Abstract
In the shallow Northern Adriatic, marine mollusks are affected by bottom trawling and seafood disturbance. Seasonal oscillations of oceanographic factors additionally influence their physiology, stress responses and survival. Tissue responses to seasonal variations in green ormer (Haliotis tuberculata L.) and Mediterranean scallop (Pecten jacobaeus L.) in the Northern Adriatic have not been reported. Hence, their biochemical and antioxidant defense properties over seasons were studied and the microanatomical structure of their tissue was correlated with function. Histological analysis of gonads revealed two peaks of gonadal maturation and spawning during the spring/summer period and winter season for scallops, and one peak during the fall for ormers. The gonadal maturation of both species was correlated with their seasonal variations of metabolic demands and antioxidant capacity. The lipid vacuoles of tubuloacinar terminations in the digestive gland differed between the two species; in scallop they are several-fold larger in size and number. Low temperatures in winter contributed to a decline in enzymatic antioxidant defense in scallop tissues, having lower superoxide dismutase (SOD) and glutathione peroxidase (GPx) activity, and higher concentrations of thiobarbituric acid reactive substances (TBARS) and total antioxidant status (TAS). In ormers, winter induced lower TAS, TBARS, SOD and GPx concentrations. The significant difference of winter TAS and TBARS levels between ormers and scallops was correlated with variations in their reproductive cycles, as well as in antioxidant defense systems. The most important factor for stress-related parameters for both species in this work was found to be the season-induced temperature change.
1. Introduction
Due to having a wide functional and taxonomic diversity, mollusks in marine environments are significant as predators, filter feeders, herbivores, scavengers and prey. By their activities, they provide and transform habitats and improve the water quality for other species. Their catch continues to increase and can be gravely affected by bottom trawling and seafood disturbance [1]. In the Northern Adriatic, Mediterranean scallop (Pecten jacobaeus L.) and tuberculate abalone (green ormer) (Haliotis tuberculata L.) are particularly threatened by such activities. Mediterranean scallops are often overfished off the northwest Istrian coast, where they naturally occur in larger numbers than in the rest of the Adriatic or Mediterranean [2]. Green ormers, on the other hand, occur in exploitable quantities only in the British Channel Islands [3], but due to benthic dredging in the Northern Adriatic, their populations face a decline. Ormers are the only species in the Haliotidae family commercially harvested in Europe [3]. Neither ormers nor scallops are currently farmed in the area, notwithstanding the efforts to start their cage rearing.
Mediterranean scallops are bivalve mollusks, having a rapid growth in their first two years of life. Their growth is intensified by the temperature increase, although temperatures above 22 °C diminish their metabolic processes [4]. Scallops can reach 120–140 mm of shell length. In the Italian part of the Northern Adriatic, they attain 100 mm of length in two years [5], while on the Croatian side they need one year more to reach that length [6]. It, thus, seems that the variation of scallop growth is location-dependent. The particular location might also affect biochemical and antioxidative properties their tissue [7].
Green ormers are slow-growing gastropod mollusks, taking three years to reach 45 mm of shell length [8]. The most important variables that govern their responses to the environment are temperature and oxygen. Temperature affects major physiological processes, while aerobic metabolism provides the majority of energy for those processes, thus creating a requirement for oxygen [9]. Although ormers are facultative anaerobes, under increased metabolic demand, they may show signs of oxidative stress [10]. Adults occur in the same habitat as juveniles. That can be attributable to the lack of their migration possibilities as they rarely move more than a few hundred meters from their settlement area and usually require a firm substrate [11]. Locality is, therefore, important for both ormer and scallop growth because of food supply, exposure to wave energy and thermal conditions.
The Northern Adriatic is characterized by a low average depth (30 m) and a weak bathymetric gradient. The most important oceanographic factors influencing the habitats of invertebrates in the Adriatic are seasonal salinity, the concentration of nutrients, light intensity, currents and temperature. Rivers are a significant source of nutrients in the Northern Adriatic, of which the largest is the River Po [12]. The phytoplankton distribution in the Northern Adriatic depends on the precipitation and snowmelt in the Alps, and the River Po influx of nutrients. The maximum influx in spring results from the snowmelt, while the fall maximum correlates with the yearly precipitation [13]. Seasonal changes of oceanographic factors, including temperature, algae and wave motion, greatly affect the growth and physiology of green ormers [14]. The oscillation in seawater seasonal properties might also negatively affect physiology, stress response and, ultimately, the survival of scallops in the Northern Adriatic [15,16]. In mollusks, the cellular immune system is composed of hemocytes and their phagocytic immune response is the first line of defense, but it is also equipped with an array of other tissue defense mechanisms [17]. The tissue responses to seasonal variations in green ormers and Mediterranean scallops in the Northern Adriatic have not been reported. As their biological reactions include rapid response to stress [18], they make ideal indicators of long-term ecological effects on invertebrates [19].
