Featured Application
β-lactolin has protective effects on reactive astrocytes and mediates dopamine metabolism; it might thus aid in improving memory.
Abstract
Astrocytes are known to regulate normal brain function. Monoamine oxidase B (MAO-B), an enzyme highly expressed in astrocytes, metabolizes dopamine (DA) and induces reactive oxygen species (ROS) production. We have previously reported that β-lactolin, a whey-derived glycine–threonine–tryptophan–tyrosine tetrapeptide, improves memory impairment in mice by regulating the dopaminergic system; however, the effects of β-lactolin on astrocytes remain unclear. Herein, we investigated the effects of β-lactolin on cultured murine astrocytes. First, we measured intracellular ROS production in lipopolysaccharide-stimulated reactive astrocytes treated with or without β-lactolin, and then determined the role of β-lactolin in DA metabolism in astrocytes by measuring MAO-B enzyme activity and the levels of DA, and its metabolites, in DA-pretreated astrocytes. We found that β-lactolin significantly suppressed ROS production in lipopolysaccharide-stimulated reactive astrocytes (p = 2.76 × 10−6), inhibited MAO-B activity (p = 2.65 × 10−2) and increased intracellular DA levels (p = 1.08 × 10−3), suggesting that β-lactolin could inhibit DA metabolism in astrocytes. These results illustrate the novel protective effects of β-lactolin on reactive astrocytes and suggest their involvement in the memory-improving effects of β-lactolin via the dopaminergic system.
Keywords:
β-lactolin; β-lactoglobulin; whey; peptide; astrocytes; reactive oxygen species; monoamine oxidase B; dopamine 1. Introduction
Astrocytes are the most abundant glial cells with fundamental physiological functions in the brain, such as supporting neurons, modulating synaptic activities, and regulating the clearance of neurotransmitters, including dopamine (DA) [1,2,3]. Recent studies have indicated that astrocytes play important and complex roles in the central nervous system (CNS); hence, astrocyte dysfunction could contribute to the development of neurodegenerative diseases [4,5]. Reactive astrocytes are induced in response to pathological conditions, such as ischemia, brain injury, infection, and neurodegenerative diseases, such as Alzheimer’s (AD), Parkinson’s (PD) and Huntington’s disease [6]. The induced astrocytes become harmful, as they lose their normal functions, upregulate the expression of inflammatory genes, and produce higher levels of reactive oxygen species (ROS) than astrocytes under resting conditions, causing further neurotoxicity and cell death [6]. Therefore, reactive astrocytes are considered as preventive and therapeutic targets in neurodegenerative diseases.
DA regulates several physiological processes, such as motor control and PD, a well-known neurodegenerative disease, is caused by DA depletion [7]. Moreover, accumulating evidence has revealed that DA is crucial for cognitive functions. DA is directly associated with frontal cortex-dependent cognitive functions, including attention, executive function, learning and memory processes [8,9]. Several studies have shown that DA depletion and neuronal loss in the brain alter cognitive impairment in mice, indicating that restoring the DA system could contribute to the improvement of impaired cognitive performance [10,11].
The main enzymes responsible for DA metabolism are monoamine oxidase B (MAO-B) and catechol-O-methyltransferase (COMT), both of which are highly expressed in astrocytes [12,13]. MAO-B inhibitors are widely used for the treatment of PD, since MAO-B inhibition increases the synaptic DA concentration [7]. Recent studies have demonstrated that some MAO-B inhibitors have preventive and therapeutic effects on the development of AD [14,15]. In contrast, MAO-B is known to be an important source of ROS in astrocytes, because it generates hydrogen peroxide (H2O2) during enzymatic reactions [16,17]. Several studies have shown that H2O2 production in reactive astrocytes is increased in an MAO-B-dependent manner and an MAO-B inhibitor suppressed ROS production and neuronal loss in transgenic mouse models of AD and PD [14,18,19].
Recently, food-derived MAO-B inhibitory peptides have been identified [20]. β-lactolin, a β-lactopeptide of glycine–threonine–tryptophan–tyrosine (GTWY), is derived from β-lactoglobulin containing tryptophan–tyrosine (WY) sequences, which is abundantly present in fermented dairy products, such as camembert cheese, and in whey proteins digested by certain enzymes. We found that β-lactolin and its core sequence dipeptide, WY, inhibit the enzymatic activity of human recombinant MAO-B [20]. We demonstrated that β-lactolin administration in mice increased DA levels in the hippocampus and improved memory function [20], which was attenuated by knocking down D1-like receptors in the hippocampus [21]. These findings indicated that β-lactolin improves memory function by regulating the dopaminergic system in mice. In addition, β-lactolin prevented neural inflammation in the mouse model of AD [22] and β-lactolin supplementation improved cognitive function in randomized clinical trials [23,24]. However, the detailed mechanisms of β-lactolin, especially its direct effects on astrocytes, remain unclear.
In the present study, we investigated the effects of β-lactolin on cultured murine astrocytes. Lipopolysaccharide (LPS) and interferon gamma (IFN-Υ) were used to induce reactive astrocytes as previously described [25]. We then evaluated the effects of β-lactolin on DA metabolism in astrocytes by measuring MAO-B enzyme activity and intracellular and extracellular monoamine levels in DA-pretreated astrocytes. This study demonstrated the novel effects of β-lactolin on astrocytes and indicates the involvement of astrocytes in the memory-improving effects of β-lactolin.
2. Materials and Methods
2.1. Cell Culture and Treatment
Murine astrocytes (CRL-2541; ATCC, Manassas, VA, USA) were maintained in Dulbecco’s Modified Eagle’s Medium (Gibco, Waltham, MA, USA) supplemented with 10% heat-inactivated fetal bovine serum (Biological Industries, Beit-Haemek, Israel) and 100 U/mL penicillin–streptomycin (Gibco) at 37 °C under 5% CO2. The cells were seeded in 6-well plates (IWAKI, Shizuoka, Japan) for reverse transcription quantitative polymerase chain reaction (RT-qPCR) and monoamine analysis or in 10-cm plates (Corning, Corning, NY, USA) for MAO and COMT activity assays at a density of 3.0 × 105 cells/mL and incubated overnight. β-lactolin (GTWY peptide; purity: 98%) was purchased from Bachem (Bubendorf, Switzerland). β-lactolin was dissolved in dimethyl sulfoxide (Wako, Osaka, Japan) and diluted in phosphate-buffered saline (PBS; TaKaRa Bio, Shiga, Japan), such that the final concentration in the culture medium was less than 1%.
2.2. ROS Analysis
Cells were seeded in 96-well plates (PerkinElmer, Waltham, MA, USA) coated with poly-D-lysine (Sigma-Aldrich, St. Louis, MO, USA) at a density of 1.5 × 104 cells/well and incubated for 8 h. Subsequently, the cells were treated with 0, 10, 50, and 100 ng/mL LPS (L3129; Sigma-Aldrich) dissolved in PBS and 50 U/L IFN-Υ (R&D systems, Minneapolis, MN, USA) for 24 h. The cells were then treated with 0, 1, 5 and 10 μM β-lactolin for 6 h prior to staining, followed by incubation with 10 μM 5-(and-6)-chloromethyl-2,7-dichlorodihydrofluorescein diacetate acetyl ester (CM-H2DCFDA; Invitrogen, Waltham, MA, USA) and 0.5 μg/mL Hoechst 33,342 (Dojindo, Kumamoto, Japan) in Hanks’ balanced salt solution (Gibco) for 30 min at 37 ℃. The culture medium was replaced with fresh Hanks’ balanced salt solution. Fluorescence was measured using an Operetta CLS™ instrument (Perkin Elmer) at excitation and emission wavelengths of 460–490 nm and 500–550 nm (CM-H2DCFDA), and 355–385 nm and 430–500 nm (Hoechst), respectively. Fluorescence intensity per cell was analyzed using the Harmony 4.9 software (Perkin Elmer) and expressed in relative fluorescence units (RFU).
