Goldmann Tonometry and Corneal Biomechanics
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
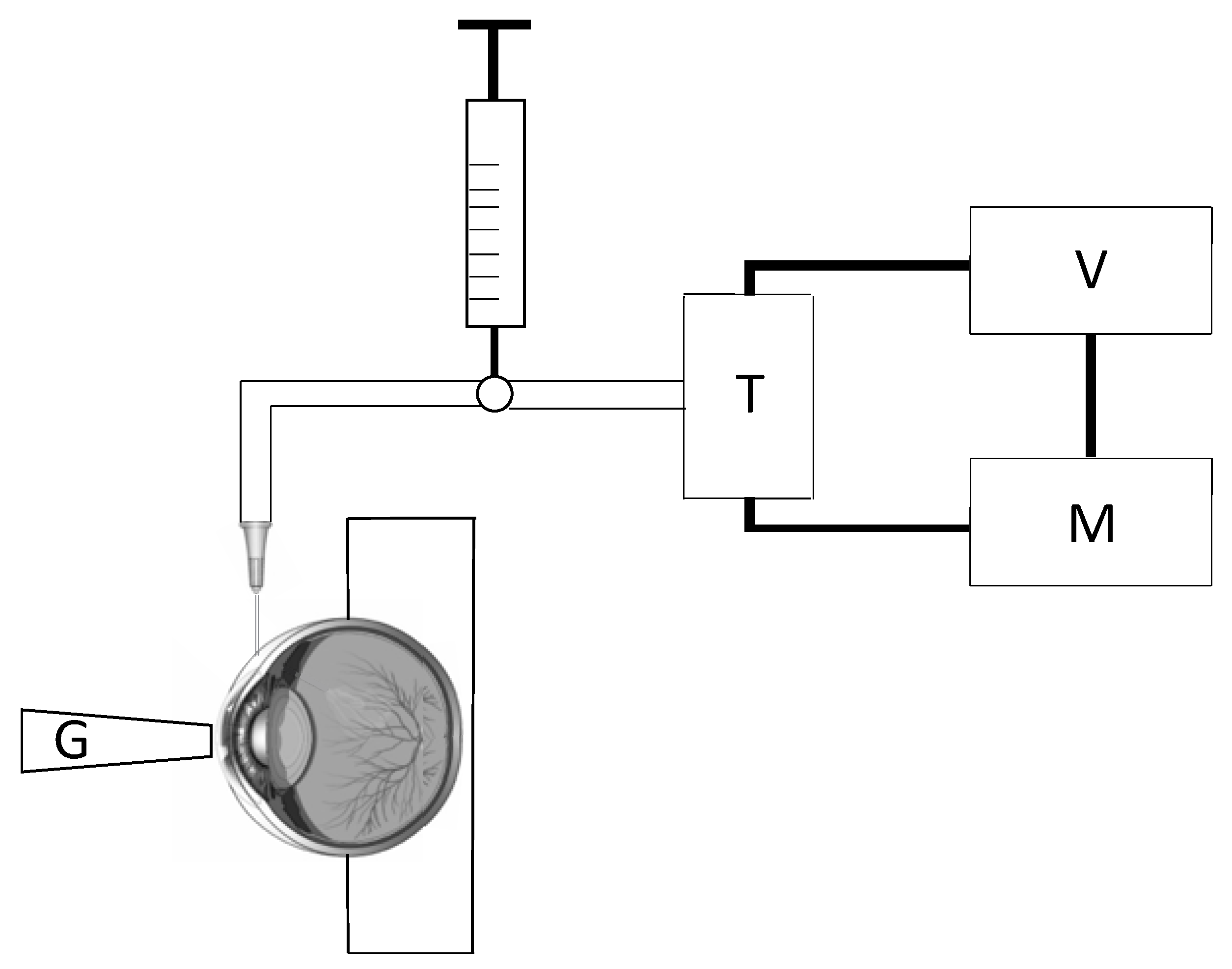
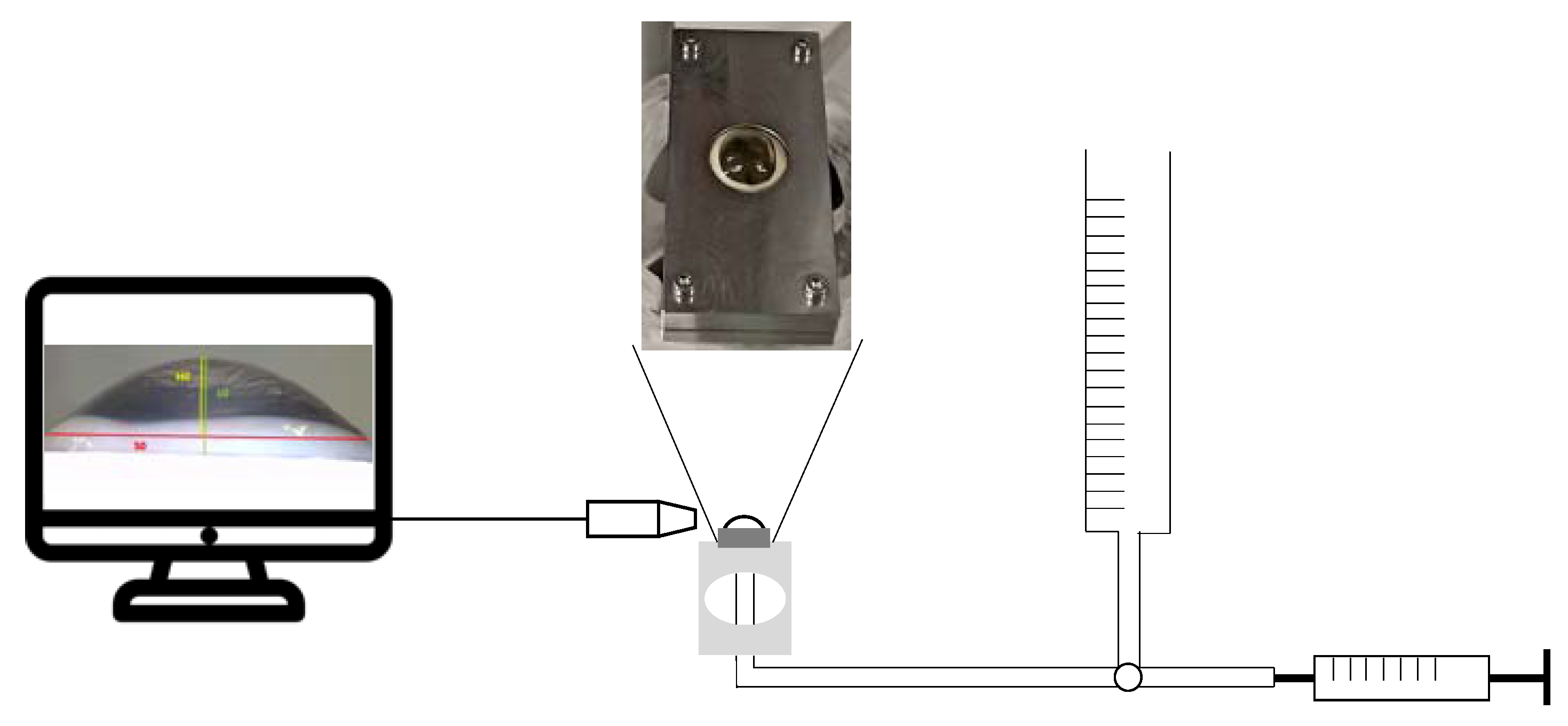
2.1. Tonometry Test
2.2. Inflation Test
2.3. Statistical Analysis
3. Results
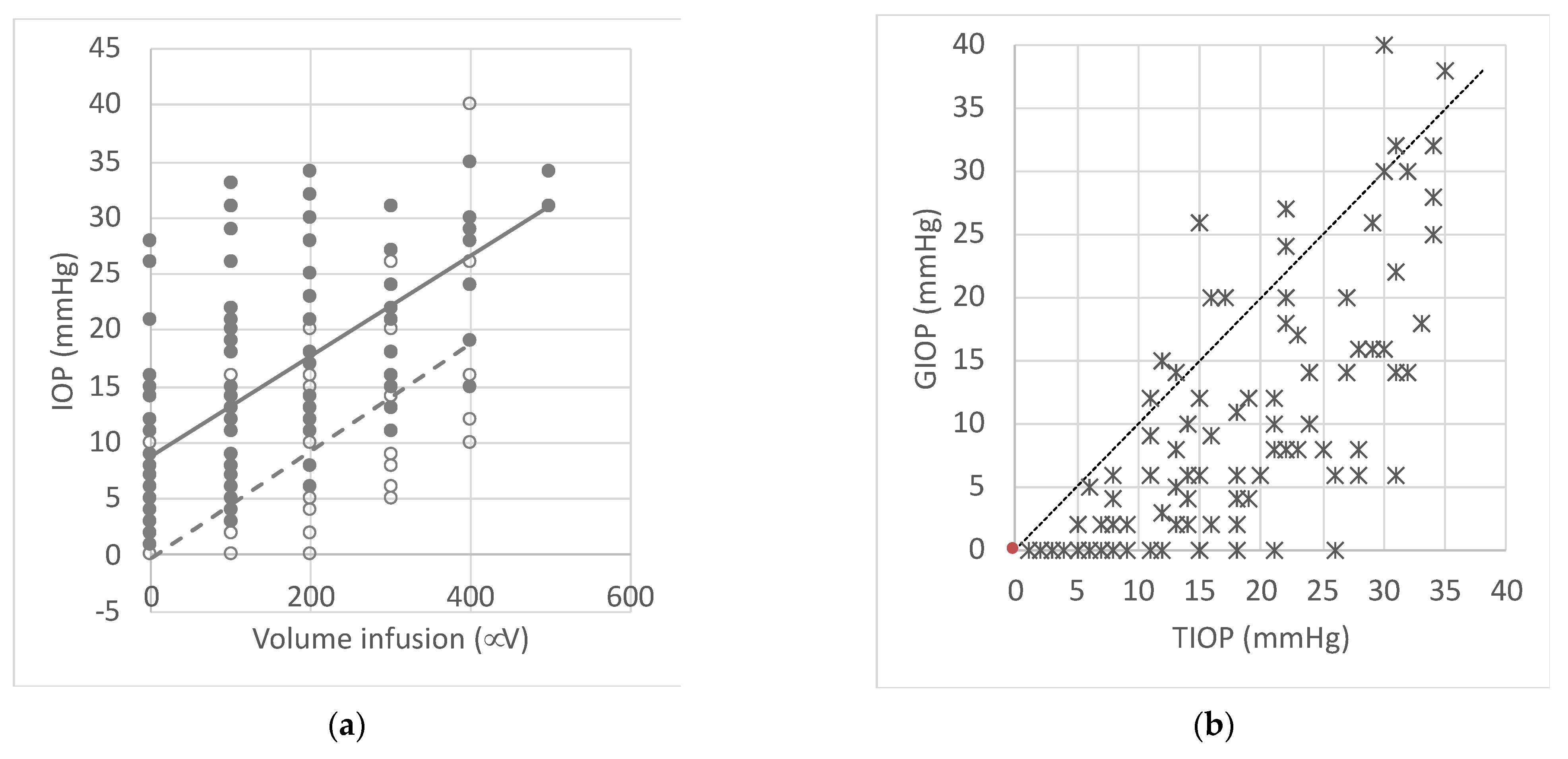
3.1. Tonometry Results
3.2. Inflation Test Results
3.3. Correlations
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.-J.; Mi, X.-S.; So, K.-F. Normal tension glaucoma: From the brain to the eye or the inverse? Neural Regen. Res. 2019, 14, 1845–1850. [Google Scholar] [CrossRef]
- Gupta, N.; Greenberg, G.; de Tilly, L.N.; Gray, B.; Polemidiotis, M.; Yücel, Y.H. Atrophy of the lateral geniculate nucleus in human glaucoma detected by magnetic resonance imaging. Br. J. Ophthalmol. 2009, 93, 56–60. [Google Scholar] [CrossRef]
- Gupta, N.; Ang, L.-C.; Noël de Tilly, L.; Bidaisee, L.; Yücel, Y.H. Human glaucoma and neural degeneration in intracranial optic nerve, lateral geniculate nucleus, and visual cortex. Br. J. Ophthalmol. 2006, 90, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; He, M.; Li, Z.; Huang, W. Epidemiological variations and trends in health burden of glaucoma worldwide. Acta Ophthalmol. 2019, 97, e349–e355. [Google Scholar] [CrossRef] [PubMed]
- Pascolini, D.; Mariotti, S.P. Global estimates of visual impairment: 2010. Br. J. Ophthalmol. 2012, 96, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Japan Glaucoma Society. The Japan Glaucoma Society Guidelines for Glaucoma (3rd Edition). Nihon Ganka Gakkai Zasshi 2012, 116, 3–46. [Google Scholar]
- The Collected Papers of Sir William Bowman, Bart., F.R.S. Nature 1893, 48, 26. [CrossRef]
- Kass, M.A.; Heuer, D.K.; Higginbotham, E.J.; Johnson, C.A.; Keltner, J.L.; Miller, J.P.; Parrish, R.K., II; Wilson, M.R.; Gordon, M.O.; Group for the O.H.T.S. The Ocular Hypertension Treatment Study: A Randomized Trial Determines That Topical Ocular Hypotensive Medication Delays or Prevents the Onset of Primary Open-Angle Glaucoma. Arch. Ophthalmol. 2002, 120, 701–713. [Google Scholar] [CrossRef]
- Leskea, M.C.; Heijl, A.; Hyman, L.; Bengtsson, B.; Komaroff, E. Factors for progression and glaucoma treatment: The Early Manifest Glaucoma Trial. Curr. Opin. Ophthalmol. 2004, 15, 102–106. [Google Scholar] [CrossRef]
