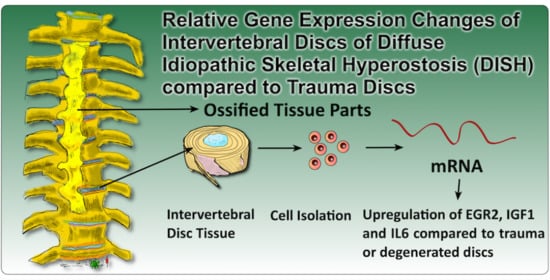
EGR2, IGF1 and IL6 Expression Are Elevated in the Intervertebral Disc of Patients Suffering from Diffuse Idiopathic Skeletal Hyperostosis (DISH) Compared to Degenerative or Trauma Discs
Abstract
1. Introduction
2. Materials and Methods
2.1. IVD Donor Materials and Cell Isolation
2.2. Analysis of Specific Gene Expression in Human Primary IVD Cells with Quantitative Polymerase Chain Reaction (qPCR)
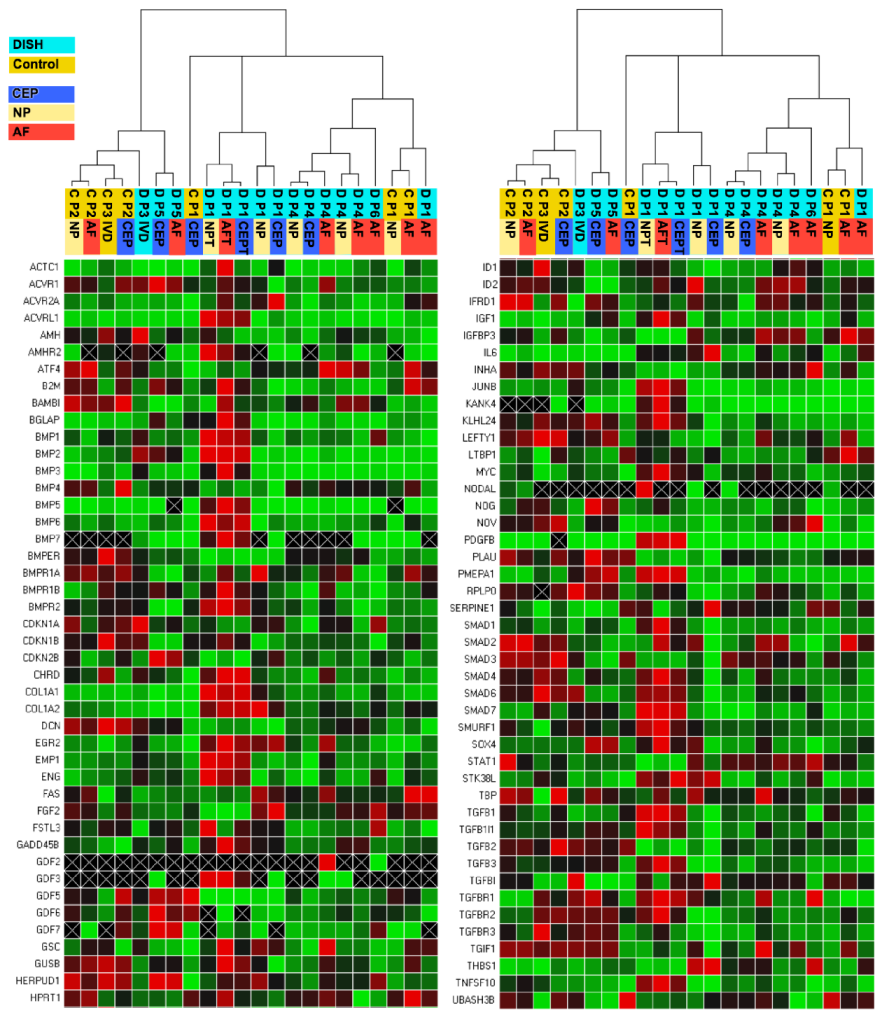