2.3. MAO Activity Assay
Cells were treated with 0, 1, 5, and 10 μM β-lactolin or 10 µM pargyline hydrochloride (Sigma-Aldrich), a selective MAO-B inhibitor, for 5 h. The cells were washed with PBS and collected by scraping the culture dishes. After centrifugation, mitochondria were isolated using the Mitochondria Isolation Kit for culture cells (Thermo Fisher Scientific, Waltham, MA, USA) as per the manufacturer’s protocol. Mitochondrial fractions were diluted in PBS and used to determine the MAO-B activity. Total protein concentration of each mitochondrial fraction was measured using the Pierce™ bicinchoninic acid (BCA) Protein Assay Kit (Thermo Fisher Scientific). Pierce™ Bovine Serum Albumin Standard Ampules (Thermo Fisher Scientific) were used as standards. MAO-B activity was measured using an MAO Assay Kit (Cell Biolabs, San Diego, CA, USA) as per the manufacturer’s protocol. The substrate reacts with MAO-B isolated from the cells and generates H2O2, which reacts with the colorimetric probe and produces a red/pink product. The color change was quantified by measuring absorbance at 570 nm using a BioTek Eon microplate spectrophotometer (BioTek, Winooski, VT, USA) and expressed as MAO-B activity.
2.4. Monoamine Analysis
To measure the intracellular and extracellular levels of DA and metabolites in astrocytes, high-performance liquid chromatography (HPLC) analysis was performed as described previously [26]. Briefly, cells were pretreated with 200 μM DA hydrochloride (Sigma-Aldrich) dissolved in PBS for 2 h, followed by treatment with 0 or 10 μM β-lactolin for 3 h. The culture supernatant was diluted in an equal volume of 0.2 M ice-cold perchloric acid (Wako) containing 10 mM ethylenediaminetetraacetic acid (EDTA; Sigma-Aldrich). The suspended cells were washed with PBS and homogenized with 0.2 M perchloric acid. After filtration through a 0.45-μm membrane (Merck Millipore, Burlington, MA, USA), the filtrate was used for HPLC analysis using an EICOMPAK SC-5ODS column (2.1 mm ø × 150 mm, 5 μm particle; Eicom, Kyoto, Japan) and a PREPAK column (Eicom) with electrochemical detection. A mobile phase containing 83% 0.1 M acetic acid (Wako) in citric acid (Nacalai Tesque, Kyoto, Japan) buffer (pH 3.5), 17% methanol (Wako), 190 mg/L sodium 1-octanesulfonate (Wako), and 5 mg/L EDTA-2Na (Dojindo) was infused at a flow rate of 0.5 mL/min. For electrochemical detection, the voltage applied was 700 mV against an Ag/AgCl reference electrode.
2.5. RT-qPCR Analysis
Total RNA was extracted using an RNeasy Mini Kit (QIAGEN, Hilden, Germany). RNA concentration and purity were assessed using a NanoDrop ND-1000 instrument (Thermo Fisher Scientific). RNA was reverse-transcribed using a SuperScript™ IV First-Strand Synthesis System (Invitrogen) as per the manufacturer’s protocol. The cDNA was then amplified using TB Green Premix Ex Taq II (TaKaRa Bio). The PCR was performed using a LightCycler 480 Instrument (Roche, Basel, Switzerland) under the following conditions: 95 °C for 10 s, followed by 45 cycles of 95 °C for 10 s, 61 °C for 5 s, and 72 °C for 9 s. The data were normalized to glyceraldehyde-3-phosphate dehydrogenase. The primer sequences used for PCR are listed in Table 1.

Table 1.
Primer list.
2.6. COMT Activity Assay
COMT activity was measured as described previously [27,28]. Briefly, cells were treated with 0 or 10 μM β-lactolin or 100 μM 3,4-dihydroxybenzoic acid (Sigma-Aldrich), a selective COMT inhibitor, for 24 h. The cells were washed with PBS and lysed in NP40 cell lysis buffer (Invitrogen) as per the manufacturer’s protocol. After centrifugation, the supernatants were collected. Total protein concentration of each supernatant was adjusted to 200 μg/mL. Esculetin (Sigma-Aldrich) was dissolved in dimethyl sulfoxide and diluted in an aqueous buffer solution [38.7 mM NaH2PO4 (Wako), 61.3 mM Na2HPO4 (Wako), 5 mM MgCl2 (Wako), 20 mM L-cysteine (Sigma-Aldrich), pH 7.4] at a final concentration of 40 μM in 120 μL of reaction mixture. A 96-well plate was placed on ice, and the cell lysate was added to achieve a final protein concentration of 100 μg/mL. The plate was preincubated in a SpectraMax M3 microplate reader (Molecular Devices, San Jose, CA, USA) preheated at 37 °C for 5 min. The reaction was initiated by the addition of S-(5′-adenosyl)-L-methionine (Sigma-Aldrich) at a final concentration of 200 μM. Fluorescence was measured at 3-min intervals for 60 min at 37 °C. The excitation and emission wavelengths were 380 and 460 nm, respectively. The change in the fluorescence intensity caused by enzymatic O-methylation of esculetin to form scopoletin was used to measure the enzyme activity. Additionally, samples without cell lysates were used as controls.
2.7. Statistical Analysis
Data are presented as mean ± standard error of the mean (SEM). Data were analyzed using the Student’s t-test or one-way analysis of variance followed by Bonferroni’s post hoc test or Dunnett’s test, as described in the figure legends. All statistical analyses were performed using the Ekuseru–Toukei Ver. 7.0 software (Social Survey Research Information, Tokyo, Japan). Values of p less than 0.05 were considered statistically significant.
