Thermometric Characterization of Fluorescent Nanodiamonds Suitable for Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. High-Pressure High-Temperature Nanodiamonds
2.2. Fluorescent Thermometry
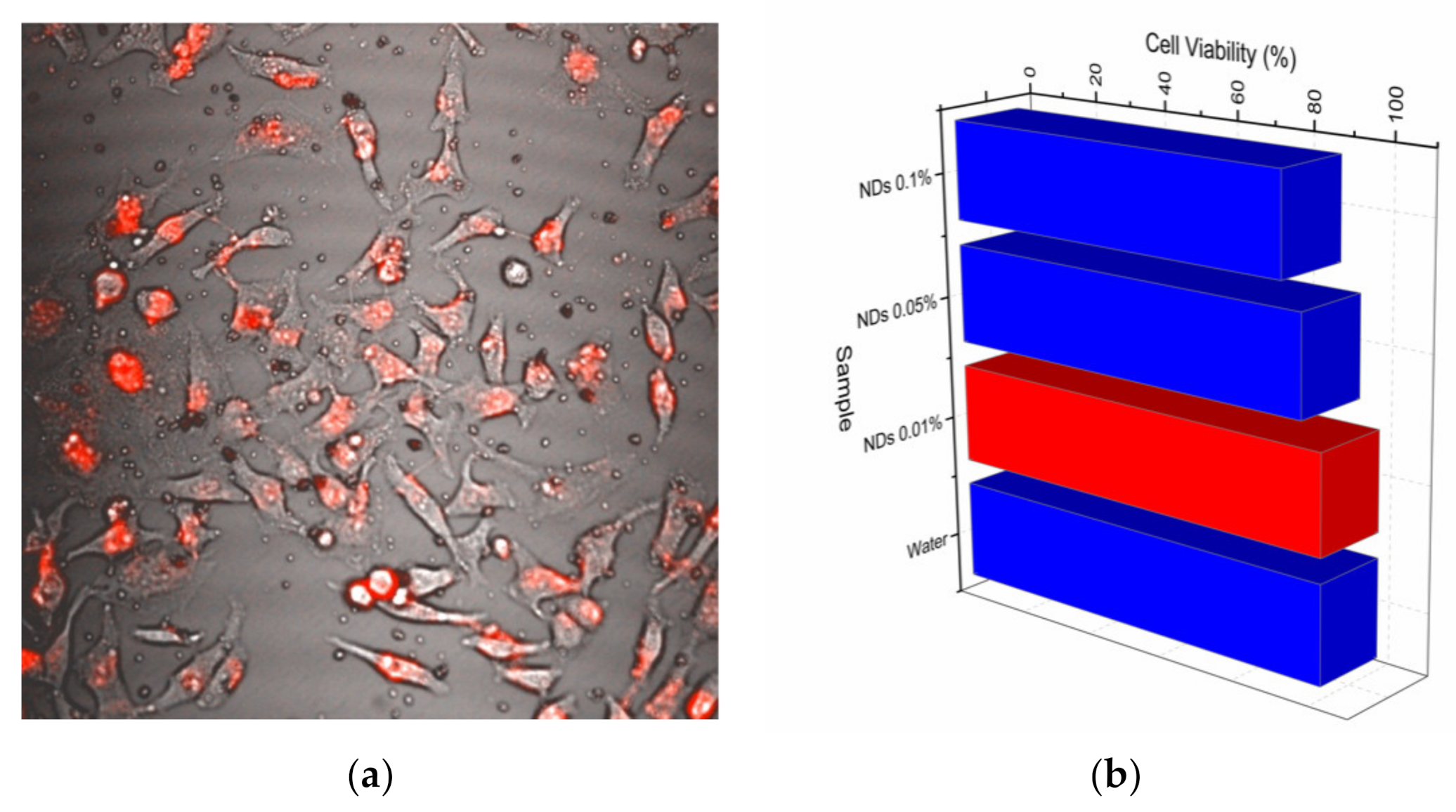
2.3. HeLa Images and Cytotoxicity Assays
3. Results and Discussion
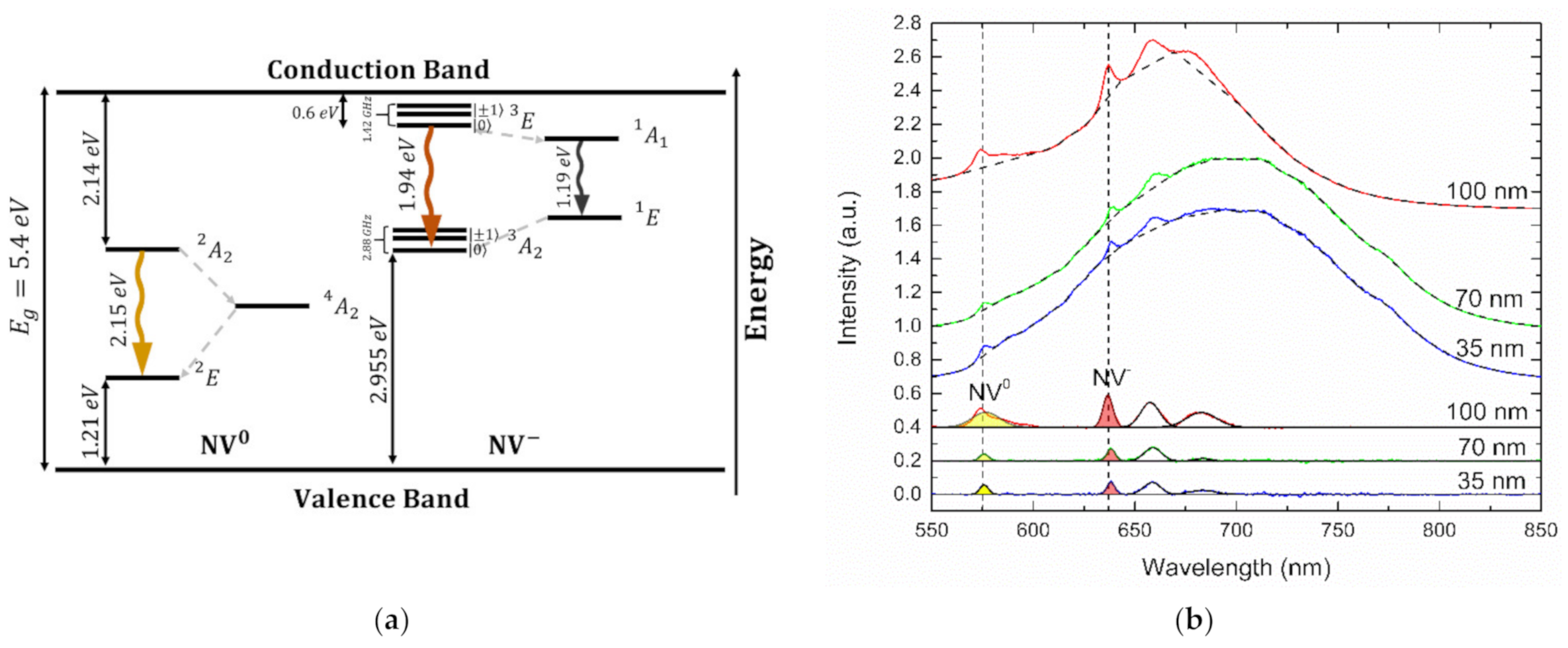
3.1. NV Centre Electronic Structure
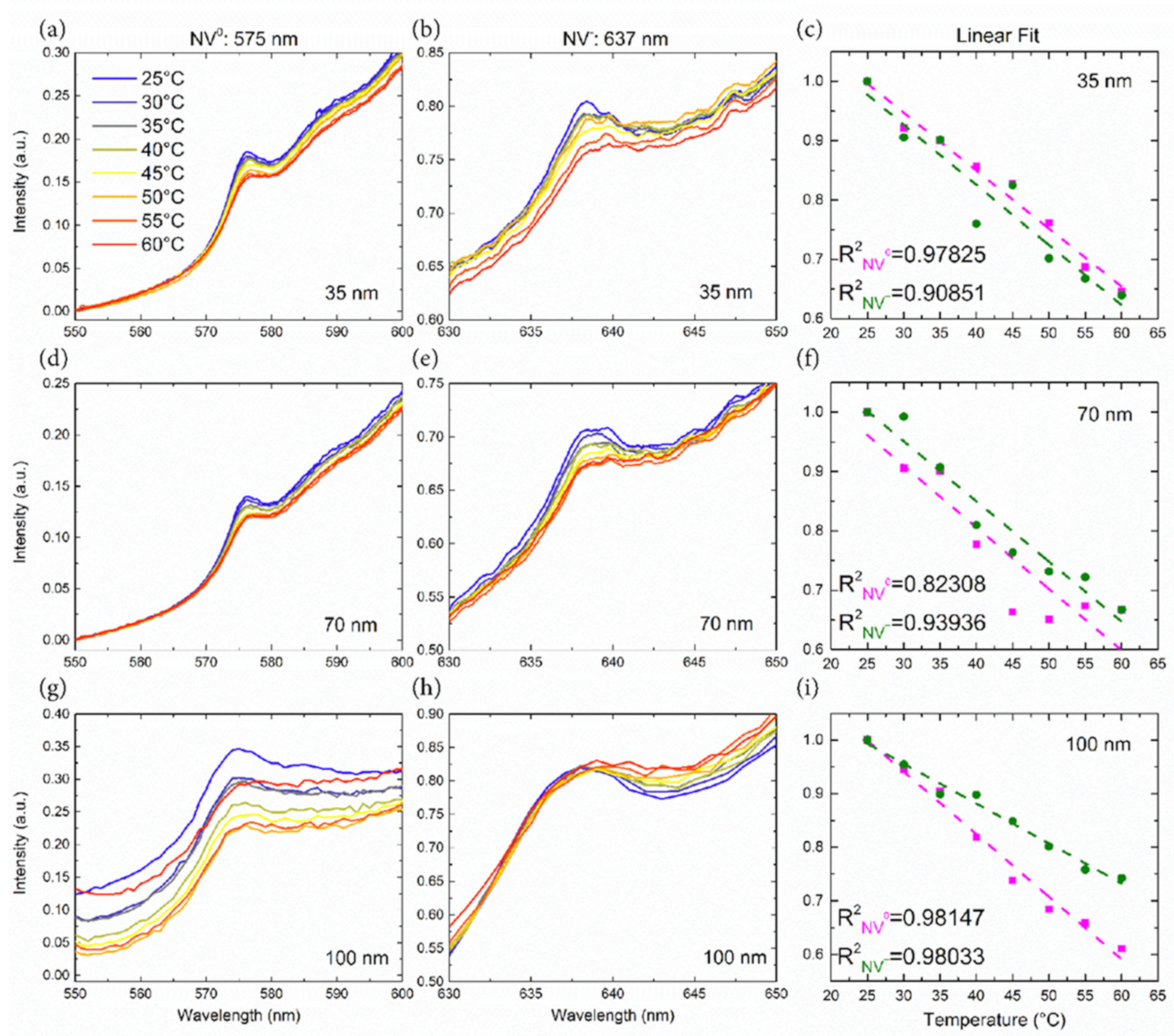
3.2. Nanothermic Scale of High-Pressure High-Temperature Nanodiamonds
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neves, A.R.; Reis, S. Nanoparticles in Life Sciences and Biomedicine; Neves, A.R., Reis, S., Eds.; Pan Stanford Publishing Pte. Ltd.: Singapore, 2018; ISBN 978-1-351-20735-5. [Google Scholar]
- Pan, M.; Annamalai, K.; Tao, Y. Applications of Nanocarbons in Bio-Medical Devices. Recent Innov. Chem. Eng. Former. Recent Pat. Chem. Eng. 2016, 8, 67–74. [Google Scholar] [CrossRef]
- Wang, X.; Nakamoto, T.; Dulińska-Molak, I.; Kawazoe, N.; Chen, G. Regulating the stemness of mesenchymal stem cells by tuning micropattern features. J. Mater. Chem. B 2016, 4, 37–45. [Google Scholar] [CrossRef]
- Panwar, N.; Soehartono, A.M.; Chan, K.K.; Zeng, S.; Xu, G.; Qu, J.; Coquet, P.; Yong, K.-T.; Chen, X. Nanocarbons for Biology and Medicine: Sensing, Imaging, and Drug Delivery. Chem. Rev. 2019, 119, 9559–9656. [Google Scholar] [CrossRef] [PubMed]
- Mitura, S. Nanodiamonds. J. Achiev. Mater. Manuf. Eng. 2007, 24, 7. [Google Scholar]
- Nunn, N.; Torelli, M.; McGuire, G.; Shenderova, O. Nanodiamond: A high impact nanomaterial. Curr. Opin. Solid State Mater. Sci. 2017, 21, 1–9. [Google Scholar] [CrossRef]
- Yu, S.-J.; Kang, M.-W.; Chang, H.-C.; Chen, K.-M.; Yu, Y.-C. Bright Fluorescent Nanodiamonds: No Photobleaching and Low Cytotoxicity. J. Am. Chem. Soc. 2005, 127, 17604–17605. [Google Scholar] [CrossRef]
- Hui, Y.Y.; Cheng, C.-L.; Chang, H.-C. Nanodiamonds for optical bioimaging. J. Phys. Appl. Phys. 2010, 43, 374021. [Google Scholar] [CrossRef]
- Vaijayanthimala, V.; Tzeng, Y.-K.; Chang, H.-C.; Li, C.-L. The biocompatibility of fluorescent nanodiamonds and their mechanism of cellular uptake. Nanotechnology 2009, 20, 425103. [Google Scholar] [CrossRef]
- Elena, P.; Jani, M.; Cheng, C.-L. Applications of Nanomaterials; Ramesh, S.C., Shrikan, C.W., Eds.; American Scientific Publishers: Valencia, CA, USA, 2013; ISBN 978-1-58883-181-1. [Google Scholar]
