Electric Stimulation of Astaxanthin Biosynthesis in Haematococcus pluvialis
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalga and Photosynthetic Cultivation
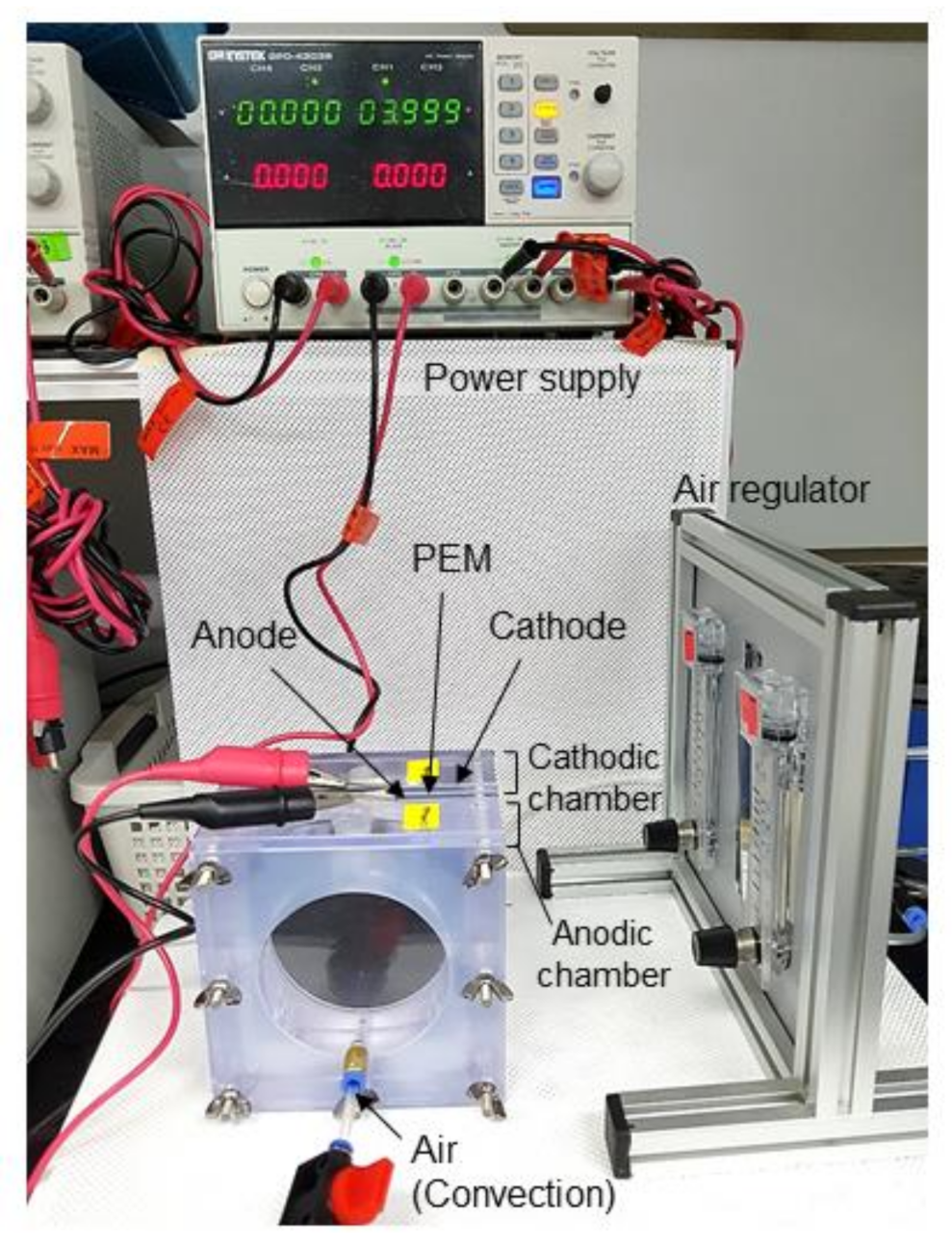
2.2. Electric Stimulation
2.3. Cyclic Voltammetry (CV) Analysis
2.4. Other Analytical Methods
3. Results and Discussion
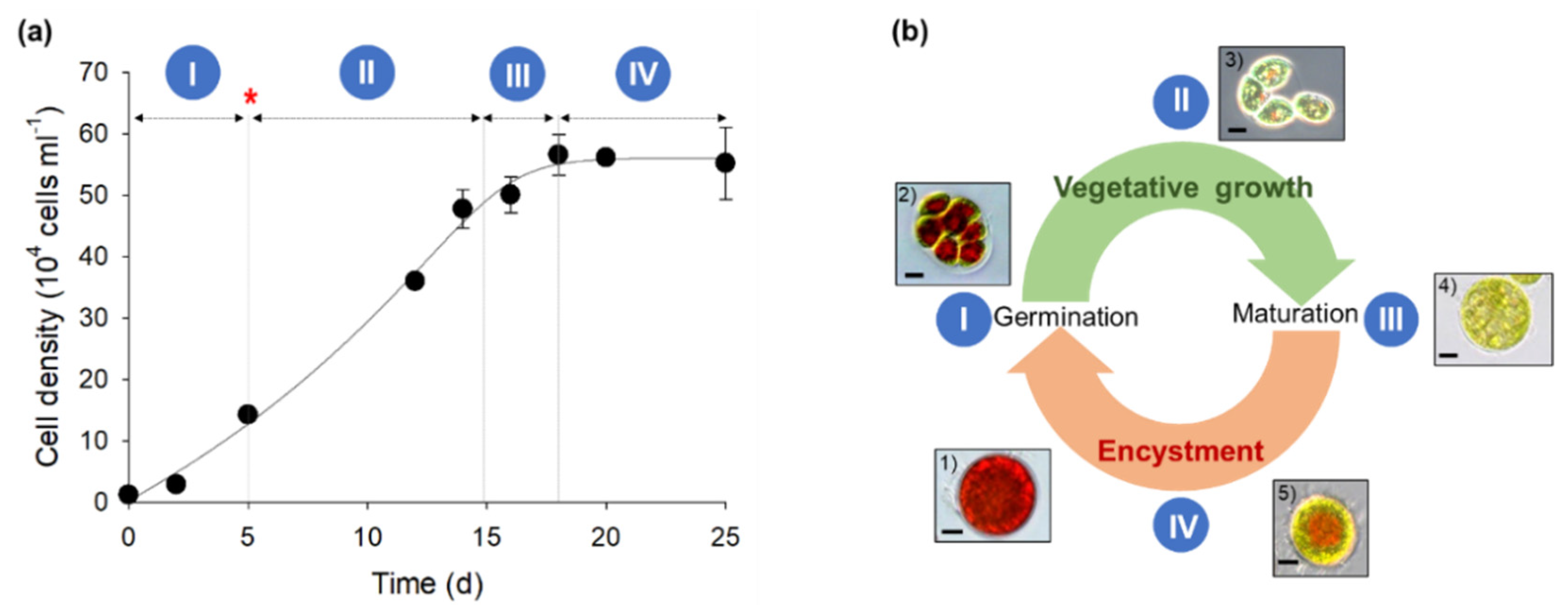
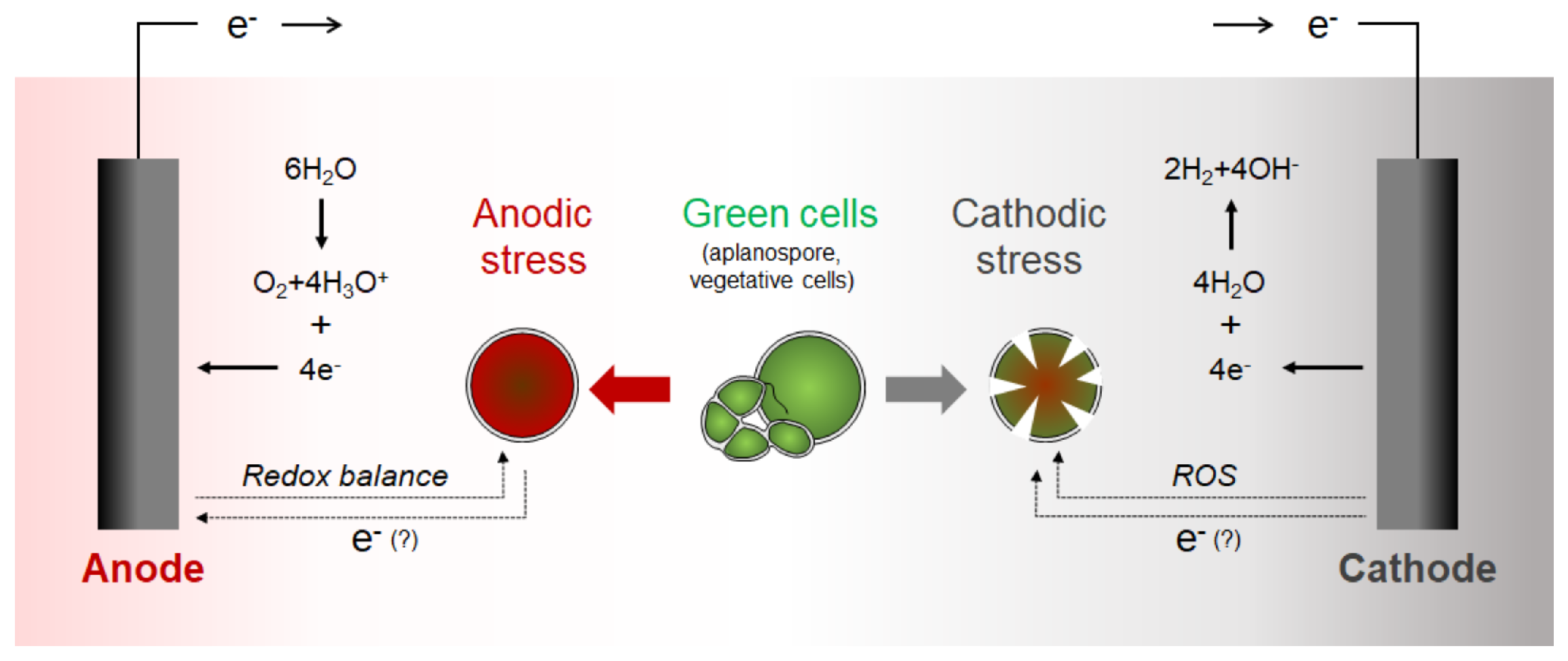
3.1. H. pluvialis Life Cycle and Astaxanthin Induction
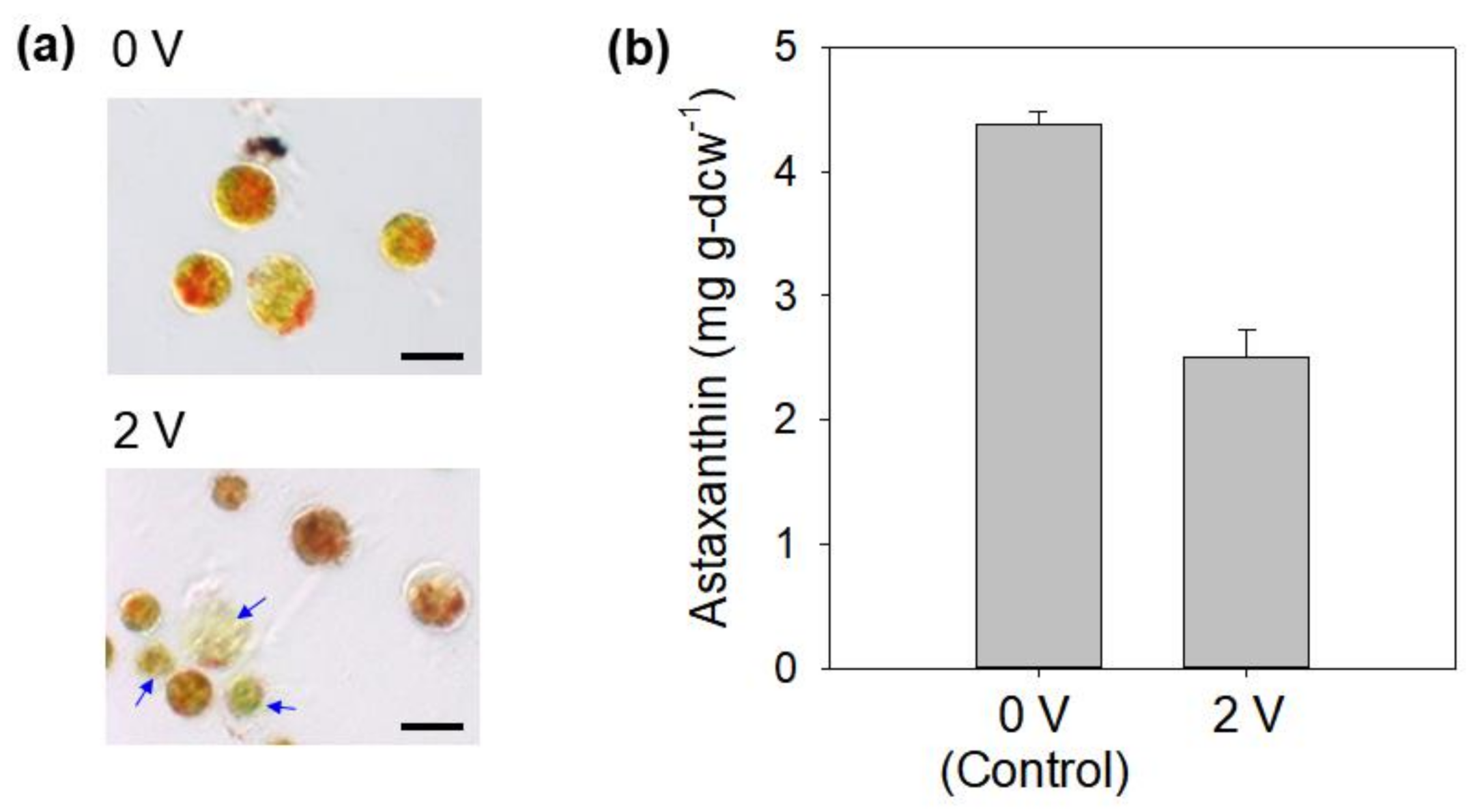
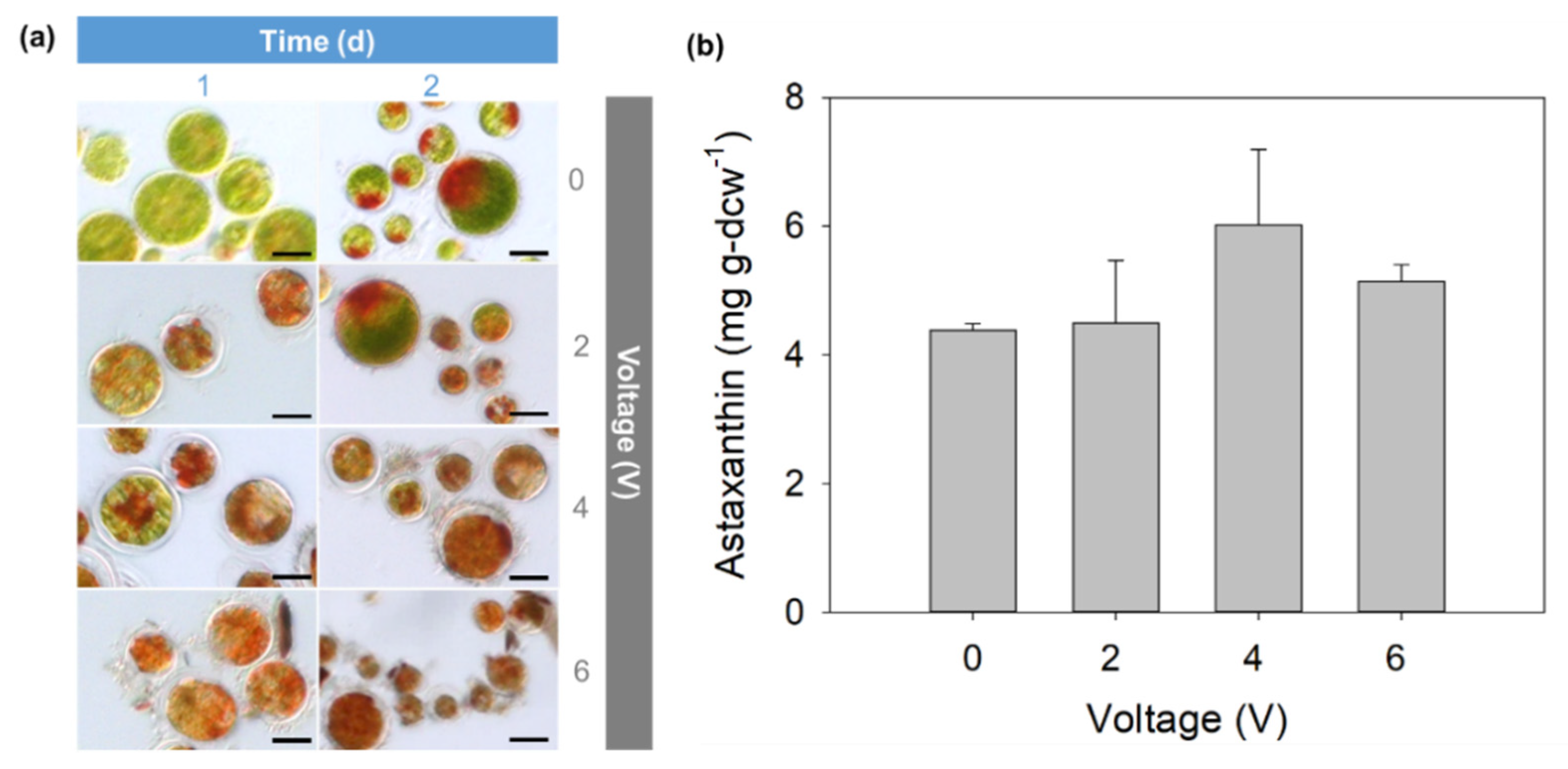
3.2. Electric Induction of Astaxanthin Production Using Cathodic and Anodic Currents
3.3. CV Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, D.Y.; Vijayan, D.; Praveenkumar, R.; Han, J.I.; Lee, K.; Park, J.Y.; Chang, W.S.; Lee, J.S.; Oh, Y.K. Cell-wall disruption and lipid/astaxanthin extraction from microalgae: Chlorella and Haematococcus. Bioresour. Technol. 2016, 199, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.M.R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-producing green microalga Haematococcus pluvialis: From single cell to high value commercial products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef]
- Lee, S.Y.; Cho, J.M.; Chang, Y.K.; Oh, Y.-K. Cell disruption and lipid extraction for microalgal biorefineries: A review. Bioresour. Technol. 2017, 244, 1317–1328. [Google Scholar] [CrossRef]
