Featured Application
Engineered Methanosarcina acetivorans can be introduced to municipal wastewater to produce renewable bioisoprene.
Abstract
Wastewater biosolids are a promising feedstock for production of value-added renewable chemicals. Methane-producing archaea (methanogens) are already used to produce renewable biogas via the anaerobic treatment of wastewater. The ability of methanogens to efficiently convert dissolved organic carbon into methane makes them an appealing potential platform for biorefining using metabolic engineering. We have engineered a strain of the methanogen Methanosarcina acetivorans to produce the volatile hemiterpene isoprene in addition to methane. The engineered strain was adapted to grow in municipal wastewater through cultivation in a synthetic wastewater medium. When introduced to municipal wastewater the engineered methanogens were able to compete with the indigenous microorganisms and produce 0.97 mM of isoprene (65.9 ± 21.3 g per m3 of effluent). The production of isoprene in wastewater appears to be dependent on the quantity of available methanogenic substrate produced during upstream digestion by heterotrophic fermenters. This shows that with minimal adaptation it is possible to drop-in engineered methanogens to existing wastewater environments and attain value-added products in addition to the processing of wastewater. This shows the potential for utilizing methanogens as a platform for low-cost production of renewable materials without expensive feedstocks or the need to build or adapt existing facilities.
1. Introduction
Methane-producing archaea (methanogens) are obligate anaerobes which inhabit a keystone niche in the global carbon cycle, utilizing the endpoint degradation products of complex organic material and liberating otherwise inaccessible carbon [1,2,3,4]. Their unique metabolism and their potential to utilize a wide array of plentiful substrates make methanogens a subject of particular interest in industrial applications such as wastewater treatment [5,6,7], and the production of value-added products (Figure 1) [8,9]. Methanogens are used worldwide to reduce dissolved organic carbon in effluent as part of the wastewater treatment process. Wastewater treatment is a multistage process which is highly variant depending on the substrate being treated, though the end goal is largely the same: The detoxification of water by degrading complex biomass and pollutants before reintroducing the effluent into the water cycle. For the purpose of this study, we focused on the anaerobic digestion of municipal wastewater which primarily aims to remove dissolved carbon and suspended solids from a city’s water supply.
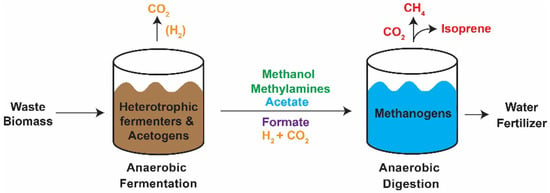
Figure 1.
Schematic representation of anaerobic digestion of waste biomass at the Theresa Street Water Resource Recovery facility in Lincoln, NE. After aerobic incubation, waste biomass is anaerobically digested in a two-step process. First, the complex biomolecules are degraded through heterotrophic fermentation to less complex substrates for methanogenic growth in the second stage. In the methanogenic bioreactor dissolved organic carbon is converted to biogas that can be recouped as a biofuel. Introduction of isoprene-synthesizing methanogens (e.g., strain NB 394 in which plasmid pJA2 expressing isoprene synthase is integrated onto the chromosome) to the second stage digester has potential to produce renewable isoprene in the captured biogas.
Municipal wastewater treatment generally occurs in three distinct stages based upon the aerobicity of the wastewater and the activity of microorganisms involved in the multistage process. In the first stage, aerobic microorganisms breakdown complex biomass into simpler organic material [10]. The deconvoluted material is further anaerobically digested by a second consortia of microbes into one- or two-carbon compounds and organic acids. These one- and two-carbon compounds are utilized by methanogens to complete the decomposition process [6,11]. In addition to removing polluting organic carbon from the water, anaerobic digestion has the added benefit of producing methane which is often captured as renewable biogas [11,12,13,14]. Due to the low energetic potential of methanogenic feedstocks, methanogens utilize a highly efficient central metabolism which greatly favors the production of methane over biomass and heat. Anaerobic treatment of wastewater results in 95% conversion of the initial substrate into available biogas with 5% being utilized for microbial biomass [15,16]. We hypothesized that the highly efficient metabolism of methanogens may have potential to produce high yields of other value-added products in addition to methane.
