Model Comparisons of Flow and Chemical Kinetic Mechanisms for Methane–Air Combustion for Engineering Applications
Abstract
1. Introduction
2. Object Description
3. Predictions with Different Turbulence Models
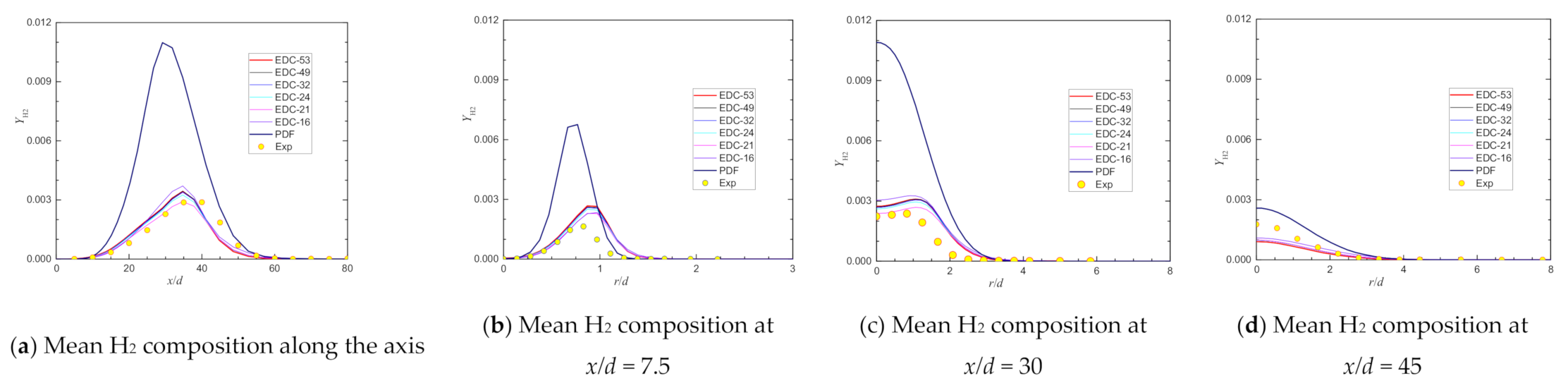
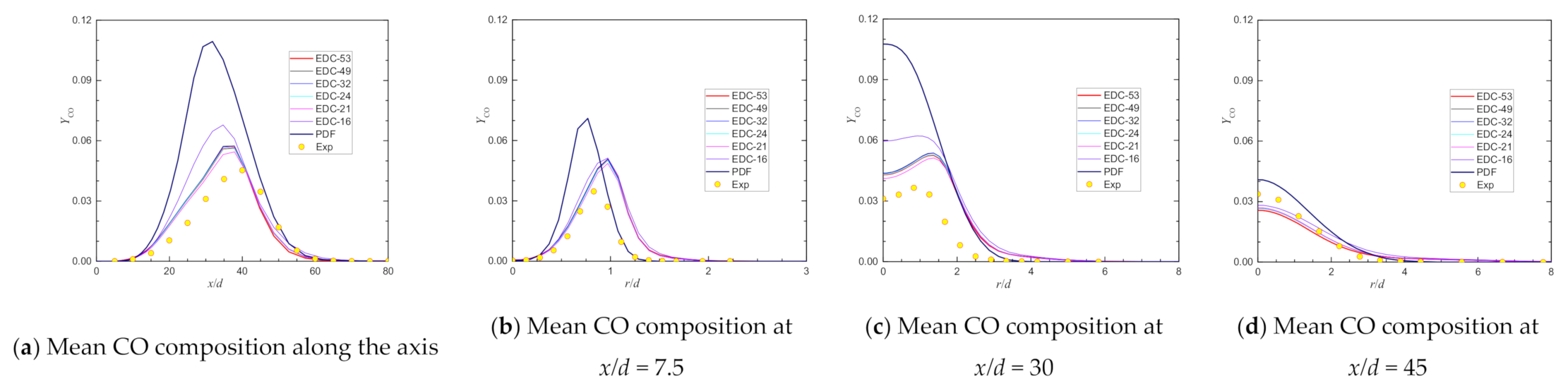
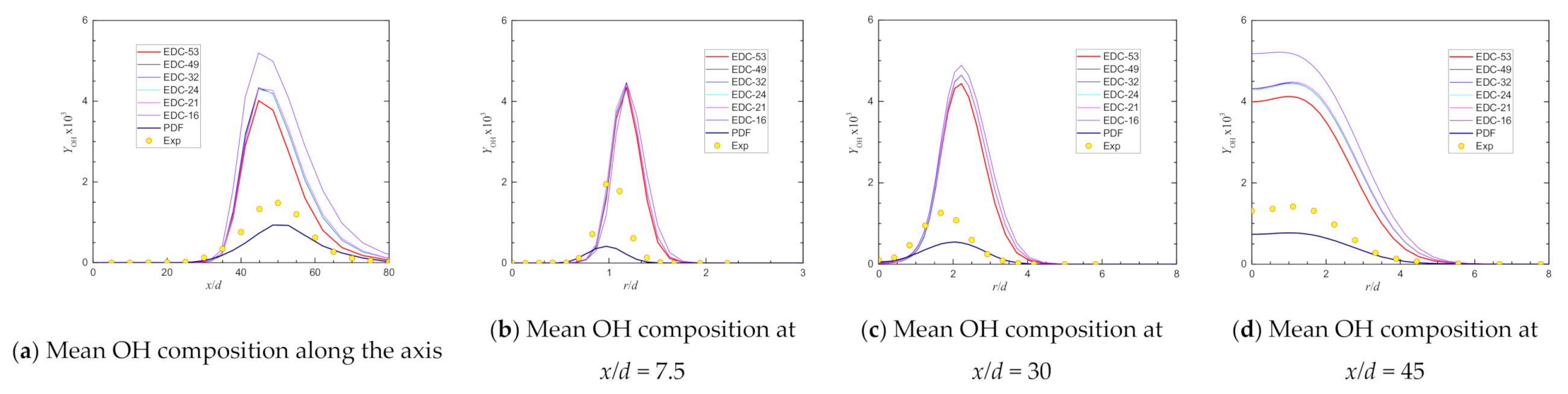
4. Predictions with Different Chemical Kinetic Mechanisms
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salarin, S.A.; Shahbaz, M. Natural gas consumption and economic growth: The role of foreign direct investment, capital formation and trade openness in Malaysia. Renew. Sustain. Energy Rev. 2015, 42, 835–845. [Google Scholar] [CrossRef]
- Birsselioglu, M.E.; Yelkenci, T.; Oz, L.O. Investigating the natural gas supply security: A new perspective. Energy 2015, 80, 168–176. [Google Scholar] [CrossRef]
- Mason, C.F.; Wilmot, N.A. Jump processes in natural gas markets. Energy Econ. 2014, 46, S69–S79. [Google Scholar] [CrossRef]
- Sayre, A.; Lallemant, N.; Dugué, J.; Weber, R. Scaling Characteristics of Aerodynamics and Low-NOx Properties of Industrial Natural Gas Burners, The SCALING 400 Study, Part IV: The 300 kW BERL Test Results, IFRF Doc No F40/y/11; International Flame Research Foundation: Ijmuiden, The Netherlands, 1994. [Google Scholar]
- Cellek, M.S.; Pınarbası, A. Investigations on performance and emission characteristics of an industrial low swirl burner while burning natural gas, methane, hydrogen-enriched natural gas and hydrogen as fuels. Int. J. Hydrog. Energy 2018, 43, 1194–1207. [Google Scholar] [CrossRef]
- Launder, B.E.; Spalding, D.B. Lectures in Mathematical Models of Turbulence; Academic Press: London, UK, 1972. [Google Scholar]
- Shih, T.H.; Liou, W.W.; Shabbir, A.; Yang, Z.; Zhu, J. A New k-epsilon Eddy-Viscosity Model for High Reynolds Number Turbulent Flows—Model Development and Validation. Comput. Fluids 1995, 24, 227–238. [Google Scholar] [CrossRef]
- Menter, F.R. Two-Equation Eddy-Viscosity Turbulence Models for Engineering Applications. AIAA J. 1994, 32, 1598–1605. [Google Scholar] [CrossRef]
- Launder, B.E.; Reece, G.J.; Rodi, W. Progress in the Development of a Reynolds-Stress Turbulence Closure. J. Fluid Mech. 1975, 68, 537–566. [Google Scholar] [CrossRef]
- Gibson, M.M.; Launder, B.E. Ground Effects on Pressure Fluctuations in the Atmospheric Boundary Layer. J. Fluid Mech. 1978, 86, 491–511. [Google Scholar] [CrossRef]
- Launder, B.E. Second-Moment Closure: Present... and Future. Int. J. Heat Fluid Flow 1989, 10, 282–300. [Google Scholar] [CrossRef]
- Shur, M.; Spalart, P.R.; Strelets, M.; Travin, A. Detached-Eddy Simulation of an Airfoil at High Angle of Attack. In Proceedings of the 4th International Symposium on Engineering Turbulence Modelling and Measurements, Ajaccio, Corsica, France, 24–26 May 1999. [Google Scholar]
- Spalart, P.R.; Deck, S.; Shur, M.L.; Squires, K.D.; Strelets, M.K.; Travin, A. A new version of detached-eddy simulation, resistant to ambiguous grid densities. Theor. Comput. Fluid Dyn. 2006, 20, 181–195. [Google Scholar] [CrossRef]
- DesJardin, P.E.; Franke, S.H.L. Large eddy simulation of a nonpremixed reacting jet: Application and assessment of subgrid-scale combustion models. Phys. Fluids 1998, 10, 2298–2314. [Google Scholar] [CrossRef]
- Pitsch, H.; Steiner, H. Large-Eddy simulation of a turbulent piloted methane/air diffusion flame (Sandia Flame D). Phys. Fluids 2000, 12, 2541–2554. [Google Scholar] [CrossRef]
- Splading, D. Mixing and chemical reaction in steady confined turbulent flames. In 13th Symposium on Combustion; The Combustion Institute: Salt Lake City, UT, USA, 1970. [Google Scholar]
- Magnussen, B.F. On the structure of turbulence and a generalized eddy dissipation concept for chemical reaction in turbulent flow. In Proceedings of the 19th American Institute of Aeronautics and Astronautics (AIAA) Aerospace Sciences Meeting, St. Louis, MO, USA, 12–15 January 1981; Available online: http://folk.ntnu.no/ivarse/edc/EDC1981.pdf (accessed on 27 April 2021).
