Abstract
High-frequency jet ventilators lacking flow monitoring systems, and information about supplied tidal volumes are still missing. This complicates the jet ventilator’s initial setting; at the same time, it complicates the line of the high-frequency ventilation itself. Therefore, an experimental flow sensor (orifice plate) was designed and placed in the ETC (endotracheal tube). Laboratory tests have confirmed the solution’s functionality, but it was necessary to verify the practical applicability for the clinical environment. The experiment shows that the flow sensor is used only for research purposes or when using functional monitoring of chest excursions.
1. Introduction
High-Frequency Ventilation (HFV) is a therapeutic technique, using a fast supply of very small breaths, comparable to the volume of the patient’s anatomical dead space [1]. The typical respiratory rate is greater than four times the normal value of spontaneous breathing; tidal volumes are in the order of milliliters. This reduces the risk of damage to lung tissue. Therefore, HFV techniques are used in premature newborns, whose lung tissue is very fragile, tends to collapse and holds excessive air in the lungs—so-called air-trapping [2,3].
Two high-frequency ventilation techniques are currently used in neonatal clinical practice—HFOV (High-Frequency Oscillatory Ventilation) and HFJV (High-Frequency Jet Ventilation), with a unique ventilator—Life Pulse (Bunnell Inc., Salt Lake City, UT, USA) [1,4]. Both techniques are widely used by neonatology centers, especially in North America. In Europe, the use of HFOV is common, but the HFJV technique is used rarely. The reason is the need for the jet ventilator and standard ventilator combination. Then, there is the absence of flow monitoring or the absence of information on the supplied tidal volumes. This complicates the initial setting of the jet ventilator (currently performed according to sufficient chest vibrations). Simultaneously, it complicates the line of the high-frequency ventilation itself, and it is not easy to maintain the required PaCO2. Given the importance of TV (Tidal Volume), it can be assumed that monitoring of this parameter must become an integral part of HFJV in the future [5,6].
In the HFJV Life Pulse ventilation circuit, it is theoretically possible to measure the flow in the Life Port fitting area, which contains the nozzle and allows the connection of a conventional ventilator, see Figure 1a. In the nozzle, the gas is accelerated and enters the ETC. However, at the same time, the fast-flowing gas from the nozzle entrains the gas from the area above the nozzle (gas from the ventilation circuit of a conventional ventilator). In the case of exhaling, gas from the patient’s respiratory system rises through the ETC and LifePort into a conventional ventilator circuit—Figure 1b.
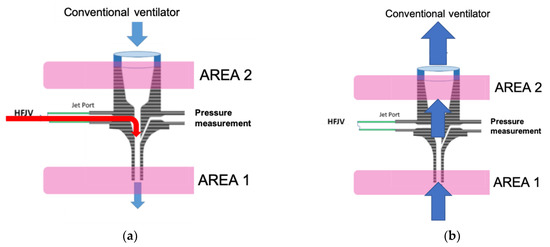
Figure 1.
Visualization of Life Port: (a) The course of gas inspiration; (b) Exhalation on the right.
There are theoretically two measurement options:
- Measurement in the patient’s ETC (AREA 1), where the flow sensor can register the entire amount of gas flowing into (from the inspiration) and from (the expiration) the patient;
- Measurement in the gas flow above the nozzle (upper part of the LifePort—AREA 2). A flow sensor located in this area can detect the total volume of expired gas. During inspiration, this area allows measurement of only the volume of gas that is entrained by the jet stream from the CV circuit. This means that the absolute value of the inspired gas cannot be measured.
Placing the flow sensor in AREA 1 makes it possible to obtain absolute values of the amount of gas during inhalation and exhalation. Thus, it represents several advantages, such as monitoring the patient’s minute ventilation and monitoring the size of gas leaks, etc.
However, in terms of possible sensors usable in pipes with a diameter of 2.5–3.5 mm, it is necessary to consider the influence of the sensor used on the specific pulsatile flow of gases generated by the HFJV, with a duration of around 20–30 ms and respiratory rates used in clinical practice reaching values of up to 660 breaths/min. Orifices and, to a limited extent, hot-wire anemometers can be used for the above conditions. The choice of a specific type of flow sensor is always a compromise between flow resistance, sensor dead volume, resistance to vapor condensation and gas composition changes [7].
