Study of Atomic Hydrogen Concentration in Grain Boundaries of Polycrystalline Diamond Thin Films
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Characterization of Morphology and Chemical States of Polycrystalline Diamond Films
3.1.1. SEM Analysis
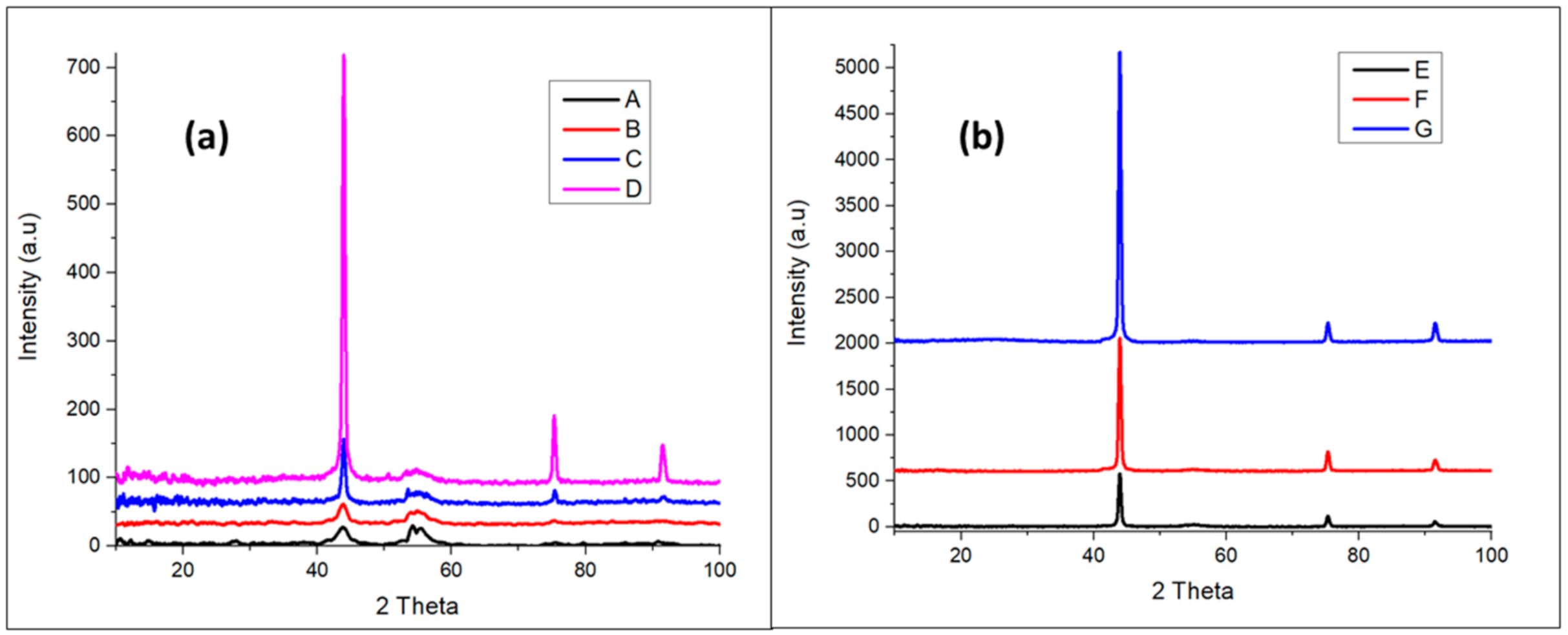
3.1.2. X-ray Diffraction (XRD) Analysis
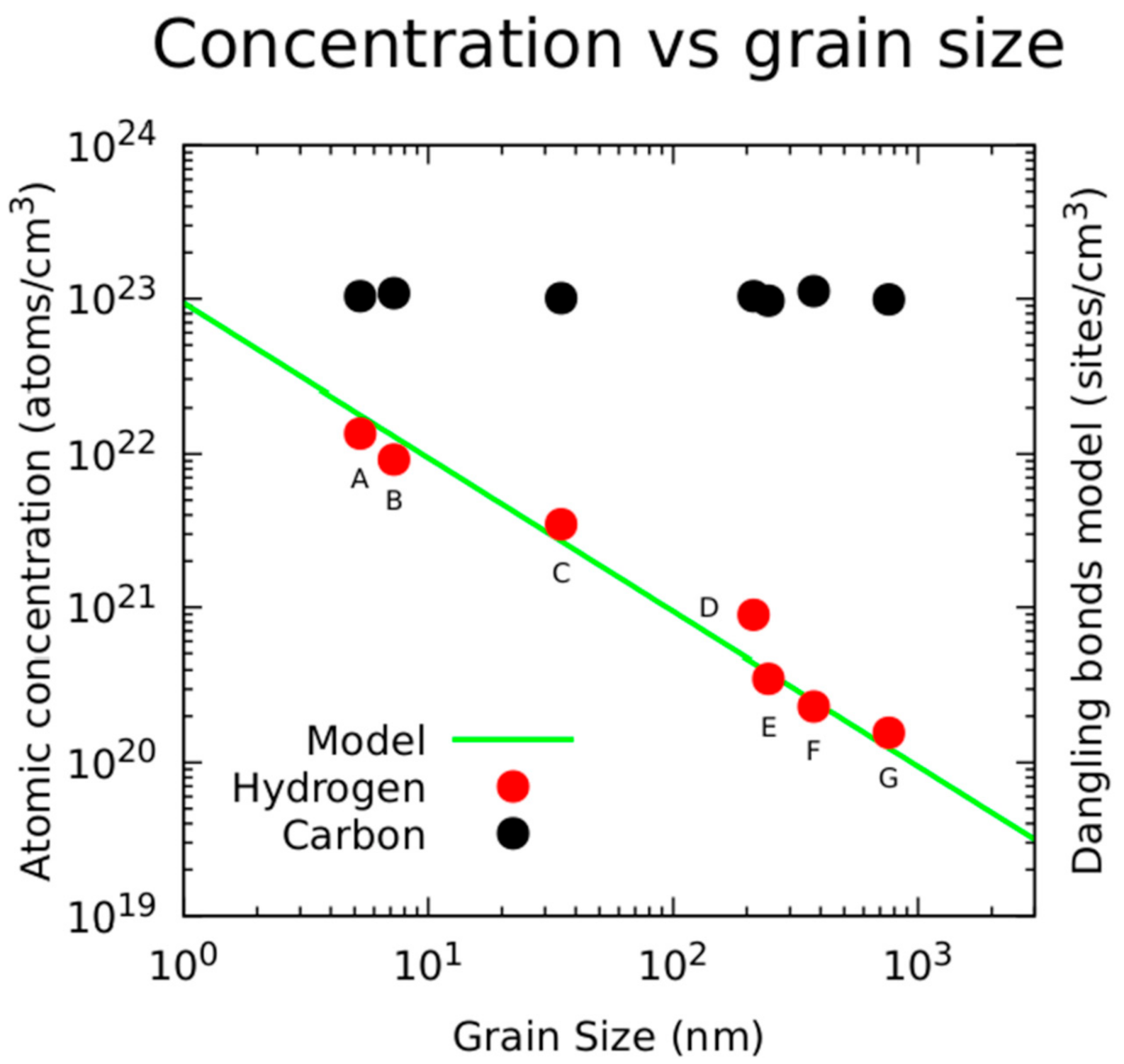
3.1.3. Secondary Ion Mass Spectrometry (SIMS) Analysis
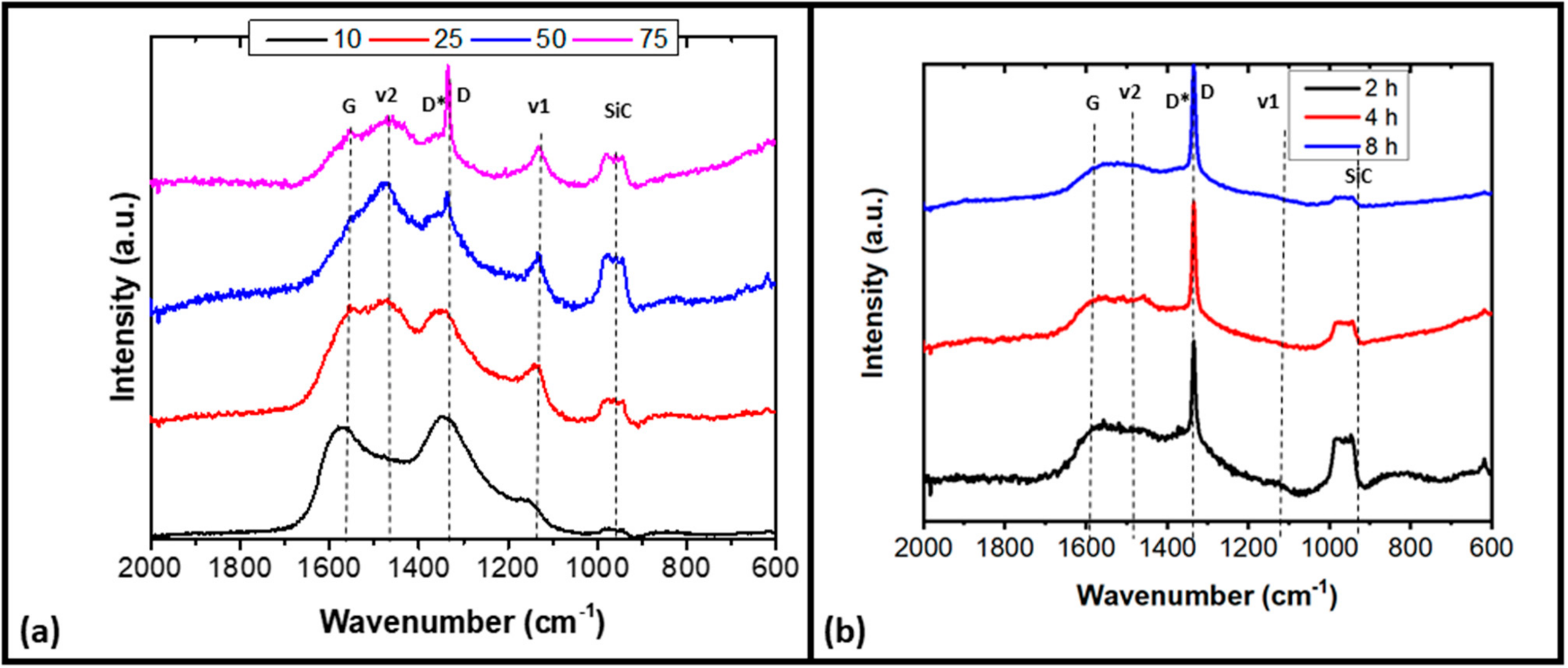
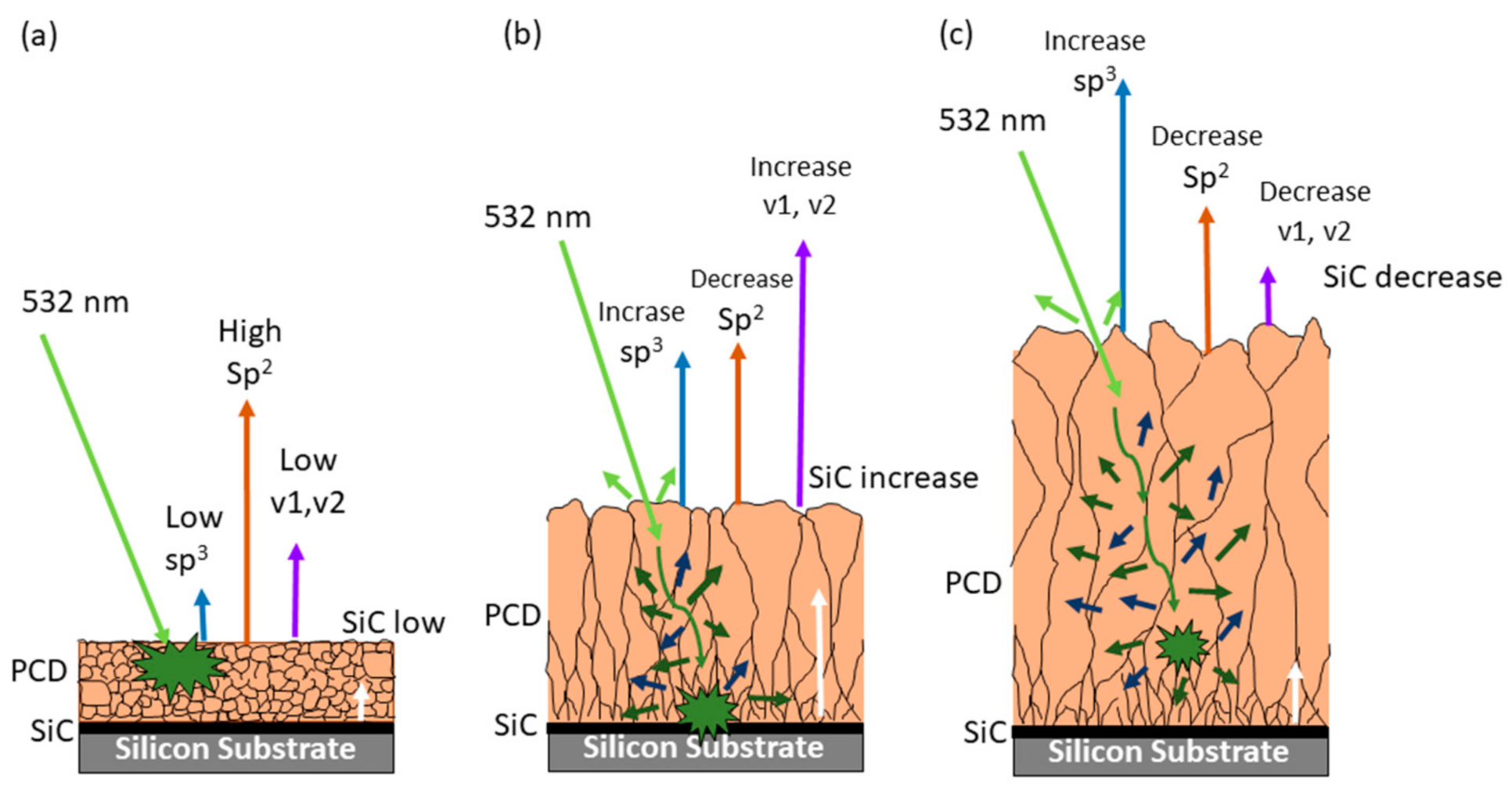
3.1.4. Raman Analysis
3.2. Atomic Force Microscopy-Based Characterization of Surface Structure and Associated Electrical Performance of PCD Films
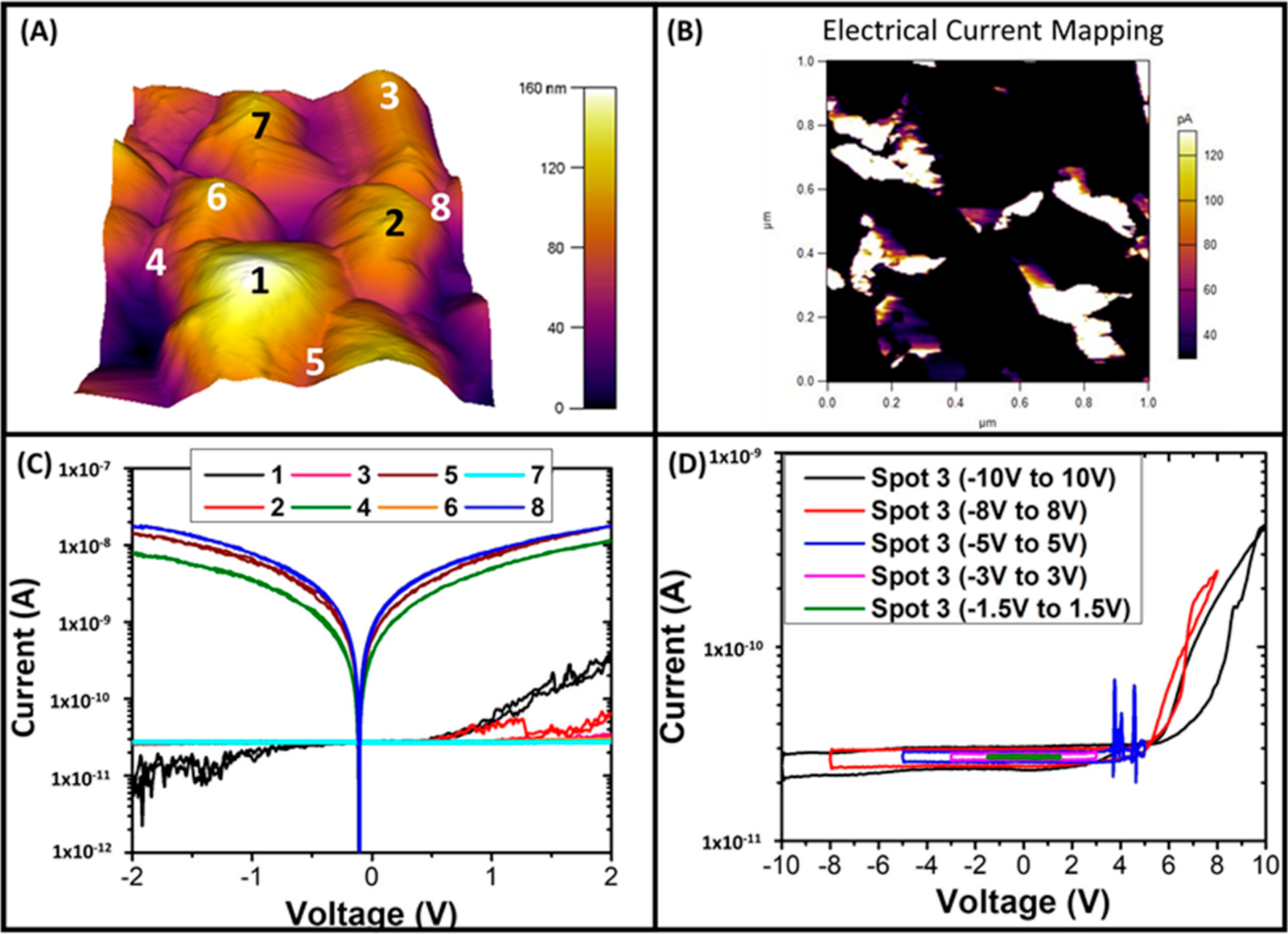
3.2.1. Conductive Atomic Force Microscopy (CAFM) Measurements of Electrical Conduction through PCD Films
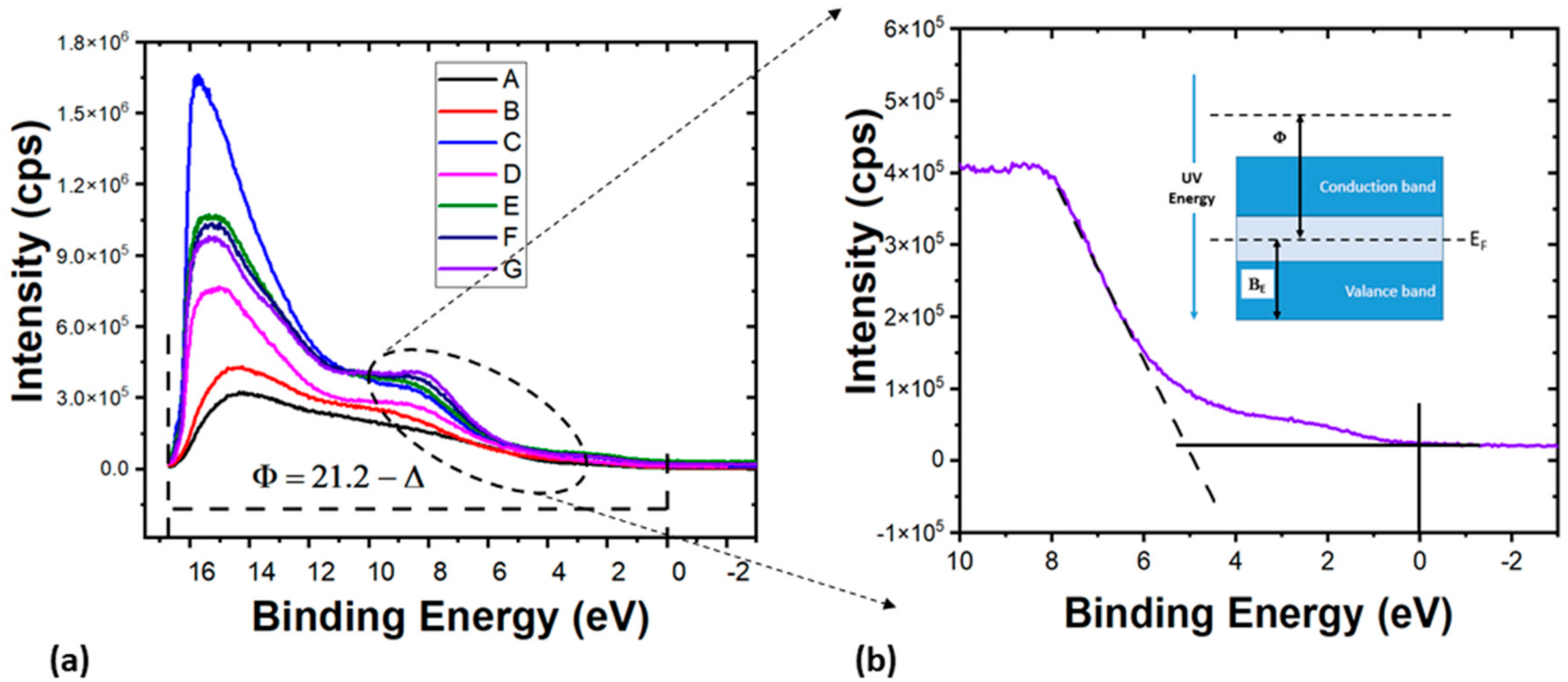
3.2.2. UPS Analysis of PCD Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Auciello, O.; Sumant, A.V. Status review of the science and technology of ultrananocrystalline diamond (UNCDTM) films and application to multifunctional devices. Diam. Relat. Mater. 2010, 19, 699–718. [Google Scholar] [CrossRef]
- Williams, O.A. Nanocrystalline diamond. Diam. Relat. Mater. 2011, 20, 621–640. [Google Scholar] [CrossRef]
- Banaszak-Piechowska, A.; Malinowski, P. Raman studies of amorphous carbon phase in diamond films grown by hot-filament CVD. Solid State Phenom. 2015, 237, 263–267. [Google Scholar] [CrossRef]