- Magnussen, B.F.; Hjertager, B.H. On mathematical modeling of turbulent combustion with special emphasis on soot formation and combustion. Proc. Combust. Inst. 1976, 16, 719–729. [Google Scholar] [CrossRef]
- Lockwood, F.; Naguib, A. The prediction of the fluctuations in the properties of free, round-jet, turbulent, diffusion flames. Combust. Flame 1975, 24, 109–124. [Google Scholar] [CrossRef]
- Gutheil, E. Multivariate PDF Closure Applied to Oxidation of CO in a Turbulent Flow. In Proceedings of the 12th International Colloquium on Dynamics of Explosions and Reactive Systems, Ann Arbor, MI, USA, 23–28 July 1989. [Google Scholar]
- Jiménez, J.; Liñán, A.; Rogers, M.; Higuera, F. A priori testing of subgrid models for chemically reacting non-premixed turbulent shear flows. J. Fluid Mech. 1997, 349, 149–171. [Google Scholar] [CrossRef]
- Cao, R.R.; Pope, S.B. The influence of chemical mechanisms on PDF calculations of nonpremixed piloted jet flames. Combust. Flame 2005, 143, 450–470. [Google Scholar] [CrossRef]
- Mastorakos, E.; Bilger, R. Second-order conditional moment closure for the autoignition of turbulent flows. Phys. Fluids 1998, 10, 1246–1248. [Google Scholar] [CrossRef]
- Klimenko, Y.; Bilger, R. Conditional moment closure for turbulent combustion. Prog. Energy Combust. Sci. 1999, 25, 595–687. [Google Scholar] [CrossRef]
- Peters, N. Laminar diffusion flamelet models in non-premixed turbulent combustion. Prog. Energy Combust. Sci. 1984, 10, 319–339. [Google Scholar] [CrossRef]
- Cook, A.; Riley, J.; Kosály, G. A laminar flamelet approach to subgrid-scale chemistry in turbulent flows. Combust. Flame 1997, 109, 332–341. [Google Scholar] [CrossRef]
- Pierce, C.D.; Moin, P. A dynamic model for subgrid-scale variance and dissipation rate of a conserved scalar. Phys. Fluids 1998, 10, 3041–3044. [Google Scholar] [CrossRef]
- Gran, I.; Melaaen, M.; Magnussen, B. Numerical simulation of local extinction effects in turbulent combustor flows of methane and air. In Twenty-Fifth Symposium (International) on Combustion; The Combustion Institute: Irvine, California, 1994. [Google Scholar]
- Ertesvåg, I.S.; Magnussen, B.F. The eddy dissipation turbulence energy cascade model. Combust. Sci. Technol. 2000, 159, 213–235. [Google Scholar] [CrossRef]
- Gran, I.R.; Magnussen, B.F. A numerical study of a bluff-body stabilized diffusion flame. Part 2. Influence of combustion modeling and finite-rate chemistry. Combust. Sci. Technol. 1996, 119, 191–217. [Google Scholar] [CrossRef]
- Myhrvold, T.; Ertesvåg, I.S.; Gran, I.R.; Cabra, R.; Chen, J.Y. A numerical investigation of a lifted H2/N2 turbulent jet flame in a vitiated coflow. Combust. Sci. Technol. 2006, 178, 1001–1030. [Google Scholar] [CrossRef]
- Lilleberg, B.; Panjwani, B.; Ertesvåg, I.S. Large Eddy Simulation of Methane Diffusion Flame: Comparison of Chemical Kinetics Mechanisms. AIP Conf. Proc. 2010, 1281, 2158–2161. [Google Scholar]
- Jones, W.P.; Prasad, V.N. Large Eddy Simulation of the Sandia Flame Series (D-F) using the Eulerian stochastic field method. Combust. Flame 2010, 157, 1621–1636. [Google Scholar] [CrossRef]
- Zahirović, S.; Scharler, R.; Kilpinen, P.; Obernberger, I. Validation of flow simulation and gas combustion sub-models for the CFD-based prediction of NOx formation in biomass grate furnaces. Combust. Theory Model. 2010, 15, 61–87. [Google Scholar] [CrossRef]
- Mustata, R.; Valiño, L.; Jiménez, C.; Jones, W.P.; Bondi, S. A probability density function Eulerian Monte Carlo field method for large eddy simulations: Application to a turbulent piloted methane/air diffusion flame (Sandia D). Combust. Flame 2006, 145, 88–104. [Google Scholar] [CrossRef]
- Lilleberg, B.; Christ, D.; Ertesvåg, I.S.; Rian, K.E.; Kneer, R. Numerical Simulation with an Extinction Database for Use with the Eddy Dissipation Concept for Turbulent Combustion. Flow Turbul. Combust. 2013, 91, 319–346. [Google Scholar] [CrossRef]
- Lysenko, D.A.; Ertesvåg, I.S.; Rian, K.E. Numerical Simulations of the Sandia Flame D Using the Eddy Dissipation Concept. Flow Turbul. Combust. 2014, 93, 665–687. [Google Scholar] [CrossRef]
- Jaravel, T.; Riber, E.; Cuenot, B.; Pepiot, P. Prediction of flame structure and pollutant formation of Sandia flame D using Large Eddy Simulation with direct integration of chemical kinetics. Combust. Flame 2018, 188, 180–198. [Google Scholar] [CrossRef]
- Cao, R.R.; Wang, H.F.; Pope, S.B. Rate-Controlled Constrained Equilibrium (RCCE) simulations of turbulent partially premixed flames. Proc. Combust. Inst. 2007, 31, 1543–1550. [Google Scholar] [CrossRef]
- Barlow, R.S.; Frank, J.H. Effects of turbulence on species mass fractions in methane/air jet flames. Proc. Combust. Inst. 1998, 27, 1087–1095. [Google Scholar] [CrossRef]
- Barlow, R.S.; Franka, J.H.; Karpetis, A.N.; Chen, J.-Y. Piloted methane/air jet flames: Transport effects and aspects of scalar structure. Combust. Flame 2005, 143, 433–449. [Google Scholar] [CrossRef]
- Barlow, R.S.; Frank, J.H. SandiaPilotDoc21.pdf. Available online: http://www.sandia.gov/TNF/DataArch/FlameD.html (accessed on 10 April 2018).
- Schneider, C.; Dreizler, A.; Janicka, J.; Hassel, E.P. Flow field measurements of stable and locally extinguishing hydrocarbon-fuelled jet flames. Combust. Flame 2003, 135, 185–190. [Google Scholar] [CrossRef]
- Smith, G.P.; Golden, D.M.; Frenklach, M.; Moriarty, N.W.; Eiteneer, B.; Goldenberg, M.; Bowman, C.T.; Hanson, R.K.; Song, S.; Gardiner, J.W.C.; et al. GRI-Mech 3.0. 1999. Available online: http://combustion.berkeley.edu/gri_mech/ (accessed on 27 April 2021).
