Elastography—A Bona Fide Non-Invasive Method for Assessing Non-Alcoholic Fatty Liver Disease in Children
Abstract
1. Introduction
2. Current Elastography Methods
3. The Opportunities and Challenges of Ultrasound-Based Elastography in Children NAFLD
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- WHO | Overweight and Obesity. Available online: http://www.who.int/gho/ncd/risk_factors/overweight_adolescents_text/en/ (accessed on 27 March 2021).
- Mǎrginean, C.O.; Mǎrginean, C.; Meliţ, L.E. New Insights Regarding Genetic Aspects of Childhood Obesity: A Minireview. Front Pediatr. 2018, 6, 271. [Google Scholar] [CrossRef]
- Mărginean, C.O.; Meliţ, L.E.; Huţanu, A.; Ghiga, D.V.; Săsăran, M.O. The Gap between Overweight and Obesity Status in Children - (STROBE-Compliant Article). Medicine (Baltimore) 2021, 100, e24520. [Google Scholar] [CrossRef]
- Pecht, T.; Gutman-Tirosh, A.; Bashan, N.; Rudich, A. Peripheral Blood Leucocyte Subclasses as Potential Biomarkers of Adipose Tissue Inflammation and Obesity Subphenotypes in Humans. Obes. Rev. 2014, 15, 322–337. [Google Scholar] [CrossRef]
- Mărginean, C.; Meliț, L.; Ghiga, D.; Mărginean, M. Early Inflammatory Status Related to Pediatric Obesity (STROBE Compliant Article). Front Pediatr. 2019, 7. [Google Scholar] [CrossRef]
- Mărginean, C.O.; Meliţ, L.E.; Huțanu, A.; Ghiga, D.V.; Săsăran, M.O. The Adipokines and Inflammatory Status in the Era of Pediatric Obesity. Cytokine 2020, 126, 154925. [Google Scholar] [CrossRef] [PubMed]
- Schwimmer, J.B.; Deutsch, R.; Kahen, T.; Lavine, J.E.; Stanley, C.; Behling, C. Prevalence of Fatty Liver in Children and Adolescents. Pediatrics 2006, 118, 1388–1393. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Noureddin, M.; Bernstein, D.; Kwo, P.; Russo, M.; Shiffman, M.L.; Younes, Z.; Abdelmalek, M. Role of Noninvasive Tests in Clinical Gastroenterology Practices to Identify Patients With Nonalcoholic Steatohepatitis at High Risk of Adverse Outcomes: Expert Panel Recommendations. Am. J. Gastroenterol. 2021, 116, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, V.W.; Stram, D.O.; Porcel, J.; Lu, S.C.; Le Marchand, L.; Noureddin, M. Prevalence of Chronic Liver Disease and Cirrhosis by Underlying Cause in Understudied Ethnic Groups: The Multiethnic Cohort. Hepatology 2016, 64, 1969–1977. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, B.; Shah, V.; Onyshchenko, M.; Elshamy, M.; Aucejo, F.; Lopez, R.; Hanouneh, I.A.; Alhaddad, R.; Alkhouri, N. Characterization of Hepatocellular Carcinoma (HCC) in Non-Alcoholic Fatty Liver Disease (NAFLD) Patients without Cirrhosis. Hepatol. Int. 2016, 10, 632–639. [Google Scholar] [CrossRef]
- Groselj, U.; Tansek, M.Z.; Smon, A.; Angelkova, N.; Anton, D.; Baric, I.; Djordjevic, M.; Grimci, L.; Ivanova, M.; Kadam, A.; et al. Newborn Screening in Southeastern Europe. Mol. Genet. Metab. 2014, 113, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Zerjav Tansek, M.; Groselj, U.; Angelkova, N.; Anton, D.; Baric, I.; Djordjevic, M.; Grimci, L.; Ivanova, M.; Kadam, A.; Kotori, V.; et al. Phenylketonuria Screening and Management in Southeastern Europe - Survey Results from 11 Countries. Orphanet J. Rare Dis. 2015, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, A.E.; Charatcharoenwitthaya, P.; Treeprasertsuk, S.; Benson, J.T.; Enders, F.B.; Angulo, P. The Natural History of Non-Alcoholic Fatty Liver Disease in Children: A Follow-up Study for up to 20 Years. Gut 2009, 58, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The Diagnosis and Management of Nonalcoholic Fatty Liver Disease: Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; S Sulkowski, M.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a Simple Noninvasive Index to Predict Significant Fibrosis in Patients with HIV/HCV Coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Vilar-Gomez, E.; Chalasani, N. Non-Invasive Assessment of Non-Alcoholic Fatty Liver Disease: Clinical Prediction Rules and Blood-Based Biomarkers. J. Hepatol. 2018, 68, 305–315. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Lawitz, E.J.; Alkhouri, N.; Wong, V.W.-S.; Romero-Gomez, M.; Okanoue, T.; Trauner, M.; Kersey, K.; Li, G.; Han, L.; et al. Noninvasive Tests Accurately Identify Advanced Fibrosis Due to NASH: Baseline Data From the STELLAR Trials. Hepatology 2019, 70, 1521–1530. [Google Scholar] [CrossRef]
- Cho, Y.; Tokuhara, D.; Morikawa, H.; Kuwae, Y.; Hayashi, E.; Hirose, M.; Hamazaki, T.; Tanaka, A.; Kawamura, T.; Kawada, N.; et al. Transient Elastography-Based Liver Profiles in a Hospital-Based Pediatric Population in Japan. PLoS ONE 2015, 10, e0137239. [Google Scholar] [CrossRef]
- Mărginean, C.O.; Meliţ, L.E.; Ghiga, D.V.; Săsăran, M.O. The Assessment of Liver Fibrosis in Children with Obesity on Two Methods: Transient and Two Dimensional Shear Wave Elastography. Sci. Rep. 2019, 9, 19800. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global Epidemiology of Nonalcoholic Fatty Liver Disease-Meta-Analytic Assessment of Prevalence, Incidence, and Outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. NAFLD: A Multisystem Disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Stepanova, M.; Afendy, M.; Fang, Y.; Younossi, Y.; Mir, H.; Srishord, M. Changes in the Prevalence of the Most Common Causes of Chronic Liver Diseases in the United States from 1988 to 2008. Clin. Gastroenterol. Hepatol. 2011, 9, 524–530.e1; quiz e60. [Google Scholar] [CrossRef] [PubMed]