- Gordon, M.O.; Beiser, J.A.; Brandt, J.D.; Heuer, D.K.; Higginbotham, E.J.; Johnson, C.A.; Keltner, J.L.; Miller, J.P.; Parrish, R.K., II; Wilson, M.R.; et al. The Ocular Hypertension Treatment Study: Baseline Factors That Predict the Onset of Primary Open-Angle Glaucoma. Arch. Ophthalmol. 2002, 120, 714–720. [Google Scholar] [CrossRef]
- Mihaylova, B.; Dimitrova, G. Evaluation of Retinal Nerve Fiber Layer and Inner Macular Layers in Primary Open-Angle Glaucoma with Spectral-Domain Optical Coherence Tomography. Opt. Nerve 2019. [Google Scholar] [CrossRef]
- Bussel, I.I.; Wollstein, G.; Schuman, J.S. OCT for glaucoma diagnosis, screening and detection of glaucoma progression. Br. J. Ophthalmol. 2014, 98 (Suppl. S2), ii15–ii19. [Google Scholar] [CrossRef]
- Michelessi, M.; Li, T.; Miele, A.; Azuara-Blanco, A.; Qureshi, R.; Virgili, G. Accuracy of optical coherence tomography for diagnosing glaucoma: An overview of systematic reviews. Br. J. Ophthalmol. 2021, 105, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Mallick, J.; Devi, L.; Malik, P.K.; Mallick, J. Update on Normal Tension Glaucoma. J. Ophthalmic Vis. Res. 2016, 11, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Killer, H.E.; Pircher, A. Normal tension glaucoma: Review of current understanding and mechanisms of the pathogenesis. Eye 2018, 32, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Messenio, D.; Marano, G.; Biganzoli, E. Electrophysiological evaluation in normal-tension glaucoma suspects: A pilot study. J. Model. Ophthalmol. 2016, 1, 9–30. [Google Scholar]
- Smedowski, A.; Weglarz, B.; Tarnawska, D.; Kaarniranta, K.; Wylegala, E. Comparison of Three Intraocular Pressure Measurement Methods Including Biomechanical Properties of the Cornea. Investig. Ophthalmol. Vis. Sci. 2014, 55, 666–673. [Google Scholar] [CrossRef]
- Hagishima, M.; Kamiya, K.; Fujimura, F.; Morita, T.; Shoji, N.; Shimizu, K. Effect of corneal astigmatism on intraocular pressure measurement using ocular response analyzer and Goldmann applanation tonometer. Graefe’s Arch. Clin. Exp. Ophthalmol. 2010, 248, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Yaoeda, K.; Fukushima, A.; Shirakashi, M.; Fukuchi, T. Comparison of intraocular pressure adjusted by central corneal thickness or corneal biomechanical properties as measured in glaucomatous eyes using noncontact tonometers and the Goldmann applanation tonometer. Clin. Ophthalmol. 2016, 10, 829–834. [Google Scholar] [CrossRef]