- Frenklach, M.; Wang, H.; Goldenberg, M.; Smith, G.P.; Golden, D.M.; Bowman, C.T.; Hanson, R.K.; Gardiner, W.C.; Lissianski, V. GRI-Mech: An Optimized Detailed Chemical Reaction Mechanism for Methane Combustion. 1 November 1995. Available online: http://combustion.berkeley.edu/gri-mech/version30/text30.html (accessed on 27 April 2021).
- Bowman, C.T.; Hanson, R.K.; Davidson, D.F.; Gardiner, J.W.C.; Lissianski, V.V.; Smith, G.P.; Golden, D.M.; Frenklach, M.; Goldenberg, M. GRI-Mech 2.11. 1999. Available online: http://combustion.berkeley.edu/gri_mech/ (accessed on 27 April 2021).
- Kazakov, A.; Frenklach, M. Reduced Reaction Sets Based on GRIMech1.2. Available online: http://combusrtion.berkeley.edu/drm./ (accessed on 27 April 2021).
- Sarras, G.; Mahmoudi, Y.; Arteaga Mendez, L.D.; van Veen, E.H.; Tummers, M.J.; Roekaerts, D.J.E.M. Modeling of Turbulent Natural Gas and Biogas Flames of the Delft Jet-in-Hot-Coflow Burner: Effects of Coflow Temperature, Fuel Temperature and Fuel Composition on the Flame Lift-Off Height. Flow Turbul. Combust 2014, 93, 607–635. [Google Scholar] [CrossRef]
- Mallampalli, H.P.; Fletcher, T.H.; Chen, J.-Y. Evaluation of CH4/NOx global mechanisms used for modelling lean premixed turbulent combustion of natural gas. J. Eng. Gas Turbines Power. 1998, 120, 703–712. [Google Scholar] [CrossRef]
- Sung, C.J.; Law, C.K.; Chen, J.-Y. Augmented reduced mechanisms for NO emission in methane oxidation. Combust. Flame 2001, 125, 906–919. [Google Scholar] [CrossRef]
- Yang, B.; Pope, S.B. An Investigation of the Accuracy of Manifold Methods and Splitting Schemes in the Computational Implementation of Combustion Chemistry. Combust. Flame 1998, 112, 16–32. [Google Scholar] [CrossRef]
- James, S.; Anand, M.S.; Razdan, M.K.; Pope, S.B. In Situ Detailed Chemistry Calculations in Combustor Flow Analyses. J. Eng. Gas Turbines Power Trans. ASME 2001, 123, 747–756. [Google Scholar] [CrossRef]
- Zahirović, S.; Scharler, R.; Kilpinen, P.; Obernberger, I. A kinetic study on the potential of a hybrid reaction mechanism for prediction of NOx formation in biomass grate furnaces. Combust. Theory Model. 2011, 15, 645–670. [Google Scholar] [CrossRef]
- Glarborg, P.; Kubel, D.; Kristenssen, P.G.; Hansen, J.; Dam-Johansen, K. Interactions of CO, NOx and H2O under post-flame conditions. Combustion Sci. Technol. 1995, 110, 461–468. [Google Scholar] [CrossRef]
- Skreiberg, Ø.; Kilpinen, P.; Glarborg, P. Ammonia chemistry below 1400 K under fuel-rich conditions in a flow reactor. Combust. Flame 2004, 136, 501–518. [Google Scholar] [CrossRef]