Isoprene (2-methyl-1,3-butadiene) is the primary component of natural rubber and an important chemical precursor utilized in the production of synthetic rubber as well as adhesives, flavorings, cosmetics, and pharmaceuticals. Traditionally, isoprene is harvested from natural rubber from tree sap or produced industrially through the thermal cracking of petroleum. By producing renewable isoprene via engineered microbes, it could be possible to reduce the need to rely on the harvesting of plant biomass or the mining of fossil fuels. Recently our laboratory demonstrated that the methanogen Methanosarcina acetivorans can be engineered to efficiently produce bioisoprene as a methane coproduct under laboratory conditions [9]. The gene for isoprene synthase, ispS, was stably inserted into the chromosome of M. acetivorans (att::ispS) and the production of isoprene as well as methane was confirmed via gas chromatography. The production of isoprene showed no detrimental effect on growth rate or metabolic efficiency of the engineered strains compared with a vector-only control. We surmised that without an obvious decrease in fitness it may be possible to drop-in these engineered methanogens into an existing anaerobic wastewater treatment consortium to produce bioisoprene. However, any inoculated methanogens would have to compete for substrate with wild methanogens in the mixed microbial community of the anaerobic digester. M. acetivorans has the largest genome of any characterized methanogen as well as the widest range of substrate utilization, allowing for growth from methanol, methyl-amines, carbon monoxide, and acetate [17,18]. We postulated that this metabolic flexibility would allow for our engineered strains to compete with the endogenous methanogens present in municipal wastewater resulting in an increase in methane production as well as the production of bioisoprene. The detection of isoprene in wastewater inoculated with our engineered strains with and without the supplementation of additional feed substrate supports our hypothesis.
2. Materials and Methods
2.1. Anaerobic Techniques
Anaerobic procedures were performed in a custom B-type Coy anaerobic chamber (Coy Labs, Grass Lake, MI, USA). The chamber was maintained at 35 °C with an atmosphere of 5% H2/20% CO2/75% N2 (±3%) (Matheson Gas, Lincoln, NE, USA). The Methanosarcina acetivorans strains used in this study are the isoprene-producing strain NB394 (Δhpt::φC31 int, att:pJA2) and the vector-only control NB452 (Δhpt::φC31 int, att:pNB730) [9]. Strains were cultured in anaerobic high-salt (HS) medium (200 mM NaCl, 45 mM NaHCO3, 13 mM KCl, 54 mM MgCl2·6H2O, 2 mM CaCl2·2H2O, 2 µM 0.1% resazurin (w/v), 5 mM KH2PO4, 19 mM NH4Cl, 2.8 mM cysteine·HCl, 0.1 mM Na2S·9H2O, trace elements, vitamin solution) [19] supplemented with a carbon and energy source (methanol, 125 mM; trimethylamine, 50 mM; sodium acetate, 120 mM) and 2 mg L−1 puromycin as needed [20,21]. Cells were incubated at 35 °C outside of anaerobic chamber in glass Balch tubes secured with butyl rubber stoppers (Belco Glass, Vineland, NJ, USA) and aluminum crimps (Wheaton, Millville, NJ, USA).
2.2. Synthetic Wastewater
Anaerobic synthetic wastewater (SWW) medium was developed to adapt cells from laboratory conditions to growth on municipal wastewater [22]. Chemical composition of SWW medium is based on OECD guidelines for testing of chemicals [23]. SWW is composed from 28 mg L−1 peptone, 100 mg L−1 meat extract, 100 mg L−1 urea, 161 µM KH2PO4, 120 µM NaCl, 27 µM CaCl2·2H2O, 8.7 µM MgCl2·6H2O, 0.23% agarose (w/v), and 3% evaporated milk (w/v), supplemented with a carbon and energy source: 125 mM of methanol, 50 mM of trimethylamine, or 120 mM of sodium acetate.
