Effect of Childbirth Experience on Cognitive Performance and Event-Related Potential Patterns
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
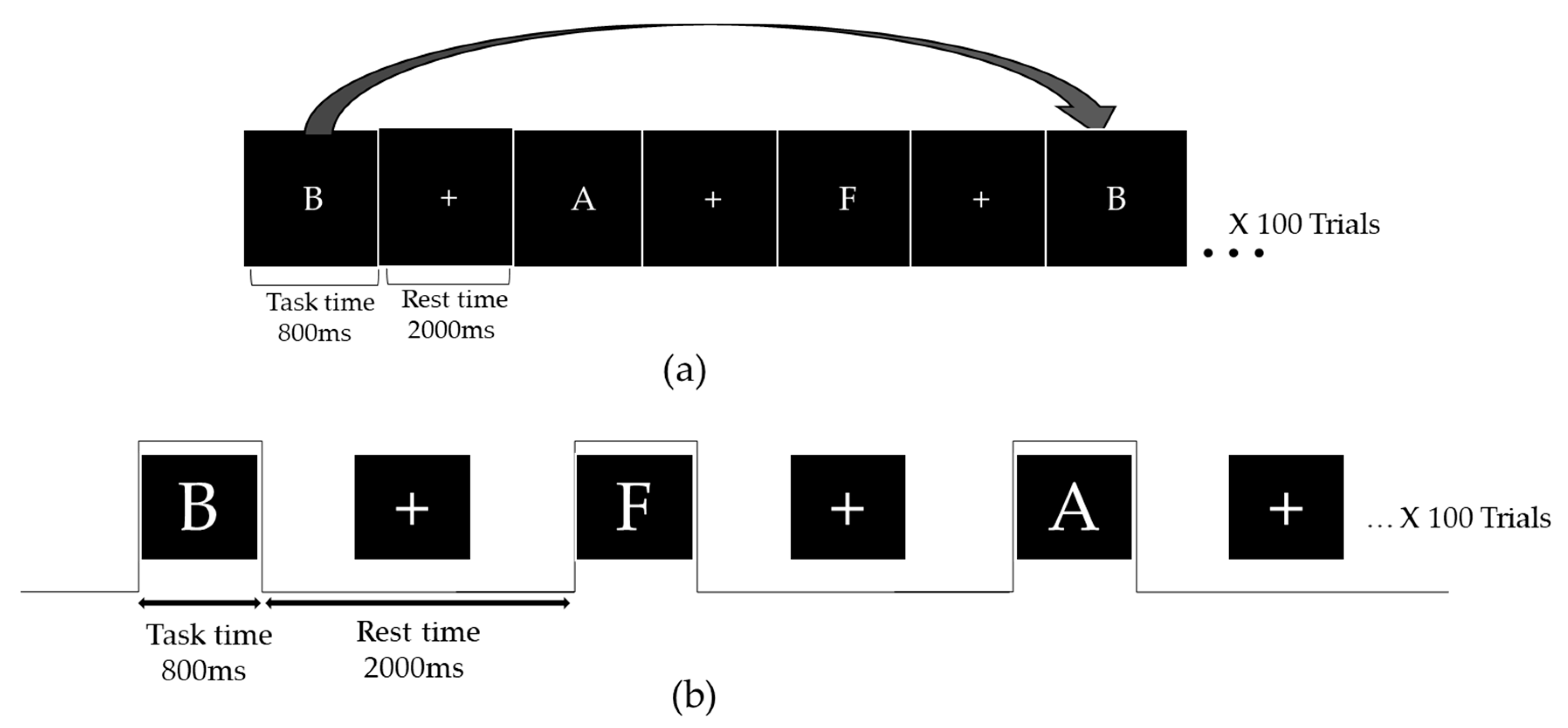
2.2. The 3-Back Task
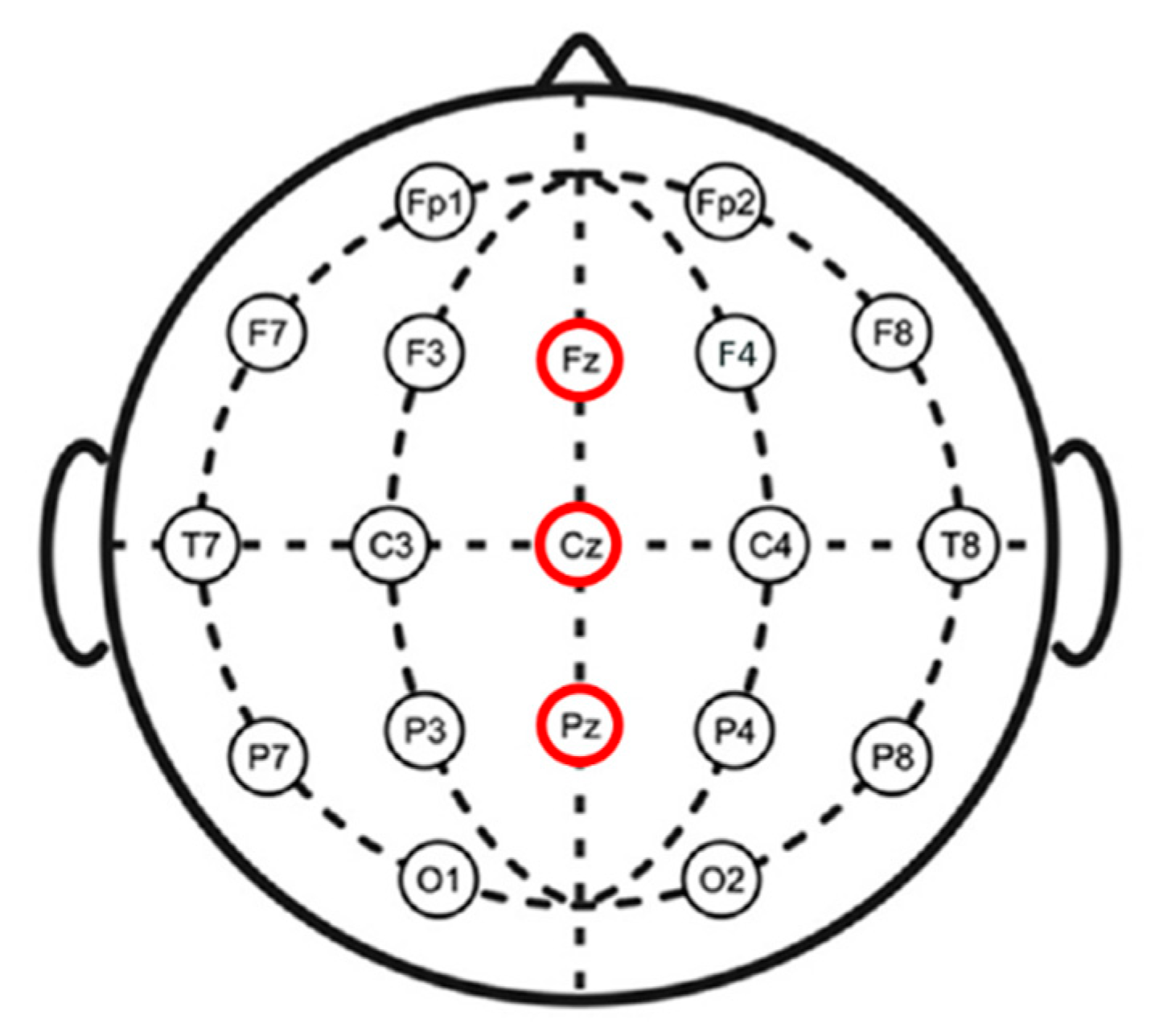
2.3. EEG Measurement and Analysis
3. Results
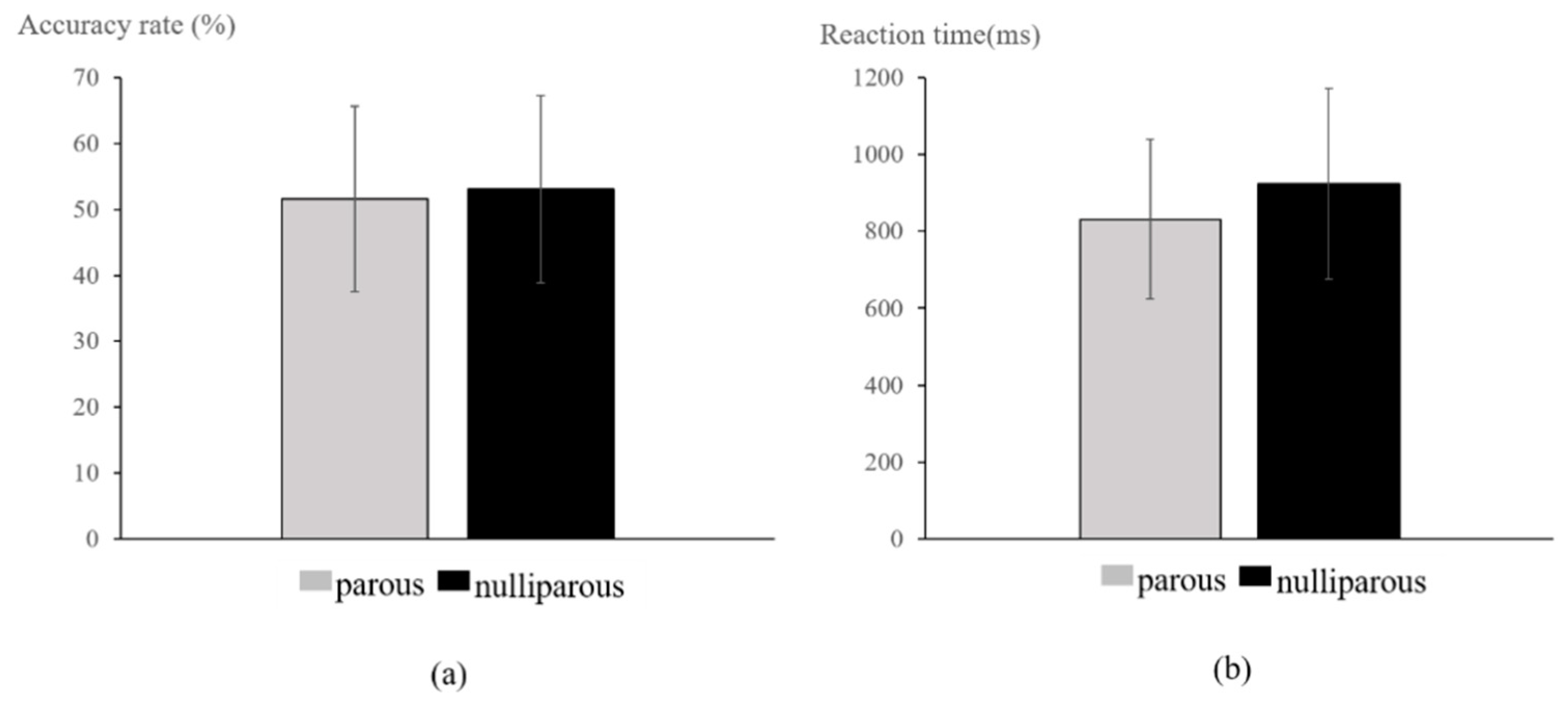
3.1. Cognitive Performance
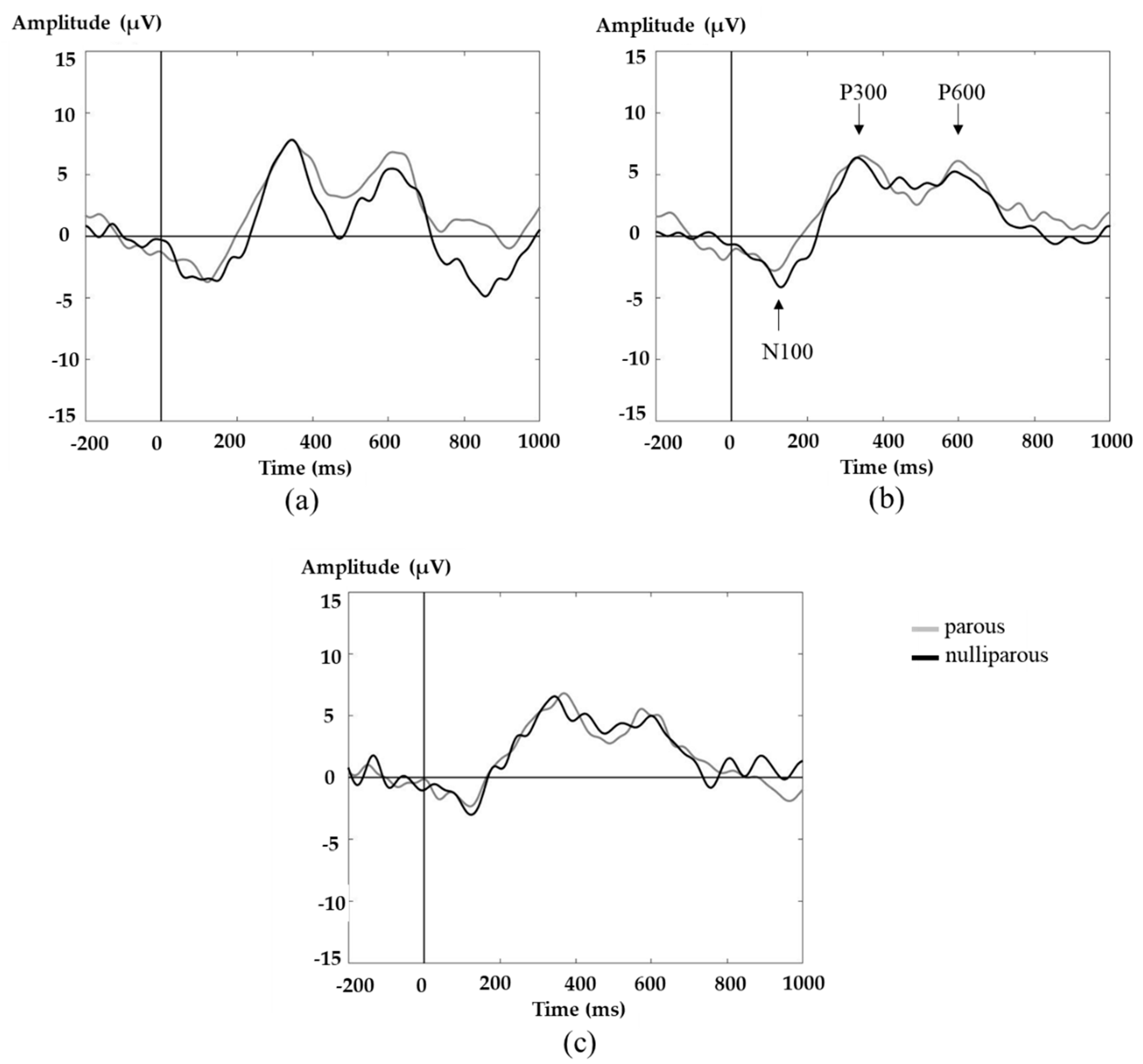
3.2. ERP Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crawley, R.A.; Dennison, K.; Carter, C. Cognition in pregnancy and the first year post-partum. Psychol. Psychother. 2003, 76, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Parsons, C.; Redman, S. Self-reported cognitive change during pregnancy. Aust. J. Adv. Nurs. Q. Publ. R. Aust. Nurs. Fed. 1991, 9, 20–29. [Google Scholar]
- Brett, M.; Baxendale, S. Motherhood and memory: A review. Psychoneuroendocrinology 2001, 26, 339–362. [Google Scholar] [CrossRef]
- Daniel, J.M.; Roberts, S.L.; Dohanich, G.P. Effects of ovarian hormones and environment on radial maze and water maze performance of female rats. Physiol. Behav. 1999, 66, 11–20. [Google Scholar] [CrossRef]
- Kinsley, C.H.; Madonia, L.; Gifford, G.W.; Tureski, K.; Griffin, G.R.; Lowry, C.; Williams, J.; Collins, J.; Mclearie, H.; Lambert, K.G. Motherhood improves learning and memory. Nature 1999, 402, 137–138. [Google Scholar] [CrossRef]
- Glynn, L.M. Giving birth to a new brain: Hormone exposures of pregnancy influence human memory. Psychoneuroendocrinology 2010, 35, 1148–1155. [Google Scholar] [CrossRef]
- Forget, H.; Lacroix, A.; Somma, M.; Cohen, H. Cognitive decline in patients with Cushing’s syndrome. J. Int. Neuropsychol. Soc. 2000, 6, 20–29. [Google Scholar] [CrossRef]