For this reason, tissues of green ormers and Mediterranean scallops were studied over the main seasonal periods for biochemical and antioxidant defense properties. Tissue concentrations of glucose (GLU), triglyceride (TRIG) and cholesterol (CHOL) were determined as the main energy sources for metabolic demands. The activities of superoxide dismutase (SOD), glutathione peroxidase (GPx), total antioxidant status (TAS) and malondialdehyde (MDA) were evaluated as parameters for antioxidant capacity and lipid peroxidation. Tissue magnesium (Mg) and calcium (Ca) were investigated as formative shell elements, crucial for ionic homeostasis and reproduction control. The microanatomical structure of ormer and scallop tissues was correlated with function in specimens of both sexes and selected tissues.
2. Materials and Methods
2.1. Animals and Study Site
The animals under study were tuberculate abalone (green ormer) (Haliotis tuberculata L.) and Mediterranean scallop (Pecten jacobaeus L.). The animals were collected from the area in the Northern Adriatic Sea, off Western Istria (Croatia), in accordance with institutional, national and international laws and guidelines.
Green ormers were collected at night by licensed divers from the rocky seabed at depths of up to 8 m. Mediterranean scallops were collected by a bottom trawl three nautical miles from shore, at depths of 15 m up to a maximum of 40 m. For each species, 60 animals were sampled in fall 2019 (samplings were conducted between September 14 and 6 October), in winter 2019 (between 25 November and 22 February) and in spring/summer 2020 (between 1 June and 6 June). Water temperature, salinity and dissolved oxygen concentrations are listed in Table 1.

Table 1.
Water temperature, salinity and dissolved oxygen concentrations at the sampling sites and respective depths for green ormer (Haliotis tuberculata L.) and Mediterranean scallop (Pecten jacobaeus L.).
Once collected, animals were wet weighed, bagged and placed on ice upon reaching the laboratory, where they were frozen and kept at −86 °C until further analyses. Ten animals from every sampling period were fixed in 4% neutral buffered formalin.
2.2. Chemicals
Thiobarbituric acid (CAS No. 504-17-6; TBA), trichloroacetic acid (CAS No. 76-03-9; TCA), butylhydroxytoluene (CAS No. 128-37-0; BHT), malondialdehyde tetraethylacetat (CAS No. 122-31-6; MDA), sodium chloride (CAS No. 7647-14-5; NaCl) and phenylmethanesulfonyl fluoride (CAS No. 329-98-6; PMSF) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Randox commercial kits (Dublin, Ireland) and Abbott commercial kits (Abbott, Germany) were used for biochemical tissue analyses. All other reagents used in this study were of chemical grade and commercially available.
2.3. Analytical Methods
Tissues for biochemical analyses and lipid peroxidation were homogenized on ice (Ultra-Turrax, IKA, Germany) in an isotonic solution of 0.9% NaCl with 0.1 mM protease inhibitor PMSF (1 g of tissue: 3 mL of isotonic solution). Obtained homogenates were subsequently centrifuged for 20 min at 4 °C (12,000 g) and supernatants were used for analyses of biochemical and antioxidative parameters. Concentrations of glucose (GLU), triglyceride (TRIG), cholesterol (CHOL), magnesium (Mg) and calcium (Ca) in supernatants were determined with Abbott commercial kits on Abbott Architect c4000 clinical chemistry analyzer (Abbott, Germany). The GLU concentration was determined in 2.0 μL of supernatant with the enzymatic method based on hexokinase/glucose-6-phosphate dehydrogenase catalytic reaction. The TRIG and CHOL concentrations were determined in 2.4 μL of supernatant using enzymatic methods with glycerol-phosphate oxidase and cholesterol esterase/cholesterol oxidase, respectively. The Mg concentration was measured in 3.2 μL of supernatant, with the isocitrate dehydrogenase enzymatic method, while the Ca concentration was measured in 2.6 μL of supernatant using the arsenazo-III method. All biochemical parameters were expressed as mg/g of tissue. Activities of superoxide dismutase (SOD), glutathione peroxidase (GPx) and total antioxidant status (TAS) were measured using Randox commercial kits (Dublin, Ireland) on Abbott Architect c4000 (Abbott, Germany) according to the manufacturers’ instructions. The SOD activity was measured by the degree of inhibition of superoxide radical formation in 5.0 μL of supernatant. The GPx method is based on the oxidation of glutathione by cumene hydroperoxide and its activity was measured in 4.0 μL of supernatant. The TAS concentration in samples was measured in 4.0 μL of supernatant by suppression of the radical production, which was visualized with specific chromogen. All enzyme activities were expressed as U/g of protein, and TAS was expressed as mmol/g of protein.
The reaction between malondialdehyde (MDA) and thiobarbituric acid (TBA) under acidic conditions resulted in the formation of thiobarbituric acid reactive substances (TBARS), as indicators of lipid peroxidation. Lipid peroxidation was measured in the entire soft body of animals, in accordance with our previous study [20]. Tissues were weighed, homogenized and centrifuged, and the supernatant was added to the reagent mixture of 10% TCA and 0.01% BHT. Samples were vortexed for 15 sec, cooled for 15 min and centrifuged for 10 min at 12,000 g (all at 4 °C). The supernatant (750 μL) was mixed with 500 μL TBA and heated at 99 °C for 30 min. The reaction stopped by cooling at 4 °C, when the absorbency of supernatant was read at 535 nm using a FLUOstar OPTIMA plate reader (Infinite M200, Tecan, Austria). The concentration of MDA-TBA complex was estimated from the calibration curve (y = 0.03x + 0.24; R2 = 0.96), correlating absorbance values and MDA dilution, and expressed as absorbance units per mg of tissue (AU/mg).