3. Results
3.1. Effects of β-Lactolin on ROS Production in Astrocytes
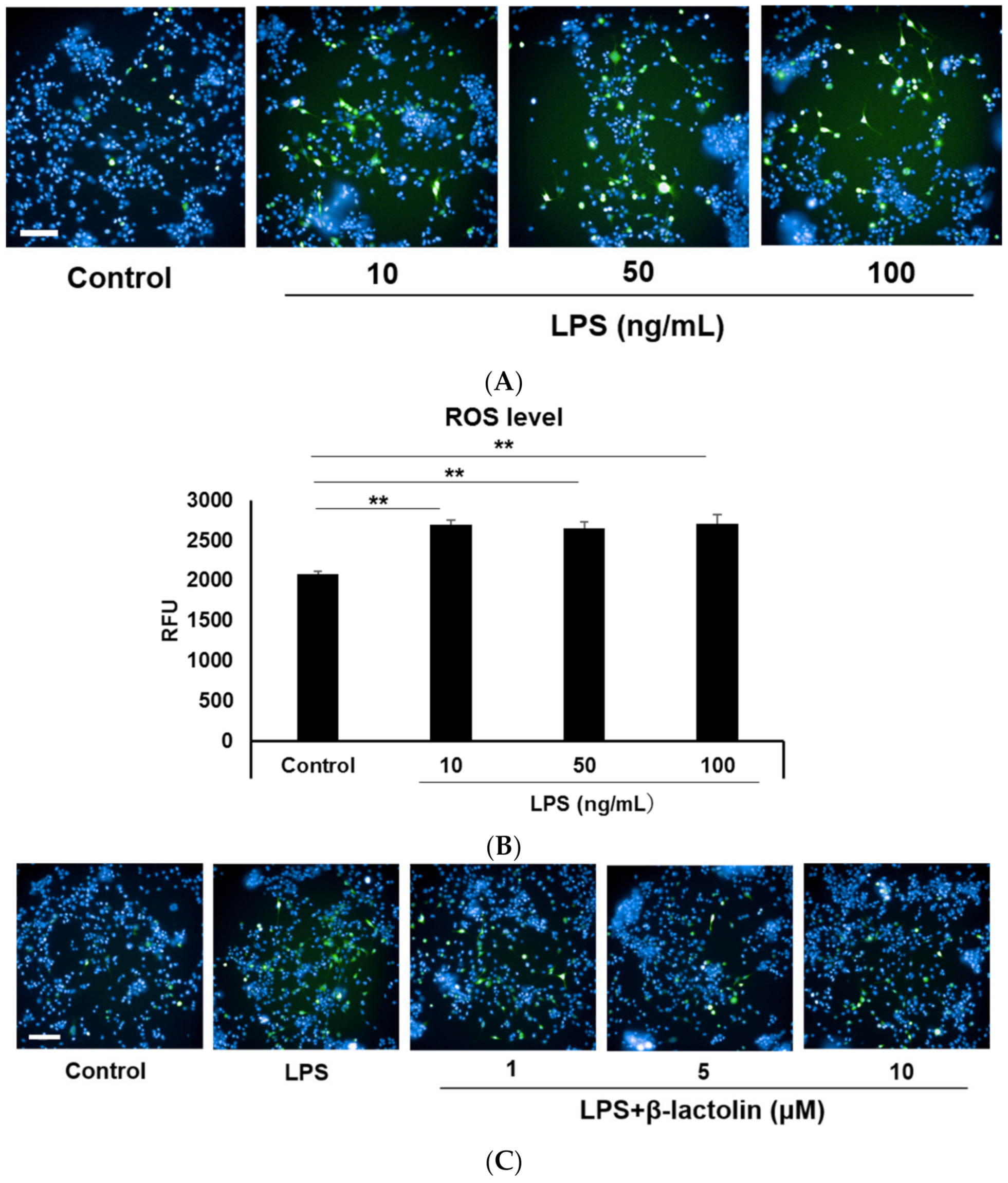
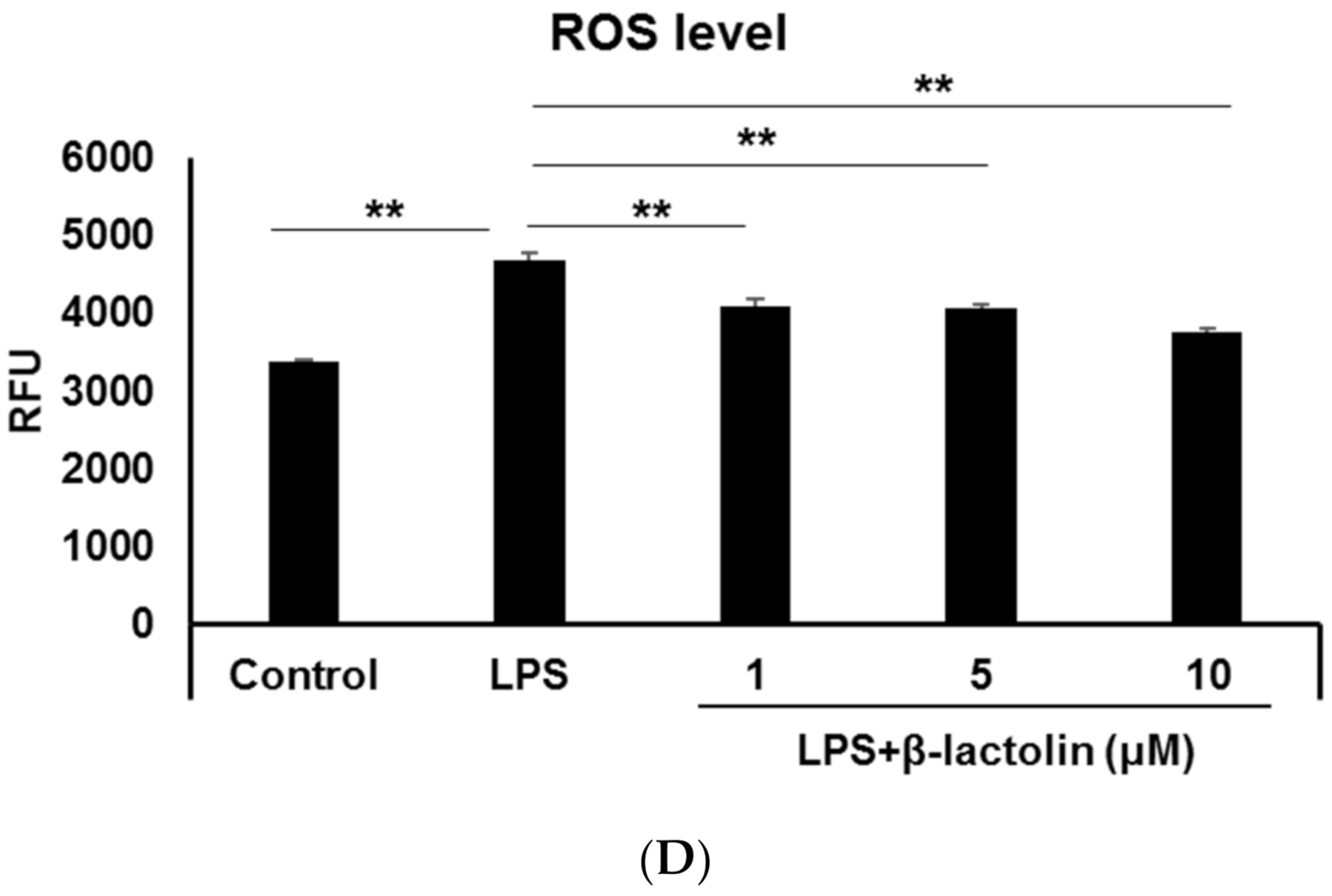
To evaluate ROS production in astrocytes, murine astrocytes were treated with LPS and IFN-Υ. Intracellular ROS levels were determined using the relative fluorescence intensity of CM-H2DCFDA. Referring to data from the literature [25,29], the used concentrations of LPS were as follows: 10, 50, and 100 ng/mL. Treatment with LPS caused a significant increase in the intracellular ROS levels compared to the control (control: 2077 ± 34.94 RFU; LPS 10 ng/mL: 2688 ± 60.58 RFU, p = 4.30 × 10−6; LPS 50 ng/mL: 2652 ± 77.46 RFU, p = 8.72 × 10−6; and LPS 100 ng/mL: 2710 ± 107.3 RFU, p = 3.41 × 10−6; Figure 1A,B). To investigate the effects of β-lactolin on ROS production in astrocytes, cells were treated with β-lactolin for 6 h after LPS stimulation. LPS treatment significantly increased the intracellular ROS levels (control: 3371 ± 38.43 RFU; LPS: 4687 ± 81.89 RFU, p = 2.76 × 10−6), whereas treatment with β-lactolin significantly reduced intracellular ROS levels (β-lactolin 1 μM: 4092 ± 89.52 RFU, p = 3.35 × 10−6; 5 μM: 4098 ± 60.45 RFU, p = 2.91 × 10−6; and 10 μM: 3771 ± 45.65 RFU, p = 2.76 × 10−6; Figure 1C,D). Of note, β-lactolin treatment alone did not induce a significant change in the intracellular ROS levels compared to the control (Supplemental Figure S1A,B). These results indicate that β-lactolin treatment suppresses intracellular ROS production in LPS-treated astrocytes.