- Silber, M.; Almkvist, O.; Larsson, B.; Uvnäs-Moberg, K. Temporary peripartal impairment in memory and attention and its possible relation to oxytocin concentration. Life Sci. 1990, 47, 57–65. [Google Scholar] [CrossRef]
- Spencer, J.L.; Waters, E.M.; Romeo, R.D.; Wood, G.E.; Milner, T.A.; Mcewen, B.S. Uncovering the mechanisms of estrogen effects on hippocampal function. Front. Neuroendocr. 2008, 29, 219–237. [Google Scholar] [CrossRef]
- Hinkelmann, K.; Moritz, S.; Botzenhardt, J.; Riedesel, K.; Wiedemann, K.; Kellner, M.; Otte, C. Cognitive impairment in major depression: Association with salivary cortisol. Biol. Psychiatry 2009, 66, 879–885. [Google Scholar] [CrossRef]
- Glynn, L.M.; Schetter, C.D.; Chicz-Demet, A.; Hobel, C.J.; Sandman, C.A. Ethnic differences in adrenocorticotropic hormone, cortisol and corticotropin-releasing hormone during pregnancy. Peptides 2007, 28, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Tulchinsky, D.; Hobel, C.J.; Yeager, E.; Marshall, J.R. Plasma estrone, estradiol, estriol, progesterone, and 17-hydroxyprogesterone in human pregnancy: I. Normal pregnancy. Am. J. Obs. Gynecol. 1972, 112, 1095–1100. [Google Scholar] [CrossRef]
- Workman, J.L.; Barha, C.K.; Galea, L.A. Endocrine substrates of cognitive and affective changes during pregnancy and postpartum. Behav. Neurosci. 2012, 126, 54. [Google Scholar] [CrossRef]
- Maggi, A.; Susanna, L.; Bettini, E.; Mantero, G.; Zucchi, I. Hippocampus: A Target for Estrogen Action in Mammalian Brain. Mol. Endocrinol. 1989, 3, 1165–1170. [Google Scholar] [CrossRef]
- Henry, J.D.; Rendell, P.G. A review of the impact of pregnancy on memory function. J. Clin. Exp. Neuropsyc. 2007, 29, 793–803. [Google Scholar] [CrossRef]
