Antimicrobials in Dentistry
Abstract
1. Introduction
2. Antimicrobials Used in Dentistry
2.1. Chlorhexidine
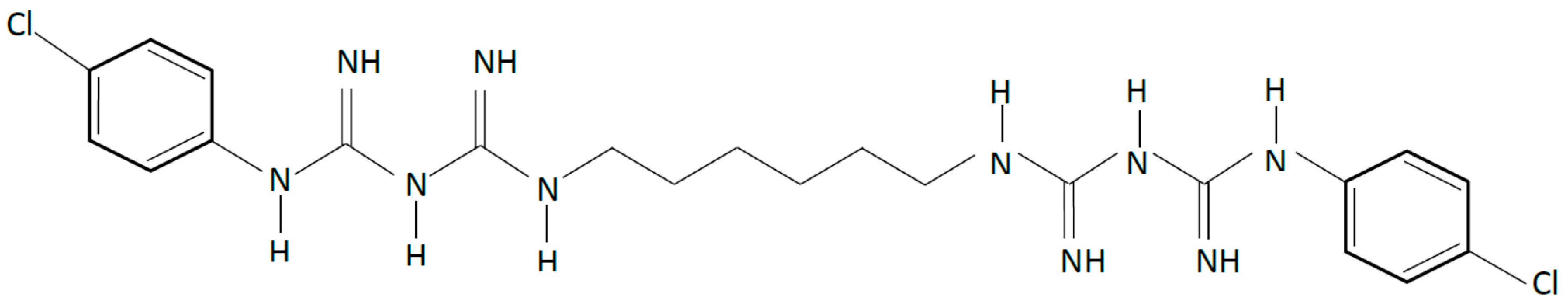
2.2. Quaternary Salts
2.3. Tetracyclines
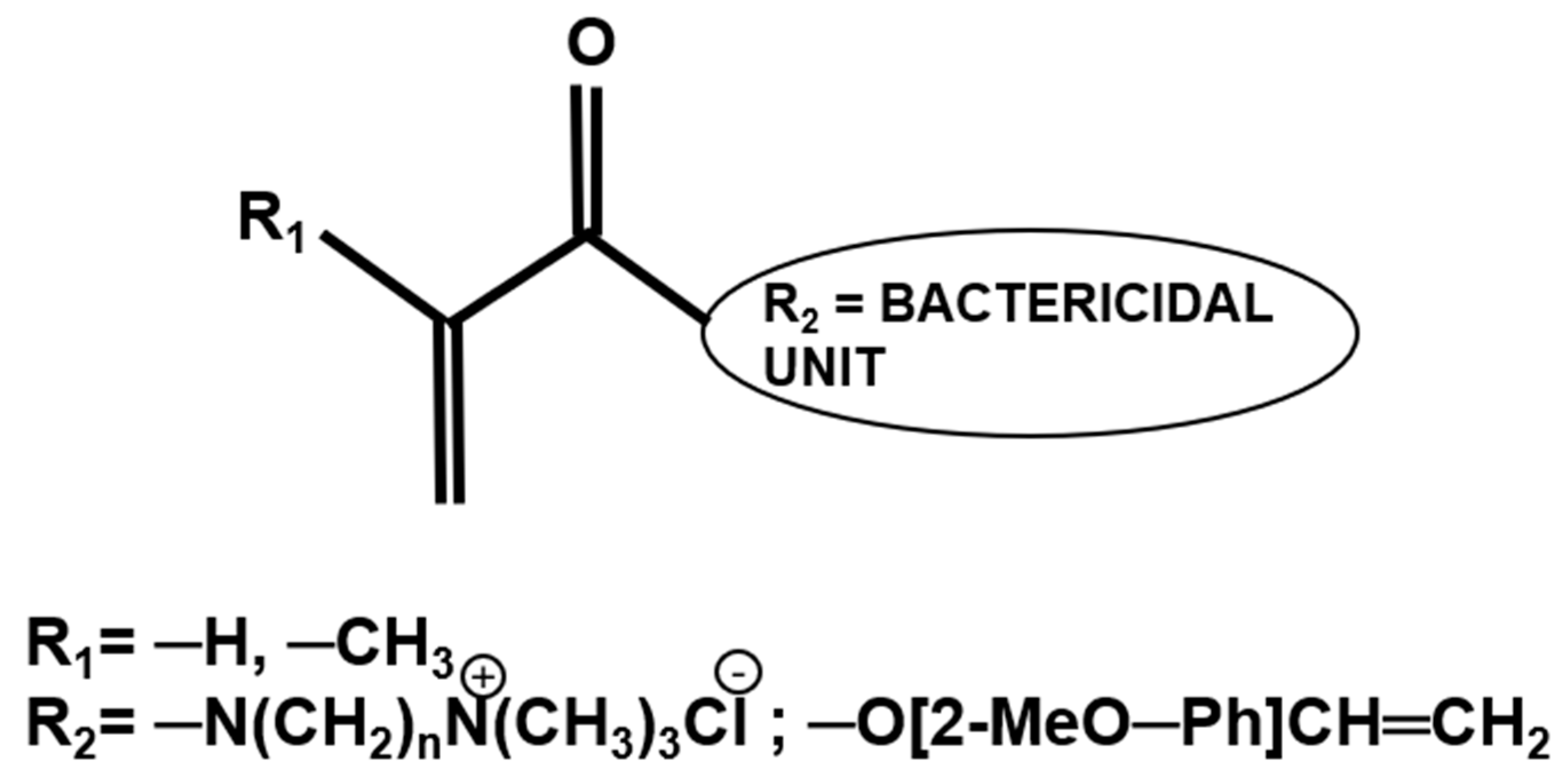
2.4. Antibacterial Monomers in Dental Restorative Systems
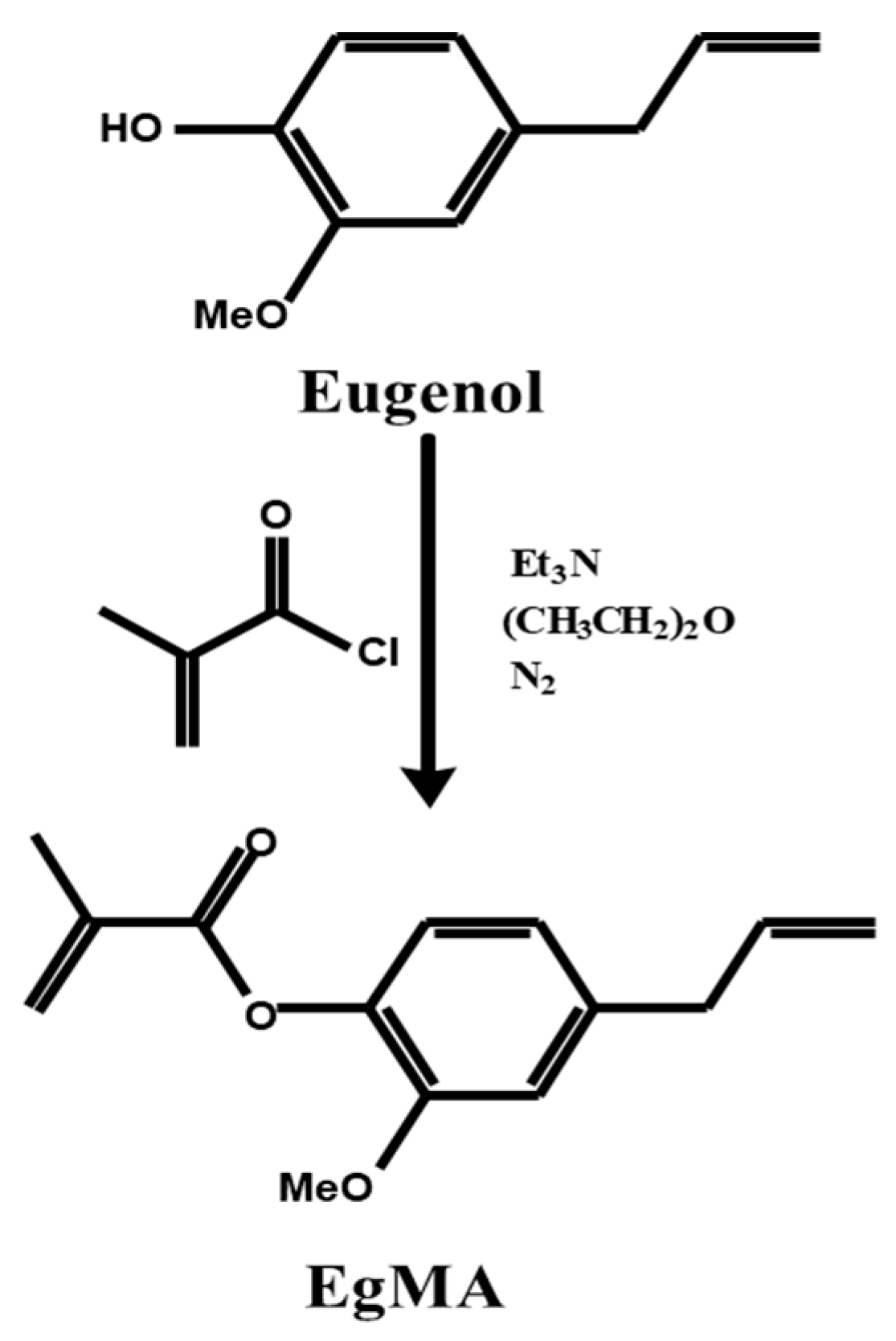
2.4.1. Eugenol and Eugenyl Methacrylate
2.4.2. Applications of Eugenyl Methacrylate
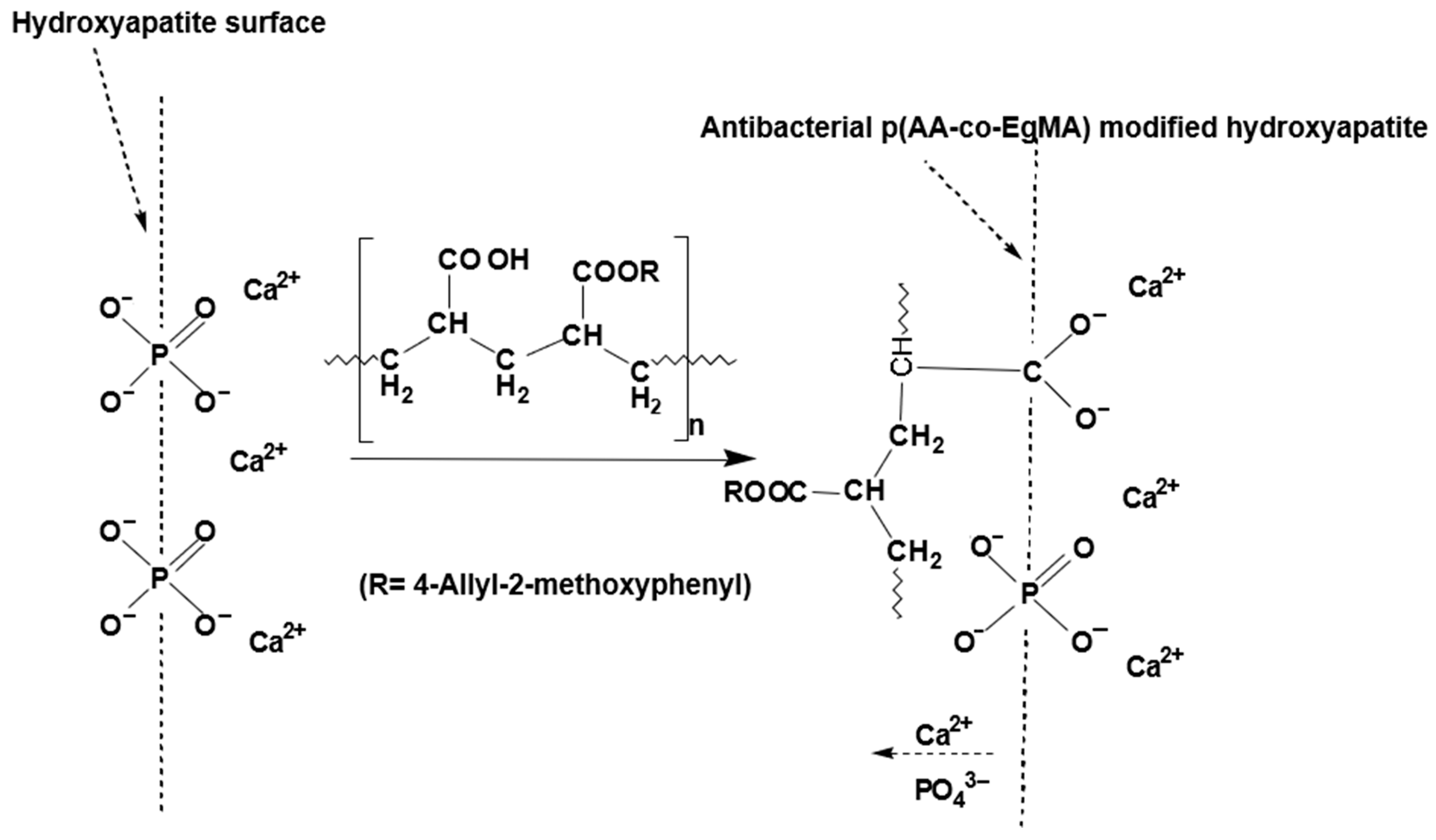
Eugenyl Methacrylate in Polyacrylic Acid for Glass-Ionomer Cements
Eugenyl Methacrylate in Resin Composites
Eugenyl Methacrylate in Dental Adhesive Systems
2.5. Chitosan
2.6. Graphene and Graphene Oxide
2.7. Antimicrobial Peptides
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). High Levels of Antibiotic Resistance Found Worldwide, New Data Shows; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- World Health Organization (WHO). Antimicrobial Resistance: Global Report on Surveillance; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- O’Neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations; Wellcome Collection: London, UK, 2014. [Google Scholar]
- Silver, L.L. Challenges of antibacterial discovery. Clin. Microbiol. Rev. 2011, 24, 71–109. [Google Scholar] [CrossRef] [PubMed]
- Coates, A.R.; Halls, G.; Hu, Y. Novel classes of antibiotics or more of the same? Br. J. Pharmacol. 2011, 163, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Frost, I.; Van Boeckel, T.P.; Pires, J.; Craig, J.; Laxminarayan, R. Global geographic trends in antimicrobial resistance: The role of international travel. J. Travel Med. 2019, 26, taz036. [Google Scholar] [CrossRef] [PubMed]
- Marcenes, W.; Kassebaum, N.J.; Bernabé, E.; Flaxman, A.; Naghavi, M.; Lopez, A.; Murray, C.J.L. Global burden of oral conditions in 1990–2010: A systematic analysis. J. Dent. Res. 2013, 92, 592–597. [Google Scholar] [CrossRef]
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers 2017, 3. [Google Scholar] [CrossRef] [PubMed]
- Martín-Del-campo, M.; Fernández-Villa, D.; Cabrera-Rueda, G.; Rojo, L. Antibacterial bio-based polymers for cranio-maxillofacial regeneration applications. Appl. Sci. 2020, 10, 8371. [Google Scholar] [CrossRef]
- Ferracane, J.L. Models of Caries Formation around Dental Composite Restorations. J. Dent. Res. 2017, 96, 364–371. [Google Scholar] [CrossRef]
- Chen, L.; Suh, B.I.; Yang, J. Antibacterial dental restorative materials: A review. Am. J. Dent. 2018, 31, 6B–12B. [Google Scholar]
- Ten Cate, J.M. Contemporary perspective on the use of fluoride products in caries prevention. Br. Dent. J. 2013, 214, 161–167. [Google Scholar] [CrossRef]
- Drummond, J.L. Degradation, fatigue, and failure of resin dental composite materials. J. Dent. Res. 2008, 87, 710–719. [Google Scholar] [CrossRef]
- Spencer, P.; Ye, Q.; Misra, A.; Goncalves, S.E.P.; Laurence, J.S. Proteins, pathogens, and failure at the composite-tooth interface. J. Dent. Res. 2014, 93, 1243–1249. [Google Scholar] [CrossRef]
- Nedeljkovic, I.; Teughels, W.; De Munck, J.; Van Meerbeek, B.; Van Landuyt, K.L. Is secondary caries with composites a material-based problem? Dent. Mater. 2015, 31, e247–e277. [Google Scholar] [CrossRef] [PubMed]
- Cocco, A.R.; De Oliveira Da Rosa, W.L.; Da Silva, A.F.; Lund, R.G.; Piva, E. A systematic review about antibacterial monomers used in dental adhesive systems: Current status and further prospects. Dent. Mater. 2015, 31, 1345–1362. [Google Scholar] [CrossRef] [PubMed]
- Dressano, D.; Salvador, M.V.; Oliveira, M.T.; Marchi, G.M.; Fronza, B.M.; Hadis, M.; Palin, W.M.; Lima, A.F. Chemistry of novel and contemporary resin-based dental adhesives. J. Mech. Behav. Biomed. Mater. 2020, 110, 103875. [Google Scholar] [CrossRef]
- Siqueira, J.F.; Rôças, I.N. Clinical Implications and Microbiology of Bacterial Persistence after Treatment Procedures. J. Endod. 2008, 34, 1291–1301.e3. [Google Scholar] [CrossRef] [PubMed]