2.3. Statistical Analysis and Visualisation of Heat Maps and Clustering
2.4. Immunocytochemistry
3. Results
3.1. Prime PCR of DISH and Traumatic/Degenerative Discs
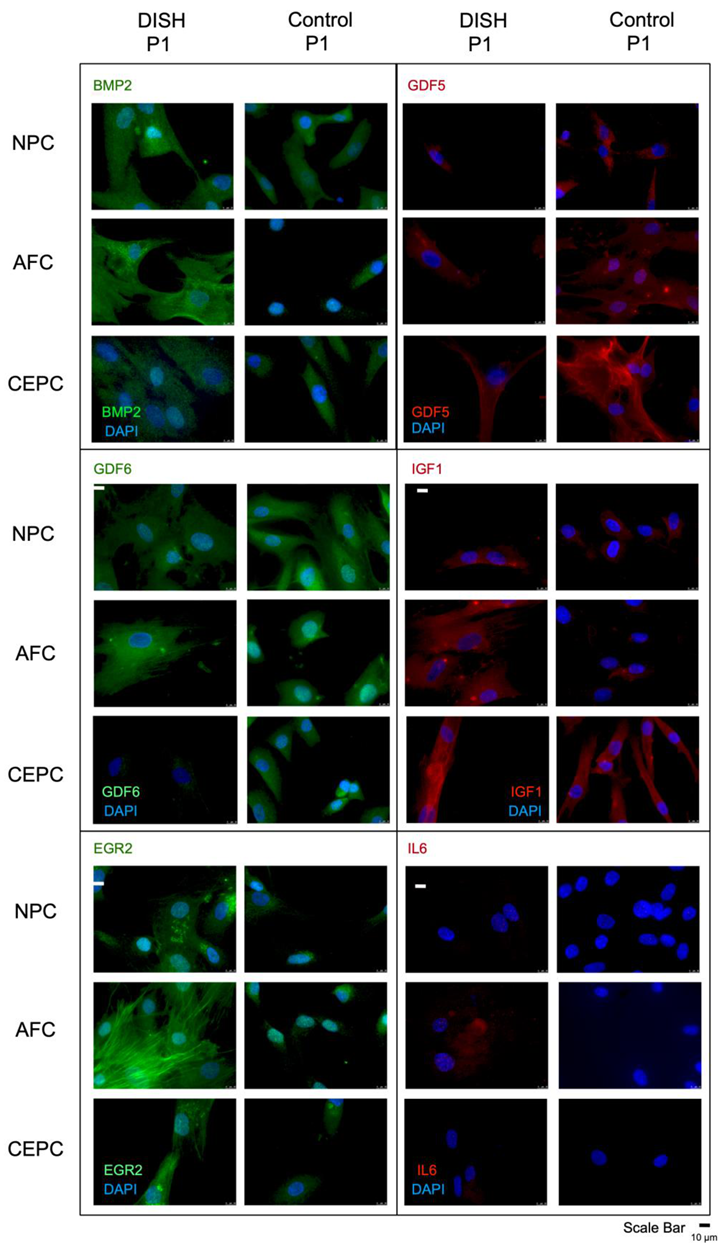
3.2. Immunocytochemistry of IGF1, IL6, EGR2, BMP2, GDF5, and GDF6
4. Discussion
5. Conclusions
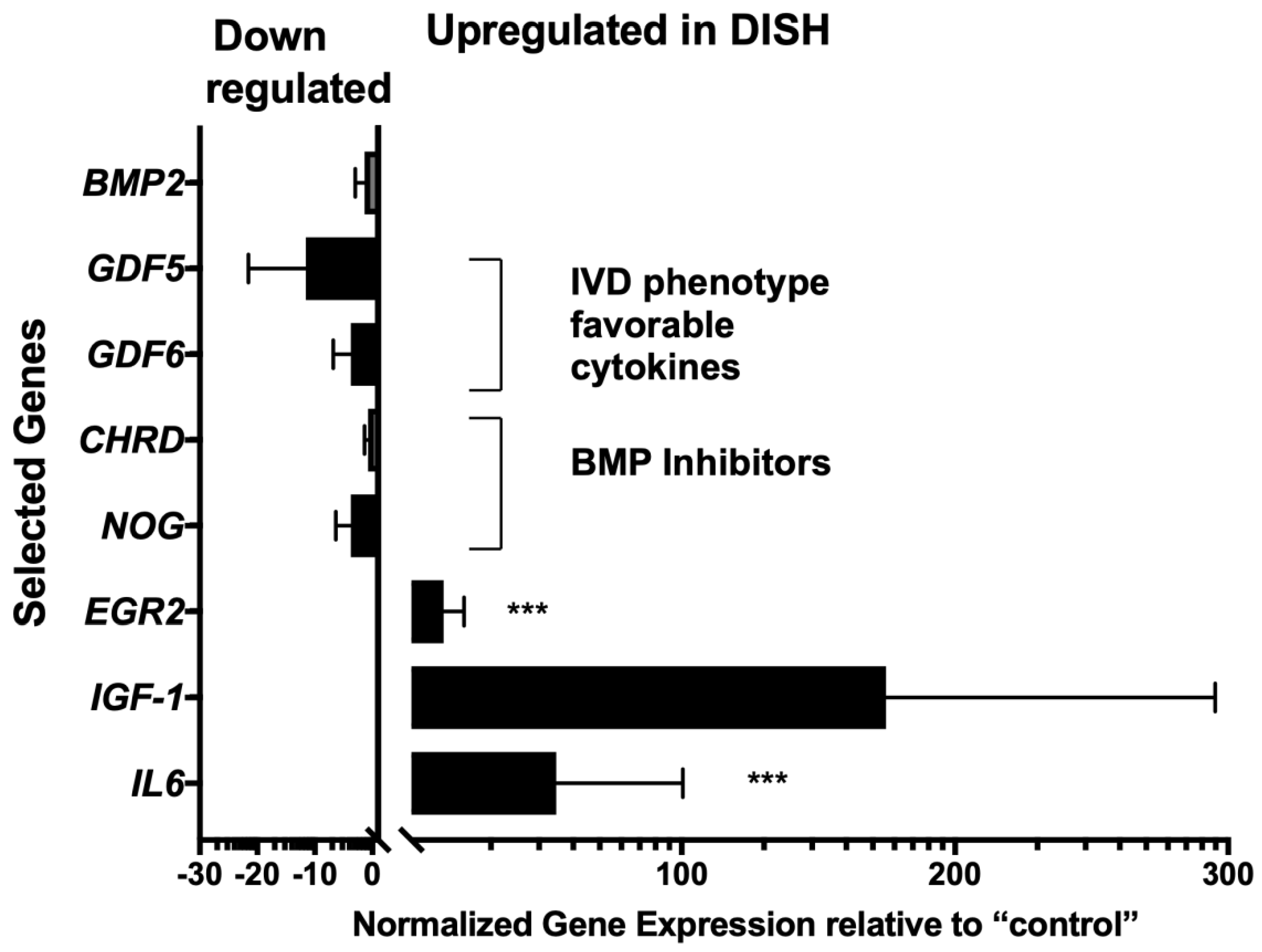
- DISH-IVD, in contrast to IVD obtained from trauma, showed an up-regulation of EGR2 and IL-6, and IGF-1 tended to be up-regulated.