- Christensen, H.; Leach, L.S.; Mackinnon, A. Cognition in pregnancy and motherhood: Prospective cohort study. Br. J. Psychiatry 2010, 196, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Keenan, P.A.; Yaldoo, D.T.; Stress, M.E.; Fuerst, D.R.; Ginsburg, K.A. Explicit memory in pregnant women. Am. J. Obs. Gynecol. 1998, 179, 731–737. [Google Scholar] [CrossRef]
- Sharp, K.; Brindle, P.M.; Brown, M.W.; Turner, G.M. Memory loss during pregnancy. BJOG 1993, 100, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Janes, C.; Casey, P.; Huntsdale, C.; Angus, G. Memory in pregnancy. I: Subjective experiences and objective assessment of implicit, explicit and working memory in primigravid and primiparous women. J. Psychosom. Obs. Gynecol. 1999, 20, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Dennerstein, L.; Armstrong, S.M.; Satohisa, E. Sleeping patterns during pregnancy in Japanese women. J. Psychosom. Obs. Gynecol. 1994, 15, 19–26. [Google Scholar] [CrossRef]
- Kim, M.S.; Kwon, J.S.; Kim, J.J. The neurophysiological mechanism of working memory: An event-related potential study. Korean J. Psychol. 2004, 23, 313–326. [Google Scholar]
- Kok, A. On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology 2001, 38, 557–577. [Google Scholar] [CrossRef]
- Olichney, J.M.; Taylor, J.R.; Gatherwright, J.; Salmon, D.P.; Bressler, A.J.; Kutas, M.; Iragui-Madoz, V.J. Patients with MCI and N400 or P600 abnormalities are at very high risk for conversion to dementia. Neurology 2008, 70, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Onton, J.; Delorme, A.; Makeig, S. Frontal midline EEG dynamics during working memory. Neuroimage 2005, 27, 341–356. [Google Scholar] [CrossRef] [PubMed]