- Jones, W.P.; Lindstedt, R.P. Global reaction schemes for hydrocarbon combustion. Combust. Flame 1988, 73, 233–249. [Google Scholar] [CrossRef]
- ANSYS Fluent Release 19.0, Fluent 19 Documentation; Fluent Inc.: Lebanon, NH, USA, 2018.
- Pope, S. Computationally efficient implementation of combustion chemistry using in-situ adaptive tabulation. Combust. Theory Model. 1997, 1, 41–63. [Google Scholar] [CrossRef]
- Masri, A.R.; Cao, R.; Pope, S.B.; Goldin, G.M. PDF calculations of turbulent lifted flames of H2/N2 fuel issuing into a vitiated co-flow. Combust. Theory Model. 2004, 8, 1–22. [Google Scholar] [CrossRef]
- Launder, B.E.; Spalding, D.B. The numerical computation of turbulent flows. Comput. Methods Appl. Mech. Eng. 1974, 3, 269–289. [Google Scholar] [CrossRef]
- IGran, R.; Ertesvåg, I.S.; Magnussen, B.F. Influence of turbulence modeling on predictions of turbulent combustion. AIAA J. 1997, 35, 106–110. [Google Scholar]
- Merci, B.; Dick, E. Influence of computational aspects on simulations of a turbulent jet diffusion flame. Int. J. Numer. Methods Heat Fluid Flow 2003, 13, 887–898. [Google Scholar] [CrossRef]
- Habibi, A.; Merci, B.; Roekaerts, D. Turbulence radiation interaction in Reynolds-averaged Navier–Stokes simulations of nonpremixed piloted turbulent laboratory-scale flames. Combust. Flame 2007, 151, 303–320. [Google Scholar] [CrossRef]
- Smith, T.F.; Shen, Z.F.; Friedman, J.N. Evaluation of Coefficients for the Weighted Sum of Gray Gases Model. J. Heat Transf. Trans. ASME 1982, 104, 602–608. [Google Scholar] [CrossRef]
- Yan, L.B.; Yue, G.X.; He, B.S. Development of an absorption coefficient calculation method potential for combustion and gasification simulations. Int. J. Heat Mass Transf. 2015, 91, 1069–1077. [Google Scholar] [CrossRef]
- Yan, L.B.; Yue, G.X.; He, B.S. Application of an efficient exponential wide band model for the natural gas combustion simulation in a 300 kW BERL burner furnace. Appl. Therm. Eng. 2016, 94, 209–220. [Google Scholar] [CrossRef]
- Yan, L.B.; Cao, Y.; Li, X.Z.; He, B.S. A Modified Exponential Wide Band Model for Gas Emissivity Prediction in Pressurized Combustion and Gasification Processes. Energy Fuels 2018, 32, 1634–1643. [Google Scholar] [CrossRef]
- Wang, C.J.; Ge, W.J.; Modest, M.F.; He, B.S. A full-spectrum k-distribution look-up table for radiative transfer in nonhomogeneous gaseous media. J. Quant. Spectrosc. Radiat. Transf. 2016, 168, 46–56. [Google Scholar] [CrossRef]