Gonads, digestive glands and hepatogonadal complexes were dissected and dehydrated in graded ethanol-xylene series. Tissues were embedded in paraplast and cut to 3–5 μm thickness as sagittal and transverse sections. They were stained with hematoxylin-eosin stains and analyzed by Olympus® BX51 microscope and AnalySIS Soft Imaging System. Six different stages of maturation were assessed in both males and females of P. jacobaeus and H. tuberculata, as described [21,22] for Pectenidae and Haliotidae, respectively.
2.4. Statistical Analysis
The results are presented as box-plots, where the boundaries indicate 25th and 75th percentiles, while a line within the box represents the median value. Whiskers above and below the box indicate the 10th and 90th percentiles. Significant differences between P. jacobeus and H. tuberculata within each season were determined using one-way analysis of variance (ANOVA) implemented in SigmaStat software version 1.0. Prior to the analysis, the data were subjected to logarithmic transformation. When the assumption for normality was violated, the Kruskal-Wallis one-way analysis of variance on ranks with post hoc Dunn’s test was performed. The p = 0.05 was used as a cutoff value of statistical significance.
3. Results
The biometric data of sampled ormers and scallops (soft body mass, shell length, shell mass) are presented in Table 2.

Table 2.
Green ormer (Haliotis tuberculata L.) and Mediterranean scallop (Pecten jacobaeus L.) soft body mass, shell length (dorso-ventral axis) and mass (mean ± SD).
The analysis of variance for each organism over seasons established significant differences between analytes and seasons. For Mediterranean scallops in particular, TAS was elevated in winter in relation to other seasons, while SOD and GPx had significantly higher activities in the spring/summer period. TBARS, on the other hand, had its peak in winter, while in the other months stayed at relatively low levels. TRIG concentrations were significantly higher in fall, and CHOL in fall and winter. GLU concentrations, albeit uniform over the year, were elevated in fall. Mg and Ca showed the highest concentrations in fall and winter when compared to other time points. For green ormers, significant differences were found between spring/summer and winter samples for TAS, with peaks in June. GPx had the highest activities in fall samples, just as SOD, CHOL, GLU, TRIG, Mg and Ca parameters. Differences in tissue biochemical and antioxidant defense properties between Mediterranean scallops and green ormers within each season are presented in Table 3.

Table 3.
Seasonal tissue biochemical and antioxidant defense properties for green ormer (Haliotis tuberculata L.) and Mediterranean scallop (Pecten jacobaeus L.).
Data are also presented in Figure 1 as a log10-based scale, showing the distribution, its central value and variability. Both organisms had similar trends in measured parameters over the seasons, generally with a greater distribution of data in scallop tissues, except in fall. In winter, only GLU in ormers had a greater data distribution. Data were grouped tightly in ormer tissues in spring/summer and winter (except for GLU), having unsymmetrical interquartile ranges. Significant differences between the two organisms were found for SOD and GPx in spring/summer, for SOD and TAS in fall, and for TAS, Mg and TBARS in winter.
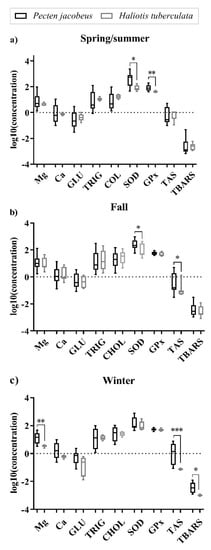
Figure 1.
Differences in tissue biochemical and antioxidant defense properties between Pecten jacobeus and Haliotis tuberculata within each season (a) spring/summer (b) fall (c) winter. The symbol * indicates a significant difference: * p < 0.05, ** p < 0.01, *** p < 0.001.
Gonadal stages of scallops and green ormers, according to seasonal cycles, are presented in Figure 2A,C,E and Figure 3A,C,E. Both species showed season-induced variation in the occurrence of different gonadal stages. During the winter, in scallops, the ripe stage and partially spawned stage were recorded in 20% and 80% of the investigated specimens, respectively. However, the occurrence of both of these stages in a sample was 80% of the total specimens examined in spring/summer, while in the fall season, the majority of gonads were at the stage of gradual maturing (Figure 4A). During the spring/summer season, gonads were at the mid-maturation stage in ormers, and in fall, the ripe and spawned stages were recorded (Figure 4B). Histological sections presented in Figure 2B,D,F and Figure 3B,D,F show ducts of the digestive gland (hepatopancreas) of both organisms. When comparing the two species, differences in the size and number of lipid vacuoles accumulated in tubuloacinar terminations were observed, whereas larger vacuoles were seen in the scallop digestive gland in all seasons.