- Perfetti, B.; Varanese, S.; Mancino, E.; Mercuri, P.; Tesse, M.; Franciotti, R.; Onofrj, M. Electrophysiological indices of interference resolution covary with individual fluid intelligence: Investigating reactive control processes in a 3-back working memory task. NeuroImage 2014, 93, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Callicott, J.H.; Ramsey, N.F.; Tallent, K.; Bertolino, A.; Knable, M.B.; Coppola, R.; Weinberger, D.R. Functional magnetic resonance imaging brain mapping in psychiatry: Methodological issues illustrated in a study of working memory in schizophrenia. Neuropsychopharmacology 1998, 18, 186–196. [Google Scholar] [CrossRef]
- Bosch, V.; Mecklinger, A.; Friederici, A.D. Slow cortical potentials during retention of object, spatial, and verbal information. Cogn. Brain Res. 2001, 10, 219–237. [Google Scholar] [CrossRef]
- Mecklinger, A.; Pfeifer, E. Event-related potentials reveal topographical and temporal distinct neuronal activation patterns for spatial and object working memory. Cogn. Brain Res. 1996, 4, 211–224. [Google Scholar] [CrossRef]
- Donchin, E.; Coles, M.G. Is the P300 component a manifestation of context updating. Behav. Brain Sci. 1988, 11, 357–427. [Google Scholar] [CrossRef]
- Gevins, A.; Smith, M.E.; McEvoy, L.; Yu, D. High-resolution EEG mapping of cortical activation related to working memory: Effects of task difficulty, type of processing, and practice. Cereb. Cortex 1997, 7, 374–385. [Google Scholar] [CrossRef]
- Kim, S.; Liu, Z.; Glizer, D.; Tannock, R.; Woltering, S. Adult ADHD and working memory: Neural evidence of impaired encoding. Clin. Neurophysiol. 2014, 125, 1596–1603. [Google Scholar] [CrossRef]