- Zandi, H.; Petronijevic, N.; Mdala, I.; Kristoffersen, A.K.; Enersen, M.; Rôças, I.N.; Siqueira, J.F.; Ørstavik, D. Outcome of Endodontic Retreatment Using 2 Root Canal Irrigants and Influence of Infection on Healing as Determined by a Molecular Method: A Randomized Clinical Trial. J. Endod. 2019, 45, 1089–1098.e5. [Google Scholar] [CrossRef] [PubMed]
- Brookes, Z.L.S.; Bescos, R.; Belfield, L.A.; Ali, K.; Roberts, A. Current uses of chlorhexidine for management of oral disease: A narrative review. J. Dent. 2020, 103, 103497. [Google Scholar] [CrossRef]
- Hoffmann, T.; Bruhn, G.; Richter, S.; Netuschil, L.; Brecx, M. Clinical controlled study on plaque and gingivitis reduction under long-term use of low-dose chlorhexidine solutions in a population exhibiting good oral hygiene. Clin. Oral Investig. 2001, 5, 89–95. [Google Scholar] [CrossRef]
- Barnett, M.L. The rationale for the daily use of an antimicrobial mouthrinse. J. Am. Dent. Assoc. 2006, 137, S16–S21. [Google Scholar] [CrossRef]
- Bellis, C.A.; Addison, O.; Nobbs, A.H.; Duckworth, P.F.; Holder, J.A.; Barbour, M.E. Glass ionomer cements with milled, dry chlorhexidine hexametaphosphate filler particles to provide long-term antimicrobial properties with recharge capacity. Dent. Mater. 2018, 34, 1717–1726. [Google Scholar] [CrossRef]
- Bellis, C.A.; Nobbs, A.H.; O’Sullivan, D.J.; Holder, J.A.; Barbour, M.E. Glass ionomer cements functionalised with a concentrated paste of chlorhexidine hexametaphosphate provides dose-dependent chlorhexidine release over at least 14 months. J. Dent. 2016, 45, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Kamarudin, Y.; Skeats, M.K.; Ireland, A.J.; Barbour, M.E. Chlorhexidine hexametaphosphate as a coating for elastomeric ligatures with sustained antimicrobial properties: A laboratory study. Am. J. Orthod. Dentofac. Orthop. 2020, 158, e73–e82. [Google Scholar] [CrossRef] [PubMed]
- Van Strydonck, D.A.C.; Slot, D.E.; Van Der Velden, U.; Van Der Weijden, F. Effect of a chlorhexidine mouthrinse on plaque, gingival inflammation and staining in gingivitis patients: A systematic review. J. Clin. Periodontol. 2012, 39, 1042–1055. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D. Chlorhexidine mouthwash reduces plaque and gingivitis. Evid. Based Dent. 2013, 14, 17–18. [Google Scholar] [CrossRef]
- Pashley, D.H.; Tay, F.R.; Yiu, C.; Hashimoto, M.; Breschi, L.; Carvalho, R.M.; Ito, S. Collagen degradation by host-derived enzymes during aging. J. Dent. Res. 2004, 83, 216–221. [Google Scholar] [CrossRef]
- Scaffa, P.M.C.; Vidal, C.M.P.; Barros, N.; Gesteira, T.F.; Carmona, A.K.; Breschi, L.; Pashley, D.H.; Tjäderhane, L.; Tersariol, I.L.S.; Nascimento, F.D.; et al. Chlorhexidine inhibits the activity of dental cysteine cathepsins. J. Dent. Res. 2012, 91, 420–425. [Google Scholar] [CrossRef]
- Carrilho, M.R.O.; Geraldeli, S.; Tay, F.; De Goes, M.F.; Carvalho, R.M.; Tjäderhane, L.; Reis, A.F.; Hebling, J.; Mazzoni, A.; Breschi, L.; et al. In vivo preservation of the hybrid layer by chlorhexidine. J. Dent. Res. 2007, 86, 529–533. [Google Scholar] [CrossRef]
- Loguercio, A.D.; Hass, V.; Gutierrez, M.F.; Luque-Martinez, I.V.; Szezs, A.; Stanislawczuk, R.; Bandeca, M.C.; Reis, A. Five-year effects of chlorhexidine on the in vitro durability of resin/dentin interfaces. J. Adhes. Dent. 2016, 18, 35–42. [Google Scholar] [CrossRef]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef]
- Jiménez, M.; Abradelo, C.; San Román, J.; Rojo, L. Bibliographic review on the state of the art of strontium and zinc based regenerative therapies. Recent developments and clinical applications. J. Mater. Chem. B 2019, 7, 1974–1985. [Google Scholar] [CrossRef]