- Ting, Y.-W.; Wong, S.-W.; Anuar Zaini, A.; Mohamed, R.; Jalaludin, M.Y. Metabolic Syndrome Is Associated With Advanced Liver Fibrosis Among Pediatric Patients With Non-Alcoholic Fatty Liver Disease. Front Pediatr. 2019, 7, 491. [Google Scholar] [CrossRef] [PubMed]
- Stål, P. Liver Fibrosis in Non-Alcoholic Fatty Liver Disease - Diagnostic Challenge with Prognostic Significance. World J. Gastroenterol. 2015, 21, 11077–11087. [Google Scholar] [CrossRef] [PubMed]
- Hoodeshenas, S.; Yin, M.; Venkatesh, S.K. Magnetic Resonance Elastography of Liver: Current Update. Top Magn. Reson. Imaging 2018, 27, 319–333. [Google Scholar] [CrossRef]
- Serai, S.D.; Trout, A.T.; Sirlin, C.B. Elastography to Assess the Stage of Liver Fibrosis in Children: Concepts, Opportunities, and Challenges. Clin. Liver Dis. (Hoboken) 2017, 9, 5–10. [Google Scholar] [CrossRef]
- DiPaola, F.W.; Schumacher, K.R.; Goldberg, C.S.; Friedland-Little, J.; Parameswaran, A.; Dillman, J.R. Effect of Fontan Operation on Liver Stiffness in Children with Single Ventricle Physiology. Eur. Radiol. 2017, 27, 2434–2442. [Google Scholar] [CrossRef]
- Barr, R.G.; Ferraioli, G.; Palmeri, M.L.; Goodman, Z.D.; Garcia-Tsao, G.; Rubin, J.; Garra, B.; Myers, R.P.; Wilson, S.R.; Rubens, D.; et al. Elastography Assessment of Liver Fibrosis: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology 2015, 276, 845–861. [Google Scholar] [CrossRef]
- Hanquinet, S.; Rougemont, A.-L.; Courvoisier, D.; Rubbia-Brandt, L.; McLin, V.; Tempia, M.; Anooshiravani, M. Acoustic Radiation Force Impulse (ARFI) Elastography for the Noninvasive Diagnosis of Liver Fibrosis in Children. Pediatr. Radiol. 2013, 43, 545–551. [Google Scholar] [CrossRef]
- Samir, A.E.; Dhyani, M.; Vij, A.; Bhan, A.K.; Halpern, E.F.; Méndez-Navarro, J.; Corey, K.E.; Chung, R.T. Shear-Wave Elastography for the Estimation of Liver Fibrosis in Chronic Liver Disease: Determining Accuracy and Ideal Site for Measurement. Radiology 2015, 274, 888–896. [Google Scholar] [CrossRef]
- Dhyani, M.; Anvari, A.; Samir, A.E. Ultrasound Elastography: Liver. Abdom. Imaging 2015, 40, 698–708. [Google Scholar] [CrossRef]
- Venkatesh, S.K.; Ehman, R.L. Magnetic Resonance Elastography of Abdomen. Abdom. Imaging 2015, 40, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Sawh, M.C.; Newton, K.P.; Goyal, N.P.; Angeles, J.E.; Harlow, K.; Bross, C.; Schlein, A.N.; Hooker, J.C.; Sy, E.Z.; Glaser, K.J.; et al. Normal Range for MR Elastography Measured Liver Stiffness in Children without Liver Disease. J. Magn. Reson. Imaging 2020, 51, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Trout, A.T.; Anupindi, S.A.; Gee, M.S.; Khanna, G.; Xanthakos, S.A.; Serai, S.D.; Baikpour, M.; Calle-Toro, J.S.; Ozturk, A.; Zhang, B.; et al. Normal Liver Stiffness Measured with MR Elastography in Children. Radiology 2020, 297, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Berzigotti, A.; Ferraioli, G.; Bota, S.; Gilja, O.H.; Dietrich, C.F. Novel Ultrasound-Based Methods to Assess Liver Disease: The Game Has Just Begun. Dig. Liver Dis. 2018, 50, 107–112. [Google Scholar] [CrossRef]
- Serai, S.D.; Wallihan, D.B.; Venkatesh, S.K.; Ehman, R.L.; Campbell, K.M.; Sticka, J.; Marino, B.S.; Podberesky, D.J. Magnetic Resonance Elastography of the Liver in Patients Status-Post Fontan Procedure: Feasibility and Preliminary Results. Congenit. Heart Dis. 2014, 9, 7–14. [Google Scholar] [CrossRef]
- Singh, S.; Venkatesh, S.K.; Wang, Z.; Miller, F.H.; Motosugi, U.; Low, R.N.; Hassanein, T.; Asbach, P.; Godfrey, E.M.; Yin, M.; et al. Diagnostic Performance of Magnetic Resonance Elastography in Staging Liver Fibrosis: A Systematic Review and Meta-Analysis of Individual Participant Data. Clin. Gastroenterol. Hepatol. 2015, 13, 440–451.e6. [Google Scholar] [CrossRef]
- Besa, C.; Wagner, M.; Lo, G.; Gordic, S.; Chatterji, M.; Kennedy, P.; Stueck, A.; Thung, S.; Babb, J.; Smith, A.; et al. Detection of Liver Fibrosis Using Qualitative and Quantitative MR Elastography Compared to Liver Surface Nodularity Measurement, Gadoxetic Acid Uptake, and Serum Markers. J. Magn. Reson. Imaging 2018, 47, 1552–1561. [Google Scholar] [CrossRef]
- Yin, M.; Glaser, K.J.; Talwalkar, J.A.; Chen, J.; Manduca, A.; Ehman, R.L. Hepatic MR Elastography: Clinical Performance in a Series of 1377 Consecutive Examinations. Radiology 2016, 278, 114–124. [Google Scholar] [CrossRef]
- Etchell, E.; Jugé, L.; Hatt, A.; Sinkus, R.; Bilston, L.E. Liver Stiffness Values Are Lower in Pediatric Subjects than in Adults and Increase with Age: A Multifrequency MR Elastography Study. Radiology 2017, 283, 222–230. [Google Scholar] [CrossRef]
- Yin, M.; Talwalkar, J.A.; Glaser, K.J.; Manduca, A.; Grimm, R.C.; Rossman, P.J.; Fidler, J.L.; Ehman, R.L. Assessment of Hepatic Fibrosis with Magnetic Resonance Elastography. Clin. Gastroenterol. Hepatol. 2007, 5, 1207–1213.e2. [Google Scholar] [CrossRef]
- Kim, B.H.; Lee, J.M.; Lee, Y.J.; Lee, K.B.; Suh, K.-S.; Han, J.K.; Choi, B.I. MR Elastography for Noninvasive Assessment of Hepatic Fibrosis: Experience from a Tertiary Center in Asia. J. Magn. Reson. Imaging 2011, 34, 1110–1116. [Google Scholar] [CrossRef]
- Venkatesh, S.K.; Wang, G.; Lim, S.G.; Wee, A. Magnetic Resonance Elastography for the Detection and Staging of Liver Fibrosis in Chronic Hepatitis B. Eur. Radiol. 2014, 24, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Huwart, L.; Peeters, F.; Sinkus, R.; Annet, L.; Salameh, N.; ter Beek, L.C.; Horsmans, Y.; Van Beers, B.E. Liver Fibrosis: Non-Invasive Assessment with MR Elastography. NMR Biomed. 2006, 19, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Talwalkar, J.A.; Yin, M.; Glaser, K.J.; Sanderson, S.O.; Ehman, R.L. Early Detection of Nonalcoholic Steatohepatitis in Patients with Nonalcoholic Fatty Liver Disease by Using MR Elastography. Radiology 2011, 259, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Morisaka, H.; Motosugi, U.; Ichikawa, S.; Nakazawa, T.; Kondo, T.; Funayama, S.; Matsuda, M.; Ichikawa, T.; Onishi, H. Magnetic Resonance Elastography Is as Accurate as Liver Biopsy for Liver Fibrosis Staging. J. Magn. Reson. Imaging 2018, 47, 1268–1275. [Google Scholar] [CrossRef] [PubMed]