2.3. Methane Production Assay
Methane in culture headspace was measured by gas chromatography using a flame ionization detector (GC-FID) as previously described [24]. Briefly, 10 mL cultures were grown to stationary phase. After growth, 100 µL of headspace was captured using a gastight Hamilton syringe and transferred to an empty crimped 2 mL autosampler serum vial (Wheaton, Millville, NJ, USA). Vial contents were analyzed by flame ionization using a custom Agilent 7890A Gas Chromatography System (Agilent Technologies, Santa Clara, CA, USA, 2010). The GC was equipped with an autosampler for consistent sample injection and utilized a GS CarbonPLOT column (Agilent Technologies, Santa Clara, CA, USA) at 145 °C for separation of volatile metabolites. Quantification of methane was achieved by comparison to a methane standard curve (Matheson, Lincoln, NE, USA) ran in parallel with experimental samples.
2.4. Isoprene Production Assay
The same GC-FID system as above was deployed to quantify isoprene [22]. M. acetivorans strains were grown in 10 mL cultures with 1 mL paraffin oil overlay in Balch tubes. Once grown to stationary phase, the oil was harvested and transferred to a 2 mL stoppered and crimped autosampler vial. The GC-FID method for isoprene quantification was as follows: 160 °C for 35 min, ramp to 200 °C at 75 °C/min for 20 min, ramp to 275 °C at 75 °C/min for 20 min, 275 °C for 5 min, ramp to 160 °C at 75 °C/min to equilibrate the system for the next run. Isoprene quantification was achieved using a standard of known volumes of isoprene injected into 1 mL of paraffin oil in a 2 mL autosampler vial.
2.5. Municipal Wastewater Handling
Municipal wastewater sludge was collected from the City of Lincoln Teresa Street Water Resource Recovery Facility (Lincoln, NE, USA) [22]. Two different sludges were collected anaerobically: One after primary anaerobic digestion, and another after secondary anaerobic digestion and settling (before dewatering, disinfection, and discharge). Aliquots (~50 g) were transferred to serum bottles and methanol (5 µL g−1) was added as appropriate. Sludge samples were inoculated with 10% (w/v) SWW starter cultures and incubated at 35 °C without shaking. Methane and isoprene synthesis were measured as described above except 10 mL paraffin oil overlay was added. Isoprene was detectable but not accurately measurable in the headspace when paraffin oil was omitted. Sludge was not autoclaved until experiments were completed.
3. Results
3.1. Adapting Methanosarcina acetivorans to Growth in Wastewater
A challenge when introducing a laboratory methanogen strain to a wastewater environment is their sensitivity to changes in osmolarity. M. acetivorans was originally isolated from marine sediment and grows best under high-salt conditions (400 mM NaCl). When introduced directly to a comparatively low solute environment such as wastewater M. acetivorans rapidly lyses. To counteract this phenomenon, strains had to first be adapted to growth in synthetic wastewater (SWW), a complex growth medium containing mixed carbohydrates, lipids, and proteins that mimics the composition of wastewater digester solids [23]. Cultures of M. acetivorans were gradually adapted by supplementing our high-salt (HS) media with 10% (v/v) of synthetic wastewater every 24 h until the media reached a ratio of 50:50 HS:SWW (8 days; final NaCl concentration was 100.06 mM). At this point the methanogens were passaged into SWW supplemented with MeOH and confirmed to grow without lysis via autofluorescence (Figure 2).
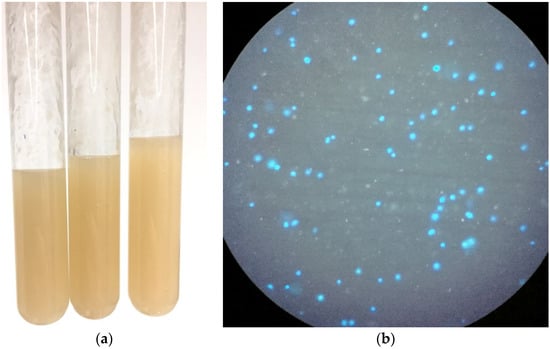
Figure 2.
Engineered methanogens adapted to synthetic wastewater. (a) Inoculated synthetic wastewater (SWW) in anaerobic Balch tubes. (b) Confirmation of live engineered methanogens in SWW under 400× optical magnification. Viable methanogens are irregular cocci (0.5–1 µm diameter) that fluoresce blue through a DAPI filter when UV illuminated.