- Kulisch, W.; Petkov, C.; Petkov, E.; Popov, C.; Gibson, P.N.; Veres, M.; Merz, R.; Merz, B.; Reithmaier, J.P. Low temperature growth of nanocrystalline and ultrananocrystalline diamond films: A comparison. Phys. Status Solidi Appl. Mater. Sci. 2012, 209, 1664–1674. [Google Scholar] [CrossRef]
- Fuentes-Fernandez, E.M.; Alcantar-Peña, J.J.; Lee, G.; Boulom, A.; Phan, H.; Smith, B.; Nguyen, T.; Sahoo, S.; Ruiz-Zepeda, F.; Arellano-Jimenez, M.J.; et al. Synthesis and characterization of microcrystalline diamond to ultrananocrystalline diamond fi lms via hot filament chemical vapor deposition for scaling to large area applications. Thin Solid Films 2016, 603, 62–68. [Google Scholar] [CrossRef]
- Liu, J.; Hei, L.F.; Chen, G.C.; Li, C.M.; Song, J.H.; Lu, F.X. Mechanical properties of ultrananocrystalline diamond films modified by hydrogen concentration in deposition atmosphere. Surf. Coat. Technol. 2013, 236, 8–12. [Google Scholar] [CrossRef]
- Sankaran, K.J.; Joseph, P.T.; Chen, H.C.; Tai, N.H.; Lin, I.N. Investigation in the role of hydrogen on the properties of diamond films grown using Ar/H2/CH4 microwave plasma. Diam. Relat. Mater. 2011, 20, 232–237. [Google Scholar] [CrossRef]
- Lin, I.N.; Chen, H.C.; Wang, C.S.; Lee, Y.R.; Lee, C.Y. Nanocrystalline diamond microstructures from Ar/H2/CH4-plasma chemical vapour deposition. CrystEngComm 2011, 13, 6082–6089. [Google Scholar] [CrossRef]