- De Munck, J.; Van Den Steen, P.E.; Mine, A.; Van Landuyt, K.L.; Poitevin, A.; Opdenakker, G.; Van Meerbeek, B. Inhibition of enzymatic degradation of adhesive-dentin interfaces. J. Dent. Res. 2009, 88, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tan, J.; Chen, L.; Li, D.; Tan, Y. The incorporation of chlorhexidine in a two-step self-etching adhesive preserves dentin bond in vitro. J. Dent. 2009, 37, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, E.M.; De Sá Rodrigues, C.U.F.; De Oliveira Matos, M.P.; De Carvalho, T.R.; Dos Santos, G.B.; Amaral, C.M. Experimental etch-and-rinse adhesive systems containing MMP-inhibitors: Physicochemical characterization and resin-dentin bonding stability. J. Dent. 2015, 43, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Ricci, H.A.; Sanabe, M.E.; de Souza Costa, C.A.; Pashley, D.H.; Hebling, J. Chlorhexidine increases the longevity of in vivo resin-dentin bonds. Eur. J. Oral Sci. 2010, 118, 411–416. [Google Scholar] [CrossRef]
- Kim, J.; Uchiyama, T.; Carrilho, M.; Agee, K.A.; Mazzoni, A.; Breschi, L.; Carvalho, R.M.; Tjäderhane, L.; Looney, S.; Wimmer, C.; et al. Chlorhexidine binding to mineralized versus demineralized dentin powder. Dent. Mater. 2010, 26, 771–778. [Google Scholar] [CrossRef]
- Sadek, F.T.; Braga, R.R.; Muench, A.; Liu, Y.; Pashley, D.H.; Tay, F.R. Ethanol wet-bonding challenges current anti-degradation strategy. J. Dent. Res. 2010, 89, 1499–1504. [Google Scholar] [CrossRef]
- Li, F.; Weir, M.D.; Xu, H.H.K. Effects of quaternary ammonium chain length on antibacterial bonding agents. J. Dent. Res. 2013, 92, 932–938. [Google Scholar] [CrossRef]
- Kwaśniewska, D.; Chen, Y.L.; Wieczorek, D. Biological activity of quaternary ammonium salts and their derivatives. Pathogens 2020, 9, 459. [Google Scholar] [CrossRef]
- Imazato, S. Bio-active restorative materials with antibacterial effects: New dimension of innovation in restorative dentistry. Dent. Mater. J. 2009, 28, 11–19. [Google Scholar] [CrossRef]
- Fik, C.P.; Konieczny, S.; Pashley, D.H.; Waschinski, C.J.; Ladisch, R.S.; Salz, U.; Bock, T.; Tiller, J.C. Telechelic Poly (2-oxazoline)s with a biocidal and a polymerizable terminal as collagenase inhibiting additive for long-term active antimicrobial dental materials. Macromol. Biosci. 2014, 14, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Caillier, L.; de Givenchy, E.T.; Levy, R.; Vandenberghe, Y.; Géribaldi, S.; Guittard, F. Synthesis and antimicrobial properties of polymerizable quaternary ammoniums. Eur. J. Med. Chem. 2009, 44, 3201–3208. [Google Scholar] [CrossRef]
- Antonucci, J.M.; Zeiger, D.N.; Tang, K.; Lin-Gibson, S.; Fowler, B.O.; Lin, N.J. Synthesis and characterization of dimethacrylates containing quaternary ammonium functionalities for dental applications. Dent. Mater. 2012, 28, 219–228. [Google Scholar] [CrossRef]
- He, J.; Söderling, E.; Vallittu, P.K.; Lassila, L.V.J. Investigation of double bond conversion, mechanical properties, and antibacterial activity of dental resins with different alkyl chain length quaternary ammonium methacrylate monomers (QAM). J. Biomater. Sci. Polym. Ed. 2013, 24, 565–573. [Google Scholar] [CrossRef]
- Huang, L.; Yu, F.; Sun, X.; Dong, Y.; Lin, P.T.; Yu, H.H.; Xiao, Y.H.; Chai, Z.G.; Xing, X.D.; Chen, J.H. Antibacterial activity of a modified unfilled resin containing a novel polymerizable quaternary ammonium salt MAE-HB. Sci. Rep. 2016, 6, 33858. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, S.; Zhou, X.; Xu, H.H.K.; Weir, M.D.; Ge, Y.; Li, M.; Wang, S.; Li, Y.; Xu, X.; et al. Effect of antibacterial dental adhesive on multispecies biofilms formation. J. Dent. Res. 2015, 94, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.H.; Ma, S.; Chen, J.H.; Chai, Z.G.; Li, F.; Wang, Y.J. Antibacterial activity and bonding ability of an adhesive incorporating an antibacterial monomer DMAE-CB. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 90 B, 813–817. [Google Scholar] [CrossRef]
- Pesci-Bardon, C.; Fosse, T.; Serre, D.; Madinier, I. In vitro antiseptic properties of an ammonium compound combined with denture base acrylic resin. Gerodontology 2006, 23, 111–116. [Google Scholar] [CrossRef]