- Schrand, A.M.; Huang, H.; Carlson, C.; Schlager, J.J.; Ōsawa, E.; Hussain, S.M.; Dai, L. Are Diamond Nanoparticles Cytotoxic? J. Phys. Chem. B 2007, 111, 2–7. [Google Scholar] [CrossRef]
- Manus, L.M.; Mastarone, D.J.; Waters, E.A.; Zhang, X.-Q.; Schultz-Sikma, E.A.; MacRenaris, K.W.; Ho, D.; Meade, T.J. Gd(III)-Nanodiamond Conjugates for MRI Contrast Enhancement. Nano Lett. 2010, 10, 484–489. [Google Scholar] [CrossRef]
- Yeap, W.S.; Chen, S.; Loh, K.P. Detonation Nanodiamond: An Organic Platform for the Suzuki Coupling of Organic Molecules. Langmuir 2009, 25, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Metwally, A.A.; Fahmy, R.H.; Osman, R. Nanodiamonds: Minuscule gems that ferry antineoplastic drugs to resistant tumors. Int. J. Pharm. 2019, 558, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Guo, Q.; Zhi, J. Nanodiamond-Based Theranostic Platform for Drug Delivery and Bioimaging. Small 2019, 15, 1902238. [Google Scholar] [CrossRef]
- Garg, S.; Garg, A.; Sahu, N.K.; Yadav, A.K. Synthesis and characterization of nanodiamond-anticancer drug conjugates for tumor targeting. Diam. Relat. Mater. 2019, 94, 172–185. [Google Scholar] [CrossRef]
- Terada, D.; Genjo, T.; Segawa, T.F.; Igarashi, R.; Shirakawa, M. Nanodiamonds for bioapplications–specific targeting strategies. Biochim. Biophys. Acta BBA Gen. Subj. 2020, 1864, 129354. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-R.; Lee, H.-Y.; Chen, K.; Chang, C.-C.; Tsai, D.-S.; Fu, C.-C.; Lim, T.-S.; Tzeng, Y.-K.; Fang, C.-Y.; Han, C.-C.; et al. Mass production and dynamic imaging of fluorescent nanodiamonds. Nat. Nanotechnol. 2008, 3, 284–288. [Google Scholar] [CrossRef]
- Schrand, A.M.; Lin, J.B.; Hens, S.C.; Hussain, S.M. Temporal and mechanistic tracking of cellular uptake dynamics with novel surface fluorophore-bound nanodiamonds. Nanoscale 2011, 3, 435–445. [Google Scholar] [CrossRef]
- Xing, Y.; Dai, L. Nanodiamonds for nanomedicine. Nanomedicine 2009, 4, 207–218. [Google Scholar] [CrossRef]
- Alkahtani, M.H.; Alghannam, F.; Jiang, L.; Almethen, A.; Rampersaud, A.A.; Brick, R.; Gomes, C.L.; Scully, M.O.; Hemmer, P.R. Fluorescent nanodiamonds: Past, present, and future. Nanophotonics 2018, 7, 1423–1453. [Google Scholar] [CrossRef]
- Jelezko, F.; Wrachtrup, J. Single defect centres in diamond: A review. Phys. Status Solidi A 2006, 203, 3207–3225. [Google Scholar] [CrossRef]