Laboratory experiments [8,9] with flow measurement during HFJV using the orifice plate in the endotracheal tube confirmed the orifice plate’s functionality. They pointed to the need for the compensation of pressure losses in the orifice.
Therefore, the work aims to verify in simulated clinical conditions the influence of the experimentally designed flow sensor for monitoring of the tidal volumes on the elimination of CO2 during HFJV of animal models, and determine the possibility of compensating negative effects resulting from the use of the orifice plate located in the ETC (endotracheal tube).
A high-frequency jet ventilator with a flow sensor is introduced in the section Materials and Methods in the following text. The measuring apparatus and the provision of an animal experiment are described, including methods for data processing. Then follows the results in the form of graphical representations of monitored parameters and statistical evaluation results. In the discussion, the author analyzes the results obtained and the limitations of the monitoring techniques used. In the end, the limitations of the current solution are summarized, and the direction of further development is indicated.
2. Materials and Methods
Achieving the set aim is based on implementing an animal experiment with appropriate instrumentation and subsequent evaluation. A description of the individual parts is given in the subchapters below.
2.1. Jet Ventilator for Newborns—Life Pulse
The only clinically used high-frequency jet ventilator for neonatology and pediatric patients in the world is Life Pulse (Bunnell Inc., Salt Lake City, UT, USA). The Life Pulse Ventilator (Figure 2) is a microprocessor-controlled ventilator for pressure-limited and time-cycled ventilation of a patient using heated and humidified gas.

Figure 2.
Neonatal jet ventilator—Life Pulse (photo by author).
The Bunnell Life Pulse Ventilator allows you to set the following parameters:
- PIP—peak inspiratory pressure, adjustable in the range 0.8–5 kPa;
- Respiratory rate—breath frequency, adjustable in the range of 240–660 breaths/min;
- Inspiratory time, adjustable in the range 0.02–0.034 s.
In clinical practice, the ventilator is normally used in tandem with a conventional ventilator (Figure 3) that sets and maintains PEEP values (positive end-expiratory pressure) at the end of exhalation while generating conventional breaths to ensure oxygenation and recruitment of the lungs.
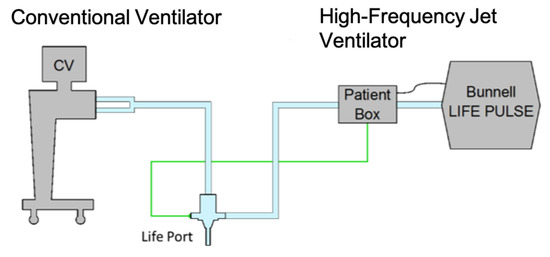
Figure 3.
Arrangement of ventilators in the experiment. The connection of the ventilation circuits is realized by means of the Life Port fitting.
2.2. Orifice Plate
The pressure differential orifice plate allows sensing of the gas flow in a section of the pipe. The orifice plate, exp. internal disc of the orifice, causes a change in the gas flow rate and its pressure in a given section (Figure 4a). There are sampling points for measuring pressures, whose difference determines the rate of the gas flow. The sampling points are located in places with the greatest pressure drop.
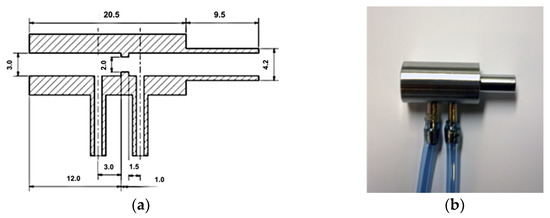
Figure 4.
Orifice plate as flow-sensor for HFJV: (a) Section and dimensions; (b) Realized flow sensor—orifice plate from stainless steel.
The orifice plate was made of stainless steel (Figure 4b), commonly used for surgical instruments. The choice of material ensured its stable properties, especially the chemical and thermal sterilization of the differential pressure orifice plate. The orifice plate design represents an increase in the dead space of the ventilation circuit by 0.212 mL. The pressure-flow characteristic of the orifice plate is given in Figure 5.
