Abstract
Cryptosporidium spp., Enterocytozoon bieneusi, and Giardia duodenalis are zoonotic pathogens commonly found in the intestinal tract of mammalian hosts including livestock and humans. The prevalence of these eukaryote microorganisms in domestic animals and their interaction with intestinal microbiota are not yet fully recognized. We analyzed the intestinal microbiota composition with metagenomics and functional characterization with Cluster of Orthologous (COG) in Bactrian camels, which were raised on Qinghai-Tibet Plateau, Northwest China. Thus, fecal samples were collected from the animals to determine the parasite infection and the profile of microbiota. Analysis of intestinal microbiota at genus level revealed important features of interaction between parasites infection and bacterial community. Coprococcus and Prevotella were more abundant while Akkermansia had lower relative abundance with E. bieneusi infection. Bacteria of Akkermansia, Lactococcus, Oxalobacter, Sphaerochaeta, Paludibacter, Fibrobacter, Anaerovibrio, Pseudomonas, Mogibacterium, Pseudoramibacter_Eubacterium, YRC22, Flexispira, SMB53, AF12, and Roseburia genera were found under-presented and Oscillospira genus over-presented when G. duodenalis infection was present. Meanwhile, Cryptosporidium spp. and E. bieneusi co-infected animals showed lower relative abundance of Allobaculum, Rikenella, Shuttleworthia, Epulopiscium, Bilophila, Dorea, Fibrobacter, and TG5. Results demonstrate important interaction between the intestinal parasites and microbiota, and provide informative link for understanding the co-evolution of zoonotic pathogens and bacteria in domestic animals.
1. Introduction
Intestinal microbiota is known to have a considerable impact on the health condition and growth of the host animals. Studies have shown that the intestinal microbiota interacts with the host immune system [1] and affects the nutritional status and metabolism [2] of the host. Many endogenous factors, such as host genotype, age, diet, and gender [3,4,5,6], are known to affect the intestinal microbiota composition and diversity. In addition, the exogenous factors including host health status [7], probiotics, and antibiotics [8,9] have effects on the intestinal microecology.
Cryptosporidium spp., Enterocytozoon bieneusi, and Giardia duodenalis as exogenous factors are single-celled eukaryote pathogens found worldwide causing a variety of enteric diseases in mammalian hosts [10,11]; they can be spread in different ways, and drinking and recreational water are the most common mode of transmission [12]. Immunocompromised hosts will be infected with these pathogens being increased susceptibility. Although these protozoan parasites and fungal microbial are an important part in the intestinal micro-ecological environment, their interaction with the intestinal microbiota is not yet fully investigated. Reciprocal restriction between intestinal parasites and microbiota may occur that would modulate host immune response to different groups of pathogens [13,14].
The Qinghai-Tibetan Plateau (QTP) in northwest China is a high-altitude geographic region characterized by low ambient temperature, air pressure, and oxygen. Previous studies have shown that infections with Cryptosporidium spp., E. bieneusi, and G. duodenalis are common in yaks, goats, sheep, and cattle on the QTP [15,16,17,18]. It has been reported that hypoxia has strong influence on the intestinal bacteria and microbiota composition in animals and humans [19,20]. Some studies analyzed the intestinal microbiota composition in ruminant animals [21,22,23], but there are few reports on the interaction between intestinal protozoan parasites and intestinal microbiota diversity of the Bactrian camel, which is one of the important economically livestock in the QTP. Thus, how common parasites perturb intestinal microbiota of Bactrian camels living in QTP is not fully understood. In the present study, we investigated the prevalence of the three intestinal eukaryote pathogen infection in Bactrian camels inhabiting in QTP, then analyzed their intestinal microbiota profile further to reveal the specific association between the zoonotic pathogen infection and microbiota diversity and composition.
2. Materials and Methods
2.1. Sampling
Fecal samples were collected from 40 individual domestic Bactrian camels (Qinghai breed, female = 35, male = 5) housed in Chaka camel camp on Mohe, Haixi Prefecture in Qinghai Province, China. All animals were raised on free range by eating natural vegetation. Two samples of the domestic Bactrian camels were collected from camels fed on wheat straw and concentrates’ mixture. Samples were collected from the rectum by sterile tubes, cryopreserved immediately in liquid nitrogen, then transported to the laboratory, and stored at −80 °C.
2.2. DNA Extraction
DNA was isolated using the TIANamp stool DNA kit (DP328-02, Tiangen, Beijing, China) according to the manufacturer’s instructions. The quality and quantity of DNA were determined as the ratio at A260/280 with a spectrophotometer (Epoch, BioTek, Winooski, USA). DNA integrity was evaluated by electrophoresis in 1% agarose gel.
2.3. Nested-PCR Analysis
Individual DNA samples were analyzed using the small-subunit rRNA-based nested PCR method, which was listed in detail in our previous study [24]. The nested PCR primers are indicated in Table S1. All positive secondary PCR products were bi-directionally sequenced to identify the parasitization.
2.4. Amplification of 16S rRNA Genes
Bacterial communities composed at taxonomic resolution were analyzed with 16S rRNA gene amplification and sequencing protocol [25]. The V3-V4 regions of bacterial 16S rRNA were amplified by using the 341F/805R primer set (341F: 5′-CCTACGGGNGGCWGCAG-3ʹ, 805R: 5′-GACTACHVGGGTATCTAATCC-3′). The PCR condition was: 94 °C for 3 min (1 cycle), 94 °C for 20 s/55 °C for 30 s/72 °C for 30 s (6 cycles), 94 °C for 15 s/68 °C for 15 s/72 °C for 20 s (30 cycles), and a last step of 72 °C for 5 min [26]. The PCR products were sequenced on an Illumina HiSeq2500 platform (Health Genomics Bioinformatics Technology Co., Ltd., Beijing, China).