- Park, C.C.; Nguyen, P.; Hernandez, C.; Bettencourt, R.; Ramirez, K.; Fortney, L.; Hooker, J.; Sy, E.; Savides, M.T.; Alquiraish, M.H.; et al. Magnetic Resonance Elastography vs Transient Elastography in Detection of Fibrosis and Noninvasive Measurement of Steatosis in Patients With Biopsy-Proven Nonalcoholic Fatty Liver Disease. Gastroenterology 2017, 152, 598–607.e2. [Google Scholar] [CrossRef]
- Loomba, R.; Cui, J.; Wolfson, T.; Haufe, W.; Hooker, J.; Szeverenyi, N.; Ang, B.; Bhatt, A.; Wang, K.; Aryafar, H.; et al. Novel 3D Magnetic Resonance Elastography for the Noninvasive Diagnosis of Advanced Fibrosis in NAFLD: A Prospective Study. Am. J. Gastroenterol. 2016, 111, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Imajo, K.; Kessoku, T.; Honda, Y.; Tomeno, W.; Ogawa, Y.; Mawatari, H.; Fujita, K.; Yoneda, M.; Taguri, M.; Hyogo, H.; et al. Magnetic Resonance Imaging More Accurately Classifies Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease Than Transient Elastography. Gastroenterology 2016, 150, 626–637.e7. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.; Dillman, J.R.; Singh, K.; Serai, S.D.; Towbin, A.J.; Xanthakos, S.; Zhang, B.; Su, W.; Trout, A.T. Quantitative MRI of Fatty Liver Disease in a Large Pediatric Cohort: Correlation between Liver Fat Fraction, Stiffness, Volume, and Patient-Specific Factors. Abdom. Radiol. (NY) 2018, 43, 1168–1179. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, K. Clinical Applications of Liver Magnetic Resonance Elastography: Chronic Liver Disease. In Magnetic Resonance Elastography New York; Springer: New York, NY, USA, 2014; pp. 39–60. [Google Scholar]
- Kim, D.; Kim, W.R.; Talwalkar, J.A.; Kim, H.J.; Ehman, R.L. Advanced Fibrosis in Nonalcoholic Fatty Liver Disease: Noninvasive Assessment with MR Elastography. Radiology 2013, 268, 411–419. [Google Scholar] [CrossRef]
- Ferraioli, G.; Wong, V.W.-S.; Castera, L.; Berzigotti, A.; Sporea, I.; Dietrich, C.F.; Choi, B.I.; Wilson, S.R.; Kudo, M.; Barr, R.G. Liver Ultrasound Elastography: An Update to the World Federation for Ultrasound in Medicine and Biology Guidelines and Recommendations. Ultrasound Med. Biol 2018, 44, 2419–2440. [Google Scholar] [CrossRef]
- Krishnamurthy, R.; Thompson, B.L.; Shankar, A.; Gariepy, C.E.; Potter, C.J.; Fung, B.R.; Hu, H.H. Magnetic Resonance Elastography of the Liver in Children and Adolescents: Assessment of Regional Variations in Stiffness. Acad Radiol. 2020, 27, e109–e115. [Google Scholar] [CrossRef] [PubMed]
- Trout, A.T.; Sheridan, R.M.; Serai, S.D.; Xanthakos, S.A.; Su, W.; Zhang, B.; Wallihan, D.B. Diagnostic Performance of MR Elastography for Liver Fibrosis in Children and Young Adults with a Spectrum of Liver Diseases. Radiology 2018, 287, 824–832. [Google Scholar] [CrossRef]
- Joshi, M.; Dillman, J.R.; Towbin, A.J.; Serai, S.D.; Trout, A.T. MR Elastography: High Rate of Technical Success in Pediatric and Young Adult Patients. Pediatr. Radiol. 2017, 47, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Schwimmer, J.B.; Behling, C.; Angeles, J.E.; Paiz, M.; Durelle, J.; Africa, J.; Newton, K.P.; Brunt, E.M.; Lavine, J.E.; Abrams, S.H.; et al. Magnetic Resonance Elastography Measured Shear Stiffness as a Biomarker of Fibrosis in Pediatric Nonalcoholic Fatty Liver Disease. Hepatology 2017, 66, 1474–1485. [Google Scholar] [CrossRef] [PubMed]
- Xanthakos, S.A.; Podberesky, D.J.; Serai, S.D.; Miles, L.; King, E.C.; Balistreri, W.F.; Kohli, R. Use of Magnetic Resonance Elastography to Assess Hepatic Fibrosis in Children with Chronic Liver Disease. J. Pediatr. 2014, 164, 186–188. [Google Scholar] [CrossRef]
- Manduca, A.; Oliphant, T.E.; Dresner, M.A.; Mahowald, J.L.; Kruse, S.A.; Amromin, E.; Felmlee, J.P.; Greenleaf, J.F.; Ehman, R.L. Magnetic Resonance Elastography: Non-Invasive Mapping of Tissue Elasticity. Med. Image Anal. 2001, 5, 237–254. [Google Scholar] [CrossRef]
- Ferraioli, G.; Barr, R.G.; Dillman, J.R. Elastography for Pediatric Chronic Liver Disease: A Review and Expert Opinion. J. Ultrasound Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Barr, R.G.; Wilson, S.R.; Rubens, D.; Garcia-Tsao, G.; Ferraioli, G. Update to the Society of Radiologists in Ultrasound Liver Elastography Consensus Statement. Radiology 2020, 296, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Ferraioli, G.; De Silvestri, A.; Lissandrin, R.; Maiocchi, L.; Tinelli, C.; Filice, C.; Barr, R.G. Evaluation of Inter-System Variability in Liver Stiffness Measurements. Ultraschall Med. 2019, 40, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Piscaglia, F.; Salvatore, V.; Mulazzani, L.; Cantisani, V.; Colecchia, A.; Di Donato, R.; Felicani, C.; Ferrarini, A.; Gamal, N.; Grasso, V.; et al. Differences in Liver Stiffness Values Obtained with New Ultrasound Elastography Machines and Fibroscan: A Comparative Study. Dig. Liver Dis. 2017, 49, 802–808. [Google Scholar] [CrossRef]