- Kutas, M.; Hillyard, S.A. Event-related brain potentials to semantically inappropriate and surprisingly large words. Biol. Psychol. 1980, 11, 99–116. [Google Scholar] [CrossRef]
- Osterhout, L.; Holcomb, P.J. Event-related brain potentials elicited by syntactic anomaly. J. Mem. Lang. 1992, 31, 785–806. [Google Scholar] [CrossRef]
- Wilson, G.F.; Swain, R.A.; Davis, I. Topographical analysis of cortical evoked activity during a variable demand spatial processing task. Aviat. Space Environ. Medicat. 1994, 65, 54–61. [Google Scholar]
- Osterhout, L.; Holcomb, P.J. Event-related potentials and language comprehension. Oxf. Univ. Press 1995, 171–215. [Google Scholar]
- Dong, S.; Reder, L.M.; Yao, Y.; Liu, Y.; Chen, F. Individual differences in working memory capacity are reflected in different ERP and EEG patterns to task difficulty. Brain Res. 2015, 1616, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Sokhadze, E.M.; Casanova, M.F.; Casanova, E.L.; Lamina, E.; Kelly, D.P.; Khachidze, I. Event-related potentials (ERP) in cognitive neuroscience research and applications. NeuroRegulation 2017, 4, 14. [Google Scholar] [CrossRef]
- Salthouse, T.; Babcock, R.L. Decomposing adult age differences in working memory. Dev. Psychol. 1991, 27, 763. [Google Scholar] [CrossRef]
- Hillyard, S.A.; Hink, R.F.; Schwent, V.L.; Picton, T.W. Electrical signs of selective attention in the human brain. Science 1973, 182, 177–180. [Google Scholar] [CrossRef]
- Polich, J. Clinical application of the P300 event-related brain potential. Phys. Med. Rehabil. Clin. 2004, 15, 133–161. [Google Scholar] [CrossRef]
- Fabiani, M.; Karis, D.; Donchin, E. Effects of mnemonic strategy manipulation in a Von Restorff paradigm. Electroencephalogr. Clin. Neurophysiol. 1990, 75, 22–35. [Google Scholar] [CrossRef]