2.5. Bioinformatics and Statistical Analysis
Sequences were searched and analyzed using NCBI BLAST, Chromas2.6 and ClustalX2.1 to confirm specific infection.
V3-V4 16S amplicon sequencing data was quality filtered using the FLASH method [27]. All sequence analyses were provided in the Quantitative Insights Into Microbial Ecology (QIIME1.9.1) [28] according to the QIIME instructions with some modification. Further error correction was performed using USEARCH with de novo models, and the remaining sequences were clustered into operational taxonomic units (OTUs) using UCLUST [29] with a cutoff of 97% similarity. Taxonomy was assigned to the representative sequence of each OTU using the Ribosomal Database Project (RDP) classifier [30] with QIIME and aligned using the Greengenes13.8 reference database. Two alpha diversity measurements including Chao1 and Shannon diversity index were calculated from OTU counts to evaluate the biodiversity of the bacterial population in samples at the genus level. A Newick formatted tree was obtained by utilizing the QIIME package, and subsequently using Unweighted Pair Group Method with Arithmetic mean (UPGMA) clustering to generate a distance matrix that was visualized by principal coordinate analysis (PCoA). The relative abundance of taxonomy was processed using STAMP2.1.3 [31] for statistical analysis.
Data were analyzed by independent-samples t-test using the SPSS21 (IBM, Armonk, New York, USA) between control (negative samples) and treatments (positive samples). Differences were considered as significant at p < 0.05.
3. Results
3.1. Parasitological Survey
The parasitological survey results were already shown in our previous study [24]. Based on the nested-PCR results, 21 of 40 camels studied were identified positive for infection with one or two of the three parasite species examined. The other 19 camels were free of infection and treated as control group. Details of parasite infection with different ages are displayed in Table 1.

Table 1.
Summary of the parasitological survey.
The positive number (n = 6) of individuals infected with Cryptosporidium spp. included 1 camel only infected with Cryptosporidium spp. and 5 camels co-infected with Cryptosporidium spp. and E. bieneusi. The positive number (n = 3) of individuals infected with G. duodenalis included 2 camels only infected with G. duodenalis, 1 camel co-infected with G. duodenalis and E. bieneusi. The positive number (n = 18) of individuals infected with E. bieneusi included 12 camels only infected with E. bieneusi, and 6 co-infection individuals.
Because 2 samples were filtered out by Fastqc, here, we used 38 samples for intestinal bacteria study. The animals were then grouped according to the status of infection with the three zoonotic pathogens: Control (negative individual, n = 17), Group A (singly infected with E. bieneusi, n = 12), Group B (singly infected with Cryptosporidium spp., n = 1), Group C (singly infected with G. duodenalis, n = 2), Group D (co-infected with Cryptosporidium spp. and E. bieneusi, n = 5), and Group E (co-infected with G. duodenalis and E. bieneusi, n = 1). This category was used for further analysis in bacterial community composition. Furthermore, we were curious about the difference of intestinal microbiota diversity between negative samples and single- or co-infected samples, we also grouped samples into 3 groups indiscriminately, regardless of which parasite was positive: Control (negative individual, n = 17), Single-infection (singly infected with one parasite, n = 15), and Co-infection (samples infected with more than one parasite, n = 6).
3.2. Microbiota Diversity
After OTU picking and chimera checking, a total of 1,180,400 reads were assigned to 23,322 non-singleton OTUs (Table S2), which resulted in the classification of 205 taxa (genus level). The richness and diversity of the bacterial in fecal samples were evaluated with the plot of Chao1 and Shannon diversity index (Figure 1a). However, no significant differences of Shannon diversity were detected between Control and other 5 groups, while Chao1 index in Control was significantly higher than that in Group A (p < 0.05). Another kind of grouping showed (Figure 1b) same results in Shannon diversity, but significant higher Chao1 index in Control compared to single-infection group (p < 0.05).
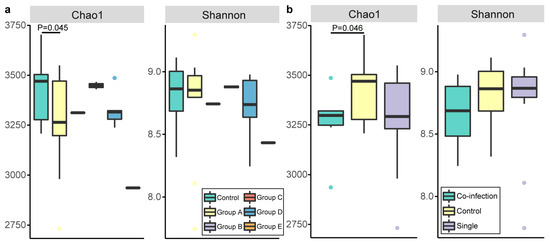
Figure 1.
Two alpha diversity measures Chao1 and Shannon’s diversity index. Community diversity represented by Chao1 and Shannon diversity index for samples from each group. Each dot stands for a discrete individual while each color represents (a) the samples from the camels infected with different parasites, (b) the samples from the camels with non-, single-, co-infection with parasites as the label showed.
3.3. Bacterial Community Composition
We analyzed the bacterial community composition at the phylum level across the six groups (Figure 2). The circlize plot represented the average relative abundance of each bacterial taxon. In all six groups, Firmicutes was the dominant phylum, as 52.49%, 56.62%, 54.69%, 54.52%, 49.33%, and 44.51% in the control, A, B, C, D, and E groups, respectively. The secondly abundant phylum was Bacteroidetes (33.18%, 30.71%, 32.31%, 35.39%, 32.63%, and 38.51%) in these six groups, respectively. In addition, the phyla Chlamydiae, SR1 and WPS-2 were present only in the Control, A, and E groups, but absent in the B, C, and D groups. OP1 was scarcely found in the Control and A groups. Fusobacteria was absent in group E, while Deferribacteres and Synergistetes were undetected in group B. Moreover, the biggest differences among the 6 groups of phyla were Verrucomicrobia and Spirochaete.