- Poonkum, W.; Powtongsook, S.; Pavasant, P. Astaxanthin induction in microalga H. pluvialis with flat panel airlift photobioreactors under indoor and outdoor conditions. Prep. Biochem. Biotechnol. 2015, 45, 1–17. [Google Scholar] [CrossRef]
- Han, D.; Li, Y.; Hu, Q. Astaxanthin in microalgae: Pathways, functions and biotechnological implications. Algae 2013, 28, 131–147. [Google Scholar] [CrossRef]
- Noh, M.H.; Cha, S.; Kim, M.; Jung, G.Y. Recent advances in microbial cell growth regulation strategies for metabolic engineering. Biotechnol. Bioprocess. Eng. 2020, 25, 810–828. [Google Scholar] [CrossRef]
- Liyanaarachchi, V.C.; Nishshanka, G.K.S.H.; Premaratne, R.G.M.M.; Ariyadasa, T.U.; Nimarshana, P.H.V.; Malik, A. Astaxanthin accumulation in the green microalga Haematococcus pluvialis: Effect of initial phosphate concentration and stepwise/continuous light stress. Biotechnol. Rep. 2020, 28, e00538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-W.; Zhou, X.-F.; Zhang, Y.-L.; Cheng, P.-F.; Ma, R.; Cheng, W.-L.; Chu, H.-Q. Enhancing astaxanthin accumulation in Haematococcus pluvialis by coupled light intensity and nitrogen starvation in column photobioreactors. J. Microbiol. Biotechnol. 2018, 28, 2019–2028. [Google Scholar] [CrossRef]