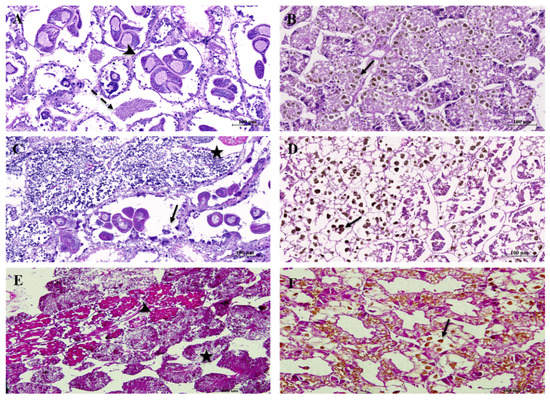
Figure 2.
Photomicrographs of the Pecten jacobaeus gonads and digestive gland. Seasonal variations of the ovarian developmental stage: (A) late active stage (spring/summer); (C) early active stage (fall); (E) partial spawn stage (winter); previtellogenic oocyte (arrow); vitellogenic oocyte (dashed arrow); mature oocyte (arrowhead); male gonads with spermatozoa (star). Changes in tubuloacinar terminations of the digestive gland: (B) spring/summer; (D) fall; (F) winter; lipid droplet (arrow).
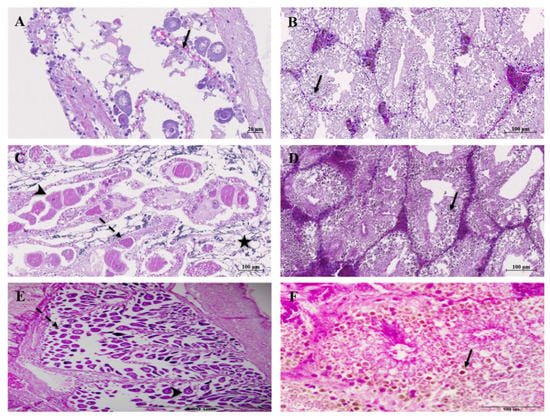
Figure 3.
Photomicrographs of sections of the Haliotis tuberculata gonads and digestive gland. Seasonal variations of the ovarian developmental stage: (A) early active stage (spring/summer); (C) partial spawn stage (fall); (E) late active stage (winter); previtellogenic oocyte (arrow); vitellogenic oocyte (dashed arrow); mature oocyte (arrowhead); male gonads with spermatozoa (star). Changes in tubuloacinar terminations of the digestive gland: (B) spring/summer; (D) fall; (F) winter; lipid droplet (arrow).
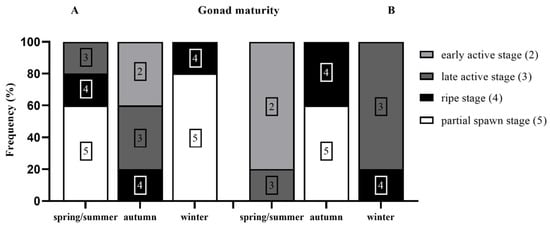
Figure 4.
Seasonal gametogenic cycles of Pecten jacobaeus (A) and Haliotis tuberculata (B). Histograms showing the relative abundance of different gonadal maturity stage: 2—early active stage; 3—late active stage; 4—ripe stage; 5—partial spawn stage.
4. Discussion
Mollusks respond differently to stressors compared to other marine organisms. They comprise species ranging from sensitive to very resilient to stress and are, thus, frequently used to establish the ecological quality of benthic communities [23,24]. The most important factor for stress-related parameters for both species in this work was found to be the season-induced temperature change. In response to temperature changes, mollusks produce reactive oxygen species (ROS) and activate antioxidant enzymes [25]. The lowest temperature (9.1 °C) was registered in winter at both sampling depths, which contributed to a decline in enzymatic antioxidant defense in scallop tissues, having lower SOD and GPx activity and higher concentrations of TBARS and TAS in this season. The scallop shell growth ceases at such low temperatures and resumes as water temperature increases [6]. Long-term temperatures between 8.5 and 9.0 °C are the lethal limit for ormers, and at low temperatures, they are limited in their ability to absorb food and grow [3,26]. In ormers, low temperatures in winter contributed to lower TAS, TBARS, SOD and GPx concentrations. The significant difference of winter TAS and TBARS levels between ormers and scallops in this work could be correlated with their variations in reproductive cycles, as well as in antioxidant defense system, since in scallop tissues, they seem inversely related to the accumulation of lipid peroxidation products. Therefore, a decrease in scallop antioxidant capacity in winter was accompanied by the MDA/TBARS increase, as previously established [27]. TAS is a quantitative representation of the total contribution from a wide range of antioxidant molecules and provides an assessment of an organism’s ability to resist oxidative stress [28]. The lower TAS in ormers in winter points to the dynamic equilibrium between different prooxidants and antioxidants in tissues, with the prevailingly low SOD and GPx. The antioxidants SOD and GPx convert superoxide anion radicals to hydrogen peroxide and catalyze the decomposition of superoxide radicals into less toxic molecules, respectively [29]. Not only antioxidant capacity, but also tissue Mg, Ca and GLU showed decreased levels in ormers in winter, which did not correlate with scallop tissues.