- Dysregulation of IGF-1 has been proposed for DISH-patients to be a serum-specific marker, and our data from IVD tissue pointed in this direction [4].
- GDF5 and GDF6 tended to be down-regulated in DISH-IVD compared to trauma-IVD.
- The observed differences in gene expression and protein differences could also be a result of factors other than DISH such as age and gender bias [58].
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AB | Antibody |
| AF | Annulus fibrosus |
| AS | Ankylosing spondylitis |
| BMP | Bone morphogenetic protein |
| BMP2 | Bone morphogenetic protein 2 |
| BSA | Bovine serum albumin |
| C | Cervical |
| CEP | Cartilaginous endplate |
| D | Degenerated |
| DISH | Diffuse idiopathic skeletal hyperostosis |
| EGR2 | Early growth response 2 |
| GDF5 | Growth and differentiation factor 5 |
| GDF6 | Growth and differentiation factor 6 |
| GH | Growth hormone |
| IGF1 | Insulin-like growth factor 1 |
| IL1 | Interleukin 1 |
| IL6 | Interleukin 6 |
| IVD | Intervertebral disc |
| L | Lumbar |
| LG-DMEM | Low-glucose Dulbecco’s Modified Eagle Medium |
| NP | Nucleus pulposus |
| OB | Osteoblast |
| TBS-T | Tris-buffered saline containing Tween 20 |
| TGF-β | Transforming growth factor β |
| P | Passage |
| PG | Pfirrmann grade |
| qPCR | Quantitative real-time Polymerase Chain Reaction |
| T | Trauma |
References
- Forestier, J.; Rotes-Querol, J. Senile Ankylosing Hyperostosis of the Spine. Ann. Rheum. Dis. 1950, 9, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, I.; D’Angelo, S.; Palazzi, C.; Padula, A.; Mader, R.; Khan, M.A. Diffuse idiopathic skeletal hyperostosis: Differentiation from ankylosing spondylitis. Curr. Rheumatol. Rep. 2009, 11, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Cammisa, M.; De Serio, A.; Guglielmi, G. Diffuse idiopathic skeletal hyperostosis. Eur. J. Radiol. 1998, 27, S7–S11. [Google Scholar] [CrossRef]
- Mader, R.; Verlaan, J.-J.; Buskila, D. Diffuse idiopathic skeletal hyperostosis: Clinical features and pathogenic mechanisms. Nat. Rev. Rheumatol. 2013, 9, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Resnick, D.; Shaul, S.R.; Robins, J.M. Diffuse Idiopathic Skeletal Hyperostosis (DISH): Forestier’s Disease with Extraspinal Manifestations. Radiology 1975, 115, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Littlejohn, G. Metabolic Factors in Diffuse Idiopathic Skeletal Hyperostosis—A Review of Clinical Data. Open Rheumatol. J. 2014, 8, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Kiss, C.; Szilágyi, M.; Paksy, A.; Poór, G. Risk factors for diffuse idiopathic skeletal hyperostosis: A case–control study. Rheumatology 2002, 41, 27–30. [Google Scholar] [CrossRef]
- Weinfeld, R.M.; Olson, P.N.; Maki, D.D.; Griffiths, H.J. The prevalence of diffuse idiopathic skeletal hyperostosis (DISH) in two large American Midwest metropolitan hospital populations. Skelet. Radiol. 1997, 26, 222–225. [Google Scholar] [CrossRef]
- Utsinger, P.D. Diffuse idiopathic skeletal hyperostosis. Clin. Rheum. Dis. 1985, 11, 325–351. [Google Scholar]
- Resnick, D.; Guerra, J.; Robinson, C.A.; Vint, V.C. Association of diffuse idiopathic skeletal hyperostosis (DISH) and calcification and ossification of the posterior longitudinal ligament. Am. J. Roentgenol. 1978, 131, 1049–1053. [Google Scholar] [CrossRef]
- Mader, R. Diffuse idiopathic skeletal hyperostosis: A distinct clinical entity. Isr. Med. Assoc. J. 2003, 5, 506–508. [Google Scholar]
- Kuperus, J.S.; Westerveld, L.A.; Rutges, J.A.; Alblas, J.; Van Rijen, M.H.; Bleys, R.L.; Oner, F.C.; Verlaan, J. Histological characteristics of diffuse idiopathic skeletal hyperostosis. J. Orthop. Res. 2016, 35, 140–146. [Google Scholar] [CrossRef]