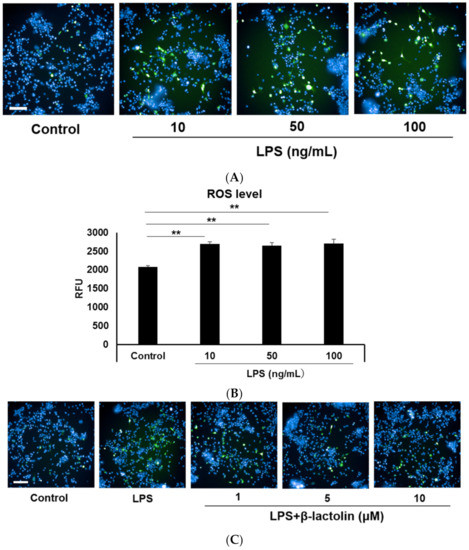
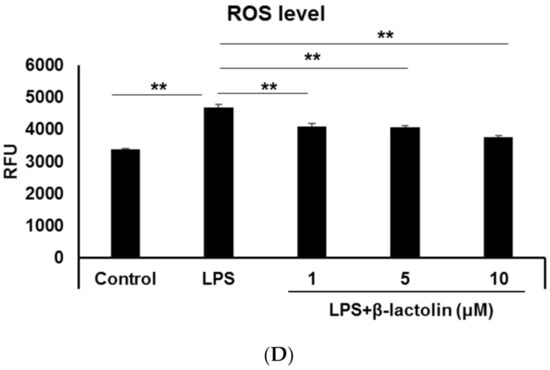
Figure 1.
Reactive oxygen species (ROS) production in astrocytes treated with LPS and β-lactolin. (A,C) Representative images show intracellular ROS detected using CM-H2DCFDA (green). The nuclei were stained with Hoechst 33,342 (blue). Scale bar is 100 μm at ×20 magnification. (A) Murine astrocytes were treated with 0, 10, 50, or 100 ng/mL lipopolysaccharide (LPS) and 50 U/L IFN-Υ for 24 h. (C) Cells were treated with PBS, 10 ng/mL LPS, and 50 U/L IFN-Υ for 24 h, then treated with 0, 1, 5, or 10 μM β-lactolin for 6 h prior to staining. (B,D) Quantification of ROS levels by measuring the relative CM-H2DCFDA fluorescence intensity per nucleus. Data represent the mean ± SEM (n = 12). The p values were calculated using one-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc test. ** p < 0.01. ROS, reactive oxygen species; CM-H2DCFDA, 5-(and-6)-chloromethyl-2,7-dichlorodihydrofluorescein diacetate acetyl ester; LPS, lipopolysaccharide; IFN-Υ, interferon gamma; PBS, phosphate-buffered saline; ANOVA, analysis of variance; SEM, standard error of the mean.
3.2. Effect of β-Lactolin on MAO-B in Astrocytes
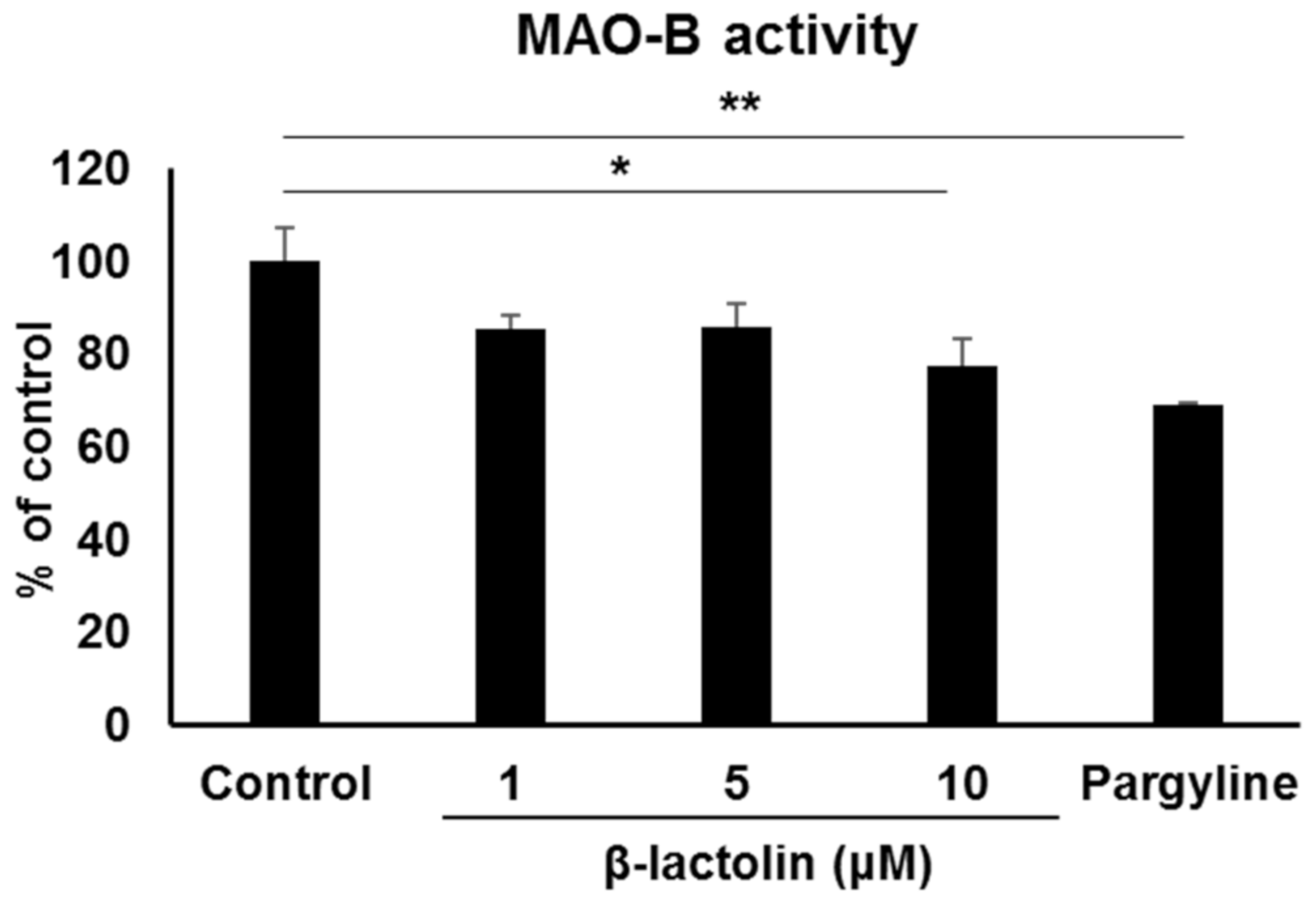
Next, we evaluated the effect of β-lactolin on MAO-B enzyme activity in astrocytes. MAO-B activity in the astrocyte mitochondria treated with 10 μM β-lactolin was significantly lower than that in the control (100 ± 7.08% vs. 77.3 ± 5.88%, p = 2.65 × 10−2). Additionally, MAO-B activity in mitochondria treated with 10 μM pargyline was significantly lower than that in the control (68.96 ± 0.31%, p = 3.90 × 10−3; Figure 2).
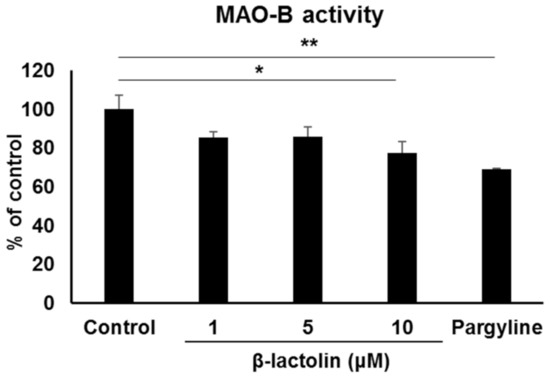
Figure 2.