- Oslan, S.N.H.; Shoparwe, N.F.; Yusoff, A.H.; Rahim, A.A.; Chang, C.S.; Tan, J.S.; Oslan, S.N.; Arumugam, K.; Ariff, A.B.; Sulaiman, A.Z.; et al. A Review on Haematococcus pluvialis bioprocess optimization of green and red stage culture conditions for the production of natural astaxanthin. Biomolecules 2021, 11, 256. [Google Scholar] [CrossRef]
- Kiperstok, A.C.; Sebestyén, P.; Podola, B.; Melkonian, M. Biofilm cultivation of Haematococcus pluvialis enables a highly productive one-phase process for astaxanthin production using high light intensities. Algal Res. 2017, 21, 213–222. [Google Scholar] [CrossRef]
- Giannelli, L.; Yamada, H.; Katsuda, T.; Yamaji, H. Effects of temperature on the astaxanthin productivity and light harvesting characteristics of the green alga Haematococcus pluvialis. J. Biosci. Bioeng. 2015, 119, 345–350. [Google Scholar] [CrossRef]
- Hong, M.-E.; Hwang, S.K.; Chang, W.S.; Kim, B.W.; Lee, J.; Sim, S.J. Enhanced autotrophic astaxanthin production from Haematococcus pluvialis under high temperature via heat stress-driven Haber–Weiss reaction. Appl. Microbiol. Biotechnol. 2015, 99, 5203–5215. [Google Scholar] [CrossRef]
- Harker, M.; Tsavalos, A.J.; Young, A.J. Factors responsible for astaxanthin formation in the Chlorophyte Haematococcus pluvialis. Bioresour. Technol. 1996, 55, 207–214. [Google Scholar] [CrossRef]