- El Miedany, Y.M.; Wassif, G.; El Baddini, M. Diffuse idiopathic skeletal hyperostosis (DISH): Is it of vascular aetiology? Clin. Exp. Rheumatol. 2000, 18, 193–200. [Google Scholar]
- Charles, W.D. Growth hormone and insulin-like growth factor-I in symptomatic and asymptomatic patients with diffuse idiopathic skeletal hyperostosis (DISH). Front. Biosci. 2002, 7, a37–a43. [Google Scholar] [CrossRef]
- Denko, C.W.; Boja, B.; Moskowitz, R.W. Growth factors, insulin-like growth factor-1 and growth hormone, in synovial fluid and serum of patients with rheumatic disorders. Osteoarthr. Cartil. 1996, 4, 245–249. [Google Scholar] [CrossRef][Green Version]
- Matteucci, B.M. Metabolic and endocrine disease and arthritis. Curr. Opin. Rheumatol. 1995, 7, 356–358. [Google Scholar] [CrossRef]
- Daragon, A.; Mejjad, O.; Czernichow, P.; Louvel, J.P.; Vittecoq, O.; Durr, A.; Le Loet, X. Vertebral hyperostosis and diabetes mellitus: A case-control study. Ann. Rheum. Dis. 1995, 54, 375–378. [Google Scholar] [CrossRef]
- Littlejohn, G.O.; Hall, S. Diffuse idiopathic skeletal hyperostosis and new bone formation in male gouty subjects. A radiologic study. Rheumatol. Int. 1982, 2, 83–86. [Google Scholar] [CrossRef]
- Vezyroglou, G.; Mitropoulos, A.; Antoniadis, C. A metabolic syndrome in diffuse idiopathic skeletal hyperostosis. A controlled study. J. Rheumatol. 1996, 23, 672–676. [Google Scholar]
- He, X.; Liu, Y.; Yuan, X.; Lu, L. Enhanced Healing of Rat Calvarial Defects with MSCs Loaded on BMP-2 Releasing Chitosan/Alginate/Hydroxyapatite Scaffolds. PLoS ONE 2014, 9, e104061. [Google Scholar] [CrossRef]
- Brazil, D.P.; Church, R.H.; Surae, S.; Godson, C.; Martin, F. BMP signalling: Agony and antagony in the family. Trends Cell Biol. 2015, 25, 249–264. [Google Scholar] [CrossRef]
- Urist, M.R. Bone: Formation by autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef]
- Ono, K.; Yonenobu, K.; Miyamoto, S.; Okada, K. Pathology of Ossification of the Posterior Longitudinal Ligament and Ligamentum Flavum. Clin. Orthop. Relat. Res. 1999, 359, 18–26. [Google Scholar] [CrossRef]
- Carragee, E.J.; Hurwitz, E.L.; Weiner, B.K. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: Emerging safety concerns and lessons learned. Spine J. 2011, 11, 471–491. [Google Scholar] [CrossRef]
- Kok, D.; Peeters, C.M.M.; Mardina, Z.; Oterdoom, D.L.M.; Bulstra, S.K.; Veldhuizen, A.G.; Kuijer, R.; Wapstra, F.H. Is remaining intervertebral disc tissue interfering with bone generation during fusion of two vertebrae? PLoS ONE 2019, 14, e0215536. [Google Scholar] [CrossRef]
- Brown, S.J.; Turner, S.A.; Balain, B.S.; Davidson, N.T.; Roberts, S. Is Osteogenic Differentiation of Human Nucleus Pulposus Cells a Possibility for Biological Spinal Fusion? Cartilage 2018, 11, 181–191. [Google Scholar] [CrossRef]
- Makino, T.; Tsukazaki, H.; Ukon, Y.; Tateiwa, D.; Yoshikawa, H.; Kaito, T. The Biological Enhancement of Spinal Fusion for Spinal Degenerative Disease. Int. J. Mol. Sci. 2018, 19, 2430. [Google Scholar] [CrossRef]
- Tekari, A.; May, R.D.; Frauchiger, D.A.; Chan, S.; Benneker, L.M.; Gantenbein, B. The BMP2 variant L51P restores the osteogenic differentiation of human mesenchymal stromal cells in the presence of intervertebral disc cells. Eur. Cells Mater. 2017, 33, 197–210. [Google Scholar] [CrossRef]
- Mader, R.; Pappone, N.; Baraliakos, X.; Eshed, I.; Sarzi-Puttini, P.; Atzeni, F.; Bieber, A.; Novofastovski, I.; Kiefer, D.; Verlaan, J.-J.; et al. Diffuse Idiopathic Skeletal Hyperostosis (DISH) and a Possible Inflammatory Component. Curr. Rheumatol. Rep. 2021, 23, 1–6. [Google Scholar] [CrossRef]