- Li, D.K.; Khan, M.R.; Wang, Z.; Chongsrisawat, V.; Swangsak, P.; Teufel-Schäfer, U.; Engelmann, G.; Goldschmidt, I.; Baumann, U.; Tokuhara, D.; et al. Normal Liver Stiffness and Influencing Factors in Healthy Children: An Individual Participant Data Meta-Analysis. Liver Int. 2020, 40, 2602–2611. [Google Scholar] [CrossRef] [PubMed]
- Raizner, A.; Shillingford, N.; Mitchell, P.D.; Harney, S.; Raza, R.; Serino, J.; Jonas, M.M.; Lee, C.K. Hepatic Inflammation May Influence Liver Stiffness Measurements by Transient Elastography in Children and Young Adults. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Wallihan, D.B.; Podberesky, D.J.; Marino, B.S.; Sticka, J.S.; Serai, S. Relationship of MR Elastography Determined Liver Stiffness with Cardiac Function after Fontan Palliation. J. Magn. Reson. Imaging 2014, 40, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Trout, A.T.; Xanthakos, S.A.; Bennett, P.S.; Dillman, J.R. Liver Shear Wave Speed and Other Quantitative Ultrasound Measures of Liver Parenchyma: Prospective Evaluation in Healthy Children and Adults. AJR Am. J. Roentgenol 2020, 214, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.S.; Youssfi, M.; Patel, M.; Hu, H.H.; Shaibi, G.Q.; Towbin, R.B. Shear-Wave Ultrasound Elastography of the Liver in Normal-Weight and Obese Children. Acta Radiol. 2017, 58, 1511–1518. [Google Scholar] [CrossRef]
- Fontanilla, T.; Cañas, T.; Macia, A.; Alfageme, M.; Gutierrez Junquera, C.; Malalana, A.; Luz Cilleruelo, M.; Roman, E.; Miralles, M. Normal Values of Liver Shear Wave Velocity in Healthy Children Assessed by Acoustic Radiation Force Impulse Imaging Using a Convex Probe and a Linear Probe. Ultrasound Med. Biol. 2014, 40, 470–477. [Google Scholar] [CrossRef]
- Matos, H.; Trindade, A.; Noruegas, M.J. Acoustic Radiation Force Impulse Imaging in Paediatric Patients: Normal Liver Values. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 684–688. [Google Scholar] [CrossRef]
- Pauleau, G.; Sandoz, B.; Thollon, L.; Serre, T.; Brunet, C. Anthropometric Characterization of the Child Liver. Surg. Radiol. Anat. 2010, 32, 767–775. [Google Scholar] [CrossRef]
- Yarpuzlu, B.; Ayyildiz, M.; Tok, O.E.; Aktas, R.G.; Basdogan, C. Correlation between the Mechanical and Histological Properties of Liver Tissue. J. Mech. Behav. Biomed. Mater. 2014, 29, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Tokuhara, D.; Cho, Y.; Shintaku, H. Transient Elastography-Based Liver Stiffness Age-Dependently Increases in Children. PLoS ONE 2016, 11, e0166683. [Google Scholar] [CrossRef]
- Mărginean, C.O.; Meliţ, L.E.; Ghiga, D.V.; Săsăran, M.O. Reference Values of Normal Liver Stiffness in Healthy Children by Two Methods: 2D Shear Wave and Transient Elastography. Sci. Rep. 2020, 10, 7213. [Google Scholar] [CrossRef]
- Goldschmidt, I.; Streckenbach, C.; Dingemann, C.; Pfister, E.D.; di Nanni, A.; Zapf, A.; Baumann, U. Application and Limitations of Transient Liver Elastography in Children. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Mjelle, A.B.; Mulabecirovic, A.; Havre, R.F.; Rosendahl, K.; Juliusson, P.B.; Olafsdottir, E.; Gilja, O.H.; Vesterhus, M. Normal Liver Stiffness Values in Children: A Comparison of Three Different Elastography Methods. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 706–712. [Google Scholar] [CrossRef]
- Galina, P.; Alexopoulou, E.; Zellos, A.; Grigoraki, V.; Siahanidou, T.; Kelekis, N.L.; Zarifi, M. Performance of Two--Dimensional Ultrasound Shear Wave Elastography: Reference Values of Normal Liver Stiffness in Children. Pediatr. Radiol. 2019, 49, 91–98. [Google Scholar] [CrossRef]
- Özkan, M.B.; Bilgici, M.C.; Eren, E.; Caltepe, G.; Yilmaz, G.; Kara, C.; Gun, S. Role of Point Shear Wave Elastography in the Determination of the Severity of Fibrosis in Pediatric Liver Diseases With Pathologic Correlations. J. Ultrasound Med. 2017, 36, 2337–2344. [Google Scholar] [CrossRef]
- Franchi-Abella, S.; Corno, L.; Gonzales, E.; Antoni, G.; Fabre, M.; Ducot, B.; Pariente, D.; Gennisson, J.-L.; Tanter, M.; Corréas, J.-M. Feasibility and Diagnostic Accuracy of Supersonic Shear-Wave Elastography for the Assessment of Liver Stiffness and Liver Fibrosis in Children: A Pilot Study of 96 Patients. Radiology 2016, 278, 554–562. [Google Scholar] [CrossRef]
- Lewindon, P.J.; Balouch, F.; Pereira, T.N.; Puertolas-Lopez, M.V.; Noble, C.; Wixey, J.A.; Ramm, G.A. Transient Liver Elastography in Unsedated Control Children: Impact of Age and Intercurrent Illness. J. Paediatr. Child Health 2016, 52, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Tutar, O.; Beşer, Ö.F.; Adaletli, I.; Tunc, N.; Gulcu, D.; Kantarci, F.; Mihmanli, I.; Cokugras, F.C.; Kutlu, T.; Ozbay, G.; et al. Shear Wave Elastography in the Evaluation of Liver Fibrosis in Children. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Honsawek, S.; Vejchapipat, P.; Payungporn, S.; Theamboonlers, A.; Chongsrisawat, V.; Poovorawan, Y. Soluble Receptor for Advanced Glycation End Products and Liver Stiffness in Postoperative Biliary Atresia. Clin. Biochem. 2013, 46, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Hanquinet, S.; Courvoisier, D.; Kanavaki, A.; Dhouib, A.; Anooshiravani, M. Acoustic Radiation Force Impulse Imaging-Normal Values of Liver Stiffness in Healthy Children. Pediatr. Radiol. 2013, 43, 539–544. [Google Scholar] [CrossRef]
- Lee, M.-J.; Kim, M.-J.; Han, K.H.; Yoon, C.S. Age-Related Changes in Liver, Kidney, and Spleen Stiffness in Healthy Children Measured with Acoustic Radiation Force Impulse Imaging. Eur. J. Radiol. 2013, 82, e290–e294. [Google Scholar] [CrossRef]
- Marginean, C.O.; Marginean, C. Elastographic Assessment of Liver Fibrosis in Children: A Prospective Single Center Experience. Eur. J. Radiol. 2012, 81, e870–e874. [Google Scholar] [CrossRef]