- Wilson, E.R.; Parker, L.M.; Orth, A.; Nunn, N.; Torelli, M.; Shenderova, O.; Gibson, B.C.; Reineck, P. The effect of particle size on nanodiamond fluorescence and colloidal properties in biological media. Nanotechnology 2019, 30, 385704. [Google Scholar] [CrossRef]
- Shershulin, V.A.; Sedov, V.S.; Ermakova, A.; Jantzen, U.; Rogers, L.; Huhlina, A.A.; Teverovskaya, E.G.; Ralchenko, V.G.; Jelezko, F.; Vlasov, I.I. Size-dependent luminescence of color centers in composite nanodiamonds. Phys. Status Solidi A 2015, 212, 2600–2605. [Google Scholar] [CrossRef]
- Fedyanin, D.Y.; Agio, M. Ultrabright single-photon source on diamond with electrical pumping at room and high temperatures. New J. Phys. 2016, 18, 073012. [Google Scholar] [CrossRef]
- Su, Z.; Ren, Z.; Bao, Y.; Lao, X.; Zhang, J.; Zhang, J.; Zhu, D.; Lu, Y.; Hao, Y.; Xu, S. Luminescence landscapes of nitrogen-vacancy centers in diamond: Quasi-localized vibrational resonances and selective coupling. J. Mater. Chem. C 2019, 7, 8086–8091. [Google Scholar] [CrossRef]
- Vervald, A.; Burikov, S.; Borisova, N.; Vlasov, I.; Laptinskiy, K.; Laptinskaya, T.; Shenderova, O.; Dolenko, T. Fluorescence properties of nanodiamonds with NV centers in water suspensions: Fluorescence properties of ND-NV in water. Phys. Status Solidi A 2016, 213, 2601–2607. [Google Scholar] [CrossRef]
- Aslam, N.; Waldherr, G.; Neumann, P.; Jelezko, F.; Wrachtrup, J. Photo induced ionization dynamics of the nitrogen vacancy defect in diamond investigated by single shot charge state detection. New J. Phys. 2013, 15, 013064. [Google Scholar] [CrossRef]
- Beha, K.; Batalov, A.; Manson, N.B.; Bratschitsch, R.; Leitenstorfer, A. Optimum Photoluminescence Excitation and Recharging Cycle of Single Nitrogen-Vacancy Centers in Ultrapure Diamond. Phys. Rev. Lett. 2012, 109, 097404. [Google Scholar] [CrossRef]
- Waldherr, G.; Beck, J.; Steiner, M.; Neumann, P.; Gali, A.; Frauenheim, T.; Jelezko, F.; Wrachtrup, J. Dark States of Single Nitrogen-Vacancy Centers in Diamond Unraveled by Single Shot NMR. Phys. Rev. Lett. 2011, 106, 157601. [Google Scholar] [CrossRef] [PubMed]
- Petrini, G.; Moreva, E.; Bernardi, E.; Traina, P.; Tomagra, G.; Carabelli, V.; Degiovanni, I.P.; Genovese, M. Is a Quantum Biosensing Revolution Approaching? Perspectives in NV-Assisted Current and Thermal Biosensing in Living Cells. Adv. Quantum Technol. 2020, 3, 2000066. [Google Scholar] [CrossRef]