- Reno, C.; Marchuk, L.; Sciore, P.; Frank, C.; Hart, D. Rapid Isolation of Total RNA from Small Samples of Hypocellular, Dense Connective Tissues. Biotechniques 1997, 22, 1082–1086. [Google Scholar] [CrossRef]
- Ginzinger, D.G. Gene quantification using real-time quantitative PCR: An emerging technology hits the mainstream. Exp. Hematol. 2002, 30, 503–512. [Google Scholar] [CrossRef]
- Levi, G.; Topilko, P.; Schneider-Maunoury, S.; Lasagna, M.; Mantero, S.; Cancedda, R.; Charnay, P. Defective bone formation in Krox-20 mutant mice. Development 1996, 122, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, N.; Noh, T.; Cogan, J.; Samarawickrama, D.B.; Smith, E.; Frenkel, B. Opposing effects of glucocorticoids and Wnt signaling on Krox20 and mineral deposition in osteoblast cultures. J. Cell. Biochem. 2008, 103, 1938–1951. [Google Scholar] [CrossRef] [PubMed]
- Zaman, G.; Sunters, A.; Galea, G.L.; Javaheri, B.; Saxon, L.K.; Moustafa, A.; Armstrong, V.J.; Price, J.S.; Lanyon, L.E. Loading-related Regulation of Transcription Factor EGR2/Krox-20 in Bone Cells Is ERK1/2 Protein-mediated and Prostaglandin, Wnt Signaling Pathway-, and Insulin-like Growth Factor-I Axis-dependent. J. Biol. Chem. 2012, 287, 3946–3962. [Google Scholar] [CrossRef]
- Verlaan, J.-J.; Boswijk, P.F.; de Ru, J.A.; Dhert, W.J.; Oner, F.C. Diffuse idiopathic skeletal hyperostosis of the cervical spine: An underestimated cause of dysphagia and airway obstruction. Spine J. 2011, 11, 1058–1067. [Google Scholar] [CrossRef]
- Sarzi-Puttini, P.; Atzeni, F. New developments in our understanding of DISH (diffuse idiopathic skeletal hyperostosis). Curr. Opin. Rheumatol. 2004, 16, 287–292. [Google Scholar] [CrossRef]
- Kobayashi, S.; Momohara, S.; Ikari, K.; Mochizuki, T.; Kawamura, K.; Tsukahara, S.; Nishimoto, K.; Okamoto, H.; Tomatsu, T. A case of Castleman’s disease associated with diffuse idiopathic skeletal hyperostosis and ossification of the posterior longitudinal ligament of the spine. Mod. Rheumatol. 2007, 17, 418–421. [Google Scholar] [CrossRef]
- De Benedetti, F.; Rucci, N.; Del Fattore, A.; Peruzzi, B.; Paro, R.; Longo, M.; Vivarelli, M.; Muratori, F.; Berni, S.; Ballanti, P.; et al. Impaired skeletal development in interleukin-6–transgenic mice: A model for the impact of chronic inflammation on the growing skeletal system. Arthritis Rheum. 2006, 54, 3551–3563. [Google Scholar] [CrossRef]
- Kang, J.D.; Georgescu, H.I.; McIntyre-Larkin, L.; Stefanovic-Racic, M.; Donaldson, W.F.; Evans, C.H. Herniated Lumbar Intervertebral Discs Spontaneously Produce Matrix Metalloproteinases, Nitric Oxide, Interleukin-6, and Prostaglandin E2. Spine 1996, 21, 271–277. [Google Scholar] [CrossRef]
- Sahin, G.; Polat, G.; Bagis, S.; Milcan, A.; Erdogan, C. Study of Axial Bone Mineral Density in Postmenopausal Women with Diffuse Idiopathic Skeletal Hyperostosis Related to Type 2 Diabetes Mellitus. J. Women’s Health 2002, 11, 801–804. [Google Scholar] [CrossRef]
- Julkunen, H.; Heinonen, O.P.; Pyorala, K. Hyperostosis of the spine in an adult population. Its relation to hyperglycaemia and obesity. Ann. Rheum. Dis. 1971, 30, 605–612. [Google Scholar] [CrossRef]
- Hájková, J.; Streda, A. Hyperostotic spondylosis in women with diabetes mellitus. Radiol. Diagn. 1973, 14, 71–80. [Google Scholar]