- Noruegas, M.J.; Matos, H.; Gonçalves, I.; Cipriano, M.A.; Sanches, C. Acoustic Radiation Force Impulse-Imaging in the Assessment of Liver Fibrosis in Children. Pediatr. Radiol. 2012, 42, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, G.; Gebhardt, C.; Wenning, D.; Wühl, E.; Hoffmann, G.F.; Selmi, B.; Grulich-Henn, J.; Schenk, J.P.; Teufel, U. Feasibility Study and Control Values of Transient Elastography in Healthy Children. Eur. J. Pediatr. 2012, 171, 353–360. [Google Scholar] [CrossRef]
- Eiler, J.; Kleinholdermann, U.; Albers, D.; Dahms, J.; Hermann, F.; Behrens, C.; Luedemann, M.; Klingmueller, V.; Alzen, G.F.P. Standard Value of Ultrasound Elastography Using Acoustic Radiation Force Impulse Imaging (ARFI) in Healthy Liver Tissue of Children and Adolescents. Ultraschall Med. 2012, 33, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Menten, R.; Leonard, A.; Clapuyt, P.; Vincke, P.; Nicolae, A.-C.; Lebecque, P. Transient Elastography in Patients with Cystic Fibrosis. Pediatr. Radiol. 2010, 40, 1231–1235. [Google Scholar] [CrossRef] [PubMed]
- Witters, P.; De Boeck, K.; Dupont, L.; Proesmans, M.; Vermeulen, F.; Servaes, R.; Verslype, C.; Laleman, W.; Nevens, F.; Hoffman, I.; et al. Non-Invasive Liver Elastography (Fibroscan) for Detection of Cystic Fibrosis-Associated Liver Disease. J. Cyst. Fibros. 2009, 8, 392–399. [Google Scholar] [CrossRef]
- Rubio, A.; Monpoux, F.; Huguon, E.; Truchi, R.; Triolo, V.; Rosenthal-Allieri, M.-A.; Deville, A.; Rosenthal, E.; Boutté, P.; Tran, A. Noninvasive Procedures to Evaluate Liver Involvement in HIV-1 Vertically Infected Children. J. Pediatr. Gastroenterol. Nutr. 2009, 49, 599–606. [Google Scholar] [CrossRef]
- Francavilla, R.; Cecinati, V.; Bucci, N.; Castellaneta, S.; Brescia, L.; Barone, M.; Indrio, F.; Cavallo, L. Normal Values of Transient Elastography (Fibroscan) in Children without Evidence of Liver Disease: Comparison of Adult versus Paediatric Probe. Dig. Liver Dis. 2008, 40, A61. [Google Scholar] [CrossRef]
- Giuffrè, M.; Fouraki, S.; Comar, M.; Masutti, F.; Crocè, L.S. The Importance of Transaminases Flare in Liver Elastography: Characterization of the Probability of Liver Fibrosis Overestimation by Hepatitis C Virus-Induced Cytolysis. Microorganisms 2020, 8, 348. [Google Scholar] [CrossRef] [PubMed]
- Popescu, A.; Bota, S.; Sporea, I.; Sirli, R.; Danila, M.; Racean, S.; Suseanu, D.; Gradinaru, O.; Ivascu Siegfried, C. The Influence of Food Intake on Liver Stiffness Values Assessed by Acoustic Radiation Force Impulse Elastography-Preliminary Results. Ultrasound Med. Biol. 2013, 39, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Talwalkar, J.A.; Glaser, K.J.; Venkatesh, S.K.; Chen, J.; Manduca, A.; Ehman, R.L. Dynamic Postprandial Hepatic Stiffness Augmentation Assessed with MR Elastography in Patients with Chronic Liver Disease. AJR Am. J. Roentgenol. 2011, 197, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Roccarina, D.; Rosselli, M.; Genesca, J.; Tsochatzis, E.A. Elastography Methods for the Non-Invasive Assessment of Portal Hypertension. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 155–164. [Google Scholar] [CrossRef]
- Giuffrè, M.; Fouraki, S.; Campigotto, M.; Colombo, A.; Visintin, A.; Buonocore, M.R.; Aversano, A.; Budel, M.; Tinè, F.; Abazia, C.; et al. Alanine Aminotransferase and Spleno-Portal Dynamics Affect Spleen Stiffness Measured by Point Shear-Wave Elastography in Patients with Chronic Hepatitis C in the Absence of Significant Liver Fibrosis. J. Ultrasound 2021, 24, 67–73. [Google Scholar] [CrossRef]
- Varbobitis, I.C.; Siakavellas, S.I.; Koutsounas, I.S.; Karagiannakis, D.S.; Ioannidou, P.; Papageorgiou, M.-V.; Pavlopoulou, I.D.; Schizas, D.; Bamias, G.; Vlachogiannakos, I.; et al. Reliability and Applicability of Two-Dimensional Shear-Wave Elastography for the Evaluation of Liver Stiffness. Eur. J. Gastroenterol. Hepatol. 2016, 28, 1204–1209. [Google Scholar] [CrossRef]
- Nobili, V.; Vizzutti, F.; Arena, U.; Abraldes, J.G.; Marra, F.; Pietrobattista, A.; Fruhwirth, R.; Marcellini, M.; Pinzani, M. Accuracy and Reproducibility of Transient Elastography for the Diagnosis of Fibrosis in Pediatric Nonalcoholic Steatohepatitis. Hepatology 2008, 48, 442–448. [Google Scholar] [CrossRef]
- Karlas, T.; Dietrich, A.; Peter, V.; Wittekind, C.; Lichtinghagen, R.; Garnov, N.; Linder, N.; Schaudinn, A.; Busse, H.; Prettin, C.; et al. Evaluation of Transient Elastography, Acoustic Radiation Force Impulse Imaging (ARFI), and Enhanced Liver Function (ELF) Score for Detection of Fibrosis in Morbidly Obese Patients. PLoS ONE 2015, 10, e0141649. [Google Scholar] [CrossRef]
- Jiang, W.; Huang, S.; Teng, H.; Wang, P.; Wu, M.; Zhou, X.; Ran, H. Diagnostic Accuracy of Point Shear Wave Elastography and Transient Elastography for Staging Hepatic Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease: A Meta-Analysis. BMJ. Open 2018, 8, e021787. [Google Scholar] [CrossRef]
- Myers, R.P.; Pomier-Layrargues, G.; Kirsch, R.; Pollett, A.; Duarte-Rojo, A.; Wong, D.; Beaton, M.; Levstik, M.; Crotty, P.; Elkashab, M. Feasibility and Diagnostic Performance of the FibroScan XL Probe for Liver Stiffness Measurement in Overweight and Obese Patients. Hepatology 2012, 55, 199–208. [Google Scholar] [CrossRef]
- Giuffrè, M.; Giuricin, M.; Bonazza, D.; Rosso, N.; Giraudi, P.J.; Masutti, F.; Palmucci, S.; Basile, A.; Zanconati, F.; de Manzini, N.; et al. Optimization of Point-Shear Wave Elastography by Skin-to-Liver Distance to Assess Liver Fibrosis in Patients Undergoing Bariatric Surgery. Diagnostics (Basel) 2020, 10, 795. [Google Scholar] [CrossRef]
