The Importance of Intraoperative Plain Radiographs during Cochlear Implant Surgery in Patients with Normal Anatomy
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Imaging Technique
3. Results
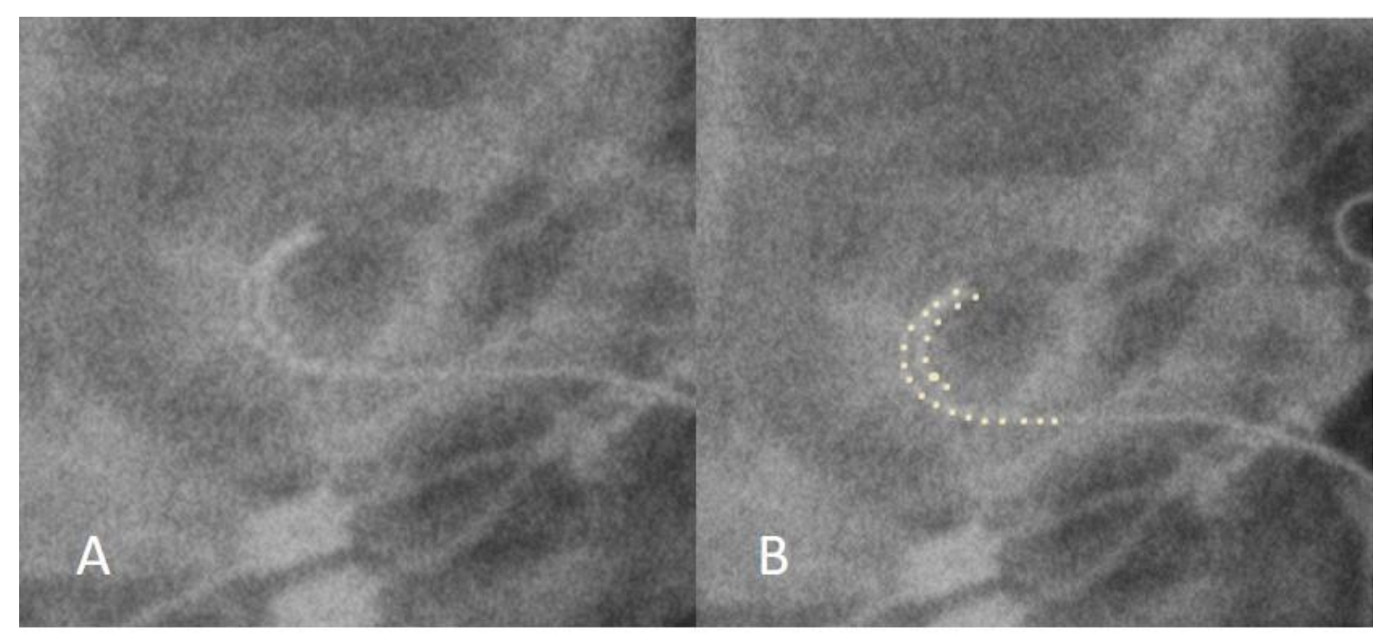
3.1. Case 1: Tip Fold-Over
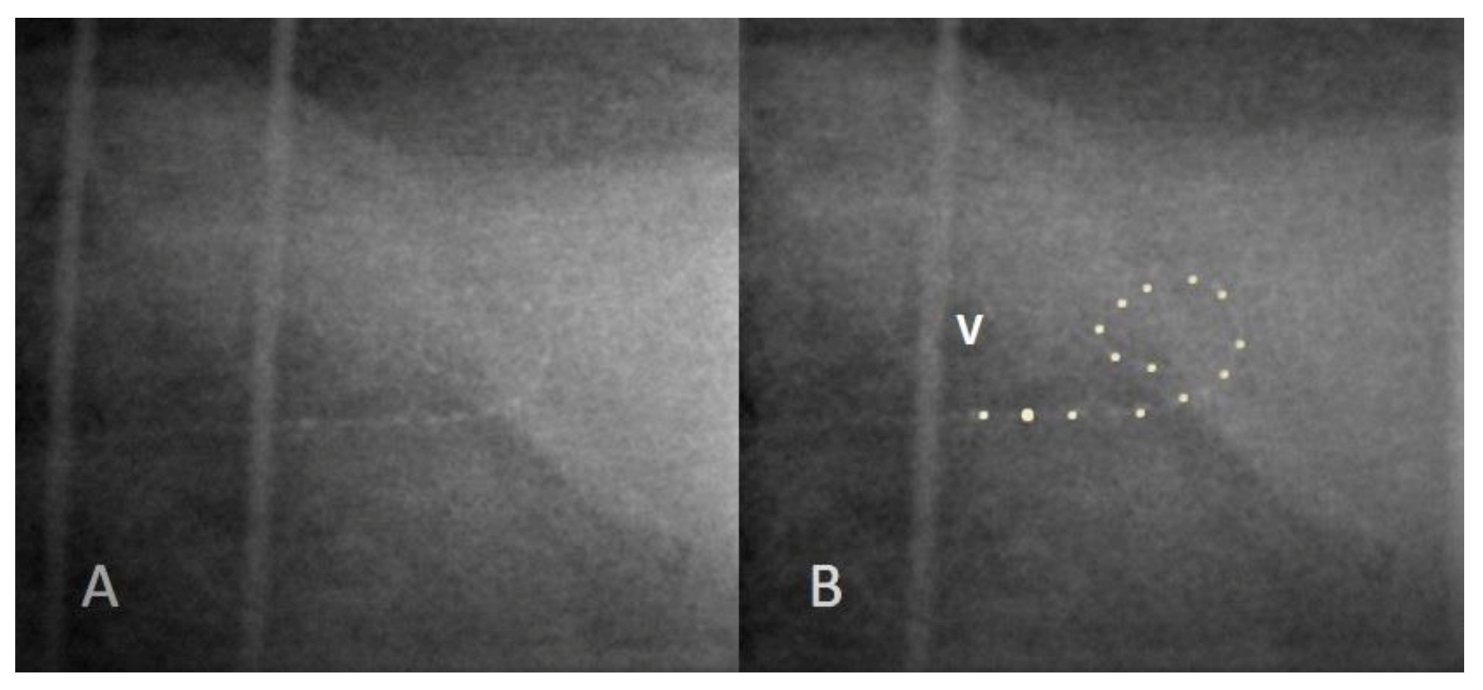
3.2. Case 2: Incomplete Insertion
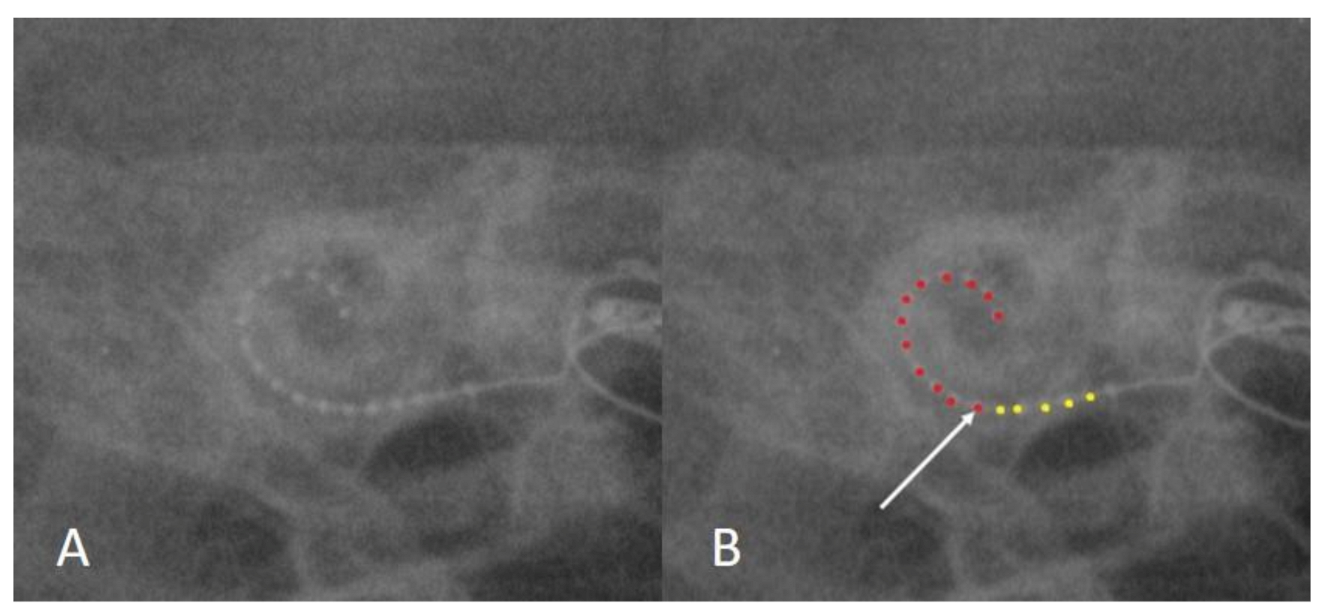
3.3. Case 3: Extracochlear Electrode Array Malposition
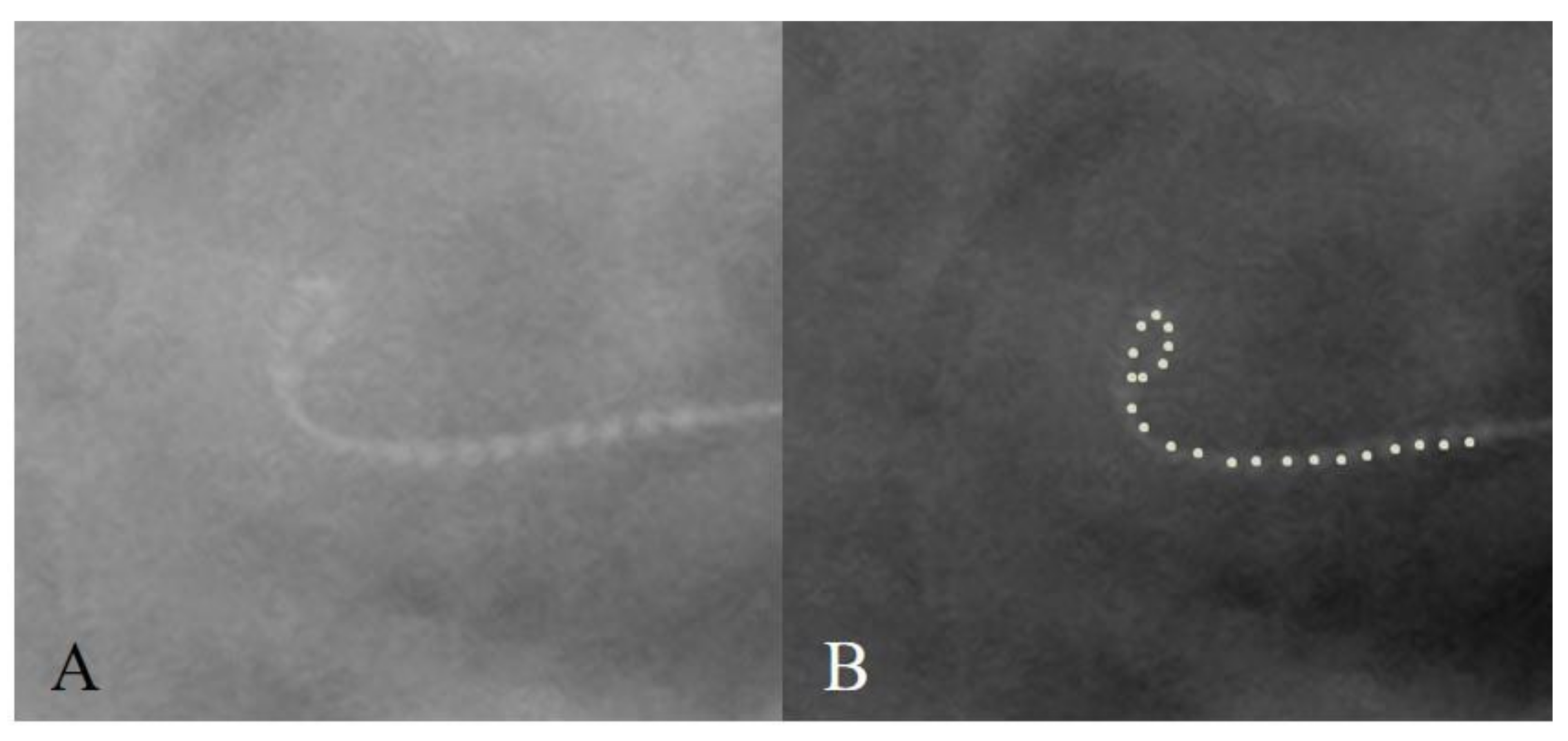
3.4. Case 4: Distal Tip Fold-Over
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Shaul, C.; Roland, J.T., Jr.; Sichel, J.Y.; Salem, R.; Perez, R. Revision cochlear implantation using a double array device in the post-meningitis ossified cochlea. Int. J. Pediatr. Otolaryngol. 2020, 139, 110446. [Google Scholar] [CrossRef] [PubMed]
- Zaltz, Y.; Bugannim, Y.; Zechoval, D.; Kishon-Rabin, L.; Perez, R. Listening in Noise Remains a Significant Challenge for Cochlear Implant Users: Evidence from Early Deafened and Those with Progressive Hearing Loss Compared to Peers with Normal Hearing. J. Clin. Med. 2020, 9, 1381. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Maxfield, A.Z.; Foyt, D.; Rapoport, R.J. Utility of intraoperative computed tomography for cochlear implantation in patients with difficult anatomy. Cochlear Implant. Int. 2018, 19, 170–179. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, B.P.; Cakir, A.; Hunter, J.B.; Francis, D.O.; Noble, J.H.; Labadie, R.F.; Zuniga, G.; Dawant, B.M.; Rivas, A.; Wanna, G.B. Electrode location and angular insertion depth are predictors of audiologic outcomes in cochlear implantation. Otol. Neurotol. 