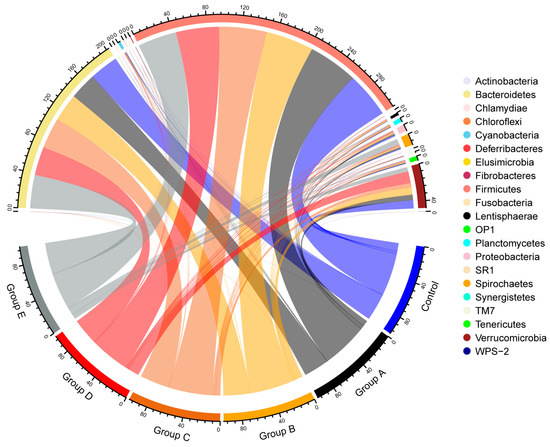
Figure 2.
Bacterial composition of different groups at the phylum level. The circlize plot shows the average relative abundance of each bacterial taxon (phylum level). There are 21 phyla for each group exhibited here, and Firmicutes and Bacteroidetes are the dominant phyla for each group.
On the PCoA plot, each symbol represented the microbiota of a camel. Overall, no significant differences in community structure were observed between the six groups, except for Group E based on weight or unweight distance analysis (Figure 3a,b). Interestingly, the microbiome of the E group was distinct from the other five groups in which almost both samples were clustered together. In addition, the two camels of Group A (A1 and A2 in Figure 3) fed with a different diet showed dispersal pattern in the PCoA plot.
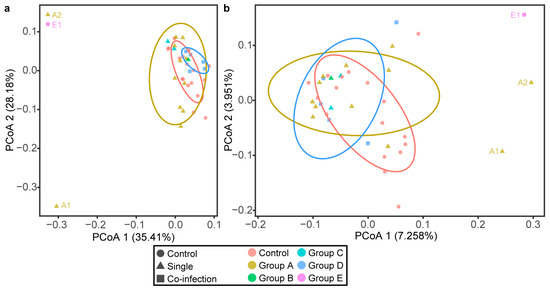
Figure 3.
Principal Coordinate Analysis (PCoA) with weighted/unweighted UniFrac distance analysis. PCoA plot of (a) the weighted UniFrac distances, (b) the unweighted UniFrac distances of fecal microbiota from each Bactrian camel. Control means negative samples of parasites, Single means samples infected with one of Cryptosporidium spp., E. bieneusi., and G. duodenalis., Co-infection means samples infected with more than one parasite. As the label shows, exactly infection and indiscriminate positive samples are differentiated by colors and shapes (circle, triangle, and square, respectively).
3.4. Taxonomic Affiliation
At the genus level, we observed significant differences in microbiota composition between control and Groups A, C, and D (Figure 4). In Group A, Coprococcus (p = 0.034) and Prevotella (p = 0.035) were more abundant, while Akkermansia (p < 0.01) showed a significantly lower relative abundance than that in the control group (Figure 4a). In comparison with the control group, Group C showed more abundant Oscillospira (p < 0.01), and less abundant Akkermansia (p < 0.01), Lactococcus (p < 0.01), Oxalobacter (p < 0.01), Sphaerochaeta (p < 0.01), Paludibacter (p < 0.01), Fibrobacter (p < 0.01), Anaerovibrio (p < 0.01), Pseudomonas (p = 0.012), Mogibacterium (p = 0.013), Pseudoramibacter_Eubacterium (p = 0.018), YRC22 (p = 0.018), Flexispira (p = 0.021), SMB53 (p = 0.025), AF12 (p = 0.031), and Roseburia (p = 0.038) (Figure 4b). For Group D, lower relative abundances were observed in Allobaculum (p < 0.01), Rikenella (p = 0.023), Shuttleworthia (p = 0.028), Epulopiscium (p = 0.029), Bilophila (p = 0.031), Dorea (p = 0.033), Fibrobacter (p = 0.047), and TG5 (p = 0.049) compared to the control group. Samples of Group B and E were not analyzed for the relative abundance due to the limited sample size.
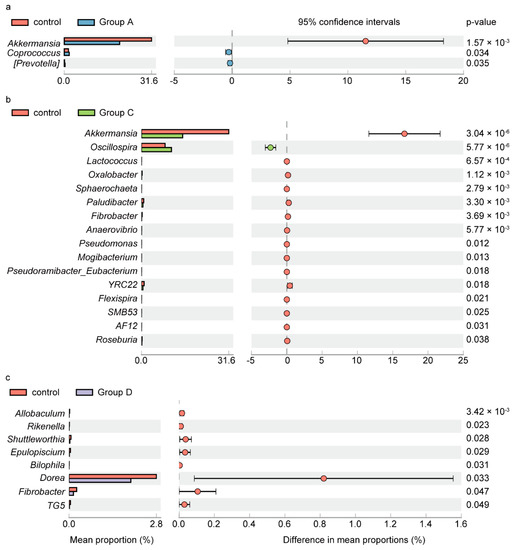
Figure 4.
Relative abundance between control and groups A, C, and D with significant difference in bacterial genera. Extended error bar plots (a–c), display the differences in mean abundance of taxa in Groups A, C, and D compared to control (p < 0.05, confidence intervals = 95%), respectively. Analysis is performed with STAMP2.1.3 and the p values are based on t-test. Prevotella with square brackets is proposed by the Greengenes curators and indicating the recommended taxonomy.
3.5. COG Annotation and Analysis
Cluster of orthologous groups (COG) were employed to identify the difference in predicted functions of intestinal microorganisms which had a symbiotic relationship with various parasites (Figure 5). Although there were no difference in COG category distribution among groups of different parasitic infection, significant differences existed between control and Group A, control and Group C, Group A and C, Group A and D, and Group C and D.
