibacterial and Anti-Inflammatory Potential of Mouthwash Composition Based on Natural Extracts. Applied Sciences, 11(9), 4227. https://doi.org/10.3390/app11094227