- Deb, S.; Doiron, R.; DiSilvio, L.; Punyani, S.; Singh, H. PMMA bone cement containing a quaternary amine comonomer with potential antibacterial properties. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 85, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Tezvergil-Mutluay, A.; Agee, K.A.; Mazzoni, A.; Carvalho, R.M.; Carrilho, M.; Tersariol, I.L.; Nascimento, F.D.; Imazato, S.; Tjäderhane, L.; Breschi, L.; et al. Can quaternary ammonium methacrylates inhibit matrix MMPs and cathepsins? Dent. Mater. 2015, 31, e25–e32. [Google Scholar] [CrossRef]
- Jaberi Ansari, Z.; Sadr, A.; Moezizadeh, M.; Aminian, R.; Ghasemi, A.; Shimada, Y.; Tagami, J.; Jaberi Ansari, S.; Moayedi, S. Effects of one-year storage in water on bond strength of self-etching adhesives to enamel and dentin. Dent. Mater. J. 2008, 27, 266–272. [Google Scholar] [CrossRef][Green Version]
- Pupo, Y.M.; Farago, P.V.; Nadal, J.M.; Simão, L.C.; Esmerino, L.A.; Gomes, O.M.M.; Gomes, J.C. Effect of a novel quaternary ammonium methacrylate polymer (QAMP) on adhesion and antibacterial properties of dental adhesives. Int. J. Mol. Sci. 2014, 15, 8998–9015. [Google Scholar] [CrossRef] [PubMed]
- De Munck, J.; Mine, A.; Van den Steen, P.E.; Van Landuyt, K.L.; Poitevin, A.; Opdenakker, G.; Van Meerbeek, B. Enzymatic degradation of adhesive-dentin interfaces produced by mild self-etch adhesives. Eur. J. Oral Sci. 2010, 118, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Osorio, R.; Yamauti, M.; Osorio, E.; Ruiz-Requena, M.E.; Pashley, D.H.; Tay, F.R.; Toledano, M. Zinc reduces collagen degradation in demineralized human dentin explants. J. Dent. 2011, 39, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Frassetto, A.; Breschi, L.; Turco, G.; Marchesi, G.; Di Lenarda, R.; Tay, F.R.; Pashley, D.H.; Cadenaro, M. Mechanisms of degradation of the hybrid layer in adhesive dentistry and therapeutic agents to improve bond durability—A literature review. Dent. Mater. 2016, 32, e41–e53. [Google Scholar] [CrossRef] [PubMed]
- Boelen, G.J.; Boute, L.; D’Hoop, J.; EzEldeen, M.; Lambrichts, I.; Opdenakker, G. Matrix metalloproteinases and inhibitors in dentistry. Clin. Oral Investig. 2019, 23, 2823–2835. [Google Scholar] [CrossRef]
- Sulkala, M.; Wahlgren, J.; Larmas, M.; Sorsa, T.; Teronen, O.; Salo, T.; Tjäderhane, L. The Effects of MMP Inhibitors on Human Salivary MMP Activity and Caries Progression in Rats. J. Dent. Res. 2001, 80, 1545–1549. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.O.; Ceballos, G.; Villarreal, F.J. Tetracycline compounds with non-antimicrobial organ protective properties: Possible mechanisms of action. Pharmacol. Res. 2011, 63, 102–107. [Google Scholar] [CrossRef] [PubMed]
- De Castilho, A.R.F.; Duque, C.; Negrini, T.D.C.; Sacono, N.T.; De Paula, A.B.; Sacramento, P.A.; De Souza Costa, C.A.; Spolidorio, D.M.P.; Puppin-Rontani, R.M. Mechanical and biological characterization of resin-modified glass-ionomer cement containing doxycycline hyclate. Arch. Oral Biol. 2012, 57, 131–138. [Google Scholar] [CrossRef]
- De Castilho, A.R.F.; Duque, C.; Kreling, P.F.; Pereira, J.A.; de Aula, A.B.; Sinhoreti, M.A.C.; Puppin-Rontani, R.M. Doxycycline-containing glass ionomer cement for arresting residual caries: An in vitro study and a pilot trial. J. Appl. Oral Sci. 2018, 26, e20170116. [Google Scholar] [CrossRef]
- Fernández-Villa, D.; Aguilar, M.R.; Rojo, L. Folic acid antagonists: Antimicrobial and immunomodulating mechanisms and applications. Int. J. Mol. Sci. 2019, 20, 4996. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, P.; Wang, X.; Kasugai, S. A doxycycline-treated hydroxyapatite implant surface attenuates the progression of peri-implantitis: A radiographic and histological study in mice. Clin. Implant Dent. Relat. Res. 2019, 21, 154–159. [Google Scholar] [CrossRef]
- Semyari, H.; Salehi, M.; Taleghani, F.; Ehterami, A.; Bastami, F.; Jalayer, T.; Semyari, H.; Hamed Nabavi, M.; Semyari, H. Fabrication and characterization of collagen–hydroxyapatite-based composite scaffolds containing doxycycline via freeze-casting method for bone tissue engineering. J. Biomater. Appl. 2018, 33, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, E.R.; Abdel-Hay, F.I.; El-Shanshoury, A.E.R.R.; El-Newehy, M.H. Biologically active polymers. V. Synthesis and antimicrobial activity of modified poly(glycidyl methacrylate-co-2-hydroxyethyl methacrylate) derivatives with quaternary ammonium and phosphonium salts. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 2384–2393. [Google Scholar] [CrossRef]