- Shim, J.; Kang, S.; Park, Y.; Kim, S.; Go, S.; Lee, E.; Seo, K. Comparative intraocular pressure measurements using three different rebound tonometers through in an ex vivo analysis and clinical trials in canine eyes. Vet. Ophthalmol. 2021, 24 (Suppl. S1), 186–193. [Google Scholar] [CrossRef]
- Elsmo, E.J.; Kiland, J.A.; Kaufman, P.L.; McLellan, G.J. Evaluation of rebound tonometry in non-human primates. Exp. Eye Res. 2011, 92, 268–273. [Google Scholar] [CrossRef][Green Version]
- McCafferty, S.; Levine, J.; Schwiegerling, J.; Enikov, E.T. Goldmann and error correcting tonometry prisms compared to intracameral pressure. BMC Ophthalmol. 2018, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Aziz, K.; Friedman, D.S. Tonometers—Which one should I use? Eye 2018, 32, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Tonnu, P.-A.; Ho, T.; Newson, T.; El Sheikh, A.; Sharma, K.; White, E.; Bunce, C.; Garway-Heath, D. The influence of central corneal thickness and age on intraocular pressure measured by pneumotonometry, non-contact tonometry, the Tono-Pen XL, and Goldmann applanation tonometry. Br. J. Ophthalmol. 2005, 89, 851–854. [Google Scholar] [CrossRef]
- Yilmaz, I.; Altan, C.; Aygit, E.D.; Alagoz, C.; Baz, O.; Ahmet, S.; Urvasizoglu, S.; Yasa, D.; Demirok, A. Comparison of three methods of tonometry in normal subjects: Goldmann applanation tonometer, non-contact airpuff tonometer, and Tono-Pen XL. Clin. Ophthalmol. 2014, 8, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Seol, B.R.; Kang, T.G.; Gu, B. Intraocular pressure according to different types of tonometry (non-contact and Goldmann applanation) in patients with different degrees of bilateral tearing. PLoS ONE 2019, 14, e0222652. [Google Scholar] [CrossRef]
- Kynigopoulos, M.; Schlote, T.; Kotecha, A.; Tzamalis, A.; Pajic, B.; Haefliger, I. Repeatability of intraocular pressure and corneal biomechanical properties measurements by the ocular response analyser. Klin. Monbl. Augenheilkd. 2008, 225, 357–360. [Google Scholar] [CrossRef]
- Takagi, D.; Sawada, A.; Yamamoto, T. Evaluation of a New Rebound Self-tonometer, Icare HOME: Comparison With Goldmann Applanation Tonometer. J. Glaucoma 2017, 26, 613–618. [Google Scholar] [CrossRef]
- Francis, B.A.; Hsieh, A.; Lai, M.-Y.; Chopra, V.; Pena, F.; Azen, S.; Varma, R. Effects of corneal thickness, corneal curvature, and intraocular pressure level on Goldmann applanation tonometry and dynamic contour tonometry. Ophthalmology 2007, 114, 20–26. [Google Scholar] [CrossRef]