- Wang, C.J.; Modest, M.F.; He, B.S. Improvement of Full-Spectrum k-Distribution Method Using Quadrature Transformation. Int. J. Therm. Sci. 2016, 108, 100–107. [Google Scholar] [CrossRef]
- Wang, C.J.; Modest, M.F.; He, B.S. Full-spectrum k-distribution look-up table for nonhomogeneous gas-soot mixtures. J. Quant. Spectrosc. Radiat. Transf. 2016, 176, 129–136. [Google Scholar] [CrossRef]
- Wang, C.J.; He, B.S.; Modest, M.F.; Ren, T. Efficient Full-Spectrum Correlated-k-distribution Look-Up Table. J. Quant. Spectrosc. Radiat. Transf. 2018, 219, 108–116. [Google Scholar] [CrossRef]
- Wang, C.J.; Modest, M.F.; He, B.S. Full-spectrum correlated-k-distribution look-up table for use with radiative Monte Carlo solvers. Int. J. Heat Mass Transf. 2019, 131, 167–175. [Google Scholar] [CrossRef]
- Wang, C.J.; He, B.S.; Modest, M.F. Full-Spectrum Correlated-k-Distribution Look-Up Table for Radiative Transfer in Nonhomogeneous Participating Media with Gas-Particle Mixtures. Int. J. Heat Mass Transf. 2019, 137, 1053–1063. [Google Scholar] [CrossRef]
- Libby, P.A.; Williams, F.A. A presumed pdf analysis of partially premixed turbulent combustion. Combust. Sci. Technol. 2000, 161, 351–390. [Google Scholar] [CrossRef]
- Salehi, M.M.; Bushe, W.K. Presumed PDF modeling for RANS simulation of turbulent premixed flames. Combust. Theory Model. 2010, 14, 381–403. [Google Scholar] [CrossRef]
- Haworth, D.C. Progress in probability density function methods for turbulent reacting flows. Prog. Energy Combust. Sci. 2010, 36, 168–259. [Google Scholar] [CrossRef]
- Yin, C.; Rosendahl, L.A.; Kaer, S.K. Grate-firing of biomass for heat and power production. Prog. Energy Combust. Sci. 2008, 34, 725–754. [Google Scholar] [CrossRef]
- Buchmayr, M.; Gruberb, J.; Hargassner, M.; Hochenauer, C. A computationally inexpensive CFD approach for small-scale biomass burners equipped with enhanced air staging. Energy Convers. Manag. 2016, 115, 32–42. [Google Scholar] [CrossRef]
- Frank, J.H.; Barlow, R.S.; Lundquist, C. Radiation and nitric oxide formation in turbulent non-premixed jet flames. Proc. Combust. Inst. 2000, 28, 447–454. [Google Scholar] [CrossRef]
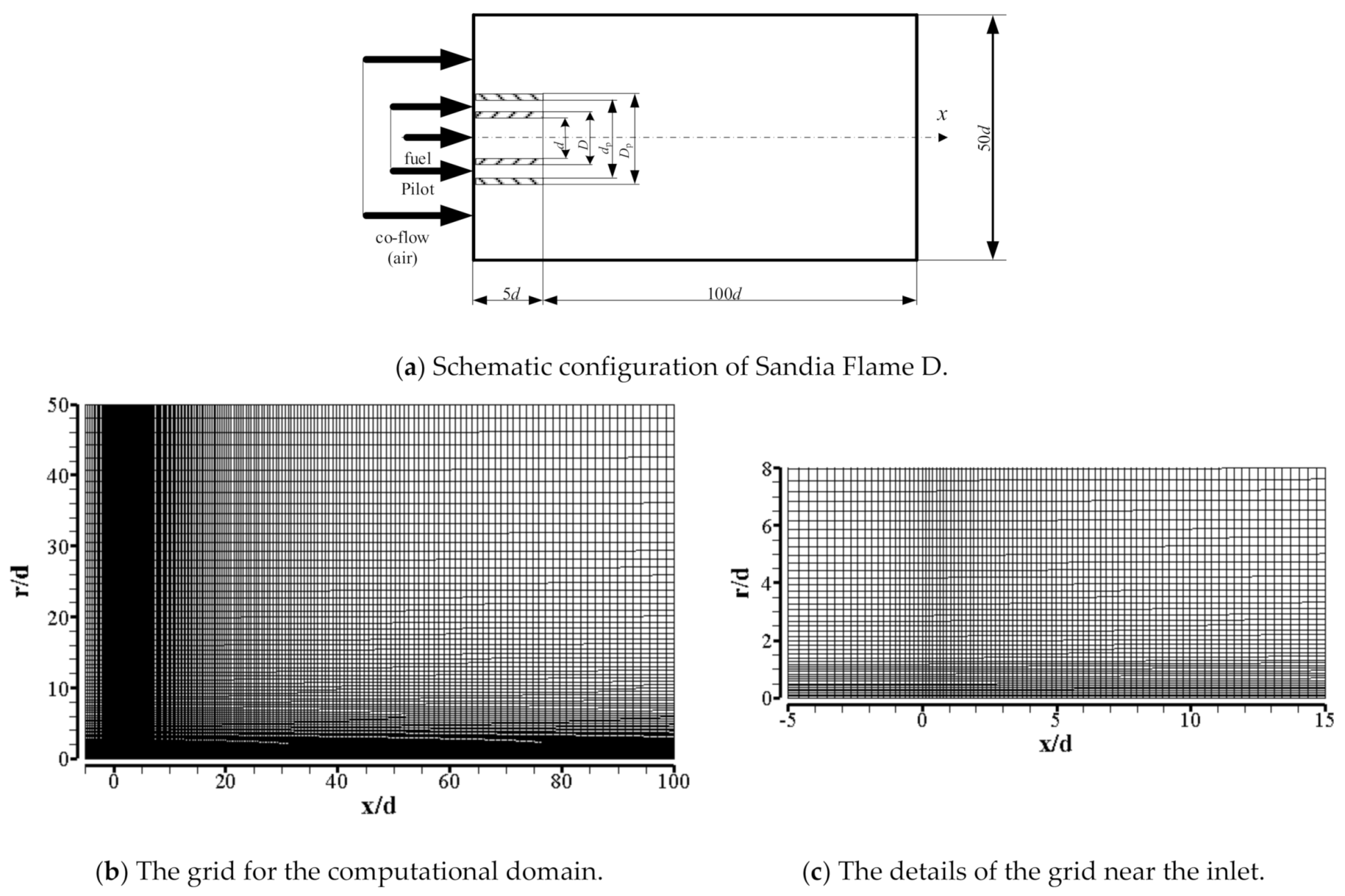

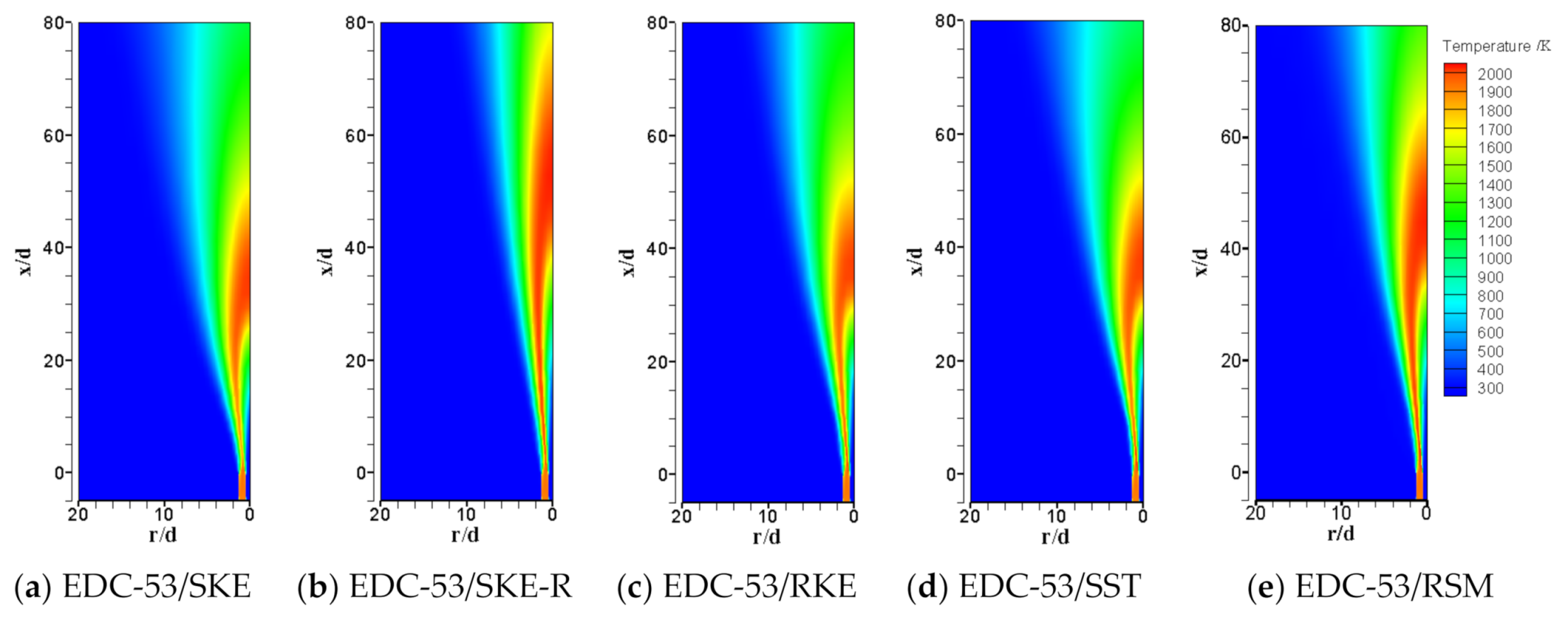
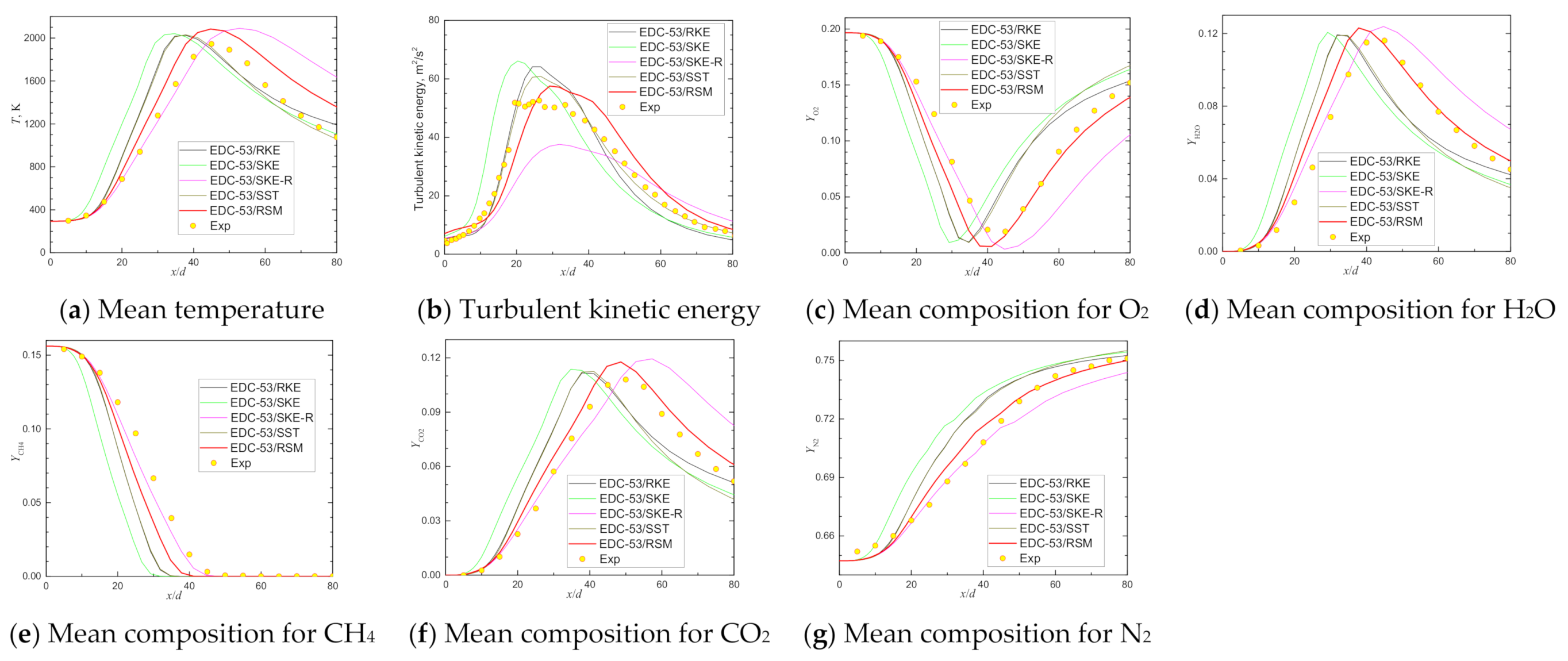

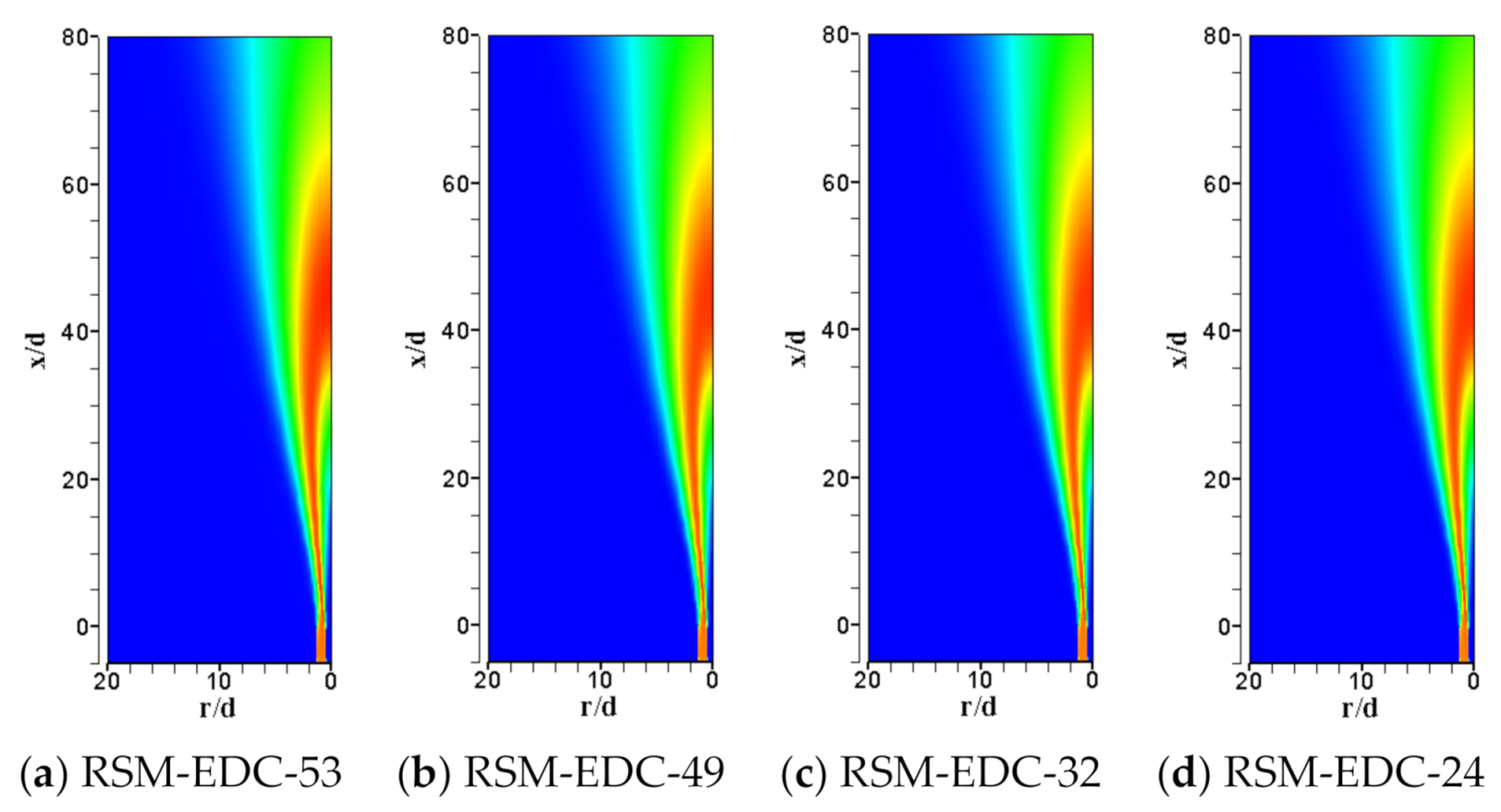
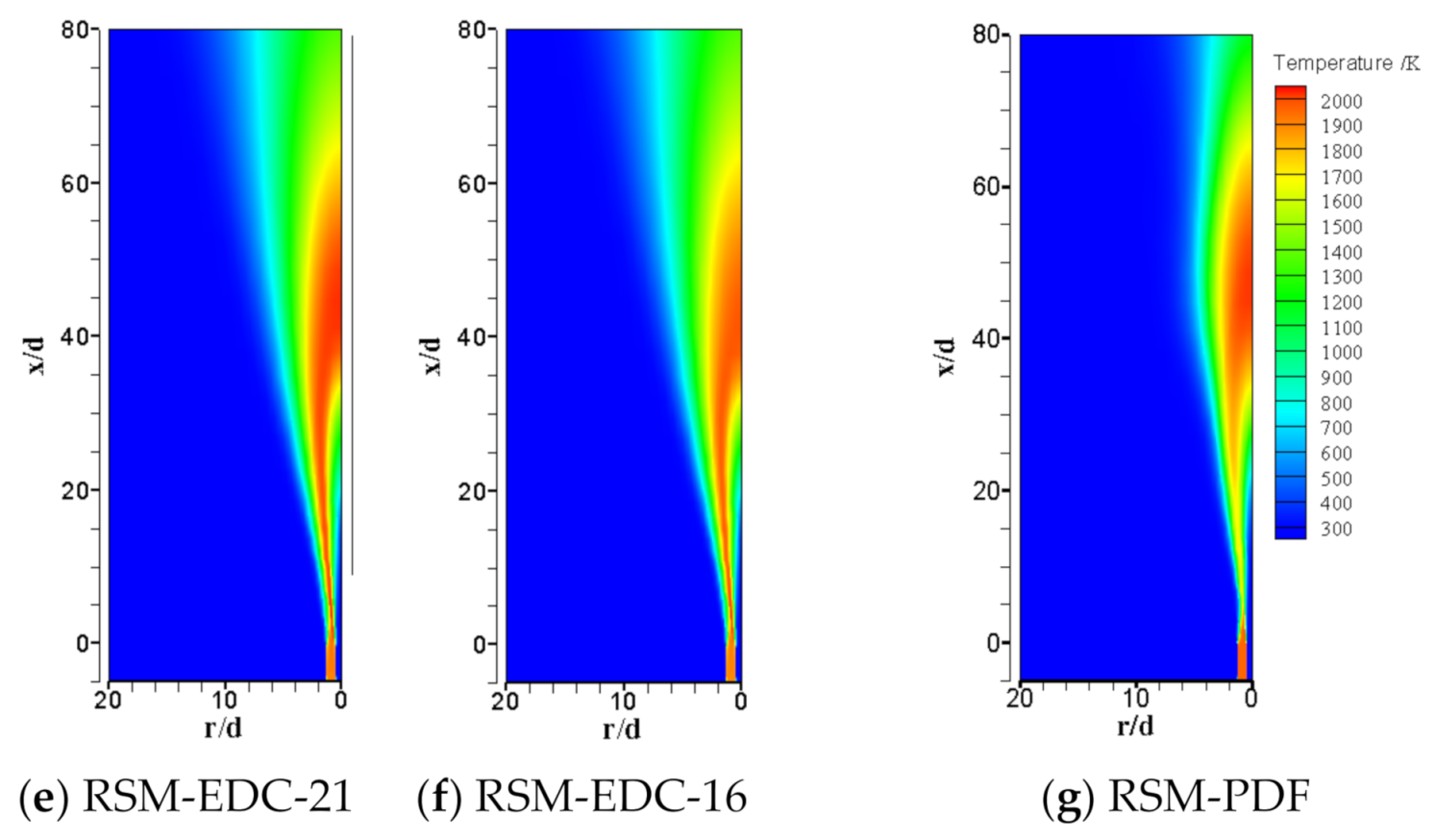
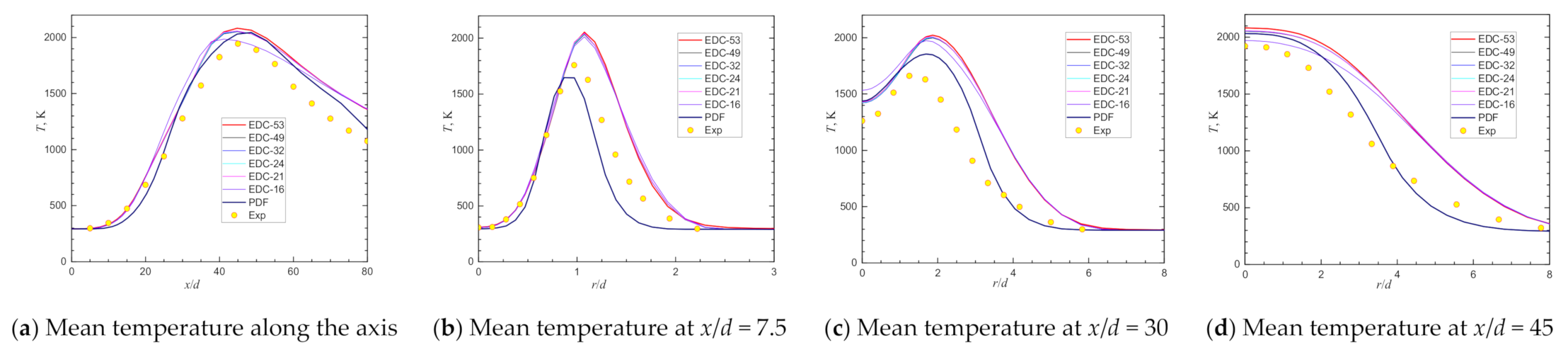
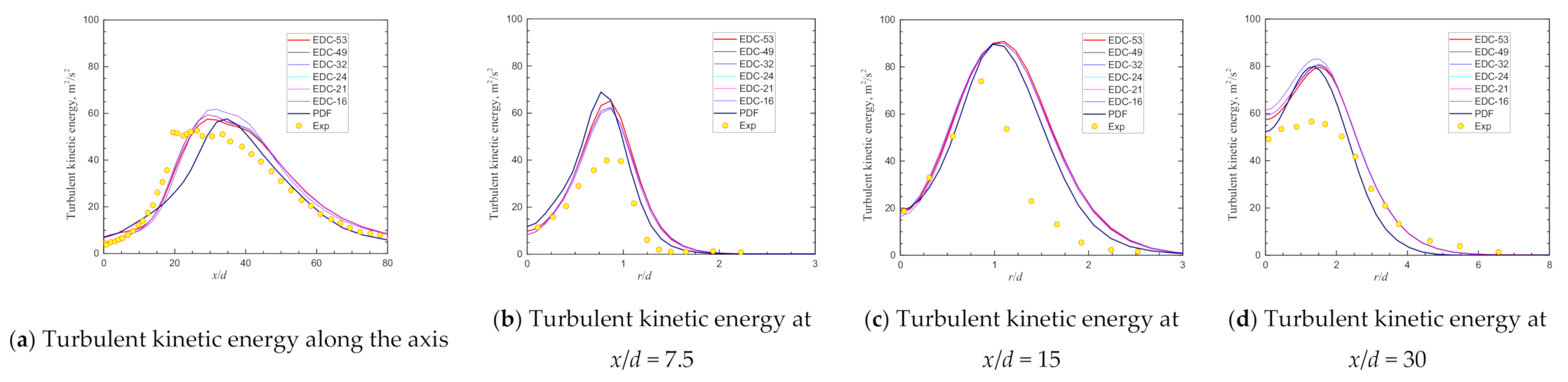
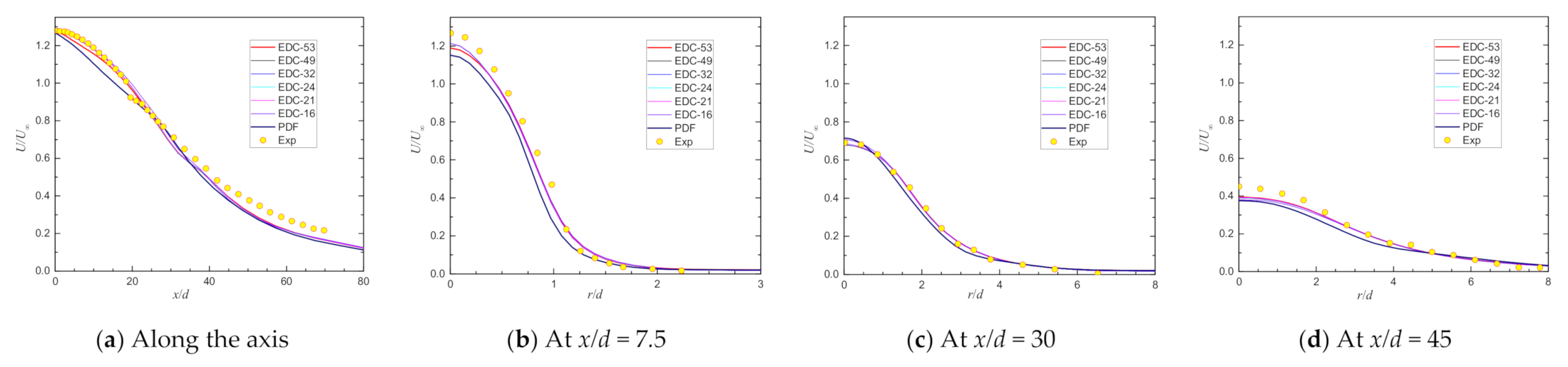
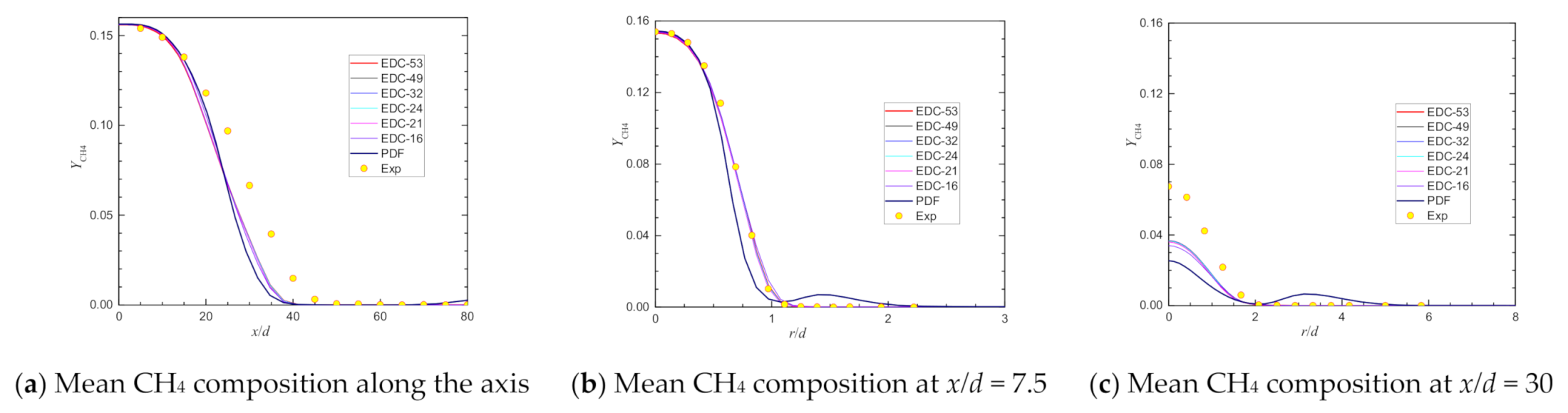
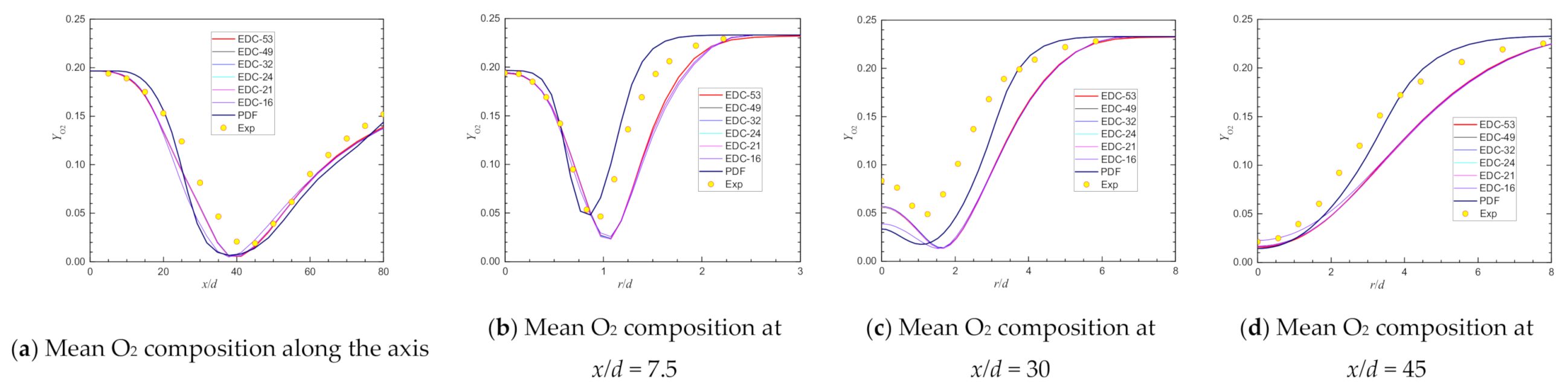
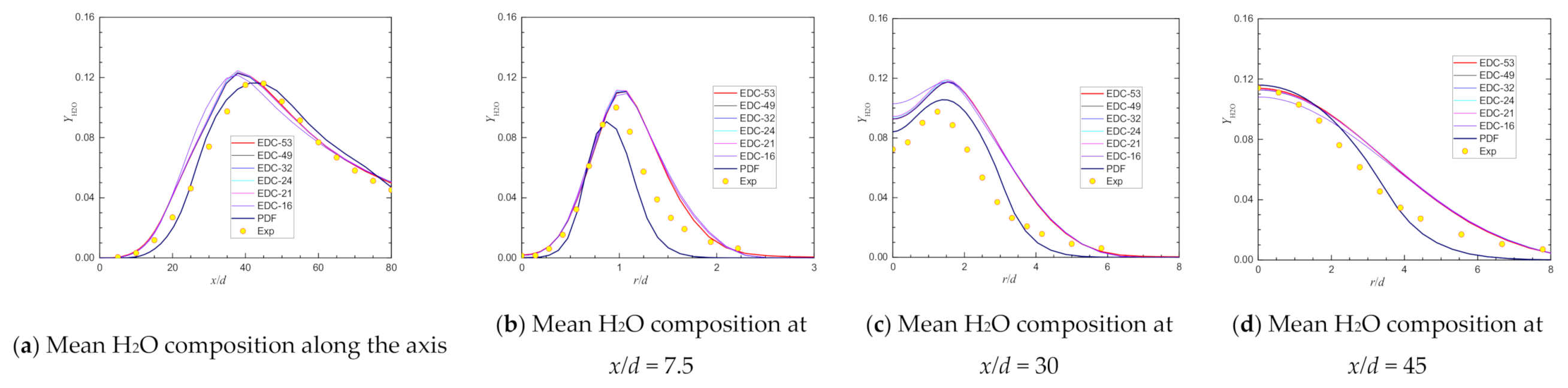