2016, 37, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.L.M.; Lin, J.W.; Oghalai, J.S.; Williamson, R.A. Cochlear implant electrode misplacement: Incidence, evaluation, and management. Laryngoscope 2013, 123, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Labadie, R.F.; Schefano, A.D.; Holder, J.T.; Dwyer, R.T.; Rivas, A.; O’Malley, M.R.; Noble, J.H.; Dawant, B.M. Use of intraoperative CT scanning for quality control assessment of cochlear implant electrode array placement. Acta. Otolaryngol. 2020, 140, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Fishman, A.J.; Roland, J.T.; Alexiades, G.; Mierzwinski, J.; Cohen, N.L. Fluoroscopically assisted cochlear implantation. Otol. Neurotol. 2003, 24, 882–886. [Google Scholar] [CrossRef] [PubMed]
- Cosetti, M.K.; Friedmann, D.R.; Heman-Ackah, S.E.; Perez, R.; Waltzman, S.B.; Roland, J.T., Jr. Surgical techniques and outcomes of cochlear implantation in patients with radiographic findings consistent with X-linked deafness. Int. J. Pediatr. Otolaryngol. 2015, 79, 1689–1693. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.; Salem, R.; Roland, J.T., Jr.; Sichel, J.Y. Fluoroscopic assisted cochlear implantation in children with inner ear malformations. Harefuah 2014, 153, 713–717. [Google Scholar] [PubMed]
- Xu, J.; Xu, S.A.; Cohen, L.T.; Clark, G.M. Cochlear view: Postoperative radiography for cochlear implantation. Otol. Neurotol. 2000, 21, 49–56. [Google Scholar]