- He, J.; Söderling, E.; Österblad, M.; Vallittu, P.K.; Lassila, L.V.J. Synthesis of methacrylate monomers with antibacterial effects against S. mutans. Molecules 2011, 16, 9755–9763. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadegan, A.; Ghahramani, Y.; Gholami, A.; Hemmateenejad, B.; Dorostkar, S.; Nabavizadeh, M.; Sharghi, H. The effect of charge at the surface of silver nanoparticles on antimicrobial activity against gram-positive and gram-negative bacteria: A preliminary study. J. Nanomater. 2015, 2015. ID 720654. [Google Scholar] [CrossRef]
- Makvandi, P.; Jamaledin, R.; Jabbari, M.; Nikfarjam, N.; Borzacchiello, A. Antibacterial quaternary ammonium compounds in dental materials: A systematic review. Dent. Mater. 2018, 34, 851–867. [Google Scholar] [CrossRef]
- Bai, X.; Lin, C.; Wang, Y.; Ma, J.; Wang, X.; Yao, X.; Tang, B. Preparation of Zn doped mesoporous silica nanoparticles (Zn-MSNs) for the improvement of mechanical and antibacterial properties of dental resin composites. Dent. Mater. 2020, 36, 794–807. [Google Scholar] [CrossRef] [PubMed]
- Rojo, L.; Deb, S. Polymer Therapeutics in Relation to Dentistry. Front. Oral Biol. 2015, 17, 13–21. [Google Scholar]
- Campos, E.; Branquinho, J.; Carreira, A.S.; Carvalho, A.; Coimbra, P.; Ferreira, P.; Gil, M.H. Designing polymeric microparticles for biomedical and industrial applications. Eur. Polym. J. 2013, 49, 2005–2021. [Google Scholar] [CrossRef]
- Jumina, J.; Mutmainah, M.; Purwono, B.; Kurniawan, Y.S.; Syah, Y.M. Antibacterial and antifungal activity of three monosaccharide monomyristate derivatives. Molecules 2019, 24, 3692. [Google Scholar] [CrossRef]
- dos Santos, A.; André, C.B.; Martim, G.C.; Schuquel, I.T.A.; Pfeifer, C.S.; Ferracane, J.L.; Tominaga, T.T.; Khalil, N.M.; Radovanovic, E.; Girotto, E.M. Methacrylate saccharide-based monomers for dental adhesive systems. Int. J. Adhes. Adhes. 2018, 87, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Huang, Q.; Liu, F.; Lin, Z.; He, J. Synthesis of antibacterial methacrylate monomer derived from thiazole and its application in dental resin. J. Mech. Behav. Biomed. Mater. 2015, 49, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Fugolin, A.P.; Dobson, A.; Huynh, V.; Mbiya, W.; Navarro, O.; Franca, C.M.; Logan, M.; Merritt, J.L.; Ferracane, J.L.; Pfeifer, C.S. Antibacterial, ester-free monomers: Polymerization kinetics, mechanical properties, biocompatibility and anti-biofilm activity. Acta Biomater. 2019, 100, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tang, Y.; Weir, M.D.; Gao, J.; Imazato, S.; Oates, T.W.; Lei, L.; Wang, S.; Hu, T.; Xu, H.H.K. Effects of S. mutans gene-modification and antibacterial monomer dimethylaminohexadecyl methacrylate on biofilm growth and acid production. Dent. Mater. 2020, 36, 296–309. [Google Scholar] [CrossRef]
- Kaplan, A.E.; Picca, M.; Gonzalez, M.I.; Macchi, R.L.; Molgatini, S.L. Antimicrobial effect of six endodontic sealers: An in vitro evaluation. Dent. Traumatol. 1999, 15, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Jendresen, M.D.; Phillips, R.W.; Swartz, M.L.; Norman, R.D. A comparative study of four zinc oxide and eugenol formulations as restorative materials. Part I. J. Prosthet. Dent. 1969, 21, 176–183. [Google Scholar] [CrossRef]
- Jendresen, M.D.; Phillips, R.W. A comparative study of four zinc oxide and eugenol formulations as restorative materials. Part II. J. Prosthet. Dent. 1969, 21, 300–309. [Google Scholar] [CrossRef]
- Millstein, P.L.; Nathanson, D. Effect of eugenol and eugenol cements on cured composite resin. J. Prosthet. Dent. 1983, 50, 211–215. [Google Scholar] [CrossRef]
- Fujisawa, S.; Kadoma, Y. Action of eugenol as a retarder against polymerization of methyl methacrylate by benzoyl peroxide. Biomaterials 1997, 18, 701–703. [Google Scholar] [CrossRef]