- Bochmann, F.; Kaufmann, C.; Thiel, M.A. Dynamic contour tonometry versus Goldmann applanation tonometry: Challenging the gold standard. Expert Rev. Ophthalmol. 2010, 5, 743–749. [Google Scholar] [CrossRef][Green Version]
- Posarelli, C.; Ortenzio, P.; Ferreras, A.; Toro, M.D.; Passani, A.; Loiudice, P.; Oddone, F.; Casini, G.; Figus, M. Twenty-Four-Hour Contact Lens Sensor Monitoring of Aqueous Humor Dynamics in Surgically or Medically Treated Glaucoma Patients. J. Ophthalmol. 2019, 2019, 9890831. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Xu, J.; Wei, A.; Deng, S.X.; Cui, X.; Yu, X.; Sun, X. A new tonometer—the Corvis ST tonometer: Clinical comparison with noncontact and Goldmann applanation tonometers. Investig. Ophthalmol. Vis. Sci. 2013, 54, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Goldmann, H.; Schmidt, T. Über Applanationstonometrie. Ophthalmologica 1957, 134, 221–242. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.A.; Botello, A.P.; Elders, A.; Fathi Ali, A.; Azuara-Blanco, A.; Fraser, C.; McCormack, K.; Margaret Burr, J. Systematic Review of the Agreement of Tonometers with Goldmann Applanation Tonometry. Ophthalmology 2012, 119, 1552–1557. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Roberts, C.J. Influence of corneal biomechanical properties on intraocular pressure measurement: Quantitative analysis. J. Cataract Refract. Surg. 2005, 31, 146–155. [Google Scholar] [CrossRef]
- Kaushik, S.; Pandav, S.S.; Banger, A.; Aggarwal, K.; Gupta, A. Relationship between corneal biomechanical properties, central corneal thickness, and intraocular pressure across the spectrum of glaucoma. Am. J. Ophthalmol. 2012, 153, 840–849.e2. [Google Scholar] [CrossRef]
- Costin, B.R.; Fleming, G.P.; Weber, P.A.; Mahmoud, A.M.; Roberts, C.J. Corneal biomechanical properties affect Goldmann applanation tonometry in primary open-angle glaucoma. J. Glaucoma 2014, 23, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Andreanos, K.; Koutsandrea, C.; Papaconstantinou, D.; Diagourtas, A.; Kotoulas, A.; Dimitrakas, P.; Moschos, M.M. Comparison of Goldmann applanation tonometry and Pascal dynamic contour tonometry in relation to central corneal thickness and corneal curvature. Clin. Ophthalmol. 2016, 10, 2477–2484. [Google Scholar] [CrossRef] [PubMed]