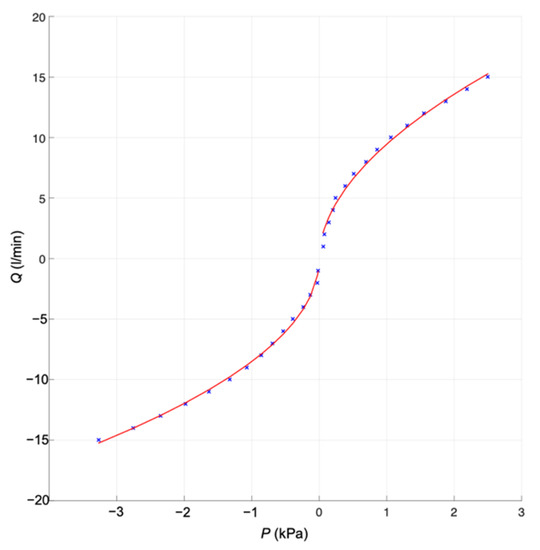
Figure 5.
Pressure-flow characteristic of the orifice plate
2.3. Measuring Apparatus
The IMON experimental measuring system is a measuring apparatus designed in the FBME CTU (Faculty of Biomedical Engineering, Czech Technical University in Prague), serving as a universal measuring device, usable for continuous monitoring and recording of up to 6 different pressures at the same time, with conventional or high-frequency lung ventilation. The system is based on an A/D converter USB NIDAQ 6009 (National Instruments, Austin, TE, USA), to which 26PC01 pressure sensors (Honeywell, Freeport, IL, USA) with a measurement range of 1 psi (6.9 kPa) are connected. Electronic circuits with INA128 amplifiers (Texas Instruments, Tucson, AZ, USA) provide impedance matching, amplification and basic filtering of the sensed signal (bandwidth 1–500 Hz). This arrangement guarantees full use of the 14-bit range of the A/D converter, with a preset sampling frequency 2 kHz. The flow sensing resolution was 2.14 mL/min, the max. non linearity is ±1.75 % span. Lab View Signal Express software (National Instruments, Austin, TX, USA) was used to visualize the course of signals and their saving to a PC.
2.4. Provision of Experiment
Animal experiments were conducted at the Institute of Physiology of the 1st Faculty of Medicine, Charles University. The Expert Commission approved them for laboratory animals of the 1st Medical Faculty of Charles University under Act No.246/1992 Coll. on the protection of animals against cruelty and its subsequent amendment according to Act No. 77/2004 Coll. of the Czech Republic, under the number MSMT—14076/2015-15 (Influence of the HFJV neonatal orifice plate on the exchange of respiratory gases).
The experiments were carried out on a set of 12 rabbits (Holland white), females of 1.1–1.5 kg. The animals were bred under standard conditions (environmental temperature 15–21 °C, relative humidity 60%, light for 12 h/dark for 12 h). The animals were anesthetized intramuscularly by injecting a solution of ketamine (Narketan) 75 mg/kg and Xylazine (Rometar) 5 mg/kg, and further maintained by regular intramuscular applications of ketamine 40 mg/kg/30 min throughout the experiment. Suppression of spontaneous respiration was ensured by injecting pipecuronium (Arduan) bolus doses of 50 μg/kg/60 min. Additionally, heparin was administered intravenously at doses of 150 IU/hour.
Arterial and venous accesses were secured using 26 G cannulas (BD Medical). The medicine was administered to v. auricularis marginalis, and blood pressure measurement (IBP) and blood samples for blood gases analysis using the AVL Compact 3 (Roche Diagnostic) were taken from a. auricularis centralis (Figure 6).
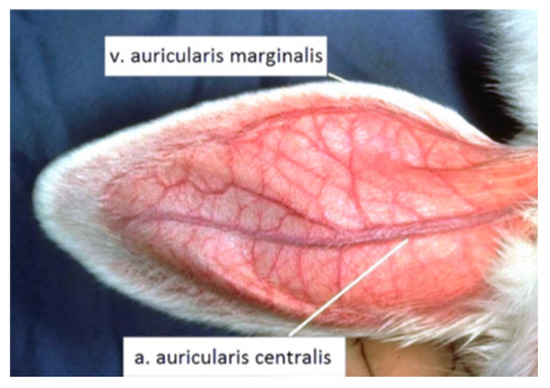
Figure 6.
Places to provide arterial and venous access.