- Rojo, L.; Vazquez, B.; Parra, J.; Bravo, A.L.; Deb, S.; San Roman, J. From natural products to polymeric derivatives of “Eugenol”: A new approach for preparation of dental composites and orthopedic bone cements. Biomacromolecules 2006, 7, 2751–2761. [Google Scholar] [CrossRef]
- Rojo, L.; Barcenilla, J.M.; Vázquez, B.; González, R.; San Román, J. Intrinsically antibacterial materials based on polymeric derivatives of eugenol for biomedical applications. Biomacromolecules 2008, 9, 2530–2535. [Google Scholar] [CrossRef] [PubMed]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Weber, F.J.; De Bont, J.A.M. Adaptation mechanisms of microorganisms to the toxic effects of organic solvents on membranes. Biochim. Biophys. Acta Rev. Biomembr. 1996, 1286, 225–245. [Google Scholar] [CrossRef]
- Markowitz, K.; Moynihan, M.; Liu, M.; Kim, S. Biologic properties of eugenol and zinc oxide-eugenol. A clinically oriented review. Oral Surg. Oral Med. Oral Pathol. 1992, 73, 729–737. [Google Scholar] [CrossRef]
- Manabe, A.; Nakayama, S.; Sakamoto, K. Effects of Essential Oils on Erythrocytes and Hepatocytes from Rats and Dipalmitoyl Phosphatidylcholine-Liposomes. Jpn. J. Pharmacol. 1987, 44, 77–84. [Google Scholar] [CrossRef]
- Rojo, L.; Borzacchiello, A.; Parra, J.; Deb, S.; Vázquez, B.; San Román, J. The preparation of high conversion polymeric systems containing eugenol residues and their rheological characterization. J. Mater. Sci. Mater. Med. 2008, 19, 1467–1477. [Google Scholar] [CrossRef]
- Sidhu, S.; Nicholson, J. A Review of Glass-Ionomer Cements for Clinical Dentistry. J. Funct. Biomater. 2016, 7, 16. [Google Scholar] [CrossRef]
- Al-Taee, L.; Deb, S.; Banerjee, A. An in vitro assessment of the physical properties of manually-mixed and encapsulated glass-ionomer cements. BDJ Open 2020, 6, 1–7. [Google Scholar] [CrossRef]
- Bielas, R.; Mielańczyk, A.; Skonieczna, M.; Mielańczyk, Ł.; Neugebauer, D. Choline supported poly(ionic liquid) graft copolymers as novel delivery systems of anionic pharmaceuticals for anti-inflammatory and anti-coagulant therapy. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Gratzl, G.; Paulik, C.; Hild, S.; Guggenbichler, J.P.; Lackner, M. Antimicrobial activity of poly(acrylic acid) block copolymers. Mater. Sci. Eng. C 2014, 38, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Purushothaman, M.; Krishnan, P.S.G.; Nayak, S.K. Effect of butyl lactate methacrylate content on the properties of acrylic acid copolymers. Polym. Sci. Ser. A 2016, 58, 368–378. [Google Scholar] [CrossRef]
- Rojo, L.; Vázquez, B.; Román, J.S.; Deb, S. Eugenol functionalized poly(acrylic acid) derivatives in the formation of glass-ionomer cements. Dent. Mater. 2008, 24, 1709–1716. [Google Scholar] [CrossRef]
- Nicholson, J.W. Maturation processes in glass-ionomer dental cements. Acta Biomater. Odontol. Scand. 2018, 4, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.C.; Ruse, N.D. Acidity of glass ionomer cements during setting and its relation to pulp sensitivity. J. Am. Dent. Assoc. 1986, 112, 654–657. [Google Scholar] [CrossRef]
- Forsten, L. Fluoride release and uptake by glass-ionomers and related materials and its clinical effect. Biomaterials 1998, 19, 503–508. [Google Scholar] [CrossRef]
- Almaroof, A.; Rojo, L.; Mannocci, F.; Deb, S. A resin composite material containing an eugenol derivative for intracanal post cementation and core build-up restoration. Dent. Mater. 2016, 32, 149–160. [Google Scholar] [CrossRef]
- Almaroof, A.; Niazi, S.A.; Rojo, L.; Mannocci, F.; Deb, S. Influence of a polymerizable eugenol derivative on the antibacterial activity and wettability of a resin composite for intracanal post cementation and core build-up restoration. Dent. Mater. 2016, 32, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Finger, W.J.; Inoue, M.; Asmussen, E. Effect of wettability of adhesive resins on bonding to dentin. Am. J. Dent. 1994, 7, 35–38. [Google Scholar]