- Su, S.; Wang, W.; Nadebaum, D.; Nicoll, A.; Sood, S.; Gorelik, A.; Lai, J.; Gibson, R. Skin-Liver Distance and Interquartile Range-Median Ratio as Determinants of Interoperator Concordance in Acoustic Radiation Force Impulse Imaging. J. Med. Ultrasound 2019, 27, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Nadebaum, D.; Gibson, R.; Howell, J.; Halliday, J.; Christie, M.; Gorelik, A.; Gib, A. Inter-Operator Concordance in Acoustic Radiation Force Impulse Imaging (ARFI): A Practical Role for Multiple Operators to Improve Diagnostic Performance. J. Gastroenterol. Hepatol. Hepatol. Clin. Abstr. 2014, 29, 68–101. [Google Scholar]
- Shen, F.; Zheng, R.-D.; Shi, J.-P.; Mi, Y.-Q.; Chen, G.-F.; Hu, X.; Liu, Y.-G.; Wang, X.-Y.; Pan, Q.; Chen, G.-Y.; et al. Impact of Skin Capsular Distance on the Performance of Controlled Attenuation Parameter in Patients with Chronic Liver Disease. Liver Int. 2015, 35, 2392–2400. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.B.; Ewertsen, C.; Carlsen, J.F.; Henriksen, B.M.; Nielsen, M.B. Ultrasound Elastography Is Useful for Evaluation of Liver Fibrosis in Children-A Systematic Review. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Nobili, V.; Monti, L.; Alisi, A.; Lo Zupone, C.; Pietrobattista, A.; Tomà, P. Transient Elastography for Assessment of Fibrosis in Paediatric Liver Disease. Pediatr. Radiol. 2011, 41, 1232–1238. [Google Scholar] [CrossRef]
- Kamble, R.; Sodhi, K.S.; Thapa, B.R.; Saxena, A.K.; Bhatia, A.; Dayal, D.; Khandelwal, N. Liver Acoustic Radiation Force Impulse (ARFI) in Childhood Obesity: Comparison and Correlation with Biochemical Markers. J. Ultrasound 2017, 20, 33–42. [Google Scholar] [CrossRef]
- Kim, J.R.; Suh, C.H.; Yoon, H.M.; Lee, J.S.; Cho, Y.A.; Jung, A.Y. The Diagnostic Performance of Shear-Wave Elastography for Liver Fibrosis in Children and Adolescents: A Systematic Review and Diagnostic Meta-Analysis. Eur. Radiol. 2018, 28, 1175–1186. [Google Scholar] [CrossRef]
- Sagir, A.; Ney, D.; Oh, J.; Pandey, S.; Kircheis, G.; Mayatepek, E.; Häussinger, D. Evaluation of Acoustic Radiation Force Impulse Imaging (ARFI) for the Determination of Liver Stiffness Using Transient Elastography as a Reference in Children. Ultrasound Int. Open 2015, 1, E2–E7. [Google Scholar] [CrossRef][Green Version]
- Garcovich, M.; Veraldi, S.; Di Stasio, E.; Zocco, M.A.; Monti, L.; Tomà, P.; Pompili, M.; Gasbarrini, A.; Nobili, V. Liver Stiffness in Pediatric Patients with Fatty Liver Disease: Diagnostic Accuracy and Reproducibility of Shear-Wave Elastography. Radiology 2017, 283, 820–827. [Google Scholar] [CrossRef]
- Lee, M.S.; Bae, J.M.; Joo, S.K.; Woo, H.; Lee, D.H.; Jung, Y.J.; Kim, B.G.; Lee, K.L.; Kim, W. Prospective Comparison among Transient Elastography, Supersonic Shear Imaging, and ARFI Imaging for Predicting Fibrosis in Nonalcoholic Fatty Liver Disease. PLoS ONE 2017, 12, e0188321. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P.; Kleiner, D.E.; Dam-Larsen, S.; Adams, L.A.; Bjornsson, E.S.; Charatcharoenwitthaya, P.; Mills, P.R.; Keach, J.C.; Lafferty, H.D.; Stahler, A.; et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-Term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2015, 149, 389–397.E10. [Google Scholar] [CrossRef] [PubMed]
- Alkhouri, N.; Sedki, E.; Alisi, A.; Lopez, R.; Pinzani, M.; Feldstein, A.E.; Nobili, V. Combined Paediatric NAFLD Fibrosis Index and Transient Elastography to Predict Clinically Significant Fibrosis in Children with Fatty Liver Disease. Liver Int. 2013, 33, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.M.; Janney, C.G.; Di Bisceglie, A.M.; Neuschwander-Tetri, B.A.; Bacon, B.R. Nonalcoholic Steatohepatitis: A Proposal for Grading and Staging the Histological Lesions. Am. J. Gastroenterol. 1999, 94, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and Validation of a Histological Scoring System for Nonalcoholic Fatty Liver Disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-Y.; Yoon, H.M.; Kim, J.R.; Lee, J.S.; Jung, A.Y.; Kim, K.M.; Cho, Y.A. Diagnostic Performance of Transient Elastography for Liver Fibrosis in Children: A Systematic Review and Meta-Analysis. AJR Am. J. Roentgenol. 2018, 211, W257–W266. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, M.; Kojima, T.; Itoh, Y.; Harano, Y.; Fujii, K.; Nakajima, T.; Kato, T.; Takeda, N.; Okuda, J.; Ida, K.; et al. The Severity of Ultrasonographic Findings in Nonalcoholic Fatty Liver Disease Reflects the Metabolic Syndrome and Visceral Fat Accumulation. Am. J. Gastroenterol. 2007, 102, 2708–2715. [Google Scholar] [CrossRef] [PubMed]
- Berná-Serna, J.D.; Sánchez-Jiménez, R.; Velázquez-Marín, F.; Sainz de Baranda, P.; Guzmán-Aroca, F.; Fernández-Hernández, C.; Doménech-Abellán, E.; Abellán-Rivero, D.; Ruiz-Merino, G.; Madrid-Conesa, J.; et al. Acoustic Radiation Force Impulse Imaging for Detection of Liver Fibrosis in Overweight and Obese Children. Acta Radiol. 2018, 59, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Hudert, C.A.; Tzschätzsch, H.; Guo, J.; Rudolph, B.; Bläker, H.; Loddenkemper, C.; Luck, W.; Müller, H.-P.; Baumgart, D.C.; Hamm, B.; et al. US Time-Harmonic Elastography: Detection of Liver Fibrosis in Adolescents with Extreme Obesity with Nonalcoholic Fatty Liver Disease. Radiology 2018, 288, 99–106. [Google Scholar] [CrossRef]