- Zhou, D.; Gruen, D.M.; Qin, L.C.; McCauley, T.G.; Krauss, A.R. Control of diamond film microstructure by Ar additions to CH4/H2 microwave plasmas. J. Appl. Phys. 1998, 84, 1981–1989. [Google Scholar] [CrossRef]
- Gan, L.; Baskin, E.; Saguy, C.; Kalish, R. Quantization of 2D hole gas in conductive hydrogenated diamond surfaces observed by electron field emission. Phys. Rev. Lett. 2006, 96, 2–5. [Google Scholar] [CrossRef]
- Auciello, O.; Aslam, D.M. Review on advances in microcrystalline, nanocrystalline and ultrananocrystalline diamond films-based micro/nano-electromechanical systems technologies. J. Mater. Sci. 2020. [Google Scholar] [CrossRef]
- Kulisch, W.; Popov, C.; Sasaki, T.; Sirghi, L.; Rauscher, H.; Rossi, F.; Reithmaier, J.P. On the development of the morphology of ultrananocrystalline diamond films. Phys. Stat. Solidi Appl. Mater. Sci. 2011, 208, 70–80. [Google Scholar] [CrossRef]
- Michaelson, S.; Lifshitz, Y.; Ternyak, O.; Akhvlediani, R.; Hoffman, A. Hydrogen incorporation in diamond films. Diam. Relat. Mater. 2007, 16, 845–850. [Google Scholar] [CrossRef]
- Dawedeit, C.; Kucheyev, S.O.; Shin, S.J.; Willey, T.M.; Bagge-Hansen, M.; Braun, T.; Wang, Y.M.; El-Dasher, B.S.; Teslich, N.E.; Biener, M.M.; et al. Grain size dependent physical and chemical properties of thick CVD diamond films for high energy density physics experiments. Diam. Relat. Mater. 2013, 40, 75–81. [Google Scholar] [CrossRef]