3.2. Measurement of Methane and Isoprene Production on Wastewater
Once growth was achieved in SWW, cells were transferred to wastewater biosolids pre- and postdigest effluent (before and after second-stage anaerobic digestion) from the Teresa Street Water Resource Recovery Facility in Lincoln, NE (Figure 3). Microcosms with and without methanol supplementation were incubated for 5 days at 35 °C, after which time methane and isoprene yields were quantified (Table 1). Methane was detected from predigest effluent whether or not methanol was added, indicating that substrates for methanogens (both wild and engineered strains) were present in the effluent (Figure 4).

Figure 3.
Municipal wastewater collected from the Theresa Street Water Resource Recovery Facility in Lincoln, Nebraska. (Left) Organic solids before anaerobic digestion. (Right) Organic solids after anaerobic digestion.

Table 1.
Methane and isoprene production on wastewater biosolids.
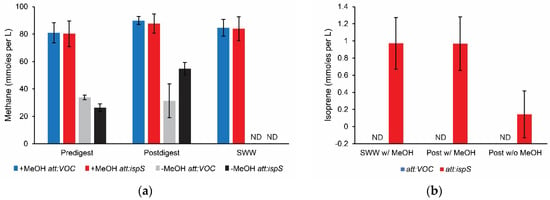
Figure 4.
Methane and isoprene production from waste biosolids. Endpoint methane assays after M. acetivorans strains were added to unsterilized wastewater biosolids digestate or synthetic wastewater (SWW) amended with methanol. (a) Methane production by isoprene-producing M. acetivorans (att:ispS) and vector only control (att:VOC) strains added to predigest effluent, postdigest effluent, or SWW with (w/MeOH) and without (w/o MeOH) added methanol. (b) Isoprene production by M. acetivorans att:VOC and att:ispS strains added to SWW or postdigest effluent amended with methanol and postdigest effluent without added substrates. Blue, att:VOC strain in which empty vector pNB730 is integrated onto the chromosome. Red, att:ispS strain in which plasmid pJA2 expressing isoprene synthase is integrated onto the chromosome. All data were obtained from triplicate biological and technical replicates (n = 9).
Methane yield was higher in the postdigest effluent than the predigest effluent, which was anticipated because wild methanogens are enriched during the anaerobic digestion treatment step. When methanol was added to SWW, pre- or postdigester effluent the methane yields were comparable to methane yields when isolated strains are grown in defined medium [9]. No methane was detected when samples were heat-killed by autoclaving microcosms before inoculating with engineered M. acetivorans, suggesting active fermentation by the digester microbial community is necessary to produce substrate unless the microcosm is supplemented with methanol. Isoprene was not detected from predigest effluent microcosms or when wastewater was heat-killed by autoclaving. Isoprene production was observed in postdigest effluent microcosms with and without addition of methanol (Figure 4). With methanol supplementation, isoprene yields were equivalent to SWW cultures, which was similar to the isoprene yield achieved by isolated strains grown in defined medium [9]. Based on these data we estimate up to 0.77 ± 0.25% of dissolved organic carbon in postdigest effluent was recovered as isoprene (Table 1). These data suggest engineered M. acetivorans can compete with wild methanogens in anaerobic digesters and isoprene can be detected in biogas from municipal waste. In pure batch culture the M. acetivorans ispS+ strain produces 0.89 mmol isoprene per mol methanol per gram of cells [9], which is 180× the yield of engineered Synechocystis cyanobacteria growing on CO2 [25]. In comparison, E. coli and Saccharomyces have been engineered to produce 352 and 175 mM isoprene, respectively, from glucose under fed-batch conditions [26,27]. It is unknown whether any of these engineered strains can synthesize isoprene from digester effluent as has been demonstrated by M. acetivorans in this work. Additional studies are needed to develop a commercially viable process that optimizes isoprene recovery from wastewater digester biogas streams.