- Fitzpatrick, E.; Quaglia, A.; Vimalesvaran, S.; Basso, M.S.; Dhawan, A. Transient Elastography Is a Useful Noninvasive Tool for the Evaluation of Fibrosis in Paediatric Chronic Liver Disease. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 72–76. [Google Scholar] [CrossRef]
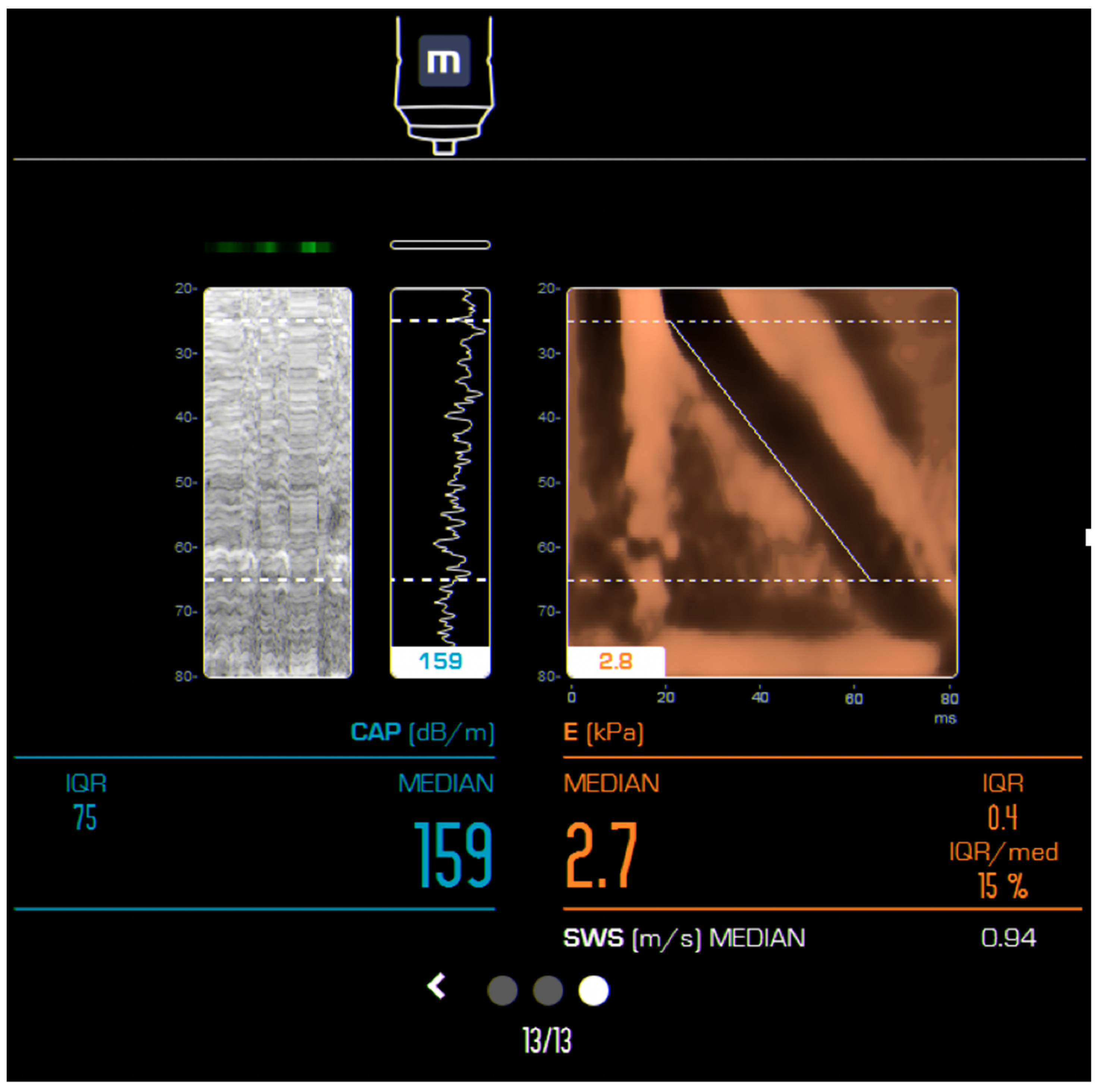
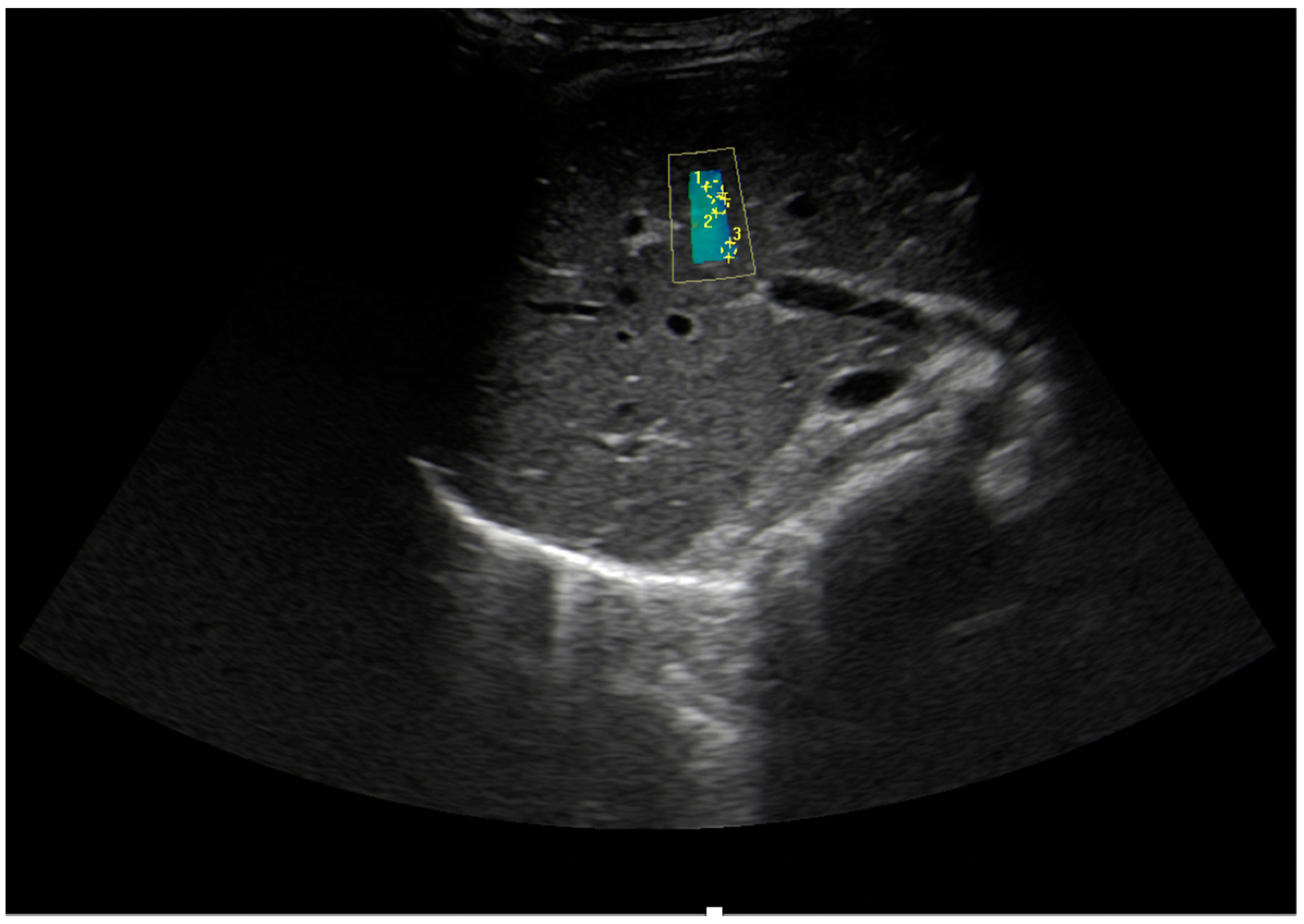
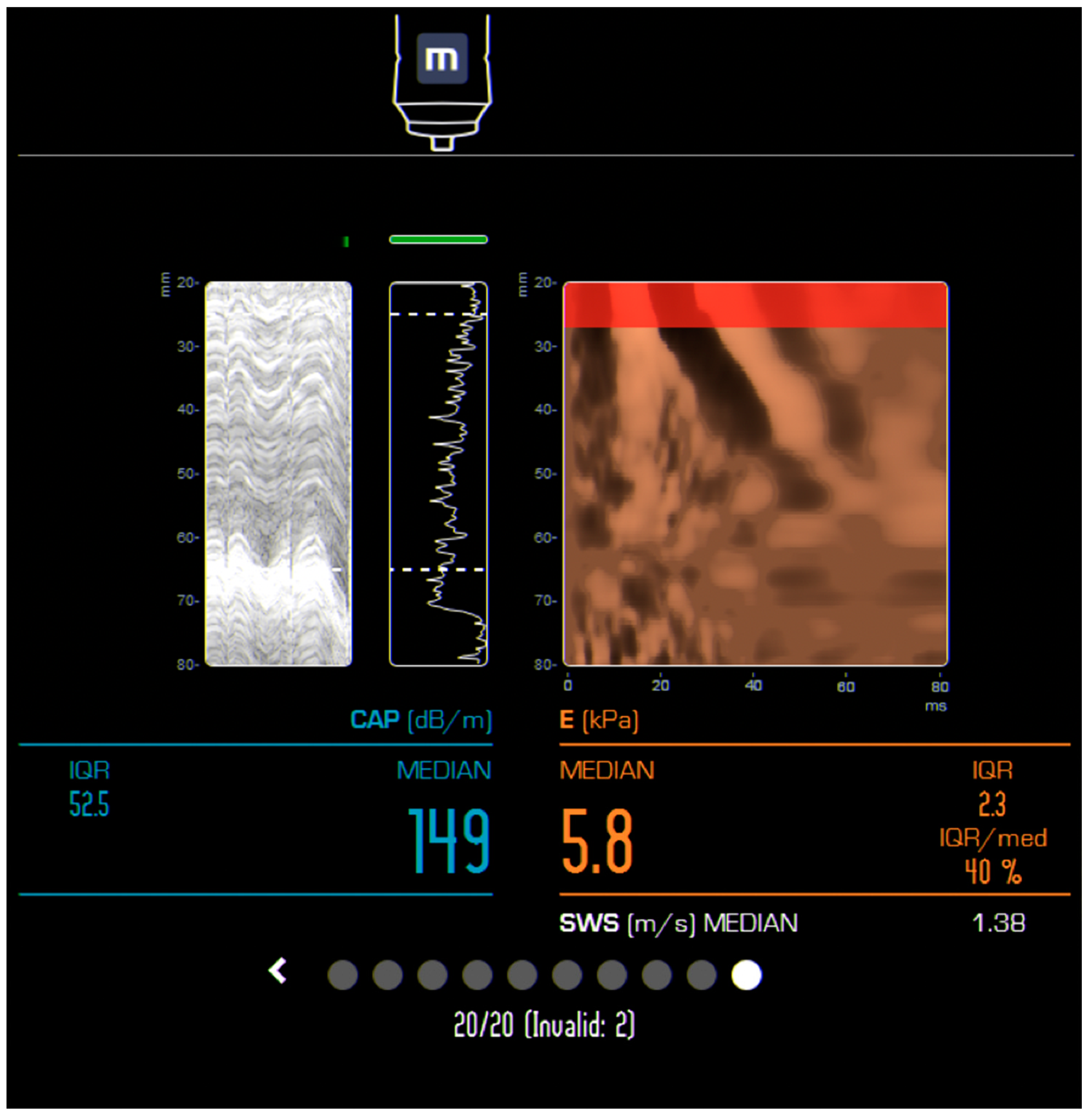

| Author, Year, and Country | Study Type/No of Patients | Age (Years) Mean/Median * | MRE (KPa) | Observations | |
|---|---|---|---|---|---|
| Normal Weight Children Mean/Median * | NAFLD/NASH Children Mean/Median * | ||||
| Sawh et al., 2020, USA [33] | Prospective/81 | 12.6 ± 2.6 | 2.45 ± 0.35 | - | The values are significant higher than in adults (2.10 ± 0.23 kPa) (p < 0.001) |
| Trout et al., 2020, USA [34] | Prospective/71 | 12 * | 2.1 ± 1.96 | - | P95 for normal liver stiffness was 2.8 kPa. Liver stiffness was independent of sex, age, or BMI |
| Krishnamurthy et al., 2020, USA [54] | Retrospective/122 | 14.7 ± 3.5 | - | 2.6 ± 0.7 kPa for boys 2.8 ± 0.8 kPa for girls | There are stiffness variations within the 8 Couinaud segments of the liver in children |
| Joshi et al., 2018, USA [50] | Prospective/202 | 13.4 ± 2.9 | - | 2.6 ± 0.6 | Relationships between stiffness, and organ volume and some patient-specific factors, including sex, age, BMI, serum ALT, and diabetic status |
| Trout et al., 2018, USA [55] | Prospective/86 | 14.2 * | F0: 2.90 ± 0.87; F1: 2.41 ± 0.51; F2: 3.64 ± 1.12; F3: 4.55 ± 1.68;F4: 4.68 ± 1.32 | F0: 2.68 ± 0.31; F1: 3.04 ± 0.63; F2: 2.71 ± 0.59; F3: 7.43 ± 2.68; F4 not aplicable | MR elastography performs significantly better for distinguishing stage 0–1 versus stage 2 or higher fibrosis in patients without steatosis than in those with steatosis. |
| Joshi et al., 2017, USA [56] | Retrospective/372 | 12.6 ± 3.7 | - | 2.94 ± 1.11 kPa | Mean patient BMI was 29.4 ± 10.1 kg/m2 |
| Schwimmer et al., 2017, USA [57] | Prospective/90 | 13.1 ± 2.4 | - | 2.35 kPa | Correlation with fibrosis (by biopsy) was 72.2% |
| Etchell et al., 2017, Australia [40] | Prospective/24 | 5–18 | - | Children: At 28 Hz: 1.2 ± 0.2/At 56 Hz: 2.2 ± 0.3/At 84 Hz: At 5.6 ± 0.8 Adolescents: At 28 Hz: 1.3 ± 0.3/At 56 Hz: 2.2 ± 0.2/At 84 Hz: 6.5 ± 1.2 | Liver stiffness values are lower and vary less with frequency in children and adolescents than in adults. |
| Xanthakos et al., 2014, USA [58] | Prospective/35 | 13 * | - | F1: 2.2 kPa; F3: 3.6 kPa; F4: 4.9 kPa | Cut-off = 2.71 kPa—8% sensitivity, 85% specificity for F2 with an AUROC of 0.92 (95% CI, 0.79–1.00; p = 0.02) |
| Author, Year, and Country | Study Type/No of Patients | Age (Years) Mean/Median * | Elastography Method (TE/p-SWE/2D-SWE/SE) | Observations | ||
|---|---|---|---|---|---|---|
| TE (KPa) Mean/Median * | p-SWE/ARFI (m/s) Mean/Median * | 2D-SWE/rSWE (Stiffness—kPa and Velocity—m/s) Mean/Median * | ||||
| Trout et al., 2020, USA [67] | Prospective/128 | 5.04 * | - | - | 1.29 ± 0.13 m/s | - |
| Ferraiolii et al., 2020, Italy [60] | Prospective/31—Siemens and 238—Samsung | 7 * | - | Siemens: 1.24 m/s (4.61 kPa) Samsung: 1.17 m/s (4.1 KPa) | - | - |
| Mărginean et al., 2020, Romania [74] | Prospective/206 | 3–18 | 3.797 ± 0.4859 | - | 3.72 ± 0.48 kPa | Stiffness cutoff: 4.13 for 3–5 y and 4.88 for 12–15 y Velocity cutoff: 1.18 m/s for 6–8 y and 1.35 m/s for 12–15 y |
| Mjelle et al., 2019, Norway [76] | Prospective/ TE and pSWE—87; rSWE—243 | 4–17 | 4.1 kPa (1.17 m/s; range 2.4–11.2 kPa) | 4.1 kPa (1.17 m/s; range 2.8–7.1 kPa | 3.3 kPa (1.05 m/s; range 2.0–7.7 kPa) | lower values using 2D-SWE versus pSWE/TE (p < 0.001); liver stiffness increases with the age |
| Galina et al., 2018, Greece [77] | Prospective/202 | 0–16 | - | - | 4.29 ± 0.59 KPa | Higher values in neonates, infants and adolescents versus preschoolers children (p < 0.001) |
| Bailey et al., 2017, USA [68] | Prospective/176 | 8.6 ± 5.6 | - | - | 1.08 ± 0.14 m/s | Comparation between normal-weight and obese groups (p < 0.001) |
| Oskan et al., 2017, Turkey [78] | Prospective/31 | 7 * | - | 1.24 m/s | - | pSWE in normal weight compared cu obese children |
| Franchi-Abella et al., 2016, France [79] | Prospective/51 | 0–17.2 | - | - | 6.58 ± 1.46 KPa | elasticity value incresed with age |
| Tokuhara et al., 2016, Japan [73] | Prospective/123 | 11.7 * | 3.4 (2.3 ± 4.6) at 1–5 y 3.8 (2.5 ± 6.1) at 6–11 y 4.1 (3.3 ± 7.9) at 12–18 y | - | - | Median LSM increased with age (p < 0.001) |
| Lewindon et al., 2016, Australia [80] | Prospective/64 | 9.3 ± 0.5 | 4.1 ± 0.1 | - | - | Comparation between normal weight and intercurrent illness groups (p < 0.001) |
| Cho et al., 2015, Japan [18] | Prospective/107 | 11.5 * | 3.9 ± 0.9 | - | - | significant differences between normal-weight and obese children (p < 0.001) |
| Fontanilla et al., 2014, Spain [69] | Prospective/60 | Max 14 (only range) | - | 4C1 transducer: 1.19 * 9L4 transducer: 1.15 * | - | |
| Matos et al., 2014, Portugal [70] | Prospective/150 | 8.9 ** | - | 1.07 * | - | - |
| Tutar et al., 2014, Turkey [81] | Prospective/50 | 7.4 * | - | - | 7.41 kPa; 1.56 m/s | - |
| Honsawek et al., 2013, Thailand [82] | Prospective/20 | 9.5 | 5.00 * | - | - | - |
| Goldschmidt et al., 2013, Germany [75] | Prospective/270 | 6.0 * (all 547) | 4.50 * (MG) | - | - | - |
| Hanquinet et al., 2013, Switzerland [83] | Prospective/103 | 6.3 * | - | 1.12 * | - | - |
| Lee et al., 2013, Republic of Korea [84] | Prospective/202 | 8.1 * | - | 1.12 * | - | - |
| Marginean et al., 2012, Romania [85] | Prospective/32 | 5.9 * | - | 1.18 * | - | - |
| Noruegas et al., 2012, Portugal [86] | Prospective/20 | 7.0 * | - | 1.11 * | - | - |