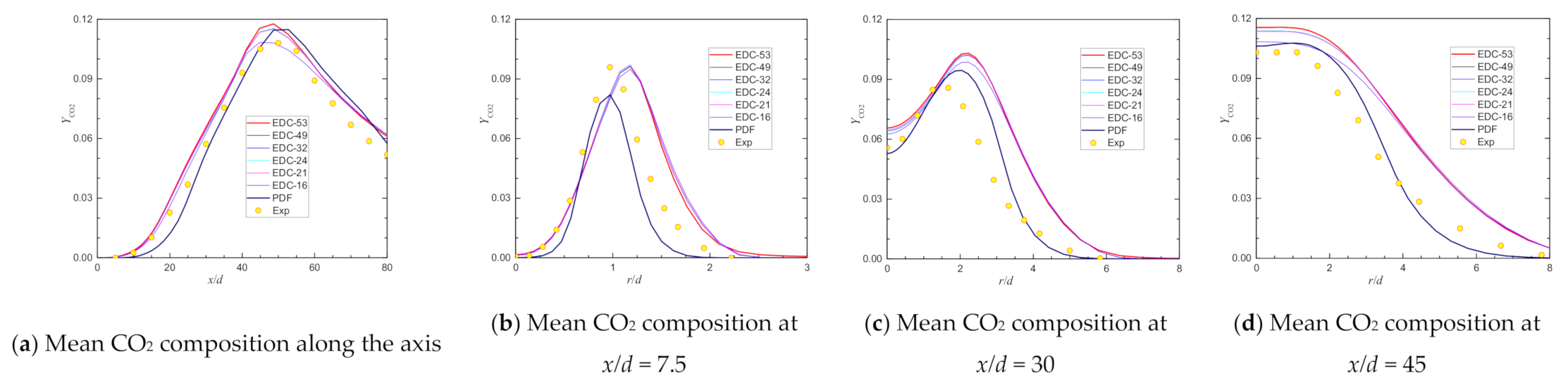

| Item | Unit | Values in Experiment | Setting Values |
|---|---|---|---|
| Jet mixture CH4/air | vol% | 25/75 | |
| Pilot mixture composition, mass fraction | % | See [42] | |
| N2 | 73.42 | ||
| O2 | 5.40 | ||
| O | 7.47 × 10−2 | ||
| H2 | 1.29 × 10−2 | ||
| H | 2.48 × 10−3 | ||
| H2O | 9.42 | ||
| CO | 0.407 | ||
| CO2 | 10.98 | ||
| OH | 0.28 | ||
| NO | 4.8 × 10−4 | ||
| Main jet inner diameter, d | mm | 7.2 | |
| Pilot annulus inner diameter, D | mm | 7.7 | |
| Pilot annulus outer diameter, dp | mm | 18.2 | |
| Burner outer diameter, Dp | mm | 18.9 | |
| Jet bulk velocity | m/s | 49.6 2 | Profile; see [42] |
| Jet inlet turbulent kinetic energy | m2/s2 | Profile; see [43] | |
| Jet inlet turbulent dissipation rate | m2/s3 | - | Profile; estimated with Equation (1) |
| Jet inlet specific dissipation rate | 1/s | Profile; estimated with Equation (2) | |
| Pilot bulk velocity | m/s | 11.4 0.5 | Profile; see [42] |
| Pilot inlet turbulent kinetic energy | m2/s2 | Profile; see [43] | |
| Pilot inlet turbulent dissipation rate | m2/s3 | - | Profile; estimated with Equation (1) |
| Pilot inlet specific dissipation rate | 1/s | Profile; estimated with Equation (2) | |
| Air co-flow velocity | m/s | 0.9 0.05 | Profile; see [42] |
| Air co-flow inlet turbulent kinetic energy | m2/s2 | Profile; see [43] | |
| Air co-flow inlet turbulent dissipation rate | m2/s3 | - | Profile; estimated with Equation (1) |
| Air co-flow inlet specific dissipation rate | 1/s | Profile; estimated with Equation (2) | |
| Jet temperature | K | 294 | |
| Pilot temperature | K | 1880 50 | 1880 |
| Co-flow temperature | K | 291 | |
| Reynolds number, Rejet | - | 22,400 | |
| Case | Turbulence Models | Options | Near-Wall Treatment | Model Constants |
|---|---|---|---|---|
| EDC-53/SKE | Standard k-ε model | Viscous heating; production limiter | Standard wall functions | Defaults |
| EDC-53/SKE-R | Standard k-ε model with changed constant C1ε | Viscous heating; production limiter | ||
| EDC-53/RKE | Realizable k-ε model | Viscous heating; production limiter | ||
| EDC-53/SST | Shear-stress transport (sst) k-ω model | Low-Re correction; viscous heating; production limiter | ||
| EDC-53/RSM | Reynolds stress model | Quadratic pressure-strain; Wall BC from k equation |
| Scalar Peak and Its Location | Exp | EDC-53/SKE | Deviation | EDC-53/SKE-R | Deviation | EDC-53/RKE | Deviation | EDC-53/SST | Deviation | EDC-53/RSM | Deviation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Max Temperature@axis | 1945 | 2040 | 4.88% | 2090 | 7.47% | 2026 | 4.16% | 2031 | 4.41% | 2083 | 7.08% |