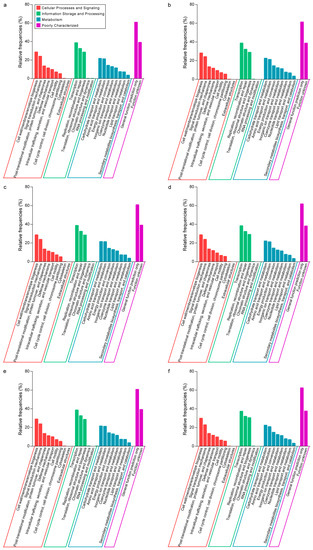
Figure 5.
The relative frequencies of matched reads assigned in cluster orthologous groups (COGs) in camel feces. Four function categories in COG are showed here; (a–f) represent for the relative frequencies of control, Group A, Group B, Group C, Group D, Group E, Group F, respectively.
The microorganisms of control showed higher relative frequency of COG in categories of Intracellular trafficking, secretion, and vesicular transport, RNA processing and modification, Replication, recombination and repair, Lipid transport and metabolism, Secondary metabolites biosynthesis, transport, and catabolism, Inorganic ion transport and metabolism, than Group A, and Cell motility, Chromatin structure and dynamics, RNA processing and modification, Transcription, Inorganic ion transport and metabolism, than Group C, while showed lower relative frequency of COG in categories of Defense mechanisms, Cell cycle control, cell division, chromosome partitioning, Carbohydrate transport and metabolism, Nucleotide transport and metabolism, than Group A, and Cell cycle control, cell division, chromosome partitioning, Translation, ribosomal structure and biogenesis, Nucleotide transport and metabolism, than Group C. It was curious that the different categories of control and Group A, Group A and D were identical.
4. Discussion
In the current study, we observed the occurrence of the zoonotic pathogens Cryptosporidium spp., E. bieneusi, and G. duodenalis in domestic Bactrian camels inhabiting on Qinghai-Tibet Plateau. High prevalence was detected in E. bieneusi (45%) infection, followed by Cryptosporidium spp. (15%) and G. duodenalis (7.5%). The intestinal-colonizing parasites G. duodenalis was considered the most common pathogenic parasite in humans and animal species [32,33,34,35,36]. Although G. duodenalis infection in yaks [37] and sheep [38] living in high-altitude environment had been reported, there were very few reports on the prevalence of G. duodenalis infection in camels. The infection rate of G. duodenalis in Bactrian camels observed in our study was comparable to that reported in yaks and sheep [38]. Inconsistency with the study to report G. duodenalis infections in sheep, which was found in the plain area with a higher infection rate of 6.7% [39] might be a result of altitude influence. The infection rate of Cryptosporidium spp. in camels observed in this study was close to that reported by others [16,40,41]. We also found that infection rate of E. bieneusi in this study was comparable to those reported by others [40,41,42].
Besides, individuals were concurrently infected with Cryptosporidium spp. and E. bieneusi (5/40), G. duodenalis and E. bieneusi (1/40), but not Cryptosporidium spp. and G. duodenalis. Even though no study on mixed infected with Cryptosporidium spp. and G. duodenalis in camels was reported, co-infection of Cryptosporidium spp. and G. duodenalis was still found in yaks [37], children [43], and goat [44]. Concurrent infection with Cryptosporidium spp. and E. bieneusi in dromedary camel [40], as well as co-infection of G. duodenalis and E. bieneusi in dairy cattle was proposed [45] in an early study.
A recent study indicated that the maintenance of homeostasis between the gut microbiota and the rest of the body was crucial for health. Intestinal parasites, particularly the protozoans, may cause an alteration in the structure of the gut microbiota, resulting in gastrointestinal upset or systemic diseases [46]. In this study, we analyzed the interaction between intestinal protozoans and bacterial community composition and diversity to understand whether eukaryote microbes modulate the microbiota in camels. The results showed that, by alpha diversity analysis, bacterial community diversity was not significantly different between Control and other groups; however, the higher Chao1 index and similar Shannon diversity index in Control indicated negative samples with higher species richness and lower evenness than Group A (E. bieneusi positive samples). It was also observed that Firmicutes and Bacteroidetes were the predominant phyla, accounting for more than 81% of the bacteriophyta presented in all groups. Although other phyla presented in low abundance, Spirochaetes was detected in relatively highest abundance in Group E than in the other five groups, followed was Group A. The phylum Verrucomicrobia was found in lower abundance in Groups A, C, E than in the other three groups. These results demonstrated that the genera of Verrucomicrobia phylum were negatively correlated with E. bieneusi and G. duodenalis, while Spirochaetes was positively correlated with co-infection with E. bieneusi and G. duodenalis in the camel host. From the literature available, there has been no report on the state of the relationship of E. bieneusi, G. duodenalis, and intestinal Spirochaetes; only a previous study mentioned that G. duodenalis provides bearable conditions for concurrent spirochetal diarrhea developing potentially [47]. According to our results, there is more possibility that infection with E. bieneusi and co-infection by G. duodenalis might contribute to the growth of bacteria that belong to Spirochaetes.
Samples from animals harboring Cryptosporidium spp. (Group B) showed the lowest microbial community diversity lacking 6 phyla: Deferribacteres, Synergistetes, Chlamydiae, SR1, OP1, and WPS-2, while animals harboring Cryptosporidium spp. and E. bieneusi (Group D) lack four phyla Chlamydiae, SR1, OP1, and WPS-2, which suggested that the four phyla, Chlamydiae, SR1, OP1, and WPS-2 responded to Cryptosporidium spp. sensitively and Cryptosporidium spp. might decrease host intestinal microbial community diversity. These results are consistent with the observations made in mice and Coquerel’s sifakas by others [48,49].