The animals were placed on the laboratory table, and their position was fixed during the entire experiment. The heated blanket RD98 (Alfamedic, Lišov, Czech Republic) was used to maintain the body temperature of the animal at T = 38.5 ± 0.2 °C. This ensured that the rate of metabolic processes remained unchanged. Using the bedside monitor of vital functions S/5 (Datex Ohmeda), monitoring 3 lead ECG, heart rate, blood oxygen saturation SpO2, body temperature and arterial blood pressure (IBP) were monitored.
A 10% lidocaine solution was applied in the form of spray into respiratory pathways of the animals; they were subsequently blindly intubated in a pronation position with the head tilted, a neonatal endotracheal cannula (ETC) without sealing sleeve of 3 mm diameter (Vygon) was used, insertion length ETC—9 cm (measured at incisors). Ventilation support was provided by a Life Pulse high-frequency jet ventilator (Bunnell Inc.) and conventional BabyLog VN 500 ventilator (Dräger Medical) connected in tandem. In the experiment, the animal model’s chest expansions were monitored using a pressure cuff designed to measure NIBP (non-invasive blood pressure). The cuff was pressurized to 0.3 kPa as standard. Pressure changes in the cuff caused by gas inflation into the animal’s lungs were continuously monitored using an experimental iMON pressure gauge (FBME CTU). A TCM transcutaneous blood gas monitor (Radiometer, Denmark) served as an additional measure of the tcpO2/CO2 trend. The specific arrangement of the devices described in the previous sub-chapter is shown in Figure 7.
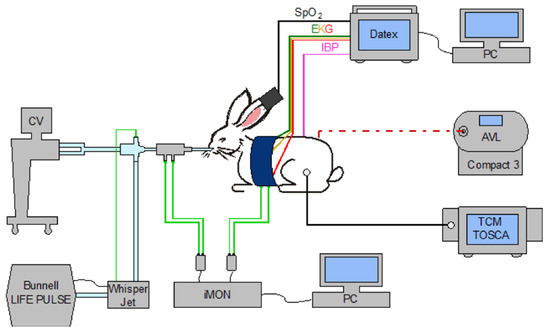
Figure 7.
Arrangement of an animal experiment. The animal model is intubated and connected to a neonatological jet and a conventional ventilator. Sensing of the pressure difference arising on the flow sensor and the cuff pressure (monitoring chest excursions) is measured synchronously using iMON and stored in a PC. The DATEX monitor monitors life functions; data are stored on a PC. A transcutaneous blood gas monitor continuously monitored the development of tcpO2 and tcpCO2; blood gas analysis was provided by an external analyzer AVL Compact3.
2.5. Experiment
After providing instrumentation and preparing the animal model according to the procedures outlined above, the experiment was conducted as follows:
Initial ventilation setting:
- PIP = 2 kPa;
- PEEP = 0.5 kPa;
- FiO2 = 30%;
- RR = 240 breaths/min;
- Inspiration time Ti = 0.02 s.
If the animal model was sufficiently oxygenated (PaO2 > 80 mmHg (10.66 kPa)) and optimally ventilated by PaCO2 35–45 mmHg (4.66–6 kPa), values stable for at least 10 min, the experimental sequence was initiated.
Experimental sequence:
- Ventilation of the animal model at a required frequency in a setting without orifice (none);
- Ventilation of the animal model at a required frequency in a setting with an orifice (Sens);
- Ventilation of the animal model at a required frequency in a setting with an orifice, including compensation (SensComp).
Ventilation of the animal model was carried out at frequencies of 240, 420 and 660 breaths/min. Blood samples for gas analysis were taken 10 min after the change was made. In the case of ventilation with the orifice plate, PIP was increased so that the animal model’s chest excursions measured by the pressure cuff were the same as for ventilation without an orifice plate. The photo from the animal lab is in Figure 8.
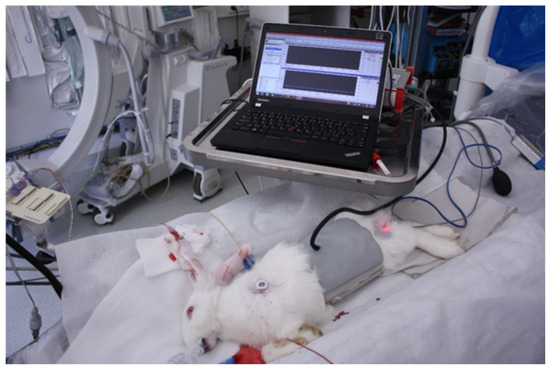
Figure 8.