- Kulisch, W.; Sasaki, T.; Rossi, F.; Popov, C.; Sippel, C.; Grambole, D. Hydrogen incorporation in ultrananocrystalline diamond/amorphous carbon films. Phys. Stat. Solidi Rapid Res. Lett. 2008, 2, 77–79. [Google Scholar] [CrossRef]
- Rakha, S.A.; Guojun, Y.; Jianqing, C. Correlation between diamond grain size and hydrogen incorporation in nanocrystalline diamond thin films. J. Exp. Nanosci. 2012, 7, 378–389. [Google Scholar] [CrossRef]
- Alcantar-Peña, J.J.; de Obaldia, E.; Montes-Gutierrez, J.; Kang, K.; Arellano-Jimenez, M.J.; Aguilar, J.E.; Suchy, G.P.; Berman-Mendoza, D.; Garcia, R.; Yacaman, M.J.; et al. Fundamentals towards large area synthesis of multifunctional Ultrananocrystalline Diamond fi LMS via large area hot fi lament chemical vapor deposition bias enhanced nucleation/bias enhanced growth for fabrication of broad range of multifunctional devices. Diam. Relat. Mater. 2017, 78, 1–11. [Google Scholar] [CrossRef]
- Alcantar-Peña, J.J.; Montes, J.; Arellano-Jimenez, M.J.; Aguilar, J.O.; Berman-Mendoza, D.; García, R.; Yacaman, M.J.; Auciello, O. Low temperature hot filament chemical vapor deposition of Ultrananocrystalline Diamond films with tunable sheet resistance for electronic power devices. Diam. Relat. Mater. 2016, 69, 207–213. [Google Scholar] [CrossRef]
- Alcantar-Peña, J.J.; de Obaldia, E.; Tirado, P.; Arellano-Jimenez, M.J.; Aguilar, J.E.; Veyan, J.F.; Yacaman, M.J.; Koudriavtsez, Y.; Auciello, O. Polycrystalline diamond films with tailored micro/nanostructure/doping for new large area film-based diamond electronics. Diam. Relat. Mater. 2019, 91, 261–271. [Google Scholar] [CrossRef]