4. Discussion
Our results confirm the hypothesis that engineered Methanosarcina acetivorans can survive in municipal wastewater and produce isoprene at detectable levels. Methane production was greater in wastewater biosolids which had undergone anaerobic digestion compared with those samples grown in preanaerobic digestion biosolids. While there is an incidental enrichment process during fermentation over time, the majority of microbes found in municipal wastewater are known bacterial gut symbionts such as Proteobacteria, Firmicutes, and Bacteroides [28], as well as a diverse collection of methanogens including Methanomicrobiales, Methanosarcinales, Methanobacteriales, and Methanobrevibacter spp. [29,30,31]. It has been well documented that these organisms exchange nutrients via syntrophy both in the gut and during wastewater treatment [32,33,34,35] and methanogen growth is dependent on metabolic byproducts of upstream microbial metabolism. The increase in methane production observed in samples grown in wastewater postanaerobic digestate suggests the engineered M. acetivorans ispS+ strains may be capable of participating in syntrophic relationships with other microbes in the digestate.
After four days of incubation at 35 °C methane was detected though no isoprene production was identified in samples grown in preanaerobic digested effluent, indicating that there may not be enough freely available methanogenic substrates for the engineered strains to compete with the existing microbial population. However, the postdigester effluent inoculated with our isoprene-producing M. acetivorans incubated under the same culture conditions yielded detectable bioisoprene (0.144 ± 0.273 mM). When this postdigest effluent was supplemented with 125 mM of MeOH the yield was increased to 0.968 ± 0.144 mM. This indicates that isoprene production from wastewater is primarily determined by the available substrate rather than environmental stressors. These results demonstrate that engineered methanogens are viable as a drop-in additive to wastewater treatment, and that the rate limiting factor for isoprene production is the rate at which the syntrophic microbial community can produce metabolites necessary for methanogen growth. By stimulating the microbial community, higher titers of bioisoprene production may be achieved.
Methanogens are a compelling source of renewable bioproducts due to their high substrate to product ratio efficiency. Here, we have demonstrated that with minor adaptation, it is possible to drop-in engineered methanogens to existing wastewater environments and attain value-added products in addition to the processing of wastewater. Due to existing capabilities for methane capture, many wastewater treatment facilities are already equipped with the infrastructure necessary for the capture of gaseous products such as isoprene. Separation of isoprene from biogas would require additional investment in biogas refining. However, isoprene separation from biogas streams is expected to be compatible with existing biogas upgrading technologies that are used to separate CO2 and enrich methane content to produce renewable natural gas. Our results suggest there may be promising potential for utilizing methanogens as a platform for low-cost production of synthetic materials without expensive feedstocks or extensive modification of existing renewable natural gas facilities.
Author Contributions
Conceptualization, N.R.B.; methodology, S.C., J.A., and N.R.B.; formal analysis, S.C., J.A., and N.R.B.; investigation, S.C. and J.A.; resources, N.R.B.; data curation, S.C. and J.A.; writing—original draft preparation, S.C. and N.R.B.; writing—review and editing, S.C., J.A., and N.R.B.; visualization, S.C., J.A., and N.R.B.; supervision, N.R.B.; project administration, N.R.B.; funding acquisition, N.R.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Water Environment and Research Foundation Grant NTRY6R14 and Nebraska Center for Energy Science awards and is described in US patent application US 20170175145 A1. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the funding agencies. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the funding agency.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Plasmids, strains, growth, and assay data that support the findings of this study are available from the corresponding author N.R.B. upon reasonable request.
Conflicts of Interest
N.R.B. has disclosed a significant financial interest in RollingCircle Biotech, LLC and Molecular Trait Evolution, LLC.