The effect of β-lactolin on MAO-B activity in astrocyte mitochondria. Cells were treated with 0, 1, 5, or 10 μM β-lactolin or 10 μM pargyline for 5 h. MAO activity assay was performed on mitochondria isolated from astrocytes. Pargyline, a selective MAO inhibitor, was used as a positive control. Data represent the mean ± SEM (n = 3). The p values were calculated using one-way ANOVA followed by Dunnett’s multiple comparison test. * p < 0.05, ** p < 0.01. MAO-B, monoamine oxidase B; ANOVA, analysis of variance; SEM, standard error of the mean.
3.3. Effects of β-Lactolin-Induced MAO-B Inhibition on DA Metabolism in Astrocytes
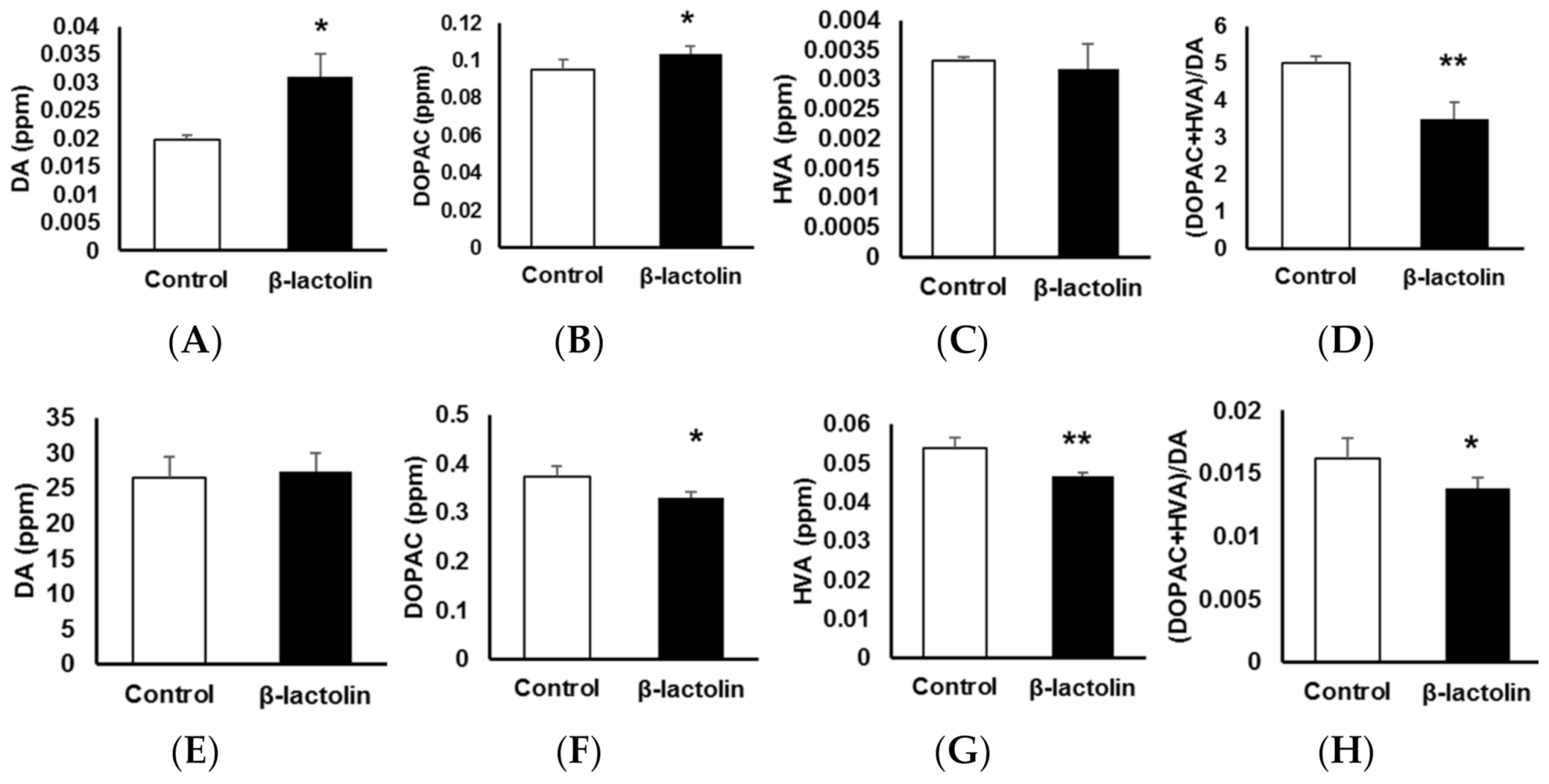
To evaluate the involvement of β-lactolin in DA metabolism in astrocytes, the levels of DA and its metabolites were measured using an HPLC-electrochemical detection system. Using RT-qPCR, we confirmed the gene expression of the two main enzymes that metabolize DA, Mao-b and Comt. The relative gene expression levels normalized to Gapdh were 1.23 ± 0.206 for Mao-b and 1.04 ± 8.4 × 10−3 for Comt (n = 3). Intracellular DA levels in astrocytes treated with β-lactolin were significantly higher than those in the control group (1.97 × 10−2 ± 8.59 × 10−4 vs. 3.10 × 10−2 ± 4.01 × 10−3 ppm, p = 1.08 × 10−3; Figure 3A). Intracellular levels of 3,4-dihydroxyphenylacetic acid (DOPAC) after treatment with β-lactolin were significantly higher than those in the control group (9.54 × 10−2 ± 5.01 × 10−3 vs. 0.104 ± 4.14 × 10−3 ppm, p = 1.05 × 10−2; Figure 3B). Homovanillic acid (HVA) levels were not changed by β-lactolin treatment (Figure 3C). The turnover of intracellular DA ([DOPAC + HVA]/DA) in β-lactolin-treated cells was significantly lower than that in control cells (5.01 ± 0.171 vs. 3.49 ± 0.443 ppm, p = 2.27 × 10−4; Figure 3D).
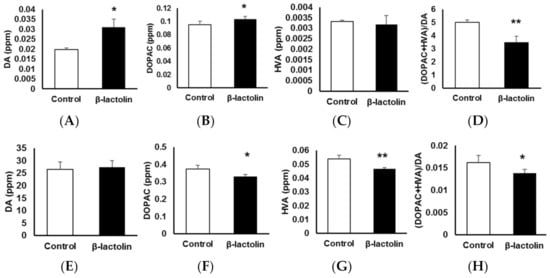
Figure 3.
The effects of β-lactolin on dopamine (DA) metabolism in astrocytes. Cells were treated with 0 or 10 μM β-lactolin for 3 h, following treatment with DA for 2 h. The following monoamine levels in the cells (A–D) and in culture media (E–H) were measured using high-performance liquid chromatography (HPLC): DA (A,E), 3,4-dihydroxyphenylacetic acid (DOPAC) (B,F), and homovanillic acid (HVA) (C,G). The (DOPAC + HVA)/DA ratio (D,H) was also determined. Data represent the mean ± SEM (n = 6). The p values were calculated using the Student’s t-test. * p < 0.05, ** p < 0.01. DA, dopamine; HPLC, high-performance liquid chromatography; DOPAC, 3,4-dihydroxyphenylacetic acid; HVA, homovanillic acid; SEM, standard error of the mean.