- Pfeiffer, R.; Kuzmany, H.; Salk, N.; Günther, B. Evidence for trans-polyacetylene in nanocrystalline diamond films from H-D isotropic substitution experiments. Appl. Phys. Lett. 2003, 82, 4149–4150. [Google Scholar] [CrossRef]
- Montaño-Figueroa, A.G.; Alcantar-Peña, J.J.; Tirado, P.; Abraham, A.; de Obaldia, E.; Auciello, O. Tailoring of polycrystalline diamond surfaces from hydrophilic to superhydrophobic via synergistic chemical plus micro-structuring processes. Carbon N. Y. 2018, 139, 361–368. [Google Scholar] [CrossRef]
- Michaelson, S.; Ternyak, O.; Akhvlediani, R.; Williams, O.A.; Gruen, D.; Hoffman, A. Hydrogen concentration and bonding in nano-diamond films of varying grain sizes grown by different chemical vapor deposition methods. Phys. Status Solidi Appl. Mater. Sci. 2007, 204, 2860–2867. [Google Scholar] [CrossRef]
- Sasinková, V.; Huran, J.; Kleinová, A.; Boháček, P.; Arbet, J.; Sekáčova, M. Raman spectroscopy study of SiC thin films prepared by PECVD for solar cell working in hard environment. Reliab. Photovolt. Cells Modul. Compon. Syst. VIII 2015, 9563, 95630V. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Origin of the 1150 cm−1 Raman mode in nanocrystalline diamond. Phys. Rev. B Condens. Matter Mater. Phys. 2001, 63, 2–5. [Google Scholar] [CrossRef]