- Tejwani, S.; Dinakaran, S.; Joshi, A.; Shetty, R.; Sinha Roy, A. A cross-sectional study to compare intraocular pressure measurement by sequential use of Goldman applanation tonometry, dynamic contour tonometry, ocular response analyzer, and Corvis ST. Indian J. Ophthalmol. 2015, 63, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Oncel, B.; Dinc, U.A.; Orge, F.; Yalvaç, B.I. Comparison of IOP measurement by ocular response analyzer, dynamic contour, Goldmann applanation, and noncontact tonometry. Eur. J. Ophthalmol. 2009, 19, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, L.; Xu, J.; Chen, X.; Gu, Y.; Ren, Y.; Wang, K. Comparability of three intraocular pressure measurement: iCare pro rebound, non-contact and Goldmann applanation tonometry in different IOP group. BMC Ophthalmol. 2019, 19, 225. [Google Scholar] [CrossRef] [PubMed]
- Feltgen, N.; Leifert, D.; Funk, J. Correlation between central corneal thickness, applanation tonometry, and direct intracameral IOP readings. Br. J. Ophthalmol. 2001, 85, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Spedding, C.; Bhojwani, R.; Kwartz, J.; Henson, D.; McLeod, D. Assessment of the diurnal variation in central corneal thickness and intraocular pressure for patients with suspected glaucoma. Ophthalmology 2000, 107, 1191–1193. [Google Scholar] [CrossRef]
- Ehlers, N.; Bramsen, T.; Sperling, S. Applanation Tonometry and Central Corneal Thickness. Acta Ophthalmol. 1975, 53, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Orssengo, G.J.; Pye, D.C. Determination of the True Intraocular Pressure and Modulus of Elasticity of the Human Cornea in vivo. Bull. Math. Biol. 1999, 61, 551–572. [Google Scholar] [CrossRef]
- Gunvant, P.; Baskaran, M.; Vijaya, L.; Joseph, I.S.; Watkins, R.J.; Nallapothula, M.; Broadway, D.C.; O’Leary, D.J. Effect of corneal parameters on measurements using the pulsatile ocular blood flow tonograph and Goldmann applanation tonometer. Br. J. Ophthalmol. 2004, 88, 518–522. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Brandt, J.D.; Garway-Heath, D.; Medeiros, F. Intraocular Pressure; Kugler Publications: Amsterdam, The Netherlands, 2007; Volume 4, ISBN 9789062992133. [Google Scholar]
- Mark, H.H. Corneal curvature in applanation tonometry. Am. J. Ophthalmol. 1973, 76, 223–224. [Google Scholar] [CrossRef]
- Nemesure, B.; Wu, S.-Y.; Hennis, A.; Leske, M.C.; Group for the B.E.S. Corneal Thickness and Intraocular Pressure in the Barbados Eye Studies. Arch. Ophthalmol. 2003, 121, 240–244. [Google Scholar] [CrossRef]