- Claveau, S.; Bertrand, J.-R.; Treussart, F. Fluorescent Nanodiamond Applications for Cellular Process Sensing and Cell Tracking. Micromachines 2018, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.C.; Shukla, S.; Pandey, G.; Narayan, R.J. Nanostructured diamond for biomedical applications. Nanotechnology 2021, 32, 132001. [Google Scholar] [CrossRef] [PubMed]
- Chrétien, D.; Bénit, P.; Ha, H.-H.; Keipert, S.; El-Khoury, R.; Chang, Y.-T.; Jastroch, M.; Jacobs, H.T.; Rustin, P.; Rak, M. Mitochondria are physiologically maintained at close to 50 °C. PLoS Biol. 2018, 16, e2003992. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.; Gu, N. Micro/Nanoscale Thermometry for Cellular Thermal Sensing. Small 2016, 12, 4590–4610. [Google Scholar] [CrossRef]
- Karnebogen, M.; Singer, D.; Kallerhoff, M.; Ringert, R.-H. Microcalorimetric investigations on isolated tumorous and non-tumorous tissue samples. Thermochim. Acta 1993, 229, 147–155. [Google Scholar] [CrossRef]
- Suzuki, M.; Tseeb, V.; Oyama, K.; Ishiwata, S. Microscopic Detection of Thermogenesis in a Single HeLa Cell. Biophys. J. 2007, 92, L46–L48. [Google Scholar] [CrossRef]
- Vetrone, F.; Naccache, R.; Zamarrón, A.; Juarranz de la Fuente, A.; Sanz-Rodríguez, F.; Martinez Maestro, L.; Martín Rodriguez, E.; Jaque, D.; García Solé, J.; Capobianco, J.A. Temperature Sensing Using Fluorescent Nanothermometers. ACS Nano 2010, 4, 3254–3258. [Google Scholar] [CrossRef]
- Drabik, J.; Cichy, B.; Marciniak, L. New Type of Nanocrystalline Luminescent Thermometers Based on Ti 3+/Ti 4+ and Ti 4+/Ln 3+ (Ln 3+ = Nd 3+, Eu 3+, Dy 3+) Luminescence Intensity Ratio. J. Phys. Chem. C 2018, 122, 14928–14936. [Google Scholar] [CrossRef]
- Elzbieciak-Piecka, K.; Matuszewska, C.; Marciniak, L. Step by step designing of sensitive luminescent nanothermometers based on Cr3+,Nd3+ co-doped La3−xLuxAl5−yGayO12 nanocrystals. New J. Chem. 2019, 43, 12614–12622. [Google Scholar] [CrossRef]
- Lupton, J.M. A molecular thermometer based on long-lived emission from platinum octaethyl porphyrin. Appl. Phys. Lett. 2002, 81, 2478–2480. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, J.; Ma, J.; Liu, Y.; Wang, Y.; Wu, D. Ratiometric Nanothermometer Based on Rhodamine Dye-Incorporated F127-Melamine-Formaldehyde Polymer Nanoparticle: Preparation, Characterization, Wide-Range Temperature Sensing, and Precise Intracellular Thermometry. ACS Appl. Mater. Interfaces 2016, 8, 14396–14405. [Google Scholar] [CrossRef]