In fall, in ormer tissues a number of parameters had their yearly peaks, such as Mg, Ca, SOD, GPx, TRIG, CHOL and GLU. Additionally, Mg, Ca, TRIG, CHOL and GLU were elevated in scallop tissues. Tissue Mg and Ca are active in ionic homeostasis; they participate in prostaglandin release from tissues and act in reproduction control [30,31,32]. They approximate the environmental ion concentrations and varying salinity of water [32]. Furthermore, tissue Mg has a regulatory role in oxidative processes and is considered an essential cofactor of glutathione (GSH) synthesis. Its decrease leads to a reduction in GSH levels and intensifies the production of ROS [33]. Calcium regulates adenosine diphosphate binding and actin affinity in mollusks. In the absence of Ca, tension generation by the striated adductor muscle and actin-dependent motility are repressed. An increase of Ca above micromolar levels significantly elevates these activities [34].
In preparation for reproduction, energy demands arise. Northern Adriatic scallops can spawn in spring or fall. Gonads are mature from May until July, while a second maturation peak can occur from November until February; however, most are spring-spawned [6]. Ormers, on the other hand, spawn for a protracted period, if not synchronously, but may also spawn twice a year. During spawning, they may cease feeding [14]. Tissue concentrations of TRIG, CHOL and GLU act as main energy sources for metabolic demands [35,36,37]. Mollusks exhibit cycles of energy storage and utilization in relation to yearly seasons and cycles of gametogenesis. Changes in biochemical tissue composition, thus, demonstrate which of these substrates act in energy metabolism in different conditions. Therefore, gonadal development is followed by a decrease of GLU in muscle, and a decrease of GLU and TRIG in the scallop digestive gland [38].
Histological analysis of scallop and ormer gonads revealed two peaks of gonadal maturation and spawning during the spring/summer and winter seasons for scallops, and one peak during fall for ormers. Spawning is an energy-demanding process and most of the energy comes from metabolism of fats stored in the digestive gland [39]. Therefore, this process could add to the production of antioxidants and TBARS in scallop and ormer gonads in order to protect fatty acids and yolk from lipoperoxidative damage [40]. Another possible reason for the discrepancy in antioxidative status in the two species could be due to species’ physiological differences. It was found that the size and number of lipid vacuoles of tubuloacinar terminations in the digestive gland differ between the two species after the microscopic examination, in scallop being several-fold larger in size and number. Similar observations relating to increased fatty acid levels of European oyster (Ostrea edulis) tissues in winter were established previously [41].
Both the decreases and increases of temperature suppress the immunity of abalone (Haliotis discus hannai) and entice higher ROS production [42]. Interestingly, in this work, ormers had elevated total antioxidant status in the warmer period, which can be attributed to the availability of food containing antioxidant compounds. In scallops, GPx had a peak in June, comparable with the work of Viarengo et al. [27], where it decreased from fall to winter, subsequently increasing in spring/summer conditions. In scallops, a relationship of increased antioxidant levels (GPx) and decreased lipid peroxidation levels measured as TBARS was evident in June, suggesting that increases in antioxidant enzyme activities could have been responsible for reducing the lipid peroxidation during the temperature increase [15]. Additionally, the feeding pattern of scallops may have an impact on elevated GPx levels in June, as they ingest relatively large phytoplankton particles [43]. Ormers, on the other hand, browse on a variety of seaweeds, but mostly on macroalgae such as Chlorophyta, Rhodophyta and Phaeophyta [44].
In the spring/summer sampling period, salinity reached its minimum of 30.88 psu. Lower salinity might have contributed to oxyradical production in the observed species, particularly ormers, which are not able to tolerate decreased salinity over a prolonged period [3]. However, the Northern Adriatic is a two-layer sea where the surface layer is enriched with a riverine fresher water, while the bottom layer consists of saltier water, and in warmer months particularly, the stratification of the water column is evident [45].
In conclusion, the results suggest that in both species, the environmental conditions and the reproductive period appear to be the main processes influencing the seasonal patterns in their biochemical parameters and tissue morphology. Both species had similar trends in biochemical and antioxidant defense parameters over the seasons, generally with a greater distribution of data in scallop tissues, except in fall. During cold months, a decline in enzymatic antioxidant defense in both species was noted, accompanied by the lipid peroxidation levels decrease in ormers only. Both species demonstrated a season-induced variation in the occurrence of different gonadal stages. The majority of investigated scallops were in the ripe or partially spawned stages in the spring/summer season. In ormers in the spring/summer season, gonads were in the mid-maturation stage, while in fall they were in the ripe or spawned stages. The cycles of gametogenesis, directly correlated with seasonal conditions, were followed by the energy storage and utilization patterns in both species.