- Kou, Y.; Liu, M.; Sun, P.; Dong, Z.; Liu, J. High light boosts salinity stress-induced biosynthesis of astaxanthin and lipids in the green alga Chromochloris zofingiensis. Algal Res. 2020, 50, 101976. [Google Scholar] [CrossRef]
- Kim, Y.-E.; Matter, I.A.; Lee, N.; Jung, M.; Lee, Y.-C.; Choi, S.-A.; Lee, S.Y.; Kim, J.R.; Oh, Y.-K. Enhancement of astaxanthin production by Haematococcus pluvialis using magnesium aminoclay nanoparticles. Bioresour. Technol. 2020, 307, 123270. [Google Scholar] [CrossRef]
- Grewe, C.; Griehl, C. Time- and media-dependent secondary carotenoid accumulation in Haematococcus pluvialis. Biotechnol. J. 2008, 3, 1232–1244. [Google Scholar] [CrossRef]
- Choi, Y.Y.; Joun, J.M.; Lee, J.; Hong, M.E.; Pham, H.-M.; Chang, W.S.; Sim, S.J. Development of large-scale and economic pH control system for outdoor cultivation of microalgae Haematococcus pluvialis using industrial flue gas. Bioresour. Technol. 2017, 244, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, C.; Jeon, M.S.; Park, J.; Choi, Y.E. Enhancement of microalga Haematococcus pluvialis growth and astaxanthin production by electrical treatment. Bioresour. Technol. 2018, 268, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-A.; Lee, S.Y.; Lee, J.; Cho, J.M.; Lee, J.-S.; Kim, S.W.; Kim, D.-Y.; Park, S.-K.; Jin, C.-S.; Oh, Y.-K. Rapid induction of edible lipids in Chlorella by mild electric stimulation. Bioresour. Technol. 2019, 292, 121950. [Google Scholar] [CrossRef] [PubMed]