- Kucsko, G.; Maurer, P.C.; Yao, N.Y.; Kubo, M.; Noh, H.J.; Lo, P.K.; Park, H.; Lukin, M.D. Nanometre-scale thermometry in a living cell. Nature 2013, 500, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Plakhotnik, T.; Doherty, M.W.; Cole, J.H.; Chapman, R.; Manson, N.B. All-Optical Thermometry and Thermal Properties of the Optically Detected Spin Resonances of the NV–Center in Nanodiamond. Nano Lett. 2014, 14, 4989–4996. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.-C.; Epperla, C.P.; Huang, J.-S.; Chen, O.Y.; Wu, C.-C.; Chang, H.-C. Measuring Nanoscale Thermostability of Cell Membranes with Single Gold-Diamond Nanohybrids. Angew. Chem. Int. Ed. 2017, 56, 3025–3030. [Google Scholar] [CrossRef] [PubMed]
- Montes-Frausto, J.B.; Juarez-Moreno, K.; Can-Uc, B.; Hirata-Flores, G.A. Synthesis and cytotoxic effects of SrAl2O4 persistent luminescence nanoparticles co-doped with Eu2+/Dy3+ ions. Opt. Mater. Express 2016, 6, 1488–1499. [Google Scholar] [CrossRef]
- Acosta-Elías, M.; Sarabia-Sainz, A.; Pedroso-Santana, S.; Silva-Campa, E.; Santacruz-Gomez, K.; Angulo-Molina, A.; Castaneda, B.; Soto-Puebla, D.; Barboza-Flores, M.; Melendrez, R.; et al. Carboxylated nanodiamond and re-oxygenation process of gamma irradiated red blood cells: Re-oxygenation process of gamma irradiated red blood cells. Phys. Status Solidi A 2015, 212, 2437–2444. [Google Scholar] [CrossRef]
- Hsiao, W.W.-W.; Hui, Y.Y.; Tsai, P.-C.; Chang, H.-C. Fluorescent Nanodiamond: A Versatile Tool for Long-Term Cell Tracking, Super-Resolution Imaging, and Nanoscale Temperature Sensing. Acc. Chem. Res. 2016, 49, 400–407. [Google Scholar] [CrossRef]
- Fujiwara, M.; Sun, S.; Dohms, A.; Nishimura, Y.; Suto, K.; Takezawa, Y.; Oshimi, K.; Zhao, L.; Sadzak, N.; Umehara, Y.; et al. Real-time nanodiamond thermometry probing in vivo thermogenic responses. Sci. Adv. 2020, 6, 9. [Google Scholar] [CrossRef]
- Yang, M.; Yuan, Q.; Gao, J.; Shu, S.; Chen, F.; Sun, H.; Nishimura, K.; Wang, S.; Yi, J.; Lin, C.-T.; et al. A Diamond Temperature Sensor Based on the Energy Level Shift of Nitrogen-Vacancy Color Centers. Nanomaterials 2019, 9, 1576. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, X. Nanoscale thermal probing. Nano Rev. 2012, 3, 11586. [Google Scholar] [CrossRef]