- Mansoori, T.; Balakrishna, N. Effect of central corneal thickness on intraocular pressure and comparison of Topcon CT-80 non-contact tonometry with Goldmann applanation tonometry. Clin. Exp. Optom. 2018, 101, 206–212. [Google Scholar] [CrossRef]
- Vinciguerra, R.; Rehman, S.; Vallabh, N.A.; Batterbury, M.; Czanner, G.; Choudhary, A.; Cheeseman, R.; Elsheikh, A.; Willoughby, C.E. Corneal biomechanics and biomechanically corrected intraocular pressure in primary open-angle glaucoma, ocular hypertension and controls. Br. J. Ophthalmol. 2020, 104, 121–126. [Google Scholar] [CrossRef]
- Langham, M.E.; Eisenlohr, J.E. A manometric study of the rate of fall of the intraocular pressure in the living and dead eyes of human subjects. Investig. Ophthalmol. 1963, 2, 72–82. [Google Scholar]
- Boschetti, F.; Triacca, V.; Spinelli, L.; Pandolfi, A. Mechanical Characterization of Porcine Corneas. J. Biomech. Eng. 2012, 134, 031003. [Google Scholar] [CrossRef] [PubMed]
- Pinsky, P.M.; van der Heide, D.; Chernyak, D. Computational modeling of mechanical anisotropy in the cornea and sclera. J. Cataract Refract. Surg. 2005, 31, 136–145. [Google Scholar] [CrossRef]
- Elsheikh, A.; Alhasso, D.; Rama, P. Assessment of the epithelium’s contribution to corneal biomechanics. Exp. Eye Res. 2008, 86, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Martin Bland, J.; Altman, D.G. Statistical Methods for Assessing Agreement Between Two Methods of Clinical Measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Giavarina, D. Understanding Bland Altman analysis. Biochem. Med. 2015, 25, 141–151. [Google Scholar] [CrossRef]
- Brunette, I.; Rosolen, S.G.; Carrier, M.; Abderrahman, M.; Nada, O.; Germain, L.; Proulx, S. Comparison of the pig and feline models for full thickness corneal transplantation. Vet. Ophthalmol. 2011, 14, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Olsen, T.W.; Sanderson, S.; Feng, X.; Hubbard, W.C. Porcine Sclera: Thickness and Surface Area. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2529–2532. [Google Scholar]
- Subasinghe, S.K.; Ogbuehi, K.C.; Mitchell, L.; Dias, G.J. Animal model with structural similarity to human corneal collagen fibrillar arrangement. Anat. Sci. Int. 2021, 96, 286–293. [Google Scholar] [CrossRef]