It is likely that due to human activities, the seasonal temperature and salinity fluctuations may increase in the oncoming years [16]. The additional stress imposed on (not only) mollusks in the Northern Adriatic Sea is the prospect of the rising levels of the sea, increased sea temperatures, salinities and ocean acidification [46]. Coupled with overfishing, global warming might enable non-indigenous alien species to migrate into the Northern Adriatic, threatening to extinct local mollusk communities. Ormers and scallops in the Northern Adriatic should, thus, become species of special interest, particularly regarding their aquaculture prospect. Understanding of tissue responses to seasonal variations in green ormers and Mediterranean scallops in the Northern Adriatic is, therefore, of utmost importance.
Author Contributions
Conceptualization, N.T.P. and D.M.; methodology, B.B.L., S.B., I.S.-P. and J.B.; validation, B.B.L., S.B., I.S.-P. and J.B.; formal analysis, B.B.L., S.B., M.K., N.T.P., V.L. and J.B.; investigation, T.T., V.L., M.K. and N.T.P.; resources, R.Č.-R.; writing—original draft preparation, N.T.P.; writing—review and editing, N.T.P.; visualization, J.B., S.B., T.T. and D.M.; supervision, N.T.P.; funding acquisition, R.Č.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Scientific Centre of Excellence for Marine Bioprospecting—BioProCro, a project co-financed by the Croatian Government and the European Union through the European Regional Development Fund—the Competitiveness and Cohesion Operational Programme (KK.01.1.1.01). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Institutional Review Board Statement
Not applicable for species in the present study.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable. All data are presented in the manuscript.
Acknowledgments
Colleagues from Croatian Institute of Oceanography and Fisheries measured sea temperature, salinity and dissolved oxygen for the Croatian Ministry of Agriculture, and the authors hereby acknowledge their support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Eddy, T.D.; Lotze, H.K.; Fulton, E.A.; Coll, M.; Ainsworth, C.H.; De Araújo, J.N.; Bulman, C.M.; Bundy, A.; Christensen, V.; Field, J.C.; et al. Ecosystem effects of invertebrate fisheries. Fish Fish. 2016, 18, 40–53. [Google Scholar] [CrossRef]
- Nerlović, V. Exploitation of scallop Pecten jacobaeus (Linneaus, 1758) in the north western coastal region of Istria. In Proceedings of the 39th Croatian Symposium on Agriculture with International Participation; Žimbrek, T., Ed.; Faculty of Agriculture, University of Zagreb: Zagreb, Croatia, 2004; pp. 606–608. ISBN 953-6135-40-X. [Google Scholar]
- Mgaya, Y.D.; Mercer, J.P. A review of the biology, ecology, fisheries and mariculture of the European abalone Haliotis tuberculata Linnaeus 1758 (Gastropoda: Haliotidae). Biol. Environ. 1994, 94B, 285–304. [Google Scholar]
- Marguš, D.; Teskeredžić, E. The reception of larvae, survival and growth of juvenile Mediterranean scallops (Pecten jacobaeus Linnaeus, 1758) in controlled breeding in the bay of Šarina Draga—The mouth of the Krka River. Croat. J. Fish. 2005, 63, 1–14. (In Croatian) [Google Scholar]
- Mattei, N.; Pellizzato, M. A population study on three stocks of a commercial Adriatic pectinid (Pecten jacobaeus). Fish. Res. 1996, 26, 49–65. [Google Scholar] [CrossRef]
- Peharda, M.; Soldo, A.; Pallaoro, A.; Matić, S.; Cetinić, P. Age and growth of the Mediterranean scallop Pecten jacobaeus (Linnaeus 1758) in the Northern Adriatic Sea. J. Shellfish Res. 2003, 22, 639–642. [Google Scholar]
- Popović, N.T.; Ljubić, B.B.; Strunjak-Perović, I.; Babić, S.; Lorencin, V.; Jadan, M.; Čižmek, L.; Matulić, D.; Bojanić, K.; Čož-Rakovac, R. Seasonal antioxidant and biochemical properties of the Northern Adriatic Pecten jacobaeus. PLoS ONE 2020, 15, e0230539. [Google Scholar] [CrossRef] [PubMed]
- Clavier, J.; Richard, O. Growth of juvenile Haliotis tuberculata (Mollusca: Gastropoda) in their natural environment. J. Mar. Biol. Assoc. UK 1986, 66, 497–503. [Google Scholar] [CrossRef]
- Morash, A.J.; Alter, K. Effects of environmental and farm stress on abalone physiology: Perspectives for abalone aquaculture in the face of global climate change. Rev. Aquac. 2015, 7, 1–27. [Google Scholar] [CrossRef]