- Brown, S. Taking the temperature of a cell. Nat. Nanotechnol. 2013, 500, 54–58. [Google Scholar] [CrossRef]
- Plakhotnik, T.; Aman, H.; Chang, H.-C. All-optical single-nanoparticle ratiometric thermometry with a noise floor of 0.3 K Hz −1/2. Nanotechnology 2015, 26, 245501. [Google Scholar]
- Plakhotnik, T. Diamonds for quantum nano sensing. Curr. Opin. Solid State Mater. Sci. 2017, 21, 25–34. [Google Scholar] [CrossRef]
- Fernández-Quiroz, D.; Loya-Duarte, J.; Silva-Campa, E.; Argüelles-Monal, W.; Sarabia-Sainz, A.; Lucero-Acuña, A.; del Castillo-Castro, T.; San Román, J.; Lizardi-Mendoza, J.; Burgara-Estrella, A.J.; et al. Temperature stimuli-responsive nanoparticles from chitosan-graft-poly(N-vinylcaprolactam) as a drug delivery system. J. Appl. Polym. Sci. 2019, 136, 47831. [Google Scholar] [CrossRef]
- Schirhagl, R.; Chang, K.; Loretz, M.; Degen, C.L. Nitrogen-Vacancy Centers in Diamond: Nanoscale Sensors for Physics and Biology. Annu. Rev. Phys. Chem. 2014, 65, 83–105. [Google Scholar] [CrossRef]
- Gali, A.; Fyta, M.; Kaxiras, E. Ab initio supercell calculations on nitrogen-vacancy center in diamond: Electronic structure and hyperfine tensors. Phys. Rev. B 2008, 77, 155206. [Google Scholar] [CrossRef]
- Smeltzer, B.; Childress, L.; Gali, A. 13 C hyperfine interactions in the nitrogen-vacancy centre in diamond. New J. Phys. 2011, 13, 025021. [Google Scholar] [CrossRef]
- Barson, M.S.J.; Krausz, E.; Manson, N.B.; Doherty, M.W. The fine structure of the neutral nitrogen-vacancy center in diamond. Nanophotonics 2019, 8, 1985–1991. [Google Scholar] [CrossRef]
- Redman, D.A.; Brown, S.; Sands, R.H.; Rand, S.C. Spin dynamics and electronic states of N-V centers in diamond by EPR and four-wave-mixing spectroscopy. Phys. Rev. Lett. 1991, 67, 4. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.P.D. Fine structure of excited _E state in nitrogen-vacancy centre of diamond. J. Lumin. 1999, 81, 237–247. [Google Scholar] [CrossRef]
- Su, L.-X.; Lou, Q.; Zang, J.-H.; Shan, C.-X.; Gao, Y.-F. Temperature-dependent fluorescence in nanodiamonds. Appl. Phys. Express 2017, 10, 025102. [Google Scholar] [CrossRef]