- Fernandez-Bueno, I.; Pastor, J.C.; Gayoso, M.J.; Alcalde, I.; Garcia, M.T. Müller and macrophage-like cell interactions in an organotypic culture of porcine neuroretina. Mol. Vis. 2008, 14, 2148–2156. [Google Scholar]
- Ruiz-Ederra, J.; García, M.; Hernández, M.; Urcola, H.; Hernández-Barbáchano, E.; Araiz, J.; Vecino, E. The pig eye as a novel model of glaucoma. Exp. Eye Res. 2005, 81, 561–569. [Google Scholar] [CrossRef]
- Blanca, M.J.; Alarcón, R.; Arnau, J.; Bono, R.; Bendayan, R. Non-normal data: Is ANOVA still a valid option? Psicothema 2017, 29, 552–557. [Google Scholar] [CrossRef]
- Bryant, M.R.; McDonnell, P.J. Constitutive laws for biomechanical modeling of refractive surgery. J. Biomech. Eng. 1996, 118, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Elsheikh, A.; Wang, D.; Pye, D. Determination of the modulus of elasticity of the human cornea. J. Refract. Surg. 2007, 23, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Susanna, B.N.; Ogata, N.G.; Jammal, A.A.; Susanna, C.N.; Berchuck, S.I.; Medeiros, F.A. Corneal Biomechanics and Visual Field Progression in Eyes with Seemingly Well-Controlled Intraocular Pressure. Ophthalmology 2019, 126, 1640–1646. [Google Scholar] [CrossRef]
- Sit, A.J.; Kazemi, A.; Zhou, B.; Zhang, X. Comparison of Ocular Biomechanical Properties in Normal and Glaucomatous Eyes Using Ultrasound Surface Wave Elastography. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1218. [Google Scholar] [CrossRef]
- Doughty, M.J.; Laiquzzaman, M.; Müller, A.; Oblak, E.; Button, N.F. Central corneal thickness in European (white) individuals, especially children and the elderly, and assessment of its possible importance in clinical measures of intra-ocular pressure. Ophthalmic Physiol. Opt. 2002, 22, 491–504. [Google Scholar] [CrossRef]
- Hayashi, S.; Osawa, T.; Tohyama, K. Comparative observations on corneas, with special reference to Bowman’s layer and Descemet’s membrane in mammals and amphibians. J. Morphol. 2002, 254, 247–258. [Google Scholar] [CrossRef]
- Doughty, M.J.; Zaman, M.L. Human corneal thickness and its impact on intraocular pressure measures: A review and meta-analysis approach. Surv. Ophthalmol. 2000, 44, 367–408. [Google Scholar] [CrossRef]