- Sokolova, I.M.; Pörtner, H.-O. Metabolic plasticity and critical temperatures for aerobic scope in a eurythermal marine invertebrate (Littorina saxatilis, Gastropoda: Littorinidae) from different latitudes. J. Exp. Biol. 2003, 206, 195–207. [Google Scholar] [CrossRef]
- Clavier, J.; Chardy, P. Investigation into the ecology of the ormer (Haliotis tuberculata L.), factors influencing spatial distribution. Aquat. Living Resour. 1989, 2, 191–197. [Google Scholar] [CrossRef]
- Polimene, L.; Pinardi, N.; Zavatarelli, M.; Colella, S. The Adriatic Sea ecosystem seasonal cycle: Validation of a three-dimensional numerical model. J. Geophys. Res. Space Phys. 2006, 112, C03S19. [Google Scholar] [CrossRef]
- Viličić, D. Specific oceanological characteristics of the Croatian part of the Adriatic. Hrvat. Vode 2014, 22, 297–314. (In Croatian) [Google Scholar]
- Mgaya, Y.D. Synopsis of Biological Data on the European abalone (ormer), Haliotis Tuberculata Linnaeus, 1758 (Gastropoda: Haliotidae); FAO Fisheries Synopsis. No 156; FAO: Rome, Italy, 1995; p. 28. [Google Scholar]
- Matozzo, V.; Chinellato, A.; Munari, M.; Bressan, M.; Marin, M.G. Can the combination of decreased pH and increased temperature values induce oxidative stress in the clam Chemelea gallina and the mussel Mytilus galloprovincialis? Mar. Pollut. Bull. 2013, 72, 34–40. [Google Scholar] [CrossRef]
- Velez, C.; Figueira, E.; Soares, A.M.; Freitas, R. Native and introduced clams biochemical responses to salinity and pH changes. Sci. Total Environ. 2016, 566–567, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Basuyaux, O.; Blin, J.-L.; Costil, K.; Richard, O.; Lebel, J.-M.; Serpentini, A. Assessing the impacts of several algae-based diets on cultured European abalone (Haliotis tuberculata). Aquat. Living Resour. 2018, 31, 28. [Google Scholar] [CrossRef]
- Lau, P.; Wong, H. Effect of size, tissue parts and location on six biochemical markers in the green-lipped mussel, Perna viridis. Mar. Pollut. Bull. 2003, 46, 1563–1572. [Google Scholar] [CrossRef]
- López-Bera, J.; Pueyo, C. Mutagen content and metabolic activation of promutagens by molluscs as biomarkers of marine pollution. Mutat. Res. 1998, 399, 3–15. [Google Scholar] [CrossRef]
- Babić, S.; Barišić, J.; Malev, O.; Klobučar, G.; Topić Popović, N.; Strunjak-Perović, I.; Krasnići, N.; Čož-Rakovac, R.; Sauerborn Klobučar, R. Sewage sludge toxicity assessment using earthworm Eisenia fetida: Can biochemical and histopathological analysis provide fast and accurate insight? Environ. Sci. Pollut. Res. 2016, 23, 12150–12163. [Google Scholar] [CrossRef]
- Narvarte, M.; Kroeck, M. Intraspecific variation in the reproductive cycle of the tehuelchus scallop Aequipecten tehuelchus (Pelecypoda, Pectinidae), in San Matias Gulf, Patagonia, Argentina. J. Shellfish Res. 2002, 21, 571–576. [Google Scholar]
- Najmudeen, T.M. Variation in biochemical composition during gonad maturation of the tropical abaloneHaliotis variaLinnaeus 1758 (Vetigastropoda: Haliotidae). Mar. Biol. Res. 2007, 3, 454–461. [Google Scholar] [CrossRef][Green Version]
- Borja, A.; Franco, J.; Pérez, V. A Marine Biotic Index to Establish the Ecological Quality of Soft-Bottom Benthos within European Estuarine and Coastal Environments. Mar. Pollut. Bull. 2000, 40, 1100–1114. [Google Scholar] [CrossRef]
- Gallmetzer, I.; Haselmair, A.; Tomašových, A.; Stachowitsch, M.; Zuschin, M. Responses of molluscan communities to centuries of human impact in the northern Adriatic Sea. PLoS ONE 2017, 12, e0180820. [Google Scholar] [CrossRef]
- Abele, D.; Heise, K.; Pörtner, H.O.; Puntarulo, S. Temperature-dependence of mitochondrial function and production of reactive oxygen species in the intertidal mud clam Mya arenaria. J. Exp. Biol. 2002, 205, 1831–1841. [Google Scholar] [PubMed]
- Peck, L.S. Feeding, growth and temperature in the ormer Haliotis tuberculata L. Prog. Underw. Sci. 1989, 14, 95–107. [Google Scholar]
- Viarengo, A.; Canesi, L.; Pertica, M.; Livingstone, D. Seasonal variations in the antioxidant defence systems and lipid peroxidation of the digestive gland of mussels. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1991, 100, 187–190. [Google Scholar] [CrossRef]
- Xie, J.J.; Chen, X.; Guo, T.Y.; Xie, S.W.; Fang, H.H.; Liu, Z.L.; Zhang, Y.M.; Tian, L.X.; Liu, Y.J.; Niu, J. Dietary values of Forsythia suspensa extract in Penaeus mondon under normal rearing and Vibrio parahaemolyticus 3HP (VP3HP) challenge conditions: Effect on growth, intestinal barrier function, immune response and immune related gene expression. Fish Shellfish Immun. 2018, 75, 316–326. [Google Scholar] [CrossRef]