| Location@x/d = | 45.00 | 34.74 | 52.79 | 37.83 | 37.83 | 44.75 | |||||
| Max Turbulent energy@axis | 52.57 | 66.07 | 25.68% | 37.58 | −28.52% | 64.12 | 21.98% | 60.87 | 15.80% | 57.50 | 9.38% |
| Location@axis, x/d = | 26.28 | 20.30 | 31.86 | 24.42 | 26.72 | 29.20 | |||||
| Min YO2@axis | 0.019 | 0.009 | −51.51% | 0.003 | −82.82% | 0.010 | −48.76% | 0.009 | −51.56% | 0.006 | −69.93% |
| Location@axis, x/d = | 45.00 | 29.20 | 44.75 | 34.74 | 34.74 | 41.16 | |||||
| Max YH2O@axis | 0.116 | 0.121 | 3.96% | 0.124 | 6.71% | 0.119 | 2.84% | 0.119 | 2.57% | 0.123 | 6.02% |
| Location@axis, x/d = | 45.00 | 29.20 | 44.75 | 31.86 | 34.74 | 37.83 | |||||
| Max YCO2@axis | 0.108 | 0.114 | 5.27% | 0.119 | 10.59% | 0.112 | 3.42% | 0.113 | 4.17% | 0.118 | 8.95% |
| Location@axis, x/d = | 50.000 | 34.74 | 57.27 | 37.83 | 41.16 | 48.62 | |||||
| Max YCO@axis | 0.0453 | 0.0514 | 13.57% | 0.0588 | 29.88% | 0.0529 | 16.73% | 0.0523 | 15.39% | 0.0565 | 24.62% |
| Location@axis, x/d = | 40.00 | 26.72 | 41.16 | 31.86 | 31.86 | 37.83 | |||||
| Max YH2@axis | 0.00288 | 0.00290 | 0.69% | 0.00346 | 20.21% | 0.00287 | −0.35% | 0.00285 | −0.96% | 0.00343 | 19.07% |
| Location@axis, x/d = | 40.00 | 26.72 | 41.16 | 29.20 | 29.20 | 34.74 | |||||
| Max YOH@axis | 0.00148 | 0.00441 | 197.74% | 0.00389 | 162.92% | 0.00433 | 192.36% | 0.00426 | 187.95% | 0.00401 | 171.18% |
| Location@axis, x/d = | 50.00 | 34.74 | 52.79 | 37.83 | 37.83 | 44.75 |
| Mechanism | No. of Species | No. of Steps | NO Species | Case | Reference |
|---|---|---|---|---|---|
| GRI3.0 | 53 | 325 | With NO | EDC-53 | [44] |
| GRI2.11 | 49 | 279 | With NO | EDC-49 | [46] |
| GRI1.2 | 32 | 177 | Without NO | EDC-32 | [45] |
| sp24 | 24 | 104 | Without NO | EDC-24 | [47] |
| sp21 | 21 | 84 | Without NO | EDC-21 | [47] |
| Skeletal | 16 | 41 | Without NO | EDC-16 | [51] |
| Scalar Peak and Its Location | Exp | RSM-EDC-53 | Deviation | RSM-EDC-49 | Deviation | RSM-EDC-32 | Deviation | RSM-EDC-24 | Deviation | RSM-EDC-21 | Deviation | RSM-EDC-16 | Deviation | Deviation | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Max Temperature@axis | 1945 | 2083 | 7.08% | 2052 | 5.52% | 2054 | 5.61% | 2057 | 5.76% | 2058 | 5.81% | 1986 | 2.12% | 2045 | 5.13% |
| Location@axis, x/d = | 45.00 | 44.75 | 44.75 | 44.75 | 44.75 | 44.75 | 41.16 | 48.62 | |||||||
| Max Turbulent energy@axis | 52.57 | 57.50 | 9.38% | 59.42 | 13.03% | 59.39 | 12.99% | 59.33 | 12.86% | 59.37 | 12.94% | 61.78 | 17.53% | 55.23 | 5.07% |