- Kiss, C.; O’Neill, T.; Mituszova, M.; Szilágyi, M.; Poór, G. The prevalence of diffuse idiopathic skeletal hyperostosis in a population-based study in Hungary. Scand. J. Rheumatol. 2002, 31, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Yakar, S.; Rosen, C.J.; Beamer, W.G.; Ackert-Bicknell, C.L.; Wu, Y.; Liu, J.-L.; Ooi, G.T.; Setser, J.; Frystyk, J.; Boisclair, Y.R.; et al. Circulating levels of IGF-1 directly regulate bone growth and density. J. Clin. Investig. 2002, 110, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Kawai, M.; Rosen, C.J. The Insulin-Like Growth Factor System in Bone: Basic and Clinical Implications. Endocrinol. Metab. Clin. North Am. 2012, 41, 323–333. [Google Scholar] [CrossRef]
- Osada, R.; Ohshima, H.; Ishihara, H.; Yudoh, K.; Sakai, K.; Matsui, H.; Tsuji, H. Autocrine/paracrine mechanism of insulin-like growth factor-1 secretion, and the effect of insulin-like growth factor-1 on proteoglycan synthesis in bovine intervertebral discs. J. Orthop. Res. 1996, 14, 690–699. [Google Scholar] [CrossRef]
- Storm, E.E.; Huynh, T.V.; Copeland, N.G.; Jenkins, N.A.; Kingsley, D.M.; Lee, S.-J. Limb alterations in brachypodism mice due to mutations in a new member of the TGFβ-superfamily. Nature 1994, 368, 639–643. [Google Scholar] [CrossRef]
- Chujo, T.; An, H.S.; Akeda, K.; Miyamoto, K.; Muehleman, C.; Attawia, M.; Andersson, G.; Masuda, K. Effects of Growth Differentiation Factor-5 on the Intervertebral Disc−In Vitro Bovine Study and In Vivo Rabbit Disc Degeneration Model Study. Spine 2006, 31, 2909–2917. [Google Scholar] [CrossRef]
- Li, X.; Leo, B.M.; Beck, G.; Balian, G.; Anderson, G.D. Collagen and Proteoglycan Abnormalities in the GDF-5-Deficient Mice and Molecular Changes When Treating Disk Cells With Recombinant Growth Factor. Spine 2004, 29, 2229–2234. [Google Scholar] [CrossRef]
- Tassabehji, M.; Fang, Z.M.; Hilton, E.N.; McGaughran, J.; Zhao, Z.; De Bock, C.E.; Howard, E.; Malass, M.; Donnai, D.; Diwan, A.; et al. Mutations in GDF6 are associated with vertebral segmentation defects in Klippel-Feil syndrome. Hum. Mutat. 2008, 29, 1017–1027. [Google Scholar] [CrossRef]
- Hodgkinson, T.; Gilbert, H.T.J.; Pandya, T.; Diwan, A.D.; Hoyland, J.A.; Richardson, S.M. Regenerative Response of Degenerate Human Nucleus Pulposus Cells to GDF6 Stimulation. Int. J. Mol. Sci. 2020, 21, 7143. [Google Scholar] [CrossRef]
- Stoyanov, J.V.; Gantenbein-Ritter, B.; Bertolo, A.; Aebli, N.; Baur, M.; Alini, M.; Grad, S. Role of hypoxia and growth and differentiation factor-5 on differentiation of human mesenchymal stem cells towards intervertebral nucleus pulposus-like cells. Eur. Cells Mater. 2011, 21, 533–547. [Google Scholar] [CrossRef]
- Peroglio, M.; Eglin, D.; Benneker, L.M.; Alini, M.; Grad, S. Thermoreversible hyaluronan-based hydrogel supports in vitro and ex vivo disc-like differentiation of human mesenchymal stem cells. Spine J. 2013, 13, 1627–1639. [Google Scholar] [CrossRef]
- Clarke, L.E.; McConnell, J.C.; Sherratt, M.J.; Derby, B.; Richardson, S.M.; Hoyland, J.A. Growth differentiation factor 6 and transforming growth factor-beta differentially mediate mesenchymal stem cell differentiation, composition, and micromechanical properties of nucleus pulposus constructs. Arthritis Res. Ther. 2014, 16, R67. [Google Scholar] [CrossRef]
- Colombier, P.; Clouet, J.; Boyer, C.; Ruel, M.; Bonin, G.; Lesoeur, J.; Moreau, A.; Fellah, B.-H.; Weiss, P.; Lescaudron, L.; et al. TGF-β1 and GDF5 Act Synergistically to Drive the Differentiation of Human Adipose Stromal Cells towardNucleus Pulposus-like Cells. STEM CELLS 2015, 34, 653–667. [Google Scholar] [CrossRef]