- Grolman, W.; Maat, A.; Verdam, F.; Simis, Y.; Carelsen, B.; Freling, N.; Tange, R.A. Spread of excitation measurements for the detection of electrode array foldovers: A prospective study comparing 3-dimensional rotational x-ray and intraoperative spread of excitation measurements. Otol. Neurotol. 2009, 30, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Finley, C.C.; Skinner, M.W. Role of electrode placement as a contributor to variability in cochlear implant outcomes. Otol. Neurotol. 2008, 29, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.; Kishon-Rabin, L. Cochlear implants-pediatric. In Encyclopedia of Otolaryngology, Head and Neck Surgery; Kountakis, S.E., Ed.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Dirr, F.; Hempel, J.M.; Krause, E.; Müller, J.; Berghaus, A.; Ertl-Wagner, B.; Braun, T. Value of routine plain x-ray position checks after cochlear implantation. Otol. Neurotol. 2013, 34, 1666–1669. [Google Scholar] [CrossRef] [PubMed]
- Cosetti, M.K.; Troob, S.H.; Latzman, J.M.; Shapiro, W.H.; Roland, J.T., Jr.; Waltzman, S.B. An evidence-based algorithm for intraoperative monitoring during cochlear implantation. Otol. Neurotol. 2012, 33, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Dhanasingh, A.; Jolly, C. Review on cochlear implant electrode array tip fold-over and scalar deviation. J. Otol. 2019, 14, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, T.; Côté, M.; Eter, E.; Laborde, M.L.; Cochard, N.; Deguine, O.; Fraysse, B. Cochlear reimplantations: Technical and surgical failures. Acta. Otolaryngol. 