- Johnson, R. Event-related potential insights into the neurobiology of memory systems. In Handbook of Neuropsychology; Boller, F., Grafman, J., Eds.; Elsevier: Amsterdam, The Netherlands, 1995; Volume 10, pp. 135–163. [Google Scholar]
- Johnson, R. On the neural generators of the P300 component of the event-related potential. Psychophysiology 1993, 30, 90–97. [Google Scholar] [CrossRef]
- Olichney, J.M.; Pak, J.; Salmon, D.P.; Yang, J.; Gahagan, T.; Nowacki, R.; Hansen, L.; Galasko, D.; Kutas, M.; Iragui-Madoz, V.J. Abnormal P600 word repetition effect in elderly persons with preclinical Alzheimer’s disease. Cogn. Neurosci. 2013, 4, 143–151. [Google Scholar] [CrossRef]
- Paller, K.A.; Kutas, M. Brain potentials during memory retrieval provide neurophysiological support for the distinction between conscious recollection and priming. J. Cogn. Neurosci. 1992, 4, 375–392. [Google Scholar] [CrossRef]
- Olichney, J.M.; Morris, S.K.; Ochoa, C.; Salmon, D.P.; Thal, L.J.; Kutas, M.; Iragui, V.J. Abnormal verbal event related potentials in mild cognitive impairment and incipient Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2002, 73, 377–384. [Google Scholar] [CrossRef]
- Kusak, G.; Grune, K.; Hagendorf, H.; Metz, A. Updating of working memory in a running memory task: An event-related potential study. Int. J. Psychophysiol. 2000, 39, 51–65. [Google Scholar] [CrossRef]
- Owen, A.M.; Evans, A.C.; Petrides, M. Evidence for a two-stage model of spatial working memory processing within the lateral frontal cortex: A positron emission tomography study. Cereb. Cortex 1996, 6, 31–38. [Google Scholar] [CrossRef]
