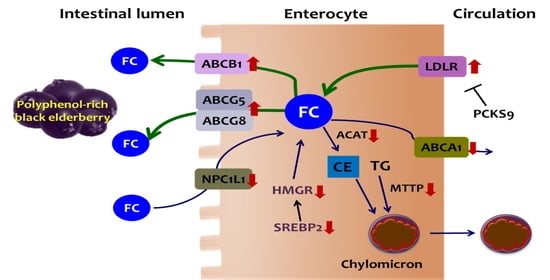
Polyphenol-Rich Black Elderberry Extract Stimulates Transintestinal Cholesterol Excretion
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Sample Treatment
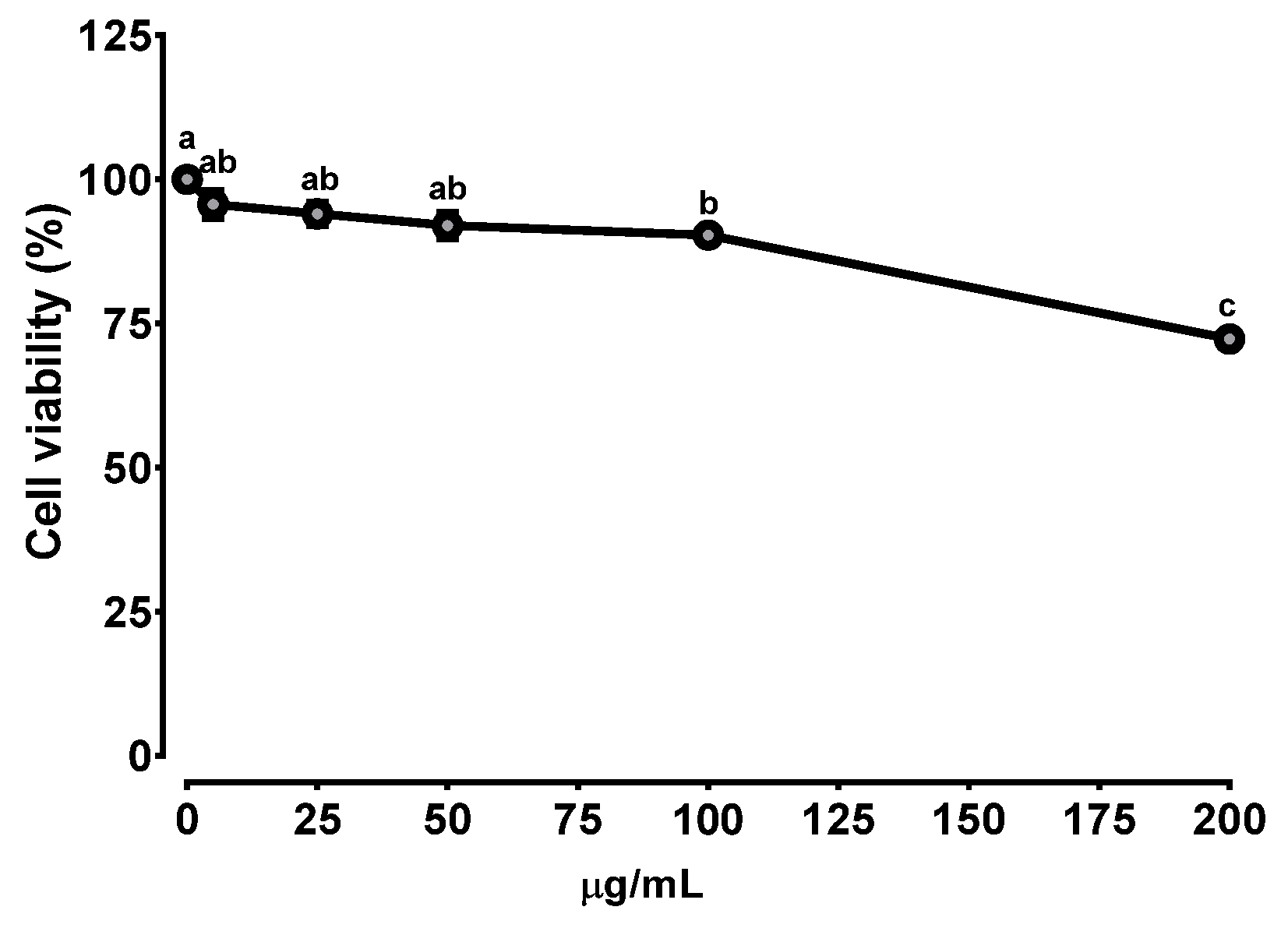
2.2. Cytotoxicity of BEE
2.3. Quantitative Real-Time PCR
2.4. Western Blot
2.5. Statistical Analysis
3. Results
3.1. Cytotoxicity of BEE in Caco-2 Cells
3.2. Effects of BEE on the Genes Involved in Cholesterol Synthesis and Absorption
3.3. Alteration of the Genes Involved in TICE by BEE
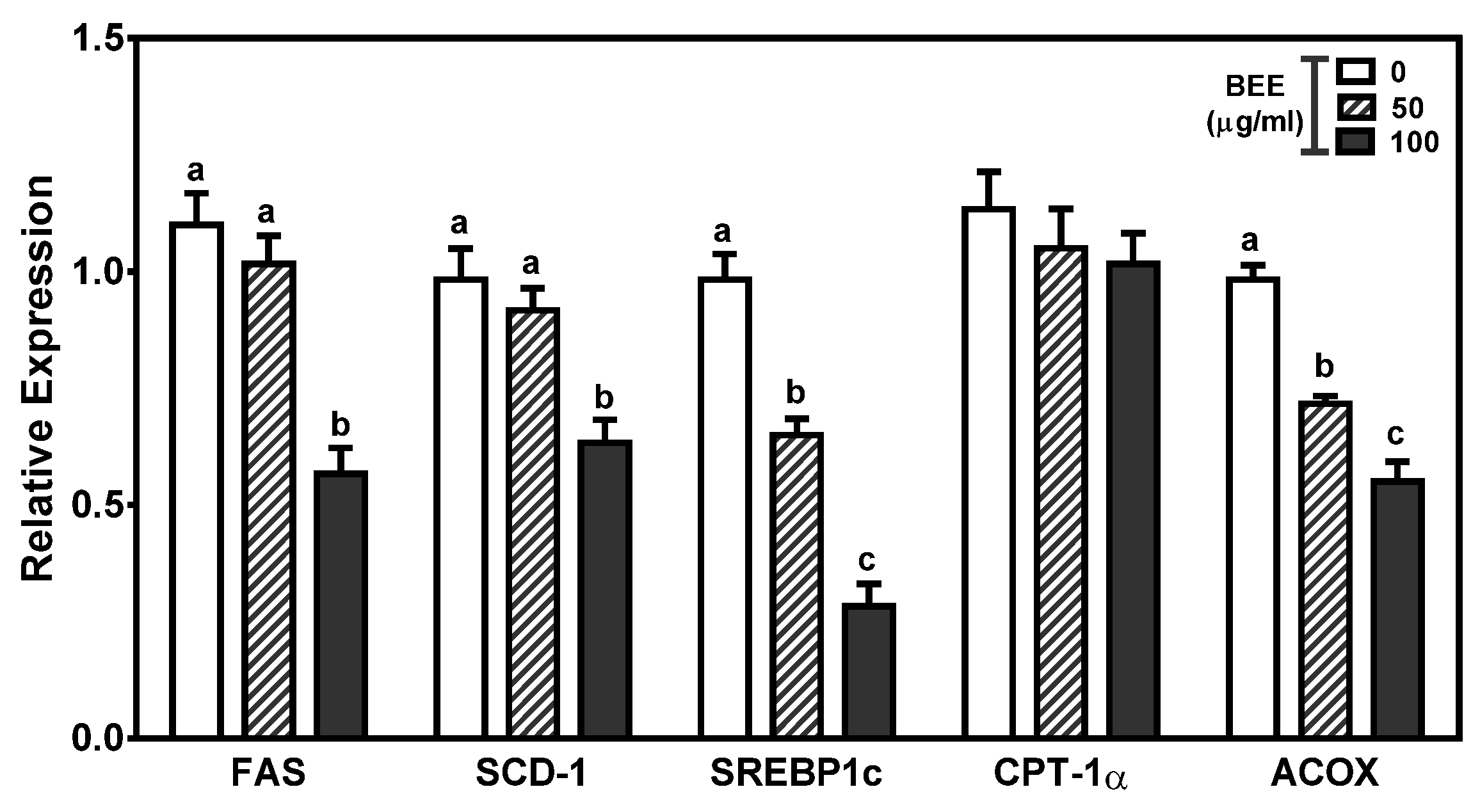
3.4. Effects of BEE on the Genes Involved in Fatty-Acid Metabolism
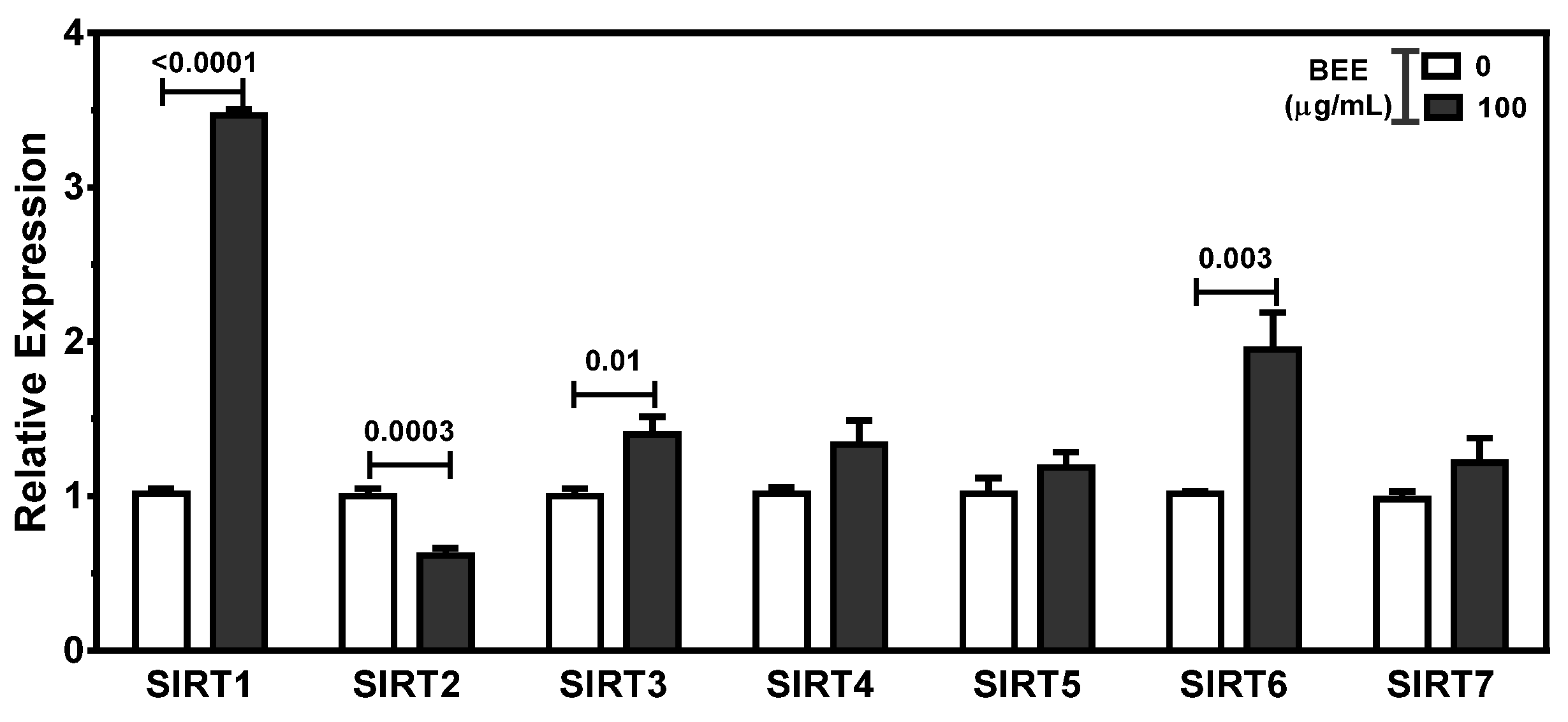
3.5. Effects of BEE on the Regulation of SIRT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef]