References
- Zinder, S.H. Physiological ecology of methanogens. In Methanogenesis; Springer: Boston, MA, USA, 1993; pp. 128–206. [Google Scholar] [CrossRef]
- Thauer, R.K.; Kaster, A.-K.; Seedorf, H.; Buckel, W.; Hedderich, R. Methanogenic archaea: Ecologically relevant differences in energy conservation. Nat. Rev. Microbiol. 2008, 6, 579–591. [Google Scholar] [CrossRef]
- Conrad, R. Microbial ecology of methanogens and methanotrophs. Adv. Agron. 2007, 96, 1–63. [Google Scholar]
- Lyu, Z.; Shao, N.; Akinyemi, T.; Whitman, W.B. Methanogenesis. Curr. Biol. 2018, 28, R727–R732. [Google Scholar] [CrossRef] [PubMed]
- Schiraldi, C.; Giuliano, M.; De Rosa, M. Perspectives on biotechnological applications of archaea. Archaea 2002, 1, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, M.; Rahim, R.A.; Abdullah, N.; Wright, A.-D.G.; Shirai, Y.; Sakai, K.; Sulaiman, A.; Hassan, M.A. Importance of the methanogenic archaea populations in anaerobic wastewater treatments. Process Biochem. 2010, 45, 1214–1225. [Google Scholar] [CrossRef]
- Chen, S.; Cheng, H.; Liu, J.; Hazen, T.C.; Huang, V.; He, Q. Unexpected competitiveness of Methanosaeta populations at elevated acetate concentrations in methanogenic treatment of animal wastewater. Appl. Microbiol. Biotechnol. 2017, 101, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Ferry, J.G.; Maranas, C.D.; Wood, T.K. Methane-to-Acetate Pathway for Producing Liquid Biofuels and Biorenewables. U.S. Patent 20150147791A1, 28 May 2015. [Google Scholar]
- Aldridge, J.; Carr, S.; Weber, K.A.; Buan, N.R. Anaerobic production of isoprene by engineered Methanosarcina spp. archaea. Appl. Environ. Microbiol. 2021. [Google Scholar] [CrossRef]
- Elalami, D.; Carrere, H.; Monlau, F.; Abdelouahdi, K.; Oukarroum, A.; Barakat, A. Pretreatment and co-digestion of wastewater sludge for biogas production: Recent research advances and trends. Renew. Sustain. Energy Rev. 2019, 114, 109287. [Google Scholar] [CrossRef]
- Vítězová, M.; Kohoutová, A.; Vítěz, T.; Hanišáková, N.; Kushkevych, I. Methanogenic Microorganisms in Industrial Wastewater Anaerobic Treatment. Processes 2020, 8, 1546. [Google Scholar] [CrossRef]
- Lettinga, G. Anaerobic digestion and wastewater treatment systems. Antonie Leeuwenhoek 1995, 67, 3–28. [Google Scholar] [CrossRef]
- Sekiguchi, Y.; Kamagata, Y.; Harada, H. Recent advances in methane fermentation technology. Curr. Opin. Biotechnol. 2001, 12, 277–282. [Google Scholar] [CrossRef]
- McCarty, P.L.; Bae, J.; Kim, J. Domestic wastewater treatment as a net energy producer—Can this be achieved? Environ. Sci. Technol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Van Lier, J.B.; Mahmoud, N.; Zeeman, G. Anaerobic wastewater treatment. In Biological Wastewater Treatment: Principles, Modelling and Design; IWA Publishing: London, UK, 2008; pp. 415–456. [Google Scholar]
- Dionisi, D. Biological Wastewater Treatment Processes: Mass and Heat Balances; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Galagan, J.E.; Nusbaum, C.; Roy, A.; Endrizzi, M.G.; Macdonald, P.; FitzHugh, W.; Calvo, S.; Engels, R.; Smirnov, S.; Atnoor, D. The genome of M. acetivorans reveals extensive metabolic and physiological diversity. Genome Res. 2002, 12, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Ferry, J.G. Methanosarcina acetivorans: A Model for Mechanistic Understanding of Aceticlastic and Reverse Methanogenesis. Front. Microbiol. 2020, 11, 1806. [Google Scholar] [CrossRef]
- Sowers, K.R.; Boone, J.E.; Gunsalus, R.P. Disaggregation of Methanosarcina spp. and Growth as Single Cells at Elevated Osmolarity. Appl. Environ. Microbiol. 1993, 59, 3832–3839. [Google Scholar] [CrossRef]
- Metcalf, W.W.; Zhang, J.K.; Apolinario, E.; Sowers, K.R.; Wolfe, R.S. A genetic system for Archaea of the genus Methanosarcina: Liposome-mediated transformation and construction of shuttle vectors. Proc. Natl. Acad. Sci. USA 1997, 94, 2626–2631. [Google Scholar] [CrossRef]