Extracellular DA levels in the culture supernatant of astrocytes were not changed by β-lactolin treatment (Figure 3E). However, extracellular DOPAC levels in the supernatant of cells treated with β-lactolin were significantly lower than those in the control (0.374 ± 2.13 × 10−2 vs. 0.329 ± 1.28 × 10−2 ppm, p = 2.20 × 10−3; Figure 3F). Furthermore, extracellular HVA levels in the supernatant of cells treated with β-lactolin were significantly lower than those in the control (5.40 × 10−2 ± 2.53 × 10−3 vs. 4.67 × 10−2 ± 1.03 × 10−3 ppm, p = 3.12 × 10−4; Figure 3G). The turnover of extracellular DA ([DOPAC + HVA]/DA) in the β-lactolin-treated group was significantly lower than that in the control group (1.62 × 10−2 ± 1.58 × 10−4 vs. 1.38 × 10−2 ± 8.15 × 10−4 ppm, p = 1.31 × 10−2; Figure 3H). Notably, COMT activity was not affected by treatment with β-lactolin but was inhibited by treating the cells with 3,4-dihydroxybenzoic acid (100 ± 12.84% vs. 68.70 ± 13.04%, p = 7.60 × 10−3; Figure S2). These results indicate that β-lactolin suppresses DA metabolism in astrocytes.
4. Discussion
Astrocytes perform various crucial functions in the central nervous system (CNS) and reactive astrocytes are considered as therapeutic targets in neurodegenerative diseases. We have previously demonstrated that orally administered β-lactolin is delivered to the brain and improves memory function in in vivo models [20]. However, the effects of β-lactolin on astrocytes have not been investigated. This study showed that β-lactolin suppresses intracellular ROS production in LPS-treated astrocytes. Several studies have shown that excessive ROS production in astrocytes is a characteristic of reactive astrocytes, which mediates the pathogenesis of neurodegenerative diseases, such as AD and PD [30,31]. The increase in ROS production in astrocytes triggers the release of pro-inflammatory cytokines, leading to neuronal dysfunction in the CNS [32]. H2O2, in particular, is an ROS that has a high membrane permeability; thus, its toxicity affects not only the astrocytes producing it but also their neighboring neurons [33]. Previous in vivo studies showed that LPS induces neuronal apoptosis, which is rescued by treatment with some bioactive products that attenuate astrocyte reactivity, including ROS production [34,35,36]. Similar neuroprotective effects of chemicals and/or bioactive compounds that suppress the reactivity of LPS-treated astrocytes were observed in ex vivo studies conducted on neuron-astrocyte co-culture models or neurons cultured in astrocyte-conditioned medium [37,38]. Hence, the present results indicate potential neuroprotective effects of β-lactolin on astrocytes. Therefore, further studies are warranted to determine the effects of β-lactolin on the crosstalk between LPS-treated astrocytes and neurons.
Next, we evaluated the inhibitory effect of β-lactolin on MAO-B in astrocytes. Consistent with the results of our previous study using human recombinant MAO-B [20], β-lactolin inhibited MAO-B enzyme activity in astrocytes. We assume that β-lactolin directly inhibits the enzyme activity because β-lactolin and WY peptide showed MAO-B inhibitory effects, whereas tryptophan did not in the previous studies [20,39]. Astrocytes highly express MAO-B, generating H2O2 as a result of its enzymatic reaction. Accumulating evidence has indicated that astrocytic MAO-B activity could regulate ROS production in astrocytes [18]. Indeed, recent studies have shown that some MAO-B inhibitors suppress intracellular ROS production in astrocytes [14,19]. Moreover, some food constituents that attenuate MAO-B activity showed protective effects by suppressing ROS production in these cells [40,41,42]. Therefore, the inhibitory effect of β-lactolin on MAO-B might also suppress ROS production. It is notable that some MAO-B inhibitory food constituents have antioxidant activity [40,41,42]. Given that whey-derived peptides containing WY showed strong antioxidant activity [43,44], the antioxidant activity of β-lactolin could be worth investigating. Furthermore, MAO-B mediates the synthesis of γ-aminobutyric acid (GABA) in astrocytes through the putrescine degradation pathway [45]. Reactive astrocytes produce excess GABA in an MAO-B-dependent manner, leading to impairment in synaptic plasticity and memory function, which is rescued by selective MAO-B inhibitors [45,46]. Therefore, the inhibitory effect of β-lactolin on MAO-B may affect GABA production in astrocytes. Further studies are warranted to investigate this possibility.
Lastly, we demonstrated that β-lactolin suppresses DA metabolism in astrocytes. The results are consistent with our previous report suggesting that β-lactolin improves memory function and depression-like behavior [47] by increasing DA levels in the hippocampus and frontal cortex of mice [20]. It has been reported that DA plays essential roles in cognitive control. Previous studies have demonstrated that DA levels in the prefrontal cortex are associated with cognitive performance, including working memory and attention [8,9]. Moreover, DA modulates synaptic plasticity in the hippocampus and enhances DA binding to DA receptors, thereby improving cognitive performance in mice [48,49]. In fact, the DA precursor levodopa improved the rate of task-based learning and task performances in elderly people [50]. Astrocytes are the main cells involved in DA metabolism in the CNS, as they express high levels of DA-metabolizing enzymes, such as MAO-B and COMT [13]. Moreover, it has been reported that DA metabolism dysfunction in astrocytes of the prefrontal cortex leads to cognitive impairment [51]. This evidence suggests that the suppressive effects of β-lactolin on DA metabolism in astrocytes might contribute to the memory-improving effects of β-lactolin. Notably, even though astrocytes express both MAO-B and COMT, the suppression of DA metabolism by β-lactolin was mainly considered to occur due to its inhibitory effect on MAO-B, since it did not significantly inhibit COMT activity, as demonstrated in this study.
However, there are some limitations to the present study. First, we did not clarify the mechanism underlying the inhibition of ROS production by β-lactolin. A mechanism that might lead to reduced ROS levels is the upregulation of antioxidant activity. Although we demonstrated that MAO-B activity was suppressed by β-lactolin, we did not evaluate the effect of MAO-B inhibitors on ROS production in this study. Therefore, further studies are warranted to investigate the mechanisms involved in the decrease in ROS production in detail. Next, since we used only cultured astrocytes in this study, the direct effects of β-lactolin on astrocytes in in vivo models have not been evaluated. For instance, it is not clear to what extent the suppressive effects of β-lactolin on DA metabolism contribute to the memory-improving effects of β-lactolin via the dopaminergic system in mice. To evaluate this possibility, astrocyte-specific MAO-B knockdown using adenovirus needs to be performed in future in vivo studies.
In conclusion, this study revealed novel effects of β-lactolin on astrocytes, including its protective effects on astrocytes under pathologic conditions, and suggested the involvement of astrocytes in the memory-improving effects of β-lactolin.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/app11073034/s1, Figure S1: ROS production in astrocytes treated with β-lactolin, Figure S2: Effect of β-lactolin on COMT activity in astrocytes.
Author Contributions
Conceptualization, Y.A., and S.A.; methodology, S.A.; investigation, S.A., C.T., and R.O.; writing—original draft preparation, S.A.; writing—review and editing, Y.A., and T.A.; visualization, S.A.; supervision, Y.A. All authors have read and agreed to the published version of the manuscript.
Funding
Kirin Holdings Company, Limited supported this study.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We thank Masato Asanuma for providing a detailed protocol for the measurement of DA and its metabolites in astrocytes.
Conflicts of Interest
The authors (S.A., T.A., C.T., R.O., Y.A.) are employed by Kirin Holdings Company, Limited. All authors declare no other competing interests.