- Kapourchali, F.R.; Surendiran, G.; Goulet, A.; Moghadasian, M.H. The Role of Dietary Cholesterol in Lipoprotein Metabolism and Related Metabolic Abnormalities: A Mini-review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2408–2415. [Google Scholar] [CrossRef]
- Millar, J.S.; Cuchel, M. Cholesterol metabolism in humans: A review of methods and comparison of results. Curr. Opin. Lipidol. 2018, 29, 1–9. [Google Scholar] [CrossRef]
- Luo, J.; Yang, H.; Song, B.L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.E.; Mooney, K.M.; Wilkinson, S.J.; Pickles, N.A.; Mc Auley, M.T. Cholesterol metabolism: A review of how ageing disrupts the biological mechanisms responsible for its regulation. Ageing Res. Rev. 2016, 27, 108–124. [Google Scholar] [CrossRef]
- Jakulj, L.; Besseling, J.; Stroes, E.S.G.; Groen, A.K. Intestinal cholesterol secretion: Future clinical implications. Neth. J. Med. 2013, 71, 459–465. [Google Scholar] [PubMed]
- von Bergmann, K.; Sudhop, T.; Lutjohann, D. Cholesterol and plant sterol absorption: Recent insights. Am. J. Cardiol. 2005, 96, 10d–14d. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Q. Regulation of intestinal cholesterol absorption. Annu. Rev. Physiol. 2007, 69, 221–248. [Google Scholar] [CrossRef]
- Black, D.D. Development and physiological regulation of intestinal lipid absorption. I. Development of intestinal lipid absorption: Cellular events in chylomicron assembly and secretion. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G519–G524