An animal experiment in the lab. An animal experiment in the lab. The animal model body is in a gray cuff to monitor the chest excursions and PC measuring apparatus.
2.6. Data Collection and Processing
To assess the effect of an experimentally designed sensor (orifice plate) on CO2 elimination and other physiological parameters of the animal model, the results of blood gas analysis and ventilator-measured values of mean pressure inside the lungs were used. Parameters evaluated within the experiment: pH, PaCO2 and OI (oxygenation index).
Oxygenation index, including a different set fraction of FiO2 and pressure parameters inside the lungs of the animal model, was determined by calculation according to the following relation.
Values used in calculating the oxygenation index for FiO2 % were MAP (mean alveolar pressure), cmH2O and PaO2 in mmHg.
Given the extent of data of individual groups from different animals in a narrow weight and age range, for which no normal distribution was demonstrably found for any monitored parameters, non-parametric tests were chosen for hypothesis testing. Based on the animal study type (cross-over), Friedman ANOVA (α = 0.05) was used for more than two dependent selections [10]. The Wilcoxon test for two dependent selections was used as a post-hoc test [10].
If the zero hypothesis was rejected during Friedman’s test (Friedman’s ANOVA), post-hoc analysis was carried out. Since each physiology parameter was tested at a given frequency three times (none—without orifice, Sens—with orifice and SensComp—with orifice and compensation), the Wilcoxon test was used for two dependent selections adjusted by the degree of relevance, the so-called Bonferroni correction. The new level of relevance was based on the original chosen level of relevance for the Friedman test (α = 0.05) and was divided by the number of tests; see the formula below, Equation (2).
3. Results
Within the animal experiment in vivo, a series of measurements were carried out on 12 rabbits. One rabbit was used to verify methodology, and at the same time, a test of appropriate anesthesia, a test of the “blind” intubation method, setting HFJV ventilator, etc. Three rabbits died during the experiment before the completion of all phases. Data from eight animals were collected in total.
The monitored physiological parameters—pH, arterial partial pressure CO2 and oxygenation index depending on the respiratory rate are given below in Figure 9, Figure 10 and Figure 11. Graphs express medians, 25 and 75% quantiles, minimums and maximum values of measured or calculated parameters. Bar graphs always show the situation without orifice (none)—No. 1, No. 2 is the setting with an inserted orifice (Sens), and No. 3 is the setting with orifice and compensation (SensComp). The results of the Friedman test and post-hoc analysis are shown in Table 1.
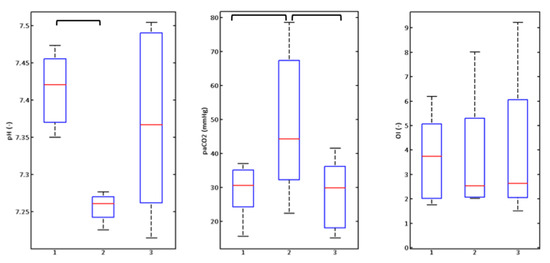
Figure 9.
Graphic illustration of monitored parameters of rabbits (n = 8) at a respiratory rate of 240 breaths/min.
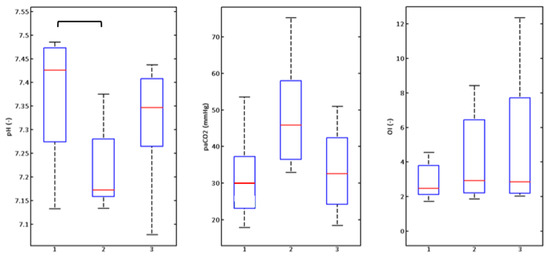
Figure 10.
Graphic illustration of monitored parameters of rabbits (n = 8) at a respiratory rate of 440 breaths/min.
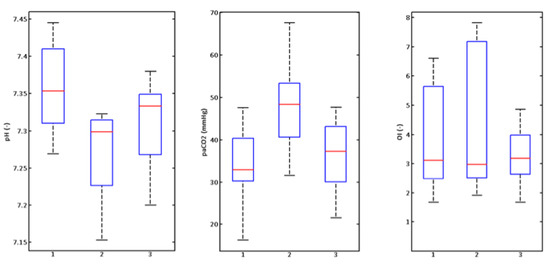
Figure 11.