- Macleod, A.K.; Buckner, R.L.; Miezin, F.M.; Petersen, S.E.; Raichle, M.E. Right anterior prefrontal cortex activation during semantic monitoring and working memory. NeuroImage 1998, 7, 41–48. [Google Scholar] [CrossRef][Green Version]
- Nystrom, L.E.; Braver, T.S.; Sabb, F.W.; Delgado, M.R.; Noll, D.C.; Cohen, J.D. Working memory for letters, shapes, and locations: fMRI evidence against stimulus-based regional organization in human prefrontal cortex. NeuroImage 2000, 11, 424–446. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, D.R.; Mattay, V.; Callicott, J.; Kotrla, K.; Santha, A.; Van Gelderen, P.; Duyn, J.; Moonen, C.; Frank, J. fMRI applications in schizophrenia research. NeuroImage 1996, 4, S118–S126. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, M.-H.; Jung, J.-J.; Lee, J.-H.; Chung, S.-C.; Park, H.-K.; Kim, H.-J. Effect of Childbirth Experience on Cognitive Performance and Event-Related Potential Patterns. Appl. Sci. 2021, 11, 3233. https://doi.org/10.3390/app11073233
Choi M-H, Jung J-J, Lee J-H, Chung S-C, Park H-K, Kim H-J. Effect of Childbirth Experience on Cognitive Performance and Event-Related Potential Patterns. Applied Sciences. 2021; 11(7):3233. https://doi.org/10.3390/app11073233
Chicago/Turabian StyleChoi, Mi-Hyun, Jin-Ju Jung, Je-Hyeop Lee, Soon-Cheol Chung, Hyun-Kyung Park, and Hyun-Jun Kim. 2021. "Effect of Childbirth Experience on Cognitive Performance and Event-Related Potential Patterns" Applied Sciences 11, no. 7: 3233. https://doi.org/10.3390/app11073233
APA StyleChoi, M.-H., Jung, J.-J., Lee, J.-H., Chung, S.-C., Park, H.-K., & Kim, H.-J. (2021). Effect of Childbirth Experience on Cognitive Performance and Event-Related Potential Patterns. Applied Sciences, 11(7), 3233. https://doi.org/10.3390/app11073233