- Labella, R.; Lambrechts, P.; Van Meerbeek, B.; Vanherle, G. Polymerization shrinkage and elasticity of flowable composites and filled adhesives. Dent. Mater. 1999, 15, 128–137. [Google Scholar] [CrossRef]
- Moreira Da Silva, E.; Dos Santos, G.O.; Guimarães, J.G.A.; Barcellos, A.D.A.L.; Sampaio, E.M. The influence of C-factor, flexural modulus and viscous flow on gap formation in resin composite restorations. Oper. Dent. 2007, 32, 356–362. [Google Scholar] [CrossRef][Green Version]
- Swift, E.J.; Triolo, P.T.; Barkmeier, W.W.; Bird, J.L.; Bounds, S.J. Effect of low-viscosity resins on the performance of dental adhesives. Am. J. Dent. 1996, 9, 100–104. [Google Scholar]
- Rojo, L.; Vázquez, B.; Deb, S.; Román, J.S. Eugenol derivatives immobilized in auto-polymerizing formulations as an approach to avoid inhibition interferences and improve biofunctionality in dental and orthopedic cements. Acta Biomater. 2009, 5, 1616–1625. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, Q. Influence of surface energy of modified surfaces on bacterial adhesion. Biophys. Chem. 2005, 117, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Absolom, D.R.; Lamberti, F.V.; Policova, Z.; Zingg, W.; van Oss, C.J.; Neumann, A.W. Surface thermodynamics of bacterial adhesion. Appl. Environ. Microbiol. 1983, 46, 90–97. [Google Scholar] [CrossRef] [PubMed]
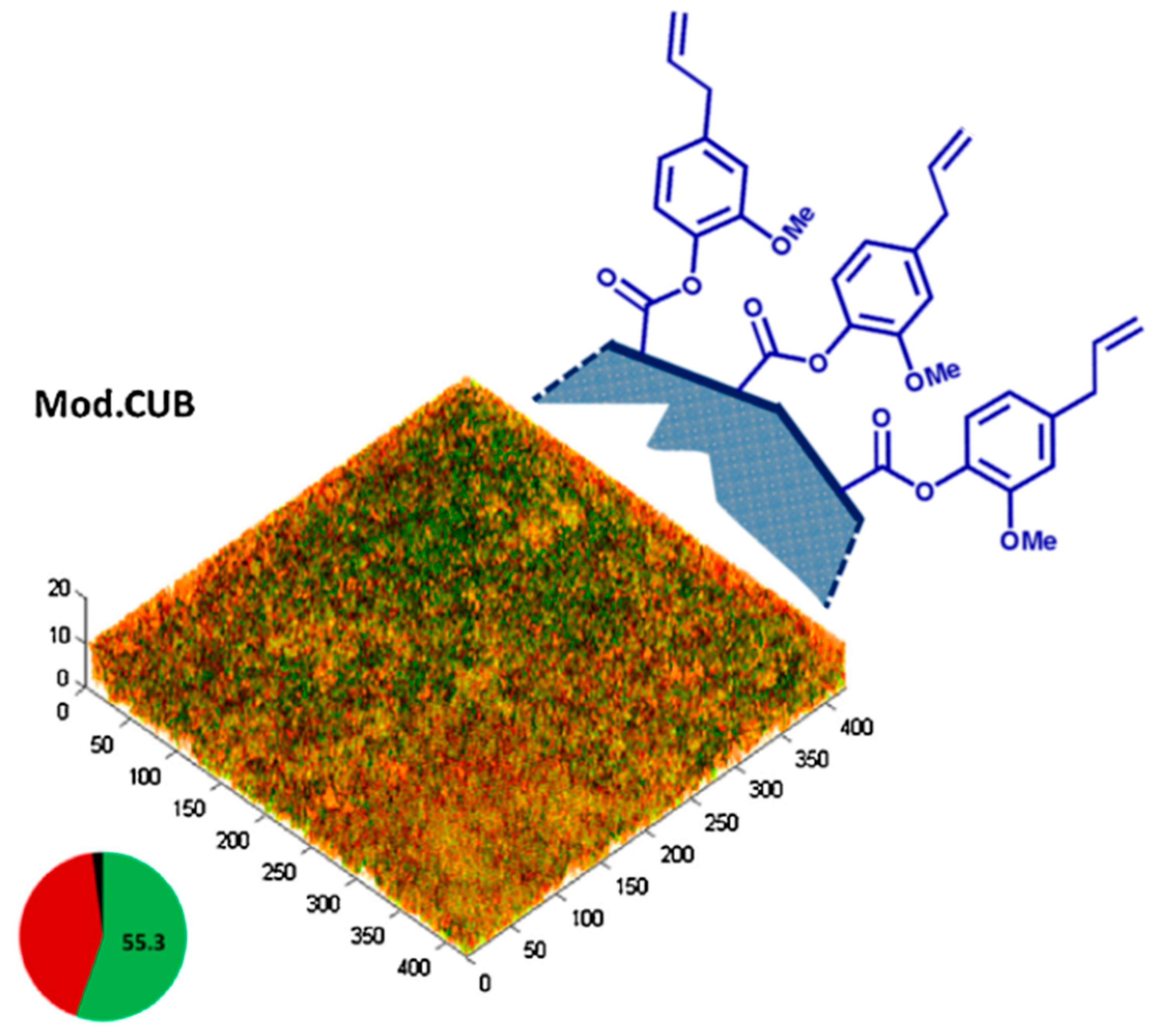
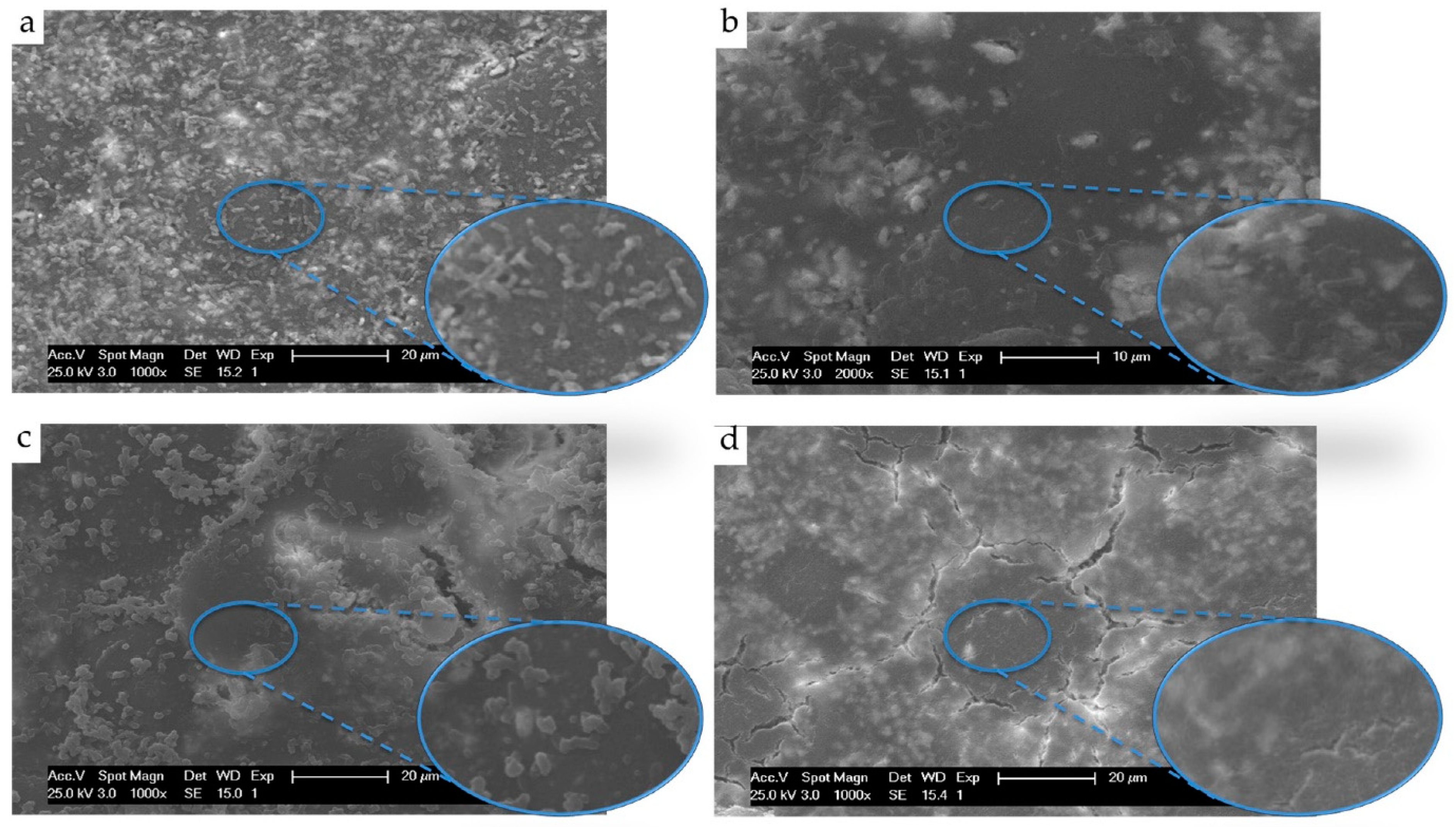
- Almaroof, A.; Niazi, S.A.; Rojo, L.; Mannocci, F.; Deb, S. Evaluation of dental adhesive systems incorporating an antibacterial monomer eugenyl methacrylate (EgMA) for endodontic restorations. Dent. Mater. 2017, 33, e239–e254. [Google Scholar] [CrossRef]
- Kurita, K. Chemistry and application of chitin and chitosan. Polym. Degrad. Stab. 1998, 59, 117–120. [Google Scholar] [CrossRef]
- Liu, X.F.; Guan, Y.L.; Yang, D.Z.; Li, Z.; Yao, K.D. Antibacterial action of chitosan and carboxymethylated chitosan. J. Appl. Polym. Sci. 2001, 79, 1324–1335. [Google Scholar] [CrossRef]
- No, H.K.; Young Park, N.; Ho Lee, S.; Meyers, S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.G.; Park, H.J.; Liu, C.G.; Liu, C.S.; Meng, X.H.; Yu, L.J. Effect of MW and concentration of chitosan on antibacterial activity of Escherichia coli. Carbohydr. Polym. 2006, 64, 60–65. [Google Scholar] [CrossRef]
- Chung, Y.C.; Chen, C.Y. Antibacterial characteristics and activity of acid-soluble chitosan. Bioresour. Technol. 2008, 99, 2806–2814. [Google Scholar] [CrossRef] [PubMed]
- Zamora, L.S.I.; Murillo, S.J.M.; Zapata, M.E.V.; Hernandez, J.H.M.; Valencia, C.H.; Rojo, L.; Grande Tovar, C.D. Influence of the chitosan morphology on the properties of acrylic cements and their biocompatibility. RSC Adv. 2020, 10, 31156–31164. [Google Scholar] [CrossRef]
- Zapata, M.E.V.; Hernandez, J.H.M.; Tovar, C.D.G.; Llano, C.H.V.; Vázquez-Lasa, B.; Román, J.S.; Rojo, L. Osseointegration of antimicrobial acrylic bone cements modified with graphene oxide and chitosan. Appl. Sci. 2020, 10, 6528. [Google Scholar] [CrossRef]
- Zhang, C.; Hui, D.; Du, C.; Sun, H.; Peng, W.; Pu, X.; Li, Z.; Sun, J.; Zhou, C. Preparation and application of chitosan biomaterials in dentistry. Int. J. Biol. Macromol. 2021, 167, 1198–1210. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, T.S.; Lussi, A. Combined effect of a fluoride-stannous-and chitosan-containing toothpaste and stannous-containing rinse on the prevention of initial enamel erosion-abrasion. J. Dent. 2014, 42, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Resende, A.H.M.; Farias, J.M.; Silva, D.D.B.; Rufino, R.D.; Luna, J.M.; Stamford, T.C.M.; Sarubbo, L.A. Application of biosurfactants and chitosan in toothpaste formulation. Colloids Surf. B Biointerfaces 2019, 181, 77–84. [Google Scholar] [CrossRef]
- Diolosà, M.; Donati, I.; Turco, G.; Cadenaro, M.; Di Lenarda, R.; Breschi, L.; Paoletti, S. Use of methacrylate-modified chitosan to increase the durability of dentine bonding systems. Biomacromolecules 2014, 15, 4606–4613. [Google Scholar] [CrossRef]