2009, 129, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Zuniga, M.G.; Rivas, A.; Hedley-Williams, A.; Gifford, R.H.; Dwyer, R.; Dawant, B.M.; Sunderhaus, L.W.; Hovis, K.L.; Wanna, G.B.; Noble, J.H.; et al. Tip fold-over in cochlear implantation: Case series. Otol. Neurotol. 2017, 38, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.M.; Patel, R.; Redleaf, M. Intra-operative skull X-ray for misdirection of the cochlear implant array into the vestibular labyrinth. J. Laryngol. Otol. 2015, 129, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Bloom, J.D.; Rizzi, M.D.; Germiller, J.A. Real-time intraoperative computed tomography to assist cochlear implant placement in the malformed inner ear. Otol. Neurotol. 2009, 30, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.R.; Selvadurai, D.; Beale, T.; Biggs, N.; Murray, B.; Gibson, P.; Risi, F.; Boyd, P. The use of cone-beam computed tomography to determine cochlear implant electrode position in human temporal bones. Otol. Neurotol. 2014, 35, 1338–1344. [Google Scholar] [CrossRef] [PubMed]

| Patient No. | Age at Implantation | Indication | Implant | NRT/NRI | Intraoperative Radiograph | Post Reinsertion Radiographs | Post Reinsertion NRT/NRI |
|---|---|---|---|---|---|---|---|
| 1 | 2 years | Congenital HL | Cochlear CI512 | Normal | Tip fold-over | Normal | Normal |
| 2 | 11 months | 21 trisomy | Ab hr90k/Hi-Focus 1j | No response at basal electrode | Incomplete insertion | Normal | Normal, including basal electrodes |