- Pick, U.; Zarka, A.; Boussiba, S.; Davidi, L. A hypothesis about the origin of carotenoid lipid droplets in the green algae Dunaliella and Haematococcus. Planta 2019, 249, 31–47. [Google Scholar] [CrossRef]
- Praveenkumar, R.; Lee, K.; Lee, J.; Oh, Y.-K. Breaking dormancy: An energy-efficient means of recovering astaxanthin from microalgae. Green Chem. 2015, 17, 1226–1234. [Google Scholar] [CrossRef]
- Choi, S.-A.; Jeong, Y.; Lee, J.; Huh, Y.H.; Choi, S.H.; Kim, H.-S.; Cho, D.-H.; Lee, J.-S.; Kim, H.; An, H.-R.; et al. Biocompatible liquid-type carbon nanodots (C-paints) as light delivery materials for cell growth and astaxanthin induction of Haematococcus pluvialis. Mater. Sci. Eng. C 2020, 109, 110500. [Google Scholar] [CrossRef] [PubMed]
- Moon, G.; Lee, N.; Kang, S.; Park, J.; Kim, Y.-E.; Lee, S.-A.; Chitumalla, R.K.; Jang, J.; Choe, Y.; Oh, Y.-K.; et al. Hydrothermal synthesis of novel two-dimensional α-quartz nanoplates and their applications in energy-saving, high-efficiency, microalgal biorefineries. Chem. Eng. J. 2020, 127467. [Google Scholar] [CrossRef]