- Miyazaki, S.; Diwan, A.D.; Kato, K.; Cheng, K.; Bae, W.C.; Sun, Y.; Yamada, J.; Muehleman, C.; Lenz, M.E.; Inoue, N.; et al. ISSLS PRIZE IN BASIC SCIENCE 2018: Growth differentiation factor-6 attenuated pro-inflammatory molecular changes in the rabbit anular-puncture model and degenerated disc-induced pain generation in the rat xenograft radiculopathy model. Eur. Spine J. 2018, 27, 739–751. [Google Scholar] [CrossRef]
- Mader, R.; Lavi, I. Diabetes mellitus and hypertension as risk factors for early diffuse idiopathic skeletal hyperostosis (DISH). Osteoarthr. Cartil. 2009, 17, 825–828. [Google Scholar] [CrossRef]
- Denko, C.W.; Malemud, C.J. Role of the Growth Hormone/Insulin-like Growth Factor-1 Paracrine Axis in Rheumatic Diseases. Semin. Arthritis Rheum. 2005, 35, 24–34. [Google Scholar] [CrossRef]
| Donor Groups Used in this Study | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DISH | Control | |||||||||
| Patient # | Cell Type | Age | Sex | Spinal Level | Patient # | Cell Type | Age | Sex | Spinal Level | Disc Condition |
| 1 | N/A/CEP | 72 | M | L4/L5 | 1 | N/A/CE | 66 | F | L4/L5 | D; PG3 |
| 2 | N/A/CEP | 65 | F | L11/T12 | 2 | N/A/CE | 52 | M | L11/T12 | T; PG3 |
| 3 | IVD | 66 | M | L5/L6 | 3 | IVD | 17 | M | C5/C6 | T |
| 4 | N/A | 68 | M | L11/T12 | 2 | N/A/CE | 52 | M | L11/T12 | T; PG3 |
| 5 | A/CEP | 79 | M | C5/C6 | 3 | IVD | 17 | M | C5/C6 | T |
| 6 | A | 84 | M | L10/T11 | 2 | N/A/CE | 52 | M | L11/T12 | T; PG3 |
| Name | Description | Name | Description | Name | Description |
|---|---|---|---|---|---|
| ACTB | Actin beta | EMP1 | Epithelial Membrane Protein 1 | NOG | Noggin |
| ACTC1 | Actin Alpha Cardiac Muscle 1 | ENG | Endoglin | NOV | Nephroblastoma overexpressed |
| ACVR1 | Activin A Receptor Type 1 | FAS | Fas Cell Surface Death Receptor | PDGFB | Platelet-Derived Growth Factor Subunit B |
| ACVR2A | Activin A Receptor Type 2A | FGF2 | Fibroblast Growth Factor 2 | PLAU | Plasminogen Activator, Urokinase |
| ACVRL1 | Activin A Receptor-Like Type 1 | FSTL3 | Follistatin Like 3 | PMEPA1 | Prostate Transmembrane Protein, Androgen Induced 1 |
| AMH | Anti-Mullerian Hormone | GADD45B | Growth Arrest and DNA Damage Inducible Beta | RPLP0 | Ribosomal Protein Lateral Stalk Subunit P0 |
| AMHR2 | Anti-Mullerian Hormone Receptor Type 2 | GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase | SERPINE1 | Serpine Family E Member 1 |
| ATF4 | Activating Transcription Factor 4 | GDF2 | Growth Differentiation Factor 2 | SMAD1 | SMAD Family Member 1 |
| B2M | Beta-2-Microglobulin | GDF3 | Growth Differentiation Factor 3 | SMAD2 | SMAD Family Member 2 |
| BAMBI | BMP and Activin Membrane Bound Inhibitor | GDF5 | Growth Differentiation Factor 5 | SMAD3 | SMAD Family Member 3 |
| BGLAP | Bone Gamma-Carboxyglutamate Protein | GDF6 | Growth Differentiation Factor 6 | SMAD4 | SMAD Family Member 4 |
| BMP1 | Bone Morphogenetic Protein 1 | GDF7 | Growth Differentiation Factor 7 | SMAD6 | SMAD Family Member 6 |
| BMP2 | Bone Morphogenetic Protein 2 | GSC | Goosecoid Homeobox | SMAD7 | SMAD Family Member 7 |
| BMP3 | Bone Morphogenetic Protein 3 | GUSB | Glucuronidase Beta | SMURF1 | SMAD Specific E3 Ubiquitin Protein Ligase 1 |
| BMP4 | Bone Morphogenetic Protein 4 | HERPUD1 | Homocystein Inducible ER Protein with Ubiquitin Like Domain 1 | SOX4 | SRY-Box 4 |
| BMP5 | Bone Morphogenetic Protein 5 | HPRT1 | Hypoxanthine Phosphoribosyltransferase 1 | STAT1 | Signal Transducer and Activator of Transcription 1 |
| BMP6 | Bone Morphogenetic Protein 6 | ID1 | Inhibitor of DNA Binding 1, HLH Protein | STK38L | Serine/Threonine Kinase 23 Like |
| BMP7 | Bone Morphogenetic Protein 7 | ID2 | Inhibitor of DNA Binding 2 | TBP | TATA-Box Binding Protein |