- Kulisch, W.; Popov, C.; Rauscher, H.; Rinke, M.; Veres, M. Investigation of the initial growth of ultrananocrystalline diamond films by multiwavelength Raman spectroscopy. Diam. Relat. Mater. 2011, 20, 1076–1080. [Google Scholar] [CrossRef]
- Villarreal, D.; Wittel, F.P.; Rajan, A.; Wittel, P.; Alcantar-Peña, J.; Auciello, O.; de Obaldia, E. Effect of nitrogen flow on the growth of nitrogen ultrananocrystalline diamond (N-UNCD) films on Si/SiO2/HfO2 substrate. In Proceedings of the 7th International Engineering Sciences and Technology Conference IESTEC 2019, Panama City, Panama, 9–11 October 2019; pp. 78–84. [Google Scholar] [CrossRef]
- Mackie, W.A.; Bell, A.E. Work function measurements of diamond film surfaces. In Proceedings of the IEEE International Vacuum Microelectronics Conference IVMC, Newport, RI, USA, 7–11 July 2003; pp. 350–354. [Google Scholar] [CrossRef]
- Michaelson, H.B. The work function of the elements and its periodicity. J. Appl. Phys. 1977, 48, 4729–4733. [Google Scholar] [CrossRef]
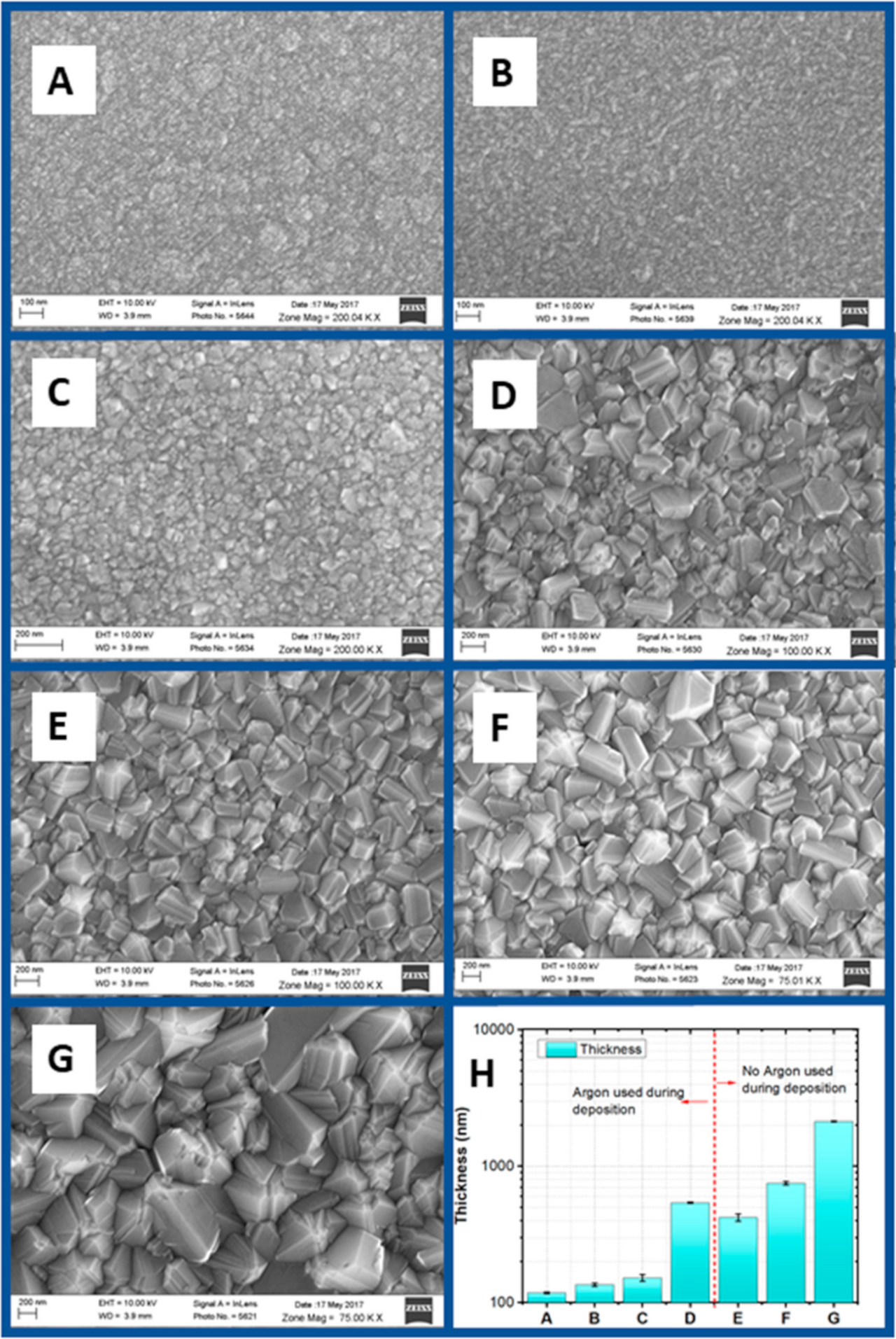


| Sample Name | H2-CH4-Ar (sccm) | Deposition Time (h) | Filament-Substrate Distance (mm) | Temperature (°C) |
|---|---|---|---|---|
| A | 10-2-90 | 2 | 20 | 550 |
| B | 25-2-75 | 2 | 20 | 575 |
| C | 50-2-50 | 2 | 20 | 600 |
| D | 75-2-25 | 2 | 20 | 675 |
| E | 200-3-0 | 2 | 10 | 725 |
| F | 200-3-0 | 4 | 10 | 725 |
| G | 200-3-0 | 8 | 10 | 725 |
| Miller Indices (hkl) | A | B | C | D | E | F | G | SCD | |
|---|---|---|---|---|---|---|---|---|---|