- Asensio, G.; Vázquez-Lasa, B.; Rojo, L. Achievements in the Topographic Design of Commercial Titanium Dental Implants: Towards Anti-Peri-Implantitis Surfaces. J. Clin. Med. 2019, 8, 1982. [Google Scholar] [CrossRef]
- Lestari, W.; Yusry, W.N.A.W.; Haris, M.S.; Jaswir, I.; Idrus, E. A glimpse on the function of chitosan as a dental hemostatic agent. Jpn. Dent. Sci. Rev. 2020, 56, 147–154. [Google Scholar] [CrossRef]
- Javed, R.; Rais, F.; Kaleem, M.; Jamil, B.; Ahmad, M.A.; Yu, T.; Qureshi, S.W.; Ao, Q. Chitosan capping of CuO nanoparticles: Facile chemical preparation, biological analysis, and applications in dentistry. Int. J. Biol. Macromol. 2021, 167, 1452–1467. [Google Scholar] [CrossRef]
- Nizami, M.Z.I.; Takashiba, S.; Nishina, Y. Graphene oxide: A new direction in dentistry. Appl. Mater. Today 2020, 19, 100576. [Google Scholar] [CrossRef]
- Tahriri, M.; Del Monico, M.; Moghanian, A.; Tavakkoli Yaraki, M.; Torres, R.; Yadegari, A.; Tayebi, L. Graphene and its derivatives: Opportunities and challenges in dentistry. Mater. Sci. Eng. C 2019, 102, 171–185. [Google Scholar] [CrossRef]
- He, J.; Zhu, X.; Qi, Z.; Wang, C.; Mao, X.; Zhu, C.; He, Z.; Li, M.; Tang, Z. Killing dental pathogens using antibacterial graphene oxide. ACS Appl. Mater. Interfaces 2015, 7, 5605–5611. [Google Scholar] [CrossRef]
- Lee, J.H.; Jo, J.K.; Kim, D.A.; Patel, K.D.; Kim, H.W.; Lee, H.H. Nano-graphene oxide incorporated into PMMA resin to prevent microbial adhesion. Dent. Mater. 2018, 34, e63–e72. [Google Scholar] [CrossRef] [PubMed]
- Zapata, M.E.V.; Hernandez, J.H.M.; Tovar, C.D.G.; Llano, C.H.V.; Escobar, J.A.D.; Vázquez-Lasa, B.; Román, J.S.; Rojo, L. Novel bioactive and antibacterial acrylic bone cement nanocomposites modified with graphene oxide and chitosan. Int. J. Mol. Sci. 2019, 20, 2938. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, K.; Niazi, S.; Mylonas, P.; Mannocci, F.; Deb, S. The synthesis of nano silver-graphene oxide system and its efficacy against endodontic biofilms using a novel tooth model. Dent. Mater. 2019, 35, 1614–1629. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Sun, L.C.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q.Y. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar] [PubMed]
- Kundu, R. Cationic Amphiphilic Peptides: Synthetic Antimicrobial Agents Inspired by Nature. Chem. Med. Chem. 2020, 15, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.Y.; Yin, I.X.; Wu, W.K.K.; Li, Q.L.; Mei, M.L.; Chu, C.H. Antimicrobial peptides for the prevention and treatment of dental caries: A concise review. Arch. Oral Biol. 2021, 122, 105022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Fang, Z.H.; Li, Q.L.; Cao, C.Y. A tooth-binding antimicrobial peptide to prevent the formation of dental biofilm. J. Mater. Sci. Mater. Med. 2019, 30, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Moussa, D.G.; Aparicio, C. Targeting the oral plaque microbiome with immobilized anti-biofilm peptides at tooth-restoration interfaces. PLoS ONE 2020, 15, e0235283. [Google Scholar] [CrossRef] [PubMed]
- Aida, K.L.; Kreling, P.F.; Caiaffa, K.S.; Calixto, G.M.F.; Chorilli, M.; Spolidorio, D.M.P.; Santos-Filho, N.A.; Cilli, E.M.; Duque, C. Antimicrobial peptide-loaded liquid crystalline precursor bioadhesive system for the prevention of dental caries. Int. J. Nanomed. 2018, 13, 3081–3091. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkattan, R.; Rojo, L.; Deb, S. Antimicrobials in Dentistry. Appl. Sci. 2021, 11, 3279. https://doi.org/10.3390/app11073279
Alkattan R, Rojo L, Deb S. Antimicrobials in Dentistry. Applied Sciences. 2021; 11(7):3279. https://doi.org/10.3390/app11073279
Chicago/Turabian StyleAlkattan, Rana, Luis Rojo, and Sanjukta Deb. 2021. "Antimicrobials in Dentistry" Applied Sciences 11, no. 7: 3279. https://doi.org/10.3390/app11073279
APA StyleAlkattan, R., Rojo, L., & Deb, S. (2021). Antimicrobials in Dentistry. Applied Sciences, 11(7), 3279. https://doi.org/10.3390/app11073279