- Pandolfi, A.; Holzapfel, G.A. Three-Dimensional Modeling and Computational Analysis of the Human Cornea Considering Distributed Collagen Fibril Orientations. J. Biomech. Eng. 2008, 130, 061006. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, A.; Boschetti, F. The influence of the geometry of the porcine cornea on the biomechanical response of inflation tests. Comput. Methods Biomech. Biomed. Engin. 2015, 18, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Sergienko, N.M.; Shargorogska, I. The scleral rigidity of eyes with different refractions. Graefe’s Arch. Clin. Exp. Ophthalmol. 2012, 250, 1009–1012. [Google Scholar] [CrossRef] [PubMed]
- Friedman, E.; Ivry, M.; Ebert, E.; Glynn, R.; Gragoudas, E.; Seddon, J. Increased scleral rigidity and age-related macular degeneration. Ophthalmology 1989, 96, 104–108. [Google Scholar] [CrossRef]
- Graebel, W.P.; van Alphen, G.W.H.M. The Elasticity of Sclera and Choroid of the Human Eye, and Its Implications on Scleral Rigidity and Accommodation. J. Biomech. Eng. 1977, 99, 203–208. [Google Scholar] [CrossRef]

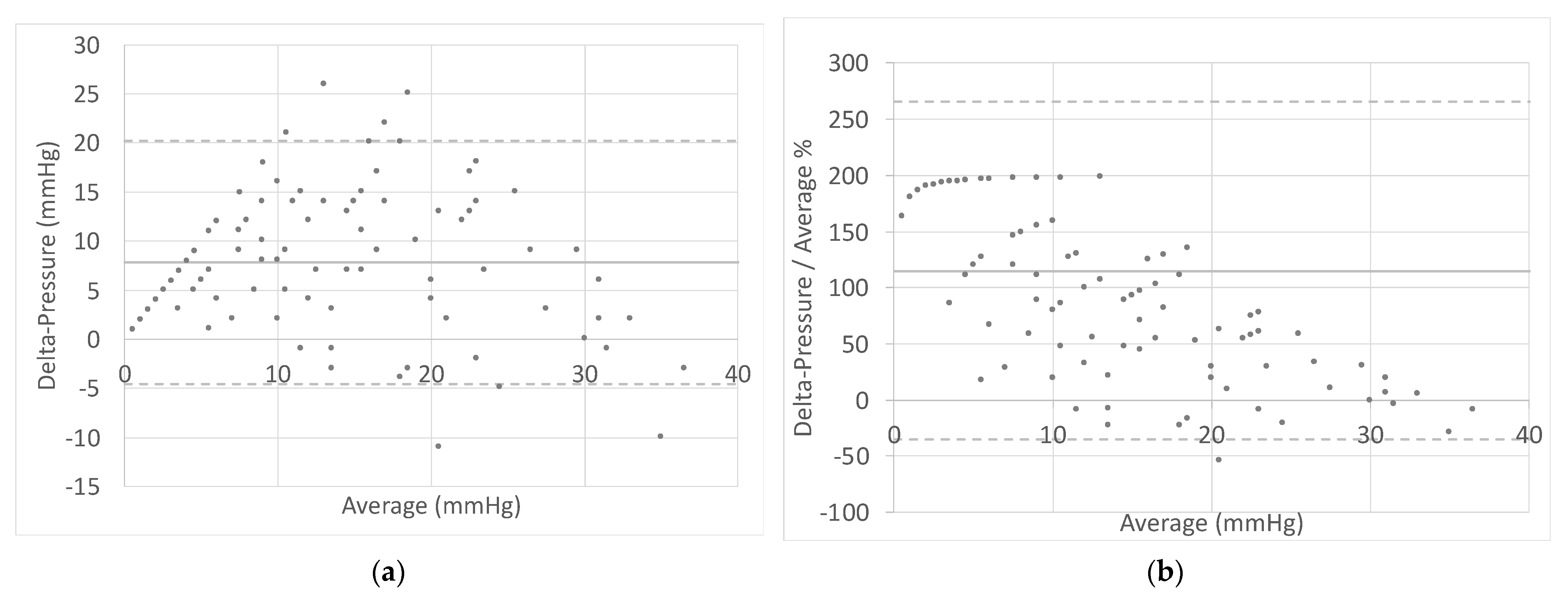
| Delta-Pressure (mmHg) | Normal Distribution | N |
|---|---|---|
| 0–7 | No | 32 |
| 8–14 | No | 37 |
| 15–21 | No | 22 |
| 22–28 | Yes | 18 |
| 29–35 | Yes | 17 |
| Delta-Pressure (mmHg) | Significance |
|---|---|
| (0–7) vs. (8–14) | p < 0.05 1 |
| (8–14) vs. (15–21) | p < 0.05 1 |
| (15–21) vs. (22–28) | p < 0.05 1 |
| (22–28) vs. (29–35) | n.s. 2 |
| Geometrical Parameter | Value (mm) ± SD |
|---|---|
| R | 8.49 ± 0.49 |
| D | 16.98 ± 0.98 |
| CCT | 1.41 ± 0.42 |
| H0 | 3.53 ± 0.65 |
| P (mmHg) | w (mm) ± SD |
|---|---|
| 3.68 | 0.26 ± 0.25 |
| 7.36 | 0.35 ± 0.29 |
| 11.03 | 0.40 ± 0.32 |
| 14.71 | 0.43 ± 0.32 |
| 18.39 | 0.47 ± 0.33 |
| 22.07 | 0.50 ± 0.34 |
| 25.74 | 0.53 ± 0.34 |
| 29.42 | 0.55 ± 0.31 |
| IOP Interval (mmHg) | Pearson Correlation |
|---|---|
| 1–35 | −0.10 |
| 1–12 | 0.09 |
| 13–21 | −0.41 |
| 22–35 | −0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messenio, D.; Ferroni, M.; Boschetti, F. Goldmann Tonometry and Corneal Biomechanics. Appl. Sci. 2021, 11, 4025. https://doi.org/10.3390/app11094025
Messenio D, Ferroni M, Boschetti F. Goldmann Tonometry and Corneal Biomechanics. Applied Sciences. 2021; 11(9):4025. https://doi.org/10.3390/app11094025
Chicago/Turabian StyleMessenio, Dario, Marco Ferroni, and Federica Boschetti. 2021. "Goldmann Tonometry and Corneal Biomechanics" Applied Sciences 11, no. 9: 4025. https://doi.org/10.3390/app11094025
APA StyleMessenio, D., Ferroni, M., & Boschetti, F. (2021). Goldmann Tonometry and Corneal Biomechanics. Applied Sciences, 11(9), 4025. https://doi.org/10.3390/app11094025