| (111) | 2 θ | 43.75 | 43.9 | 43.94 | 43.95 | 43.94 | 43.95 | 43.95 | 43.93 |
| d (Å) | 2.07 | 2.06 | 2.06 | 2.06 | 2.06 | 2.06 | 2.06 | 2.06 | |
| (220) | 2 θ | 75.5 | 75.33 | 75.37 | 75.32 | 75.33 | 75.31 | 75.34 | 75.3 |
| d (Å) | 1.26 | 1.26 | 1.26 | 1.26 | 1.26 | 1.26 | 1.26 | 1.26 | |
| (311) | 2 θ | 90.75 | 91.41 | 91.71 | 91.51 | 91.48 | 91.5 | 91.49 | 91.5 |
| d (Å) | 1.08 | 1.08 | 1.08 | 1.08 | 1.08 | 1.08 | 1.08 | 1.08 |
| Samples | Fit Parameters | v1 1150 cm−1 | Diamond 1332 cm−1 | D* 1350 cm−1 | v2 1480 cm−1 | G 1580 cm−1 | (v1 + v2) % | (D* + G) % |
|---|---|---|---|---|---|---|---|---|
| A | Center | 1161 | 1345 | 1487 | 1572 | 14% | 86% | |
| FWHM | 80 | 160 | 100 | 110 | ||||
| B | Center | 1147 | 1343 | 1474 | 1559 | 40% | 60% | |
| FWHM | 90 | 110 | 120 | 120 | ||||
| C | Center | 1136 | 1333 | 1358 | 1473 | 1537 | 38% | 60% |
| FWHM | 50 | 10 | 130 | 100 | 114 | |||
| D | Center | 1134 | 1334 | 1358 | 1466 | 1553 | 56% | 36% |
| FWHM | 40 | 12 | 80 | 120 | 80 | |||
| E | Center | 1263 | 1334 | 1374 | 1467 | 1566 | 40% | 48% |
| FWHM | 44 | 15 | 69 | 120 | 100 | |||
| F | Center | 1270 | 1332 | 1376 | 1460 | 1562 | 31% | 61% |
| FWHM | 54 | 8 | 80 | 120 | 160 | |||
| G | Center | 1285 | 1332 | 1367 | 1460 | 1555 | 30% | 62% |
| FWHM | 120 | 8 | 80 | 120 | 160 |
| Sample | BE (eV) | E cut-off (eV) | Φ (eV) |
|---|---|---|---|
| A | 3.3 | 16.6 | 4.6 |
| B | 4.3 | 16.7 | 4.5 |
| C | 4.9 | 16.6 | 4.6 |