- Imamoglu, E.; Dalay, M.C.; Sukan, F.V. Influences of different stress media and high light intensities on accumulation of astaxanthin in the green alga Haematococcus pluvialis. New Biotechnol. 2009, 26, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.-K.; Hwang, K.-R.; Kim, C.; Kim, J.R.; Lee, J.-S. Recent developments and key barriers to advanced biofuels: A short review. Bioresour. Technol. 2018, 257, 320–333. [Google Scholar] [CrossRef]
- La, H.-J.; Choi, G.-G.; Cho, C.; Seo, S.-H.; Srivastava, A.; Jo, B.-H.; Lee, J.-Y.; Jin, Y.-S.; Oh, H.-M. Increased lipid productivity of Acutodesmus dimorphus using optimized pulsed electric field. J. Appl. Phycol. 2016, 28, 931–938. [Google Scholar] [CrossRef]
- Liang, J.; Tang, D.; Huang, L.; Chen, Y.; Ren, W.; Sun, J. High oxygen reduction reaction performance nitrogen-doped biochar cathode: A strategy for comprehensive utilizing nitrogen and carbon in water hyacinth. Bioresour. Technol. 2018, 267, 524–531. [Google Scholar] [CrossRef]
- Özcan, A.; Şahin, Y.; Savaş Koparal, A.; Oturan, M.A. Carbon sponge as a new cathode material for the electro-Fenton process: Comparison with carbon felt cathode and application to degradation of synthetic dye basic blue 3 in aqueous medium. J. Electroanal. Chem. 2008, 616, 71–78. [Google Scholar] [CrossRef]
- Wu, Y.; Guan, K.; Wang, Z.; Xu, B.; Zhao, F. Isolation, identification and characterization of an electrogenic microalgae strain. PLoS ONE 2013, 8, e73442. [Google Scholar] [CrossRef]