| Engelmann et al., 2012, Germany [87] | Prospective/240 | 9.3 (female) * 7.9 (male) * | 4.70 * | - | - | - |
| Eiler et al., 2012, Germany [88] | Prospective/132 | 9.2 * | - | 1.16 ± 0.14 | - | - |
| Menten et al., 2010, Belgium [89] | Prospective/31 | 8.5 * | 4.30 (M) | - | - | - |
| Witters et al., 2009, Belgium [90] | Prospective/59 | 10.2 * | <12 y > 5.63; >12 y > 6.50 | - | - | - |
| Rubio et al., 2009, France [91] | Prospective/19 | 12.7 * | 4.34 * (M) | - | - | |
| Francavilla et al., 2008, Italy [92] | Prospective/175 | 8 ± 3.6 | 3–7 | - | - | 4.5 ± 1.4 with M probe; 4.8 ± 1.9 with P probe |
| Author, Year, and Country | Study Type and No Patients | Age (Years) Mean/Median * | Diagnosis | Gold Standard and Score Type | Elastography Method (TE/p-SWE/2D-SWE (pSWE) | Elastography Values Mean/Median | Correlation to Gold Standard/Cut-off AUROC |
|---|---|---|---|---|---|---|---|
| Hudert et al., 2018, Germany [121] | Prospective/47 | 14.1 ± 2.2 | NAFLD/NASH | Biopsy, Kleiner | Harmonic SWE | F0: 1.45 ± 0.05 m/s F1: 1.49 ± 0.07 m/s F2: 1.78 ± 0.13 m/s F3: 1.81 ± 0.11 m/s | Usefull for detection of moderate fibrosis in extreme obese children Cutoffs: F1: 1.52 m/s; F2: 1.62 m/s; F3: 1.64 m/s |
| Oskan et al., 2017, Turkey [78] | Prospective/11 | 9 * | NAFLD | Biopsy, Knodell | pSWE | 1.56 m/s (range, 1.32–2.03 m/s) | pSWE—increased in fibrosis; Cutoff: 2.09 m/s VTQ; pSWE cutoff: 1.67m/s |
| Garcovich et al., 2016, Italy [112] | Prospective/68 | 12.6 ± 2.48 | NASH | Biopsy, Brunt | rSWE | F0: 4.4 ± 0.6 KPa F1: 5.6 ± 0.6 KPa F2: 7.1 ±0.7 KPa | Significant correlation with liver fibrosis (r = 0.84, p < 0.001) |
| Cho et al., 2015, Japan [18] | Prospective/52 | 11.5 * | NAFLD | Biopsy only in 8 children, Kleiner | TE | 5.5 ± 2.3 kPa | highly correlated with fibrosis stage (Spearman’s ρ = 0.920) |
| Alkhouri et al., 2013, USA [115] | Prospective/67 | Min 5.5; Max 11.3 (only range) | NAFLD | Biopsy, Kleiner | TE (S) | F0-F1: 5.7 ± 1.2 kPa F2-F3: 10.9 ± 3.3 kPa | Significant difference between TE at F0–1 and F2–3 (p < 0.001) ≥ F2: 8.6 (1.00) |
| Marginean et al., 2012, Romania [85] | Prospective/13 | 7.9 * | NAFLD | Biopsy, (-) | pSWE | 1.65 ± 0.49 m/s | significant difference between NAFLD and healthy controls (p < 0.05) |
| Nobili et al., 2008, Italy [99] | Prospective/50 | 13.6 * | NASH | Biopsy, Brunt | TE (M) | F0: 4.4 kPa; F1: 6.1 kPa; F2: 8.6 kPa; F3–4: 20.4 kPa | Cut-off: ≥F1: 5.1 (0.97); ≥F2: 7.4 (0.99); ≥F3: 10.2 (1.00) |
| Author, Year, and Country | Study Type and No Patients (Normal Weight/Obese Children) | Age (Years) Mean/Median * | Elastography Method (TE/p-SWE/2D-SWE (rSWE) | Elastogpraphy in Healty Children (Mean/Median) | Elastography Values in NAFLD/NASH (Mean/Median) | Correlations/Observations |
|---|---|---|---|---|---|---|
| Mjelle et al., 2019, Norway [76] | Prospective 243/27 | 4–17 | 2D-SWE pSWE TE | 8–11 y: 3.35 kPa 15–17 y: 3.78 kPa 4.1 kPa 15–17 y: 4.52 kPa | 8–11 y: 4.45 kPa 15–17 y: 4.64 kPa 8–11 y: 4.1 kPa 15–17 y: 3.4 kPa | Significant corellation (p < 0.001/p < 0.008) Significant corellation, p = 0.003 TE lower LSM values in the overweight |
| Mărginean et al., 2019, Romania [19] | Prospective 210/77 | 11.29 ± 3.83 y—normal weigh 10.44 ± 3.38 y—obese | TE 2D-SWE | 3.80 ± 0.48 kPa 3.73 ± 0.48 kPa 1.09 ± 0.09 m/s | 4.23 ± 0.53 kPa 3.84 ± 0.35 kPa 1.18 ± 0.09 m/s | p < 0.0001 p = 0.0314/p < 0.0001 AUC: V median: 0.817 ± 0.028 (p = 0.0001); TE: 0.730± 0.033 (p = 0.0001) |
| Bailey et al., 2017, USA [68] | Prospective 176/124 | 9.9 ± 5.3 | 2D-SWE | 1.08 ± 0.14 m/s | 1.44 ± 0.39 m/s | significant differences between normal-weight and obese children (p < 0.001); Kappa coefficient = 0.64 |
| Berna’-Serna et al., 2017, Spain [120] | Prospective -/148 | 8.02 ± 1.64 | pSWE (ARFI) | - | F0: 1.03 ± 0.13; F1:1.24 ± 0.04; F2: 1.40 ± 0.07; F3: 1.75 ± 0.08; F4: 2.21 ± 0.28 | Significant difference between boys and girls (p = 0.0003) |
| Oskan et al., 2017, Turkey [78] | Prospective 31/11 | 7 * 9 * | pSWE | 1.24 m/s | 1.56 m/s (range, 1.32–2.03 m/s) | pSWE significant higher in obese versus normal weight (p < 0.001) |
| Cho et al., 2015, Japan [18] | Prospective 107/52 | 11.5 * | TE | 3.9 ± 0.9 kPa | 5.5 ± 2.3 kPa | significant differences between normal-weight and obese children (p < 0.001) |
| Fitzpatrick et al., 2013, Italy [122] | Prospective/ -/37 | 13.5 * | TE | - | F0: 6.1 kPa; F1: 5.1 kPa; F2: 5.8 kPa; F3: 7.4 kPa | For severe fibrosis, the AUROC was 0.8 (p = 0.003); a cutoff of 6.9 kPa had a 72% sensitivity and a 85% specificity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mărginean, C.O.; Meliț, L.E.; Săsăran, M.O. Elastography—A Bona Fide Non-Invasive Method for Assessing Non-Alcoholic Fatty Liver Disease in Children. Appl. Sci. 2021, 11, 3240. https://doi.org/10.3390/app11073240
Mărginean CO, Meliț LE, Săsăran MO. Elastography—A Bona Fide Non-Invasive Method for Assessing Non-Alcoholic Fatty Liver Disease in Children. Applied Sciences. 2021; 11(7):3240. https://doi.org/10.3390/app11073240
Chicago/Turabian StyleMărginean, Cristina Oana, Lorena Elena Meliț, and Maria Oana Săsăran. 2021. "Elastography—A Bona Fide Non-Invasive Method for Assessing Non-Alcoholic Fatty Liver Disease in Children" Applied Sciences 11, no. 7: 3240. https://doi.org/10.3390/app11073240
APA StyleMărginean, C. O., Meliț, L. E., & Săsăran, M. O. (2021). Elastography—A Bona Fide Non-Invasive Method for Assessing Non-Alcoholic Fatty Liver Disease in Children. Applied Sciences, 11(7), 3240. https://doi.org/10.3390/app11073240