| D | 4.6 | 15.8 | 5.5 |
| E | 5.0 | 16.1 | 5.2 |
| F | 5.2 | 16.4 | 4.8 |
| G | 5.1 | 15.9 | 5.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Obaldía, E.I.; Alcantar-Peña, J.J.; Wittel, F.P.; Veyan, J.F.; Gallardo-Hernadez, S.; Koudriavtsev, Y.; Berman-Mendoza, D.; Auciello, O. Study of Atomic Hydrogen Concentration in Grain Boundaries of Polycrystalline Diamond Thin Films. Appl. Sci. 2021, 11, 3990. https://doi.org/10.3390/app11093990
de Obaldía EI, Alcantar-Peña JJ, Wittel FP, Veyan JF, Gallardo-Hernadez S, Koudriavtsev Y, Berman-Mendoza D, Auciello O. Study of Atomic Hydrogen Concentration in Grain Boundaries of Polycrystalline Diamond Thin Films. Applied Sciences. 2021; 11(9):3990. https://doi.org/10.3390/app11093990
Chicago/Turabian Stylede Obaldía, Elida I., Jesus J. Alcantar-Peña, Frederick P. Wittel, Jean François Veyan, Salvador Gallardo-Hernadez, Yury Koudriavtsev, Dainet Berman-Mendoza, and Orlando Auciello. 2021. "Study of Atomic Hydrogen Concentration in Grain Boundaries of Polycrystalline Diamond Thin Films" Applied Sciences 11, no. 9: 3990. https://doi.org/10.3390/app11093990
APA Stylede Obaldía, E. I., Alcantar-Peña, J. J., Wittel, F. P., Veyan, J. F., Gallardo-Hernadez, S., Koudriavtsev, Y., Berman-Mendoza, D., & Auciello, O. (2021). Study of Atomic Hydrogen Concentration in Grain Boundaries of Polycrystalline Diamond Thin Films. Applied Sciences, 11(9), 3990. https://doi.org/10.3390/app11093990