- Lee, S.Y.; Oh, Y.-K.; Lee, S.; Fitriana, H.N.; Moon, M.; Kim, M.-S.; Lee, J.; Min, K.; Park, G.W.; Lee, J.-P.; et al. Recent developments and key barriers to microbial CO2 electrobiorefinery. Bioresou. Technol. 2021, 320, 124350. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fitriana, H.-N.; Lee, S.-Y.; Choi, S.-A.; Lee, J.-Y.; Kim, B.-L.; Lee, J.-S.; Oh, Y.-K. Electric Stimulation of Astaxanthin Biosynthesis in Haematococcus pluvialis. Appl. Sci. 2021, 11, 3348. https://doi.org/10.3390/app11083348
Fitriana H-N, Lee S-Y, Choi S-A, Lee J-Y, Kim B-L, Lee J-S, Oh Y-K. Electric Stimulation of Astaxanthin Biosynthesis in Haematococcus pluvialis. Applied Sciences. 2021; 11(8):3348. https://doi.org/10.3390/app11083348
Chicago/Turabian StyleFitriana, Hana-Nur, Soo-Youn Lee, Sun-A Choi, Ji-Ye Lee, Bo-Lam Kim, Jin-Suk Lee, and You-Kwan Oh. 2021. "Electric Stimulation of Astaxanthin Biosynthesis in Haematococcus pluvialis" Applied Sciences 11, no. 8: 3348. https://doi.org/10.3390/app11083348
APA StyleFitriana, H.-N., Lee, S.-Y., Choi, S.-A., Lee, J.-Y., Kim, B.-L., Lee, J.-S., & Oh, Y.-K. (2021). Electric Stimulation of Astaxanthin Biosynthesis in Haematococcus pluvialis. Applied Sciences, 11(8), 3348. https://doi.org/10.3390/app11083348