- Alkauskas, A.; Buckley, B.B.; Awschalom, D.D.; Van de Walle, C.G. First-principles theory of the luminescence lineshape for the triplet transition in diamond NV centres. New J. Phys. 2014, 16, 073026. [Google Scholar] [CrossRef]
- Davies, G. The Jahn-Teller effect and vibronic coupling at deep levels in diamond. Rep. Prog. Phys. 1981, 44, 787–830. [Google Scholar] [CrossRef]
- Treussart, F.; Vlasov, I.I. Photoluminescence of color centers in nanodiamonds. In Nanodiamonds; Elsevier: Amsterdam, The Netherlands, 2017; pp. 155–181. ISBN 978-0-323-43029-6. [Google Scholar]
- Rondin, L.; Dantelle, G.; Slablab, A.; Grosshans, F.; Treussart, F.; Bergonzo, P.; Perruchas, S.; Gacoin, T.; Chaigneau, M.; Chang, H.-C.; et al. Surface-induced charge state conversion of nitrogen-vacancy defects in nanodiamonds. Phys. Rev. B 2010, 82, 115449. [Google Scholar] [CrossRef]
- Shenderova, O.; Nunn, N.; Oeckinghaus, T.; Torelli, M.; McGuire, G.; Smith, K.; Danilov, E.; Reuter, R.; Wrachtrup, J.; Shames, A.; et al. Commercial Quantities of Ultrasmall Fluorescent Nanodiamonds Containing Color Centers; Hasan, Z.U., Hemmer, P.R., Lee, H., Migdall, A.L., Eds.; International Society for Optics and Photonics: San Francisco, CA, USA, 2017; Volume 10118, pp. 1–17. [Google Scholar]
- Robinson, M.E.; Ng, J.D.; Zhang, H.; Buchman, J.T.; Shenderova, O.A.; Haynes, C.L.; Ma, Z.; Goldsmith, R.H.; Hamers, R.J. Optically Detected Magnetic Resonance for Selective Imaging of Diamond Nanoparticles. Anal. Chem. 2018, 90, 769–776. [Google Scholar] [CrossRef]
- Yanagi, T.; Kaminaga, K.; Kada, W.; Hanaizumi, O.; Igarashi, R. Optimization of Wide-Field ODMR Measurements Using Fluorescent Nanodiamonds to Improve Temperature Determination Accuracy. Nanomaterials 2020, 10, 2282. [Google Scholar] [CrossRef]
- Sekiguchi, T.; Sotoma, S.; Harada, Y. Fluorescent nanodiamonds as a robust temperature sensor inside a single cell. Biophys. Physicobiology 2018, 15, 229–234. [Google Scholar] [CrossRef]
- Basso, L.; Cazzanelli, M.; Orlandi, M.; Miotello, A. Nanodiamonds: Synthesis and Application in Sensing, Catalysis, and the Possible Connection with Some Processes Occurring in Space. Appl. Sci. 2020, 10, 4094. [Google Scholar] [CrossRef]
- 72. Bradac, C.; Gaebel, T.; Pakes, C.I.; Say, J.M.; Zvyagin, A.V.; Rabeau, J.R. Effect of the Nanodiamond Host on a Nitrogen-Vacancy Color-Centre Emission State. Small 2013, 9, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef]