| Idiopathic, congenital HL | |||||||
| 3 | 22 years | Prematurity | AB HR90K/Hi-Focus MS | No response | Oval curve, acute-angled | Normal | No response |
| Congenital progressive HL | |||||||
| 4 | 4 years | Familial progressive HL | Cochlear CI632 | Normal | Tip fold-over | Normal | Normal |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cohen, O.; Sichel, J.-Y.; Shaul, C.; Chen, I.; Roland, J.T., Jr.; Perez, R. The Importance of Intraoperative Plain Radiographs during Cochlear Implant Surgery in Patients with Normal Anatomy. Appl. Sci. 2021, 11, 4144. https://doi.org/10.3390/app11094144
Cohen O, Sichel J-Y, Shaul C, Chen I, Roland JT Jr., Perez R. The Importance of Intraoperative Plain Radiographs during Cochlear Implant Surgery in Patients with Normal Anatomy. Applied Sciences. 2021; 11(9):4144. https://doi.org/10.3390/app11094144
Chicago/Turabian StyleCohen, Ohad, Jean-Yves Sichel, Chanan Shaul, Itay Chen, J. Thomas Roland, Jr., and Ronen Perez. 2021. "The Importance of Intraoperative Plain Radiographs during Cochlear Implant Surgery in Patients with Normal Anatomy" Applied Sciences 11, no. 9: 4144. https://doi.org/10.3390/app11094144
APA StyleCohen, O., Sichel, J.-Y., Shaul, C., Chen, I., Roland, J. T., Jr., & Perez, R. (2021). The Importance of Intraoperative Plain Radiographs during Cochlear Implant Surgery in Patients with Normal Anatomy. Applied Sciences, 11(9), 4144. https://doi.org/10.3390/app11094144