From the study, most Bactrian camels tended to share a similar intestinal microbiota regardless of the weighted or unweighted UniFrac distance metric (PCoA plot). Compared with the unweighted UniFrac metric, weighted UniFrac metric can distinguish the difference of OTU abundance. Meanwhile, after considering OTU abundance, the maximum explanation of principal coordinate component increased. The dispersal of two camels of Group A (A1 and A2) fed with wheat straw and concentrates mixture to others of Group A indicated that the diet might influence bacterial communities [50]. Although the animal in Group E (1 sample) shared the same dietary and environment with others, it was scattered significantly. Therefore, the combined effect of E. bieneusi and G. duodenalis may have a considerable influence on microbiota composition of hosts.
Analysis of microbiota at the genus level revealed a notable difference in bacterial genera between Groups A, C, D, and the Control group. The significant bacterial genera in Groups A, C, and D compared to Control were different from each group except for Akkermansia and Fibrobacter. The bacterial genera that exhibited the greatest statistical significance in Groups A, C, and D were Akkermansia, Oscillospira, Allobaculum, respectively, with all having a higher predicted relative abundance. Compared with the control group, a bacterial genus underrepresented both in Group A and C was Akkermansia. Bacteria in this genus were known play an important role in provision of nutrition to intestinal mucosa and control of function of intestinal barrier [51,52].
E. bieneusi and G. duodenalis were known as the intestinal epithelial barrier breaker [53,54], but the pathogenesis of the two parasites was not clear. It was possible that E. bieneusi or G. duodenalis break intestinal mucosa of the host by reducing the relative abundance of Akkermansia. Fibrobacter bacteria were known for solubilizing cellulose [55,56]. The abundance of Fibrobacter was significantly reduced in Group C and D compare to the Control animals. It was speculated that Cryptosporidium spp., G. duodenalis, and E. bieneusi may impair the host nutrition efficiency by reducing Fibrobacter bacteria abundance.
A study on patients with inflammatory bowel diseases showed Oscillospira was negatively associated with colonic disease of patients [57]. We observed that Oscillospira genus in individuals with G. duodenalis infection was more abundant than Control. It might be possible, when G. duodenalis existed, that the increased Oscillospira could exhaust the nutrient of hosts. In agreement with the current finding, a study identified that Oscillospira had been detected in the intestines of vegetarians and can be able to utilize host glycans by producing a diverse glycoside hydrolase repertoire [58].
A recent study showed that feeding mice with Bactrian camel milk resulted in establishment of predominant Allobaculum and as Akkermansia genus in mouse intestinal [59]. Allobaculum bacteria were beneficial to the host by supplying short-chain fatty acids and providing control of inflammation [60,61,62]. We observed that Allobaculum was significantly decreased by co-infection with Cryptosporidium spp. and E. bieneusi in Group D. The decreased abundance of bacteria of this genus by co-infection with Cryptosporidium spp. and E. bieneusi may have important implication in malnutrition and dysregulation of the inflammatory response.
The association of relative abundance of Prevotella with high altitude had been reported in yaks and sheep [63]. The bacteria in this genus produce short-chain fatty acids as an energy source to the host under hypoxia environment [64]. Though the abundance of Prevotella was lower in control compared to Group A in this study, no evidence was found for explaining the relationship between E. bieneusi and Prevotella.
Compared with samples in control, there were more energy metabolism categories changed in samples infected with E. bieneusi (Group A) than infected with G. duodenalis (Group C), which led to more changes of cellular processes and signaling, information storage, and processing in intestinal microorganisms. As in previous studies, E. bieneusi had no full pathways of carbon metabolism and depended on transporting ATP from hosts [65], while G. duodenalis generated enzymes to degrade the epithelial mucus of hosts [66]. Due to only 1 sample in Group B, we could not make a statistical analysis between control and Group B in COG annotation and analysis. Then, it was a bit hard to explain why samples co-infected with Cryptosporidium spp. and E. bieneusi, infected with E. bieneusi had the same impact on the function of intestinal microorganisms.
In summary, we investigated the interaction between three main intestinal parasites and microbiota composition in Bactrian camels and observed some important link between the parasites and the microbiota feature. The observation made in the study may help understand the co-evolution of intestinal microbiota and the three zoonotic parasites that occur in both humans and animals.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/app11083595/s1, Table S1: Primers of small-subunit rRNA nested PCR, Table S2: OTU table summary.