- Maruyama, H.; Kimura, T.; Liu, H.; Ohtsuki, S.; Miyake, Y.; Isogai, M.; Arai, F.; Honda, A. Influenza virus replication raises the temperature of cells. Virus Res. 2018, 257, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Kratochvílová, I.; Šebera, J.; Ashcheulov, P.; Golan, M.; Ledvina, M.; Míčová, J.; Mravec, F.; Kovalenko, A.; Zverev, D.; Yavkin, B.; et al. Magnetical and Optical Properties of Nanodiamonds Can Be Tuned by Particles Surface Chemistry: Theoretical and Experimental Study. J. Phys. Chem. C 2014, 118, 25245–25252. [Google Scholar] [CrossRef]
| Part Number | Concentration | NV Centres/Particle | Size |
|---|---|---|---|
| 900172-5 mL | 1 mg/mL | ≤4 | 35 nm |
| 798169-5 mL | 1 mg/mL | >300 | 70 nm |
| 900174-5 mL | 1 mg/mL | >900 | 100 nm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedroza-Montero, F.; Santacruz-Gómez, K.; Acosta-Elías, M.; Silva-Campa, E.; Meza-Figueroa, D.; Soto-Puebla, D.; Castaneda, B.; Urrutia-Bañuelos, E.; Álvarez-Bajo, O.; Navarro-Espinoza, S.; et al. Thermometric Characterization of Fluorescent Nanodiamonds Suitable for Biomedical Applications. Appl. Sci. 2021, 11, 4065. https://doi.org/10.3390/app11094065
Pedroza-Montero F, Santacruz-Gómez K, Acosta-Elías M, Silva-Campa E, Meza-Figueroa D, Soto-Puebla D, Castaneda B, Urrutia-Bañuelos E, Álvarez-Bajo O, Navarro-Espinoza S, et al. Thermometric Characterization of Fluorescent Nanodiamonds Suitable for Biomedical Applications. Applied Sciences. 2021; 11(9):4065. https://doi.org/10.3390/app11094065
Chicago/Turabian StylePedroza-Montero, Francisco, Karla Santacruz-Gómez, Mónica Acosta-Elías, Erika Silva-Campa, Diana Meza-Figueroa, Diego Soto-Puebla, Beatriz Castaneda, Efraín Urrutia-Bañuelos, Osiris Álvarez-Bajo, Sofía Navarro-Espinoza, and et al. 2021. "Thermometric Characterization of Fluorescent Nanodiamonds Suitable for Biomedical Applications" Applied Sciences 11, no. 9: 4065. https://doi.org/10.3390/app11094065
APA StylePedroza-Montero, F., Santacruz-Gómez, K., Acosta-Elías, M., Silva-Campa, E., Meza-Figueroa, D., Soto-Puebla, D., Castaneda, B., Urrutia-Bañuelos, E., Álvarez-Bajo, O., Navarro-Espinoza, S., Riera, R., & Pedroza-Montero, M. (2021). Thermometric Characterization of Fluorescent Nanodiamonds Suitable for Biomedical Applications. Applied Sciences, 11(9), 4065. https://doi.org/10.3390/app11094065