| BMPER | BMP Binding Endothelial Regulator | IFRD1 | Interferon Related Developmental Regulator 1 | TGFB1 | Transforming Growth Factor Beta 1 |
| BMPR1A | Bone Morphogenetic Protein Receptor, Type IA | IGF1 | Insulin-Like Growth Factor 1 | TGFB1l1 | Transforming Growth Gactor Beta-1-induced Transcript 1 |
| BMPR1B | Bone Morphogenetic Protein Receptor, Type IB | IGFBP3 | Insulin-Like Growth Factor Binding Protein 3 | TGFB2 | Transforming Growth Factor Beta 2 |
| BMPR2 | Bone Morphogenetic Protein Receptor, Type II | IL6 | Interleukin 6 | TGFB3 | Transforming Growth Factor Beta 3 |
| CDKN1A | Cyclin-Dependent Kinase Inhibitor 1A | INHA | Inhibin Subunit Alpha | TGFBI | Transforming Growth Factor Beta Induced |
| CDKN1B | Cyclin-Dependent Kinase Inhibitor 1B | JUNB | JunB Proto-Oncogene, AP-1 Transcription Factor Subunit | TGFBR1 | Transforming Growth Factor Beta Receptor 1 |
| CDKN2B | Cyclin-Dependent Kinase Inhibitor 2B | KANK4 | KN Motif and Ankyrin Repeat Domains 4 | TGFBR2 | Transforming Growth Factor Beta Receptor 2 |
| CHRD | Chordin | KLHL24 | Kelch Like Family Member 24 | TGFBR3 | Transforming Growth Factor Beta Receptor 3 |
| COL1A1 | Collagen Type I Alpha 1 Chain | LEFTY1 | Left-Right Determination Factor 1 | TGIF1 | TGFB Induced Factor Homeobox 1 |
| COL1A2 | Collagen Type I Alpha 2 Chain | LTBP1 | Latent Transforming Growth Factor Beta Binding Protein 1 | THBS1 | Thrombospondin 1 |
| DCN | Decorin | MYC | MYC Proto-Oncogene, BHLH Transcription Factor | TNFSF10 | TNF Superfamily Member 10 |
| EGR2 | Early Growth Response 2 | NODAL | Nodal Growth Differentiation Factor | UBASH3B | Ubiquitin Associated and SH3 Domain Containing B |
| Primary AB | Source | Concentration ICC |
|---|---|---|
| Mouse GDF5 | Polyclonal Goat IgG (Biotechne, Zug, Switzerland) | 5 µg/mL |
| Human IL6 | Monoclonal mouse IgG (Biotechne) | 16 µg/mL |
| Human IGF1 | Polyclonal Goat IgG (Biotechne) | 5 µg/mL |
| Human BMP2 | Polyclonal Rabbit (Novusbio, Zug, Switzerland) | 5.75 µg/mL |
| Human EGR2 | Polyclonal Rabbit (Novusbio) | 5 µg/mL |
| Human GDF6 | Rabbit polyclonal–C terminal (Abcam, Cambridge, UK) | 1:100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gantenbein, B.; May, R.D.; Bermudez-Lekerika, P.; Oswald, K.A.C.; Benneker, L.M.; Albers, C.E. EGR2, IGF1 and IL6 Expression Are Elevated in the Intervertebral Disc of Patients Suffering from Diffuse Idiopathic Skeletal Hyperostosis (DISH) Compared to Degenerative or Trauma Discs. Appl. Sci. 2021, 11, 4072. https://doi.org/10.3390/app11094072
Gantenbein B, May RD, Bermudez-Lekerika P, Oswald KAC, Benneker LM, Albers CE. EGR2, IGF1 and IL6 Expression Are Elevated in the Intervertebral Disc of Patients Suffering from Diffuse Idiopathic Skeletal Hyperostosis (DISH) Compared to Degenerative or Trauma Discs. Applied Sciences. 2021; 11(9):4072. https://doi.org/10.3390/app11094072
Chicago/Turabian StyleGantenbein, Benjamin, Rahel D. May, Paola Bermudez-Lekerika, Katharina A. C. Oswald, Lorin M. Benneker, and Christoph E. Albers. 2021. "EGR2, IGF1 and IL6 Expression Are Elevated in the Intervertebral Disc of Patients Suffering from Diffuse Idiopathic Skeletal Hyperostosis (DISH) Compared to Degenerative or Trauma Discs" Applied Sciences 11, no. 9: 4072. https://doi.org/10.3390/app11094072
APA StyleGantenbein, B., May, R. D., Bermudez-Lekerika, P., Oswald, K. A. C., Benneker, L. M., & Albers, C. E. (2021). EGR2, IGF1 and IL6 Expression Are Elevated in the Intervertebral Disc of Patients Suffering from Diffuse Idiopathic Skeletal Hyperostosis (DISH) Compared to Degenerative or Trauma Discs. Applied Sciences, 11(9), 4072. https://doi.org/10.3390/app11094072