Graphic illustration of monitored parameters of rabbits (n = 8) at a respiratory rate of 660 breaths/min.

Table 1.
Results of Friedman test and post-hoc analysis.
4. Discussion
The main observation of the in vivo experiment is a disruption of CO2 elimination from the animals’ lungs, which affects the internal environment of the animals’ organism using an orifice plate. Compensation for the pressure loss caused by the used differential orifice plate is possible by increasing the PIP inspiratory pressure on the Life Pulse ventilator, but only in case of external monitoring of chest excursions.
A hypothesis determined before the experiment indicates that a negative impact on the animal model’s physiological parameters using a pressure orifice plate (flow sensor) at high-frequency jet ventilation is expected. This assumption is given mainly because the phenomenon of permanent pressure loss in these flow sensors is known in the literature [6,7,11,12] and confirmed by the work [8,9]. On the other hand, the described experiments showed that all observed negative manifestations (decrease in pressure amplitude in the lung model and deterioration of CO2 elimination) could be compensated for by increasing the jet breath’s pressure amplitude using the PIP parameter.
Based on these findings, PaCO2 and pH parameters were monitored in animal models, and CO2 was expected to increase and pH to decrease (Figure 9, Figure 10 and Figure 11). The PaO2 parameter was also monitored as the oxygenation indicator of the animal models. Since the same fraction of oxygen in the ventilation mixture was not always used in experiments, the oxygenation index was used for the determination of the oxygenation, and to see the relationship (1), taking into account not only FiO2 but also the mean pressure in the airways, measured by the LifePulse ventilator.
Boxplots express the graphic illustration of the results in Figure 9, Figure 10 and Figure 11. The Friedman test (results in Table 1) confirmed changes in PaCO2 and pH values at respiratory rates of 240 and 420 (Figure 9 and Figure 10) breaths/min and changes to the oxygenation index at the rate of 420 breaths/minute (Figure 10). Subsequent post-hoc analysis by the Wilcoxon test (Table 1) with the adjusted degree of relevance then confirmed statistically significant changes between groups without the orifice plate (none) and with the orifice plate (Sens) for PaCO2 and pH parameters at rates of 240 and 420 breaths/min (Figure 9 and Figure 10, Table 1). The difference in PaCO2 at a respiratory rate of 240 breaths/min (Figure 9) between groups with an orifice (Sens) and with orifice plate and compensation (SensComp) was also statistically significant. These results point to the significant impact on PaCO2 and pH parameters when inserting the orifice plate into the ventilation circuit. After increasing PIP pressure amplitude (compensating pressure loss—SensComp situation), a statistically significant change was observed only in the PaCO2 parameter at a respiratory rate of 240 breaths/min.
Statistically significant changes to the oxygenation index were observed at 420 breaths/min between the groups none and SensComp (Figure 10). This situation was not expected in advance. Pressure increases to achieve consistent CO2 elimination during the setting of the ventilation circuit without an orifice plate (none) and with the orifice plate (SensComp) were carried out within the option of the LifePulse ventilator with a satisfactory result. Still, this setting caused a considerable response in changes to the OI oxygenation index. It can be accepted that orifice compensation based on the monitoring of volume changes to the animal models’ chests using a pressure cuff is not ideal, and the current position of the cuff to the chest has a direct effect on the sensitivity of the entire device (Figure 8). The mechanics of the thorax of the animal models with respect to the flexibility of the animal models’ chest walls had another effect on the accuracy of the indirect method of measuring the chest’s volume changes. As a result, it acts as a mechanical filter that does not transmit the rapid pressure generated by a high-frequency jet ventilator inside the alveolar space. Therefore, pressure loss was likely to be offset on the orifice plate, but this fact was not observable on the monitor of the chest’s volume changes. A more sensitive method used in recent years and in clinical practice is electric impedance tomography [13]. This method can non-invasively monitor lung aeration and determine the size of breath volumes. However, available systems have a relatively low sampling frequency that does not monitor high-frequency ventilation in the full range of the experiment discussed [14].
At the highest possible respiratory rate of 660 breaths/min, no changes to the parameters were confirmed at the 0.05 level of relevance.
5. Conclusions
The laboratory experiment results confirmed the applicability of the orifice plate for measuring the gas flow at HFJV, but only in compensating for undesired pressure losses caused by a permanent pressure drop on the orifice plate. The compensation can be conveniently set if the amount of pressure loss is known to the ventilator operator, which can be ensured mainly in lung simulators’ laboratory conditions.