References
- Sidoryk-Wegrzynowicz, M.; Wegrzynowicz, M.; Lee, E.; Bowman, A.B.; Aschner, M. Role of Astrocytes in Brain Function and Disease. Toxicol. Pathol. 2010, 39, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Tabata, H. Diverse subtypes of astrocytes and their development during corticogenesis. Front. Neurosci. 2015, 9, 114. [Google Scholar] [CrossRef] [PubMed]
- Ota, Y.; Zanetti, A.T.; Hallock, R.M. The Role of Astrocytes in the Regulation of Synaptic Plasticity and Memory Formation. Neural. Plast. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef]
- Dossi, E.; Vasile, F.; Rouach, N. Human astrocytes in the diseased brain. Brain Res. Bull. 2018, 136, 139–156. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Prim. 2017, 3, 1–21. [Google Scholar] [CrossRef]
- Epuig, M.V.; Erose, J.; Eschmidt, R.; Efreund, N. Dopamine modulation of learning and memory in the prefrontal cortex: Insights from studies in primates, rodents, and birds. Front. Neural Circuits 2014, 8, 93. [Google Scholar] [CrossRef]
- Arnsten, A.F.; Li, B.-M. Neurobiology of Executive Functions: Catecholamine Influences on Prefrontal Cortical Functions. Biol. Psychiatry 2005, 57, 1377–1384. [Google Scholar] [CrossRef]
- Morgan, R.G.; Gibbs, J.T.; Melief, E.J.; Postupna, N.O.; Sherfield, E.E.; Wilson, A.; Keene, C.D.; Montine, T.J.; Palmiter, R.D.; Darvas, M. Relative contributions of severe dopaminergic neuron ablation and dopamine depletion to cognitive impairment. Exp. Neurol. 2015, 271, 205–214. [Google Scholar] [CrossRef]
- Nobili, A.; Latagliata, E.C.; Viscomi, M.T.; Cavallucci, V.; Cutuli, D.; Giacovazzo, G.; Krashia, P.; Rizzo, F.R.; Marino, R.; Federici, M.; et al. Dopamine neuronal loss contributes to memory and reward dysfunction in a model of Alzheimer’s disease. Nat. Commun. 2017, 8, 1–14. [Google Scholar] [CrossRef]
- Hansson, E.; Sellström, Å. MAO COMT, and GABA-T Activities in Primary Astroglial Cultures. J. Neurochem. 1983, 40, 220–225. [Google Scholar] [CrossRef]
- Levitt, P.; Pintar, J.E.; Breakefield, X.O. Immunocytochemical demonstration of monoamine oxidase B in brain astrocytes and serotonergic neurons. Proc. Natl. Acad. Sci. USA 1982, 79, 6385–6389. [Google Scholar] [CrossRef]
- Borroni, E.; Bohrmann, B.; Grueninger, F.; Prinssen, E.; Nave, S.; Loetscher, H.; Chinta, S.J.; Rajagopalan, S.; Rane, A.; Siddiqui, A.; et al. Sembragiline: A Novel, Selective Monoamine Oxidase Type B Inhibitor for the Treatment of Alzheimer’s Disease. J. Pharmacol. Exp. Ther. 2017, 362, 413–423. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Guo, Y.; Wang, Z.; Huang, L.; Li, X. Dual functional cholinesterase and MAO inhibitors for the treatment of Alzheimer’s disease: Synthesis, pharmacological analysis and molecular modeling of homoisoflavonoid derivatives. J. Enzym. Inhib. Med. Chem. 2015, 31, 1–9. [Google Scholar] [CrossRef]
- Vindis, C.; Séguélas, M.-H.; Lanier, S.; Parini, A.; Cambon, C. Dopamine induces ERK activation in renal epithelial cells through H2O2 produced by monoamine oxidase. Kidney Int. 2001, 59, 76–86. [Google Scholar] [CrossRef]
- Cohen, G.; Farooqui, R.; Kesler, N. Parkinson disease: A new link between monoamine oxidase and mitochondrial electron flow. Proc. Natl. Acad. Sci. USA 1997, 94, 4890–4894. [Google Scholar] [CrossRef]
- Pizzinat, N.; Copin, N.; Vindis, C.; Parini, A.; Cambon, C. Reactive oxygen species production by monoamine oxidases in intact cells. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1999, 359, 428–431. [Google Scholar] [CrossRef]
- Mallajosyula, J.K.; Kaur, D.; Chinta, S.J.; Rajagopalan, S.; Rane, A.; Nicholls, D.G.; Di Monte, D.A.; MacArthur, H.; Andersen, J.K. MAO-B Elevation in Mouse Brain Astrocytes Results in Parkinson’s Pathology. PLoS ONE 2008, 3, e1616. [Google Scholar] [CrossRef]
- Ano, Y.; Ayabe, T.; Kutsukake, T.; Ohya, R.; Takaichi, Y.; Uchida, S.; Yamada, K.; Uchida, K.; Takashima, A.; Nakayama, H. Novel lactopeptides in fermented dairy products improve memory function and cognitive decline. Neurobiol. Aging 2018, 72, 23–31. [Google Scholar] [CrossRef]
- Ayabe, T.; Ano, Y.; Ohya, R.; Kitaoka, S.; Furuyashiki, T. The Lacto-Tetrapeptide Gly–Thr–Trp–Tyr, β-Lactolin, Improves Spatial Memory Functions via Dopamine Release and D1 Receptor Activation in the Hippocampus. Nutrition 2019, 11, 2469. [Google Scholar] [CrossRef]
- Ano, Y.; Ozawa, M.; Kutsukake, T.; Sugiyama, S.; Uchida, K.; Yoshida, A.; Nakayama, H. Preventive Effects of a Fermented Dairy Product against Alzheimer’s Disease and Identification of a Novel Oleamide with Enhanced Microglial Phagocytosis and Anti-Inflammatory Activity. PLoS ONE 2015, 10, e0118512. [Google Scholar] [CrossRef]
- Kita, M.; Obara, K.; Kondo, S.; Umeda, S.; Ano, Y. Effect of Supplementation of a Whey Peptide Rich in Tryptophan-Tyrosine-Related Peptides on Cognitive Performance in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrition 2018, 10, 899. [Google Scholar] [CrossRef]
- Kita, M.; Kobayashi, K.; Obara, K.; Koikeda, T.; Umeda, S.; Ano, Y. Supplementation with Whey Peptide Rich in β-Lactolin Improves Cognitive Performance in Healthy Older Adults: A Randomized, Double-Blind, Placebo-Controlled Study. Front. Neurosci. 2019, 13, 399. [Google Scholar] [CrossRef]
- Jang, E.; Kim, J.-H.; Lee, S.; Kim, J.-H.; Seo, J.-W.; Jin, M.; Lee, M.-G.; Jang, I.-S.; Lee, W.-H.; Suk, K. Phenotypic Polarization of Activated Astrocytes: The Critical Role of Lipocalin-2 in the Classical Inflammatory Activation of Astrocytes. J. Immunol. 2013, 191, 5204–5219. [Google Scholar] [CrossRef]
- Asanuma, M.; Miyazaki, I.; Murakami, S.; Diaz-Corrales, F.J.; Ogawa, N. Striatal Astrocytes Act as a Reservoir for L-DOPA. PLoS ONE 2014, 9, e106362. [Google Scholar] [CrossRef]
- Landis-Piwowar, K.; Chen, D.; Chan, T.H.; Dou, Q.P. Inhibition of catechol-O-methyltransferase activity in human breast cancer cells enhances the biological effect of the green tea polyphenol (-)-EGCG. Oncol. Rep. 2010, 24, 563–569. [Google Scholar] [CrossRef]
- Kurkela, M.; Siiskonen, A.; Finel, M.; Tammela, P.; Taskinen, J.; Vuorela, P. Microplate screening assay to identify inhibitors of human catechol-O-methyltransferase. Anal. Biochem. 2004, 331, 198–200. [Google Scholar] [CrossRef]