Author Contributions
Methodology, software, data curation, writing—original draft preparation, X.W.; investigation, Z.Z.; resources, Q.Z. and W.Y.; writing—review and editing, Z.D. and R.W.; funding acquisition, Z.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant number XDA2004010305), and the Key Projects of Chinese Academy of Sciences (Grant number KFZD-SW-219).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We thank Zhao Kai, doctoral candidates Ding Ning and Tian Dehong (Northwest Institute of Plateau Biology, Chinese Academy of Sciences) for helping collect samples on the plateau.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mazmanian, S.K.; Liu, C.H.; Tzianabos, A.O.; Kasper, D.L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005, 122, 107–118. [Google Scholar] [CrossRef]
- Cummings, J.H.; Beatty, E.R.; Kingman, S.M.; Bingham, S.A.; Englyst, H.N. Digestion and physiological properties of resistant starch in the human large bowel. Br. J. Nutr. 1996, 75, 733–747. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.L.; Nkrumah, J.D.; Basarab, J.A.; Moore, S.S. Linkage of microbial ecology to phenotype: Correlation of rumen microbial ecology to cattle’s feed efficiency. FEMS Microbiol. Lett. 2008, 288, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Penner, G.B.; Li, M.; Oba, M.; Guan, L.L. Changes in bacterial diversity associated with epithelial tissue in the beef cow rumen during the transition to a high-grain diet. Appl. Environ. Microbiol. 2011, 77, 5770–5781. [Google Scholar] [CrossRef] [PubMed]
- Claesson, M.J.; Cusack, S.; O’Sullivan, O.; Greene-Diniz, R.; de Weerd, H.; Flannery, E.; Marchesi, J.R.; Falush, D.; Dinan, T.; Fitzgerald, G.; et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4586–4591. [Google Scholar] [CrossRef]
- Jami, E.; Israel, A.; Kotser, A.; Mizrahi, I. Exploring the bovine rumen bacterial community from birth to adulthood. ISME J. 2013, 7, 1069–1079. [Google Scholar] [CrossRef]
- Riva, A.; Borgo, F.; Lassandro, C.; Verduci, E.; Morace, G.; Borghi, E.; Berry, D. Pediatric obesity is associated with an altered gut microbiota and discordant shifts in Firmicutes populations. Environ. Microbiol. 2017, 19, 95–105. [Google Scholar] [CrossRef]
- Arboleya, S.; Binetti, A.; Salazar, N.; Fernandez, N.; Solis, G.; Hernandez-Barranco, A.; Margolles, A.; de Los Reyes-Gavilan, C.G.; Gueimonde, M. Establishment and development of intestinal microbiota in preterm neonates. FEMS Microbiol. Ecol. 2012, 79, 763–772. [Google Scholar] [CrossRef]
- Wang, J.; Tang, H.; Zhang, C.; Zhao, Y.; Derrien, M.; Rocher, E.; van-Hylckama Vlieg, J.E.; Strissel, K.; Zhao, L.; Obin, M.; et al. Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. ISME J. 2015, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, R.M.; Davies, A.P. Minireview: Clinical cryptosporidiosis. Exp. Parasitol. 2010, 124, 138–146. [Google Scholar] [CrossRef]
- Wright, S.G. Protozoan infections of the gastrointestinal tract. Infect. Dis. Clin. N. Am. 2012, 26, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Lobo, M.L.; Xiao, L.; Antunes, F.; Matos, O. Occurrence of Cryptosporidium and Giardia genotypes and subtypes in raw and treated water in Portugal. Lett. Appl. Microbiol. 2009, 48, 732–737. [Google Scholar]
- Bancroft, A.J.; Hayes, K.S.; Grencis, R.K. Life on the edge: The balance between macrofauna, microflora and host immunity. Trends Parasitol. 2012, 28, 93–98. [Google Scholar] [CrossRef]
- Lantier, L.; Drouet, F.; Guesdon, W.; Mancassola, R.; Metton, C.; Lo-Man, R.; Werts, C.; Laurent, F.; Lacroix-Lamande, S. Poly(I:C)-induced protection of neonatal mice against intestinal Cryptosporidium parvum infection requires an additional TLR5 signal provided by the gut flora. J. Infect. Dis. 2014, 209, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Karanis, P.; Plutzer, J.; Halim, N.A.; Igori, K.; Nagasawa, H.; Ongerth, J.; Liqing, M. Molecular characterization of Cryptosporidium from animal sources in Qinghai province of China. Parasitol. Res. 2007, 101, 1575–1580. [Google Scholar] [CrossRef] [PubMed]
- Sazmand, A.; Rasooli, A.; Nouri, M.; Hamidinejat, H.; Hekmatimoghaddam, S. Prevalence of Cryptosporidium spp. in Camels and Involved People in Yazd Province, Iran. Iran. J. Parasitol. 2012, 7, 80–84. [Google Scholar] [PubMed]
- Ma, J.G.; Zhang, N.Z.; Hou, J.L.; Zou, Y.; Hu, G.X.; Zhu, X.Q.; Zhou, D.H. Detection of Enterocytozoon bieneusi in White Yaks in Gansu Province, China. Biomed. Res. Int. 2017, 2017, 5790181. [Google Scholar] [CrossRef]
- Jian, Y.; Zhang, X.; Li, X.; Karanis, G.; Ma, L.; Karanis, P. Prevalence and molecular characterization of Giardia duodenalis in cattle and sheep from the Qinghai-Tibetan Plateau Area (QTPA), northwestern China. Vet. Parasitol. 2018, 250, 40–44. [Google Scholar] [CrossRef]
- Adak, A.; Maity, C.; Ghosh, K.; Mondal, K.C. Alteration of predominant gastrointestinal flora and oxidative damage of large intestine under simulated hypobaric hypoxia. Z. fur Gastroenterol. 2014, 52, 180–186. [Google Scholar] [CrossRef]