| Location@axis, x/d = | 26.28 | 29.20 | 29.20 | 29.20 | 29.20 | 29.20 | 31.86 | 34.74 | |||||||
| Min YO2@axis | 0.019 | 0.006 | −69.93% | 0.006 | −66.91% | 0.006 | −67.21% | 0.006 | −68.54% | 0.006 | −70.40% | 0.005 | −75.24% | 0.007 | −64.81% |
| Location@axis, x/d = | 45.00 | 41.16 | 37.83 | 37.83 | 37.83 | 37.83 | 37.83 | 37.83 | |||||||
| Max YH2O@axis | 0.116 | 0.123 | 6.02% | 0.123 | 5.74% | 0.123 | 6.07% | 0.124 | 6.92% | 0.125 | 7.40% | 0.122 | 4.82% | 0.116 | 0.23% |
| Location@axis, x/d = | 45.00 | 37.83 | 37.83 | 37.83 | 37.83 | 37.83 | 37.83 | 44.75 | |||||||
| Max YCO2@axis | 0.108 | 0.118 | 8.95% | 0.115 | 6.78% | 0.115 | 6.81% | 0.115 | 6.83% | 0.115 | 6.52% | 0.108 | 0.28% | 0.115 | 6.29% |
| Location@axis, x/d = | 50.00 | 48.62 | 48.62 | 48.62 | 48.62 | 48.62 | 44.75 | 52.79 | |||||||
| Max YCO@axis | 0.0453 | 0.0565 | 24.62% | 0.0574 | 26.80% | 0.0572 | 26.36% | 0.0561 | 23.81% | 0.0545 | 20.33% | 0.0678 | 49.76% | 0.1094 | 141.57% |
| Location@axis, x/d = | 40.00 | 37.83 | 37.83 | 37.83 | 37.83 | 37.83 | 34.74 | 31.86 | |||||||
| Max YH2@axis | 0.0029 | 0.0034 | 19.07% | 0.0034 | 19.44% | 0.0034 | 17.64% | 0.0032 | 12.78% | 0.0029 | 0.92% | 0.0037 | 28.80% | 0.0110 | 281.44% |
| Location@axis, x/d = | 40.00 | 34.74 | 34.74 | 34.74 | 34.74 | 34.74 | 34.74 | 29.20 | |||||||
| Max YOH@axis | 0.0015 | 0.0040 | 171.18% | 0.0043 | 192.88% | 0.0043 | 191.90% | 0.0043 | 190.11% | 0.0043 | 191.66% | 0.0052 | 251.23% | 0.0009 | −36.82% |
| Location@axis, x/d = | 50.00 | 44.75 | 44.75 | 44.75 | 44.75 | 44.75 | 44.75 | 48.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, D.; Yu, Y.; Kuang, Y.; Wang, C. Model Comparisons of Flow and Chemical Kinetic Mechanisms for Methane–Air Combustion for Engineering Applications. Appl. Sci. 2021, 11, 4107. https://doi.org/10.3390/app11094107
He D, Yu Y, Kuang Y, Wang C. Model Comparisons of Flow and Chemical Kinetic Mechanisms for Methane–Air Combustion for Engineering Applications. Applied Sciences. 2021; 11(9):4107. https://doi.org/10.3390/app11094107
Chicago/Turabian StyleHe, Di, Yusong Yu, Yucheng Kuang, and Chaojun Wang. 2021. "Model Comparisons of Flow and Chemical Kinetic Mechanisms for Methane–Air Combustion for Engineering Applications" Applied Sciences 11, no. 9: 4107. https://doi.org/10.3390/app11094107
APA StyleHe, D., Yu, Y., Kuang, Y., & Wang, C. (2021). Model Comparisons of Flow and Chemical Kinetic Mechanisms for Methane–Air Combustion for Engineering Applications. Applied Sciences, 11(9), 4107. https://doi.org/10.3390/app11094107