- Tarassishin, L.; Suh, H.-S.; Lee, S.C. LPS and IL-1 differentially activate mouse and human astrocytes: Role of CD14. Glia 2014, 62, 999–1013. [Google Scholar] [CrossRef]
- Rizor, A.; Pajarillo, E.; Johnson, J.; Aschner, M.; Lee, E. Astrocytic Oxidative/Nitrosative Stress Contributes to Parkinson’s Disease Pathogenesis: The Dual Role of Reactive Astrocytes. Antioxidants 2019, 8, 265. [Google Scholar] [CrossRef] [PubMed]
- González-Reyes, R.E.; Nava-Mesa, M.O.; Vargas-Sánchez, K.; Ariza-Salamanca, D.; Mora-Muñoz, L. Involvement of Astrocytes in Alzheimer’s Disease from a Neuroinflammatory and Oxidative Stress Perspective. Front. Mol. Neurosci. 2017, 10, 427. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.-L.; Yang, C.-M. Role of Redox Signaling in Neuroinflammation and Neurodegenerative Diseases. BioMed. Res. Int. 2013, 2013, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Reactive Oxygen Species and the Central Nervous System. J. Neurochem. 1992, 59, 1609–1623. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, S.; Kanzaki, T.; Kotani, Y.; Katoh, M.; Sawamoto, A.; Nakajima, M.; Furukawa, Y. Continual Treatment with the Peels of Citrus kawachiensis (Kawachi Bankan) Protects against Dopaminergic Neuronal Cell Death in a Lipopolysaccharide-Induced Model of Parkinson’s Disease. J. Nutr. Sci. Vitaminol. 2019, 65, 205–208. [Google Scholar] [CrossRef]
- Park, W.H.; Kang, S.; Piao, Y.; Pak, C.J.; Oh, M.S.; Kim, J.; Kang, M.S.; Pak, Y.K. Ethanol extract of Bupleurum falcatum and saikosaponins inhibit neuroinflammation via inhibition of NF-κB. J. Ethnopharmacol. 2015, 174, 37–44. [Google Scholar] [CrossRef]
- Lee, Y.J.; Choi, I.S.; Park, M.H.; Lee, Y.M.; Song, J.K.; Kim, Y.H.; Kim, K.H.; Hwang, D.Y.; Jeong, J.H.; Yun, Y.P.; et al. 4-O-Methylhonokiol attenuates memory impairment in presenilin 2 mutant mice through reduction of oxidative damage and inactivation of astrocytes and the ERK pathway. Free Radic. Biol. Med. 2011, 50, 66–77. [Google Scholar] [CrossRef]
- McNaught, K.S.P.; Jenner, P. Altered Glial Function Causes Neuronal Death and Increases Neuronal Susceptibility to 1-Methyl-4-Phenylpyridinium- and 6-Hydroxydopamine-Induced Toxicity in Astrocytic/Ventral Mesencephalic Co-Cultures. J. Neurochem. 2002, 73, 2469–2476. [Google Scholar] [CrossRef]
- Lin, M.-S.; Sun, Y.-Y.; Chiu, W.-T.; Hung, C.-C.; Chang, C.-Y.; Shie, F.-S.; Tsai, S.-H.; Lin, J.-W.; Hung, K.-S.; Lee, Y.-H. Curcumin Attenuates the Expression and Secretion of RANTES after Spinal Cord Injury In Vivo and Lipopolysaccharide-Induced Astrocyte Reactivation In Vitro. J. Neurotrauma 2011, 28, 1259–1269. [Google Scholar] [CrossRef]
- Ano, Y.; Ayabe, T.; Ohya, R.; Kondo, K.; Kitaoka, S.; Furuyashiki, T. Tryptophan-Tyrosine Dipeptide, the Core Sequence of β-Lactolin, Improves Memory by Modulating the Dopamine System. Nutrition 2019, 11, 348. [Google Scholar] [CrossRef]
- Mazzio, E.; Harris, N.; Soliman, K. Food Constituents Attenuate Monoamine Oxidase Activity and Peroxide Levels in C6 Astrocyte Cells. Planta Med. 1998, 64, 603–606. [Google Scholar] [CrossRef]
- Lim, D.W.; Han, T.; Jung, J.; Song, Y.; Um, M.Y.; Yoon, M.; Kim, Y.T.; Cho, S.; Kim, I.-H.; Han, D.; et al. Chlorogenic Acid from Hawthorn Berry (Crataegus pinnatifida Fruit) Prevents Stress Hormone-Induced Depressive Behavior, through Monoamine Oxidase B-Reactive Oxygen Species Signaling in Hippocampal Astrocytes of Mice. Mol. Nutr. Food Res. 2018, 62, e1800029. [Google Scholar] [CrossRef]
- Lim, D.W.; Park, J.; Jung, J.; Kim, S.-H.; Um, M.Y.; Yoon, M.; Kim, Y.T.; Han, D.; Lee, C.; Lee, J. Dicaffeoylquinic acids alleviate memory loss via reduction of oxidative stress in stress-hormone-induced depressive mice. Pharmacol. Res. 2020, 161, 105252. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; Amigo, L.; Recio, A.I.; Bartolomé, B. ACE-Inhibitory and Radical-Scavenging Activity of Peptides Derived from β-Lactoglobulin f(19−25). Interactions with Ascorbic Acid. J. Agric. Food Chem. 2007, 55, 3392–3397. [Google Scholar] [CrossRef]
- Zhang, Q.-X.; Wu, H.; Ling, Y.-F.; Lu, R.-R. Isolation and identification of antioxidant peptides derived from whey protein enzymatic hydrolysate by consecutive chromatography and Q-TOF MS. J. Dairy Res. 2013, 80, 367–373. [Google Scholar] [CrossRef]
- Jo, S.; Yarishkin, O.; Hwang, Y.J.; Chun, Y.E.; Park, M.; Woo, D.H.; Bae, J.Y.; Kim, T.; Lee, J.; Chun, H.; et al. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer’s disease. Nat. Med. 2014, 20, 886–896. [Google Scholar] [CrossRef]
- Park, J.-H.; Ju, Y.H.; Choi, J.W.; Song, H.J.; Jang, B.K.; Woo, J.; Chun, H.; Kim, H.J.; Shin, S.J.; Yarishkin, O.; et al. Newly developed reversible MAO-B inhibitor circumvents the shortcomings of irreversible inhibitors in Alzheimer’s disease. Sci. Adv. 2019, 5, eaav0316. [Google Scholar] [CrossRef]
- Yasuhisa, A.; Rena, O.; Keiji, K. Antidepressant-Like Effect of b -Lactolin, a Glycine-Threonine-Tryptophan-Tyrosine Peptide. J. Nutr. Sci. Vitaminol. 2019, 65, 430–434. [Google Scholar]
- Broussard, J.I.; Yang, K.; Levine, A.T.; Tsetsenis, T.; Jenson, D.; Cao, F.; Garcia, I.; Arenkiel, B.R.; Zhou, F.-M.; De Biasi, M.; et al. Dopamine Regulates Aversive Contextual Learning and Associated In Vivo Synaptic Plasticity in the Hippocampus. Cell Rep. 2016, 14, 1930–1939. [Google Scholar] [CrossRef]
- McNamara, C.G.; Tejero-Cantero, Á.; Trouche, S.; Campo-Urriza, N.; Dupret, D. Dopaminergic neurons promote hippocampal reactivation and spatial memory persistence. Nat. Neurosci. 2014, 17, 1658–1660. [Google Scholar] [CrossRef]
- Chowdhury, R.; Guitart-Masip, M.; Lambert, C.; Dayan, P.; Huys, Q.; Düzel, E.; Dolan, R.J. Dopamine restores reward prediction errors in old age. Nat. Neurosci. 2013, 16, 648–653. [Google Scholar] [CrossRef]
- Petrelli, F.; Dallérac, G.; Pucci, L.; Calì, C.; Zehnder, T.; Sultan, S.; Lecca, S.; Chicca, A.; Ivanov, A.; Asensio, C.S.; et al. Dysfunction of homeostatic control of dopamine by astrocytes in the developing prefrontal cortex leads to cognitive impairments. Mol. Psychiatry 2020, 25, 732–749. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).