Animal experiments further point out that installing an orifice as a flow meter directly in the endotracheal cannula is hazardous for clinical practice and, without knowledge of the currently necessary pressure compensation, dangerous for the animal model patient.
Based on the obtained results and knowledge, it is recommended to place the flow sensor for monitoring tidal volumes during high-frequency jet ventilation with the Bunnell Life Pulse device in another place, especially into the expiratory part of the ventilation circuit, even at the cost of inaccurate inspiratory volume measurements. The amount of total exhaled gas will be measured accurately.
Future work in this area will lead to the development of a sensitive flowmeter with minimized resistance and a minimal increase in the volume of the LifePort armature. This will probably be a hot-wire anemometer implementation.
Funding
This work was supported by projects SGS19/202/OHK4/3T/17.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the 1st Medical Faculty of Charles University in Prague (protocol code MSMT—14076/2015-15 (Influence of the HFJV neonatal membrane on the exchange of respiratory gases), date of approval: 13 May 2015.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The project was supported by grant CTU in Prague with number SGS19/202/OHK4/3T/17—Processing and interpretation of biomedical data for neonatology.
Conflicts of Interest
The authors declare no conflict of interest.
References
- White, G. Equipment Theory for Respiratory Care, 5th ed.; CENGAGE: Boston, MA, USA, 2015. [Google Scholar]
- Gomella, T.L. Neonatology Management, Procedures, On-Call Problems, Diseases, and Drugs, 8th ed.; Mc Graw-Hill Profesional: New York, NY, USA, 2020. [Google Scholar]
- Voynow, J.A. “New” bronchopulmonary dysplasia and chronic lung disease. Paediatr. Respir. Rev. 2017, 24, 17–18. [Google Scholar] [CrossRef] [PubMed]
- Jobe, A.H.; Steinhorn, R. Can We Define Bronchopulmonary Dysplasia? J. Pediatr. 2017, 188, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Polin, R.A.; Fox, W.W.; Abman, S.H. Fetal and Neonatal Physiology: Expert Consult-Online and Print; Elsevier Health Sciences: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Rimensberger, P.C.; Heulitt, M.J.; Meliones, J.; Pons, M.; Bronicki, R.A. Mechanical ventilation in the pediatric cardiac intensive care unit: The essentials. World J. Pediatr. Congenit. Heart Surg. 2011, 2, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Schena, E.; Massaroni, C.; Saccomandi, P.; Cecchini, S. Flow measurement in mechanical ventilation: A review. Med. Eng. Phys. 2015, 37, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Kudrna, P.; Rožánek, M. The influence of an orifice plates as a flow sensors on the removal of carbon dioxide in high fre-quency oscillatory and jet ventilation. In Proceedings of the International Conference on Advancements of Medicine and Health Care through Technology, Cluj-Napoca, Romania, 12–15 October 2016; pp. 137–140. [Google Scholar]
- Kudrna, P.; Rožánek, M.; Hřibalová, B. Flow measuring during neonatal high frequency jet ventilation using orifice plate. Biomed. Tech. 2014, 59, 252–254. [Google Scholar]
- Moore, D.S.; Kirkland, S. The Basic Practice of Statistics; WH Freeman: New York, NY, USA, 2007; Volume 2. [Google Scholar]
- Courtney, S.E.; Asselin, J.M. High-frequency jet and oscillatory ventilation for neonates: Which strategy and when? Respir. Care Clin. N. Am. 2006, 12, 453–467. [Google Scholar] [PubMed]
- Rimensberger, P.C.; Schulzke, S.; Tingay, D.; Von Ungern-Sternberg, B. Pediatric and Neonatal Mechanical Ventilation—From Basics to Clinical; Springer: Berlin, Germany, 2015. [Google Scholar]
- Tingay, D.G.; Waldmann, A.D.; Frerichs, I.; Ranganathan, S.; Adler, A. Electrical Impedance Tomography Can Identify Ventilation and Perfusion Defects: A Neonatal Case. Am. J. Respir. Crit. Care Med. 2019, 199, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Sobota, V.; Müller, M.; Roubík, K. Intravenous administration of normal saline may be misinterpreted as a change of end-expiratory lung volume when using electrical impedance tomography. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).