- Karl, J.P.; Berryman, C.E.; Young, A.J.; Radcliffe, P.N.; Branck, T.A.; Pantoja-Feliciano, I.G.; Rood, J.C.; Pasiakos, S.M. Associations between the gut microbiota and host responses to high altitude. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G1003–G1015. [Google Scholar] [CrossRef]
- Al-Masaudi, S.; El Kaoutari, A.; Drula, E.; Al-Mehdar, H.; Redwan, E.M.; Lombard, V.; Henrissat, B. A Metagenomics Investigation of Carbohydrate-Active Enzymes along the Gastrointestinal Tract of Saudi Sheep. Front. Microbiol. 2017, 8, 666. [Google Scholar] [CrossRef]
- Alipour, M.J.; Jalanka, J.; Pessa-Morikawa, T.; Kokkonen, T.; Satokari, R.; Hynonen, U.; Iivanainen, A.; Niku, M. The composition of the perinatal intestinal microbiota in cattle. Sci. Rep. 2018, 8, 10437. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Zhu, L.; Xu, Y.; Liu, N.; Sun, X.; Hu, L.; Huang, H.; Wei, K.; Zhu, R. Dynamic Distribution of Gut Microbiota in Goats at Different Ages and Health States. Front. Microbiol. 2018, 9, 2509. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Z.; Ai, S.; Wang, X.; Zhang, R.; Duan, Z. Cryptosporidium spp., Enterocytozoon bieneusi, and Giardia duodenalis from animal sources in the Qinghai-Tibetan Plateau Area (QTPA) in China. Comp. Immunol. Microbiol. Infect. Dis. 2019, 67, 101346. [Google Scholar] [CrossRef] [PubMed]
- Singer, E.; Bushnell, B.; Coleman-Derr, D.; Bowman, B.; Bowers, R.M.; Levy, A.; Gies, E.A.; Cheng, J.F.; Copeland, A.; Klenk, H.P.; et al. High-resolution phylogenetic microbial community profiling. ISME J. 2016, 10, 2020–2032. [Google Scholar] [CrossRef] [PubMed]
- Herlemann, D.P.; Labrenz, M.; Jurgens, K.; Bertilsson, S.; Waniek, J.J.; Andersson, A.F. Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. ISME J. 2011, 5, 1571–1579. [Google Scholar] [CrossRef]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Cole, J.R.; Wang, Q.; Cardenas, E.; Fish, J.; Chai, B.; Farris, R.J.; Kulam-Syed-Mohideen, A.S.; McGarrell, D.M.; Marsh, T.; Garrity, G.M.; et al. The Ribosomal Database Project: Improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009, 37, D141–D145. [Google Scholar] [CrossRef]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef]
- Thompson, R.C. Giardiasis as a re-emerging infectious disease and its zoonotic potential. Int. J. Parasitol. 2000, 30, 1259–1267. [Google Scholar] [CrossRef]
- Feng, Y.; Xiao, L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin. Microbiol. Rev. 2011, 24, 110–140. [Google Scholar] [CrossRef] [PubMed]
- Huete-Pérez, J.A.; Zhang, W.; Zhang, X.; Wang, R.; Liu, A.; Shen, Y.; Ling, H.; Cao, J.; Yang, F.; Zhang, X.; et al. Genetic Characterizations of Giardia duodenalis in Sheep and Goats in Heilongjiang Province, China and Possibility of Zoonotic Transmission. PLoS Negl. Trop. Dis. 2012, 6, e1826. [Google Scholar]
- Ye, J.; Xiao, L.; Ma, J.; Guo, M.; Liu, L.; Feng, Y. Anthroponotic enteric parasites in monkeys in public park, China. Emerg. Infect. Dis. 2012, 18, 1640–1643. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, C.; Yu, Y.; Li, J.; Gong, P.; Song, M.; Xiao, L.; Zhang, X. Molecular characterization of Giardia duodenalis isolates from police and farm dogs in China. Exp. Parasitol. 2013, 135, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Cai, J.; Wang, R.; Li, J.; Jian, F.; Huang, J.; Zhou, H.; Zhang, L. Molecular characterization of Cryptosporidium spp. and Giardia duodenalis from yaks in the central western region of China. BMC Microbiol 2015, 15, 1–7. [Google Scholar] [CrossRef]
- Wu, Y.; Chang, Y.; Chen, Y.; Zhang, X.; Li, D.; Zheng, S.; Wang, L.; Li, J.; Ning, C.; Zhang, L. Occurrence and molecular characterization of Cryptosporidium spp., Giardia duodenalis, and Enterocytozoon bieneusi from Tibetan sheep in Gansu, China. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2018, 64, 46–51. [Google Scholar] [CrossRef]
- Wang, H.; Qi, M.; Zhang, K.; Li, J.; Huang, J.; Ning, C.; Zhang, L. Prevalence and genotyping of Giardia duodenalis isolated from sheep in Henan Province, central China. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2016, 39, 330–335. [Google Scholar] [CrossRef]
- Baroudi, D.; Zhang, H.; Amer, S.; Khelef, D.; Roellig, D.M.; Wang, Y.; Feng, Y.; Xiao, L. Divergent Cryptosporidium parvum subtype and Enterocytozoon bieneusi genotypes in dromedary camels in Algeria. Parasitol. Res. 2018, 117, 905–910. [Google Scholar] [CrossRef]
- Zahedi, A.; Lee, G.K.C.; Greay, T.L.; Walsh, A.L.; Blignaut, D.J.C.; Ryan, U.M. First report of Cryptosporidium parvum in a dromedary camel calf from Western Australia. Acta Parasitol. 2018, 63, 422–427. [Google Scholar] [CrossRef]
- Qi, M.; Li, J.; Zhao, A.; Cui, Z.; Wei, Z.; Jing, B.; Zhang, L. Host specificity of Enterocytozoon bieneusi genotypes in Bactrian camels (Camelus bactrianus) in China. Parasites Vectors 2018, 11, 219. [Google Scholar] [CrossRef]
- Wang, Y.; Li, N.; Guo, Y.; Wang, L.; Wang, R.; Feng, Y.; Xiao, L. Persistent Occurrence of Cryptosporidium hominis and Giardia duodenalis Subtypes in a Welfare Institute. Front. Microbiol. 2018, 9, 2830. [Google Scholar] [CrossRef]