- Zhang, J.K.; White, A.K.; Kuettner, H.C.; Boccazzi, P.; Metcalf, W.W. Directed mutagenesis and plasmid-based complementation in the methanogenic archaeon Methanosarcina acetivorans C2A demonstrated by genetic analysis of proline biosynthesis. J. Bacteriol. 2002, 184, 1449–1454. [Google Scholar] [CrossRef]
- Aldridge, J.; Carr, S.; Weber, K.A.; Buan, N.R. Production of Bioisoprene from Wastewater; NTRY6R14/4822; The Water Research Foundation: Denver, CO, USA, 2018. [Google Scholar]
- OECD. Test No. 314: Simulation Tests to Assess the Biodegradability of Chemicals Discharged in Wastewater; OECD: Paris, France, 2008. [Google Scholar]
- Aldridge, J.T.; Catlett, J.L.; Smith, M.L.; Buan, N.R. Methods for Detecting Microbial Methane Production and Consumption by Gas Chromatography. Bio Protoc. 2016, 6, e1779. [Google Scholar] [CrossRef]
- Pade, N.; Erdmann, S.; Enke, H.; Dethloff, F.; Duhring, U.; Georg, J.; Wambutt, J.; Kopka, J.; Hess, W.R.; Zimmermann, R.; et al. Insights into isoprene production using the cyanobacterium Synechocystis sp. PCC 6803. Biotechnol. Biofuels 2016, 9, 89. [Google Scholar] [CrossRef]
- Yang, C.; Gao, X.; Jiang, Y.; Sun, B.; Gao, F.; Yang, S. Synergy between methylerythritol phosphate pathway and mevalonate pathway for isoprene production in Escherichia coli. Metab. Eng. 2016, 37, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Zhou, P.; Su, B.; Su, S.; Ye, L.; Yu, H. Enhanced Isoprene Production by Reconstruction of Metabolic Balance between Strengthened Precursor Supply and Improved Isoprene Synthase in Saccharomyces cerevisiae. ACS Synth. Biol. 2018, 7, 2308–2316. [Google Scholar] [CrossRef]
- Cabezas, A.; de Araujo, J.C.; Callejas, C.; Galès, A.; Hamelin, J.; Marone, A.; Sousa, D.Z.; Trably, E.; Etchebehere, C. How to use molecular biology tools for the study of the anaerobic digestion process? Rev. Environ. Sci. Bio/Technol. 2015, 14, 555–593. [Google Scholar] [CrossRef]
- Weiss, A.; Jérôme, V.; Freitag, R.; Mayer, H.K. Diversity of the resident microbiota in a thermophilic municipal biogas plant. Appl. Microbiol. Biotechnol. 2008, 81, 163–173. [Google Scholar] [CrossRef]
- Karakashev, D.; Batstone, D.J.; Angelidaki, I. Influence of environmental conditions on methanogenic compositions in anaerobic biogas reactors. Appl. Environ. Microbiol. 2005, 71, 331–338. [Google Scholar] [CrossRef]
- Ozgun, H.; Tao, Y.; Ersahin, M.E.; Zhou, Z.; Gimenez, J.B.; Spanjers, H.; van Lier, J.B. Impact of temperature on feed-flow characteristics and filtration performance of an upflow anaerobic sludge blanket coupled ultrafiltration membrane treating municipal wastewater. Water Res. 2015, 83, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Catlett, J.L.; Catazaro, J.; Cashman, M.; Carr, S.; Powers, R.; Cohen, M.B.; Buan, N.R. Metabolic Feedback Inhibition Influences Metabolite Secretion by the Human Gut Symbiont Bacteroides thetaiotaomicron. mSystems 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- McInerney, M.J.; Sieber, J.R.; Gunsalus, R.P. Microbial syntrophy: Ecosystem-level biochemical cooperation. Microbe Mag. 2011, 6, 479–485. [Google Scholar] [CrossRef][Green Version]
- Sieber, J.R.; McInerney, M.J.; Gunsalus, R.P. Genomic insights into syntrophy: The paradigm for anaerobic metabolic cooperation. Annu. Rev. Microbiol. 2012, 66, 429–452. [Google Scholar] [CrossRef]
- Worm, P.; Koehorst, J.J.; Visser, M.; Sedano-Núñez, V.T.; Schaap, P.J.; Plugge, C.M.; Sousa, D.Z.; Stams, A.J. A genomic view on syntrophic versus non-syntrophic lifestyle in anaerobic fatty acid degrading communities. Biochim. Biophys. Acta BBA Bioenerg. 2014, 1837, 2004–2016. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).