- Wang, J.; Dong, B.; Yu, Z.-X.; Yao, C.-L. The impact of acute thermal stress on green mussel Perna viridis: Oxidative damage and responses. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2018, 222, 7–15. [Google Scholar] [CrossRef]
- Freas, W.; Grollman, S. Ionic and osmotic influence on prostaglandin release from the gill tissue of a marine bivalve, Modiolus demissus. J. Exp. Biol. 1980, 84, 169–185. [Google Scholar]
- Deridovich, I.; Reunova, O. Prostaglandins: Reproduction control in bivalve molluscs. Comp. Biochem. Physiol. Part A: Physiol. 1993, 104, 23–27. [Google Scholar] [CrossRef]
- Carregosa, V.; Velez, C.; Soares, A.M.V.M.; Figueira, E.; Freitas, R. Physiological and biochemical responses of three Veneridae clams exposed to salinity changes. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2014, 177–178, 1–9. [Google Scholar] [CrossRef]
- Brucka-Jastrzebska, E.; Kawczuga, D.; Grzelak, A.; Bartosz, G. Magnesium content, total antioxidant status and lipid peroxidation in rainbow trout (Onchorhynchus mykiss Walbaum). Magnes. Res. 2009, 22, 273–279. [Google Scholar]
- Chantler, P.D. Scallop Adductor Muscles: Structure and Function. In Scallops: Biology, Ecology, Aquaculture, and Fisheries, 3rd ed.; Shumway, S.E., Parsons, G.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 161–218. [Google Scholar]
- Palacios, E.; Racotta, I.S.; Arjona, O.; Marty, Y.; Le Coz, J.R.; Moal, J.; Samain, J.F. Lipid composition of the pacific lion-paw scallop, Nodipecten subnodosus, in relation to gametogenesis 2. Lipid classes and sterols. Aquaculture 2007, 266, 266–273. [Google Scholar] [CrossRef]
- Pernet, F.; Tremblay, R.; Comeau, L.; Guderley, H. Temperature adaptation in two bivalve species from different thermal habitats: Energetics and remodelling of membrane lipids. J. Exp. Biol. 2007, 210, 2999–3014. [Google Scholar] [CrossRef]
- Martínez-Pita, I.; Sánchez-Lazo, C.; Ruíz-Jarabo, I.; Herrera, M.; Mancera, J.M. Biochemical composition, lipid classes, fatty acids and sexual hormones in the mussel Mytilus galloprovincialis from cultivated populations in south Spain. Aquaculture 2012, 358–359, 274–283. [Google Scholar] [CrossRef]
- Barber, B.J.; Blake, N.J. Reproductive Physiology. In Scallops: Biology, Ecology, Aquaculture, and Fisheries, 3rd ed.; Shumway, S.E., Parsons, G.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 253–299. [Google Scholar]
- Le Pennec, G.; Le Pennec, M.; Beninger, G. Seasonal digestive gland dynamics of the scallop Pecten maximus in the Bay of Brest (France). J. Mar. Biol. Assoc. UK 2001, 81, 663–671. [Google Scholar] [CrossRef]
- Besnard, J.Y.; Lubet, P.; Nouvelot, A. Seasonal variations of the fatty acid content of the neutral lipids and phospholipids in the female gonad of Pecten maximus L. Comp. Biochem. Physiol. 1989, 93B, 21–26. [Google Scholar] [CrossRef]
- Josip, B.; Čož-Rakovac, R.; Delaš, I.; Popović, N.T.; Gavrilović, A.; Jug-Dujaković, J.; Brailo, M.; Sauerborn-Klobučar, R.; Babić, S.; Strunjak-Perović, I. Predictive modeling of European flat oyster (Ostrea edulis L.) fatty acid composition. Aquac. Int. 2017, 25, 805–825. [Google Scholar] [CrossRef]
- Ding, J.; Li, L.; Wu, F.; Zhang, G. Effect of chronic temperature exposure on the immunity of abalone, Haliotis discus hannai. Aquac. Res. 2015, 47, 2861–2873. [Google Scholar] [CrossRef]
- MacDonald, B.A.; Bricelj, V.M.; Shumway, S.E. Physiology: Energy Acquisition and Utilisation. In Scallops: Biology, Ecology, Aquaculture, and Fisheries, 3rd ed.; Shumway, S.E., Parsons, G.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 301–353. [Google Scholar]
- Marchais, V.; Jolivet, A.; Hervé, S.; Roussel, S.; Schöne, B.R.; Grall, J.; Chauvaud, L.; Clavier, J. New tool to elucidate the diet of the ormer Haliotis tuberculata (L.): Digital shell color analysis. Mar. Biol. 2017, 164, 213. [Google Scholar] [CrossRef]
- Jeffries, M.A.; Lee, C.M. A climatology of the northern Adriatic Sea’s response to bora and river forcing. J. Geophys. Res. 2007, 112, 1–18. [Google Scholar] [CrossRef]
- Furlan, E.; Torresan, S.; Critto, A.; Lovato, T.; Solidoro, C.; Lazzari, P.; Marcomini, A. Cumulative Impact Index for the Adriatic Sea: Accounting for interactions among climate and anthropogenic pressures. Sci. Total Environ. 2019, 670, 379–397. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).