- Peng, X.Q.; Tian, G.R.; Ren, G.J.; Yu, Z.Q.; Lok, J.B.; Zhang, L.X.; Wang, X.T.; Song, J.K.; Zhao, G.H. Infection rate of Giardia duodenalis, Cryptosporidium spp. and Enterocytozoon bieneusi in cashmere, dairy and meat goats in China. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2016, 41, 26–31. [Google Scholar] [CrossRef]
- Fayer, R.; Santin, M.; Macarisin, D. Detection of concurrent infection of dairy cattle with Blastocystis, Cryptosporidium, Giardia, and Enterocytozoon by molecular and microscopic methods. Parasitol. Res. 2012, 111, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Partida-Rodriguez, O.; Serrano-Vazquez, A.; Nieves-Ramirez, M.E.; Moran, P.; Rojas, L.; Portillo, T.; Gonzalez, E.; Hernandez, E.; Finlay, B.B.; Ximenez, C. Human Intestinal Microbiota: Interaction Between Parasites and the Host Immune Response. Arch. Med. Res. 2017, 48, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Duhamel, G.E.; Hunsaker, B.D.; Mathiesen, M.R.; Moxley, R.A. Intestinal spirochetosis and giardiasis in a beagle pup with diarrhea. Vet. Pathol. 1996, 33, 360–362. [Google Scholar] [CrossRef] [PubMed]
- Ras, R.; Huynh, K.; Desoky, E.; Badawy, A.; Widmer, G. Perturbation of the intestinal microbiota of mice infected with Cryptosporidium parvum. Int. J. Parasitol. 2015, 45, 567–573. [Google Scholar] [CrossRef] [PubMed]
- McKenney, E.A.; Greene, L.K.; Drea, C.M.; Yoder, A.D. Down for the count: Cryptosporidium infection depletes the gut microbiome in Coquerel’s sifakas. Microb. Ecol. Health Dis. 2017, 28, 1335165. [Google Scholar] [CrossRef]
- Ming, L.; Yi, L.; Hasi, S.; He, J.; Hai, L.; Wang, Z.; Guo, F.; Qiao, X. Comparative analysis of fecal microbial communities in cattle and Bactrian camels. PLoS ONE 2017, 12, e0173062. [Google Scholar] [CrossRef]
- Belzer, C.; de Vos, W.M. Microbes inside--from diversity to function: The case of Akkermansia. ISME J. 2012, 6, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Ankarklev, J.; Jerlstrom-Hultqvist, J.; Ringqvist, E.; Troell, K.; Svard, S.G. Behind the smile: Cell biology and disease mechanisms of Giardia species. Nat. Rev. Microbiol. 2010, 8, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Santin, M.; Fayer, R. Microsporidiosis: Enterocytozoon bieneusi in domesticated and wild animals. Res. Vet. Sci. 2011, 90, 363–371. [Google Scholar] [CrossRef]
- Daly, K.; Proudman, C.J.; Duncan, S.H.; Flint, H.J.; Dyer, J.; Shirazi-Beechey, S.P. Alterations in microbiota and fermentation products in equine large intestine in response to dietary variation and intestinal disease. Br. J. Nutr. 2012, 107, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Neumann, A.P.; McCormick, C.A.; Suen, G. Fibrobacter communities in the gastrointestinal tracts of diverse hindgut-fermenting herbivores are distinct from those of the rumen. Environ. Microbiol. 2017, 19, 3768–3783. [Google Scholar] [CrossRef] [PubMed]
- Walters, W.A.; Xu, Z.; Knight, R. Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett. 2014, 588, 4223–4233. [Google Scholar] [CrossRef]
- Kohl, K.D.; Amaya, J.; Passement, C.A.; Dearing, M.D.; McCue, M.D. Unique and shared responses of the gut microbiota to prolonged fasting: A comparative study across five classes of vertebrate hosts. FEMS Microbiol. Ecol. 2014, 90, 883–894. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, W.; Wang, B.; Zhang, F.; Shao, Y. Influence of Bactrian camel milk on the gut microbiota. J. Dairy Sci. 2018, 101, 5758–5769. [Google Scholar] [CrossRef]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell. Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef]
- Ravussin, Y.; Koren, O.; Spor, A.; LeDuc, C.; Gutman, R.; Stombaugh, J.; Knight, R.; Ley, R.E.; Leibel, R.L. Responses of gut microbiota to diet composition and weight loss in lean and obese mice. Obesity 2011, 20, 738–747. [Google Scholar] [CrossRef]
- Tachon, S.; Zhou, J.; Keenan, M.; Martin, R.; Marco, M.L. The intestinal microbiota in aged mice is modulated by dietary resistant starch and correlated with improvements in host responses. FEMS Microbiol. Ecol. 2013, 83, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, D.; Wang, L.; Hao, J.; Wang, J.; Zhou, X.; Wang, W.; Qiu, Q.; Huang, X.; Zhou, J.; et al. Convergent Evolution of Rumen Microbiomes in High-Altitude Mammals. Curr. Biol. CB 2016, 26, 1873–1879. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.A.; Martins, F.M.; Nachman, M.W. Altitudinal variation of the gut microbiota in wild house mice. Mol. Ecol. 2018, 27, 2378–2390. [Google Scholar] [CrossRef] [PubMed]
- Keeling, P.J.; Corradi, N.; Morrison, H.G.; Haag, K.L.; Ebert, D.; Weiss, L.M.; Akiyoshi, D.E.; Tzipori, S. The reduced genome of the parasitic microsporidian Enterocytozoon bieneusi lacks genes for core carbon metabolism. Genome Biol. Evol. 2010, 2, 304–309. [Google Scholar] [CrossRef]
- Hicks, S.J.; Theodoropoulos, G.; Carrington, S.D.; Corfield, A.P. The role of mucins in host-parasite interactions. Part I-protozoan parasites. Parasitol. Today 2000, 16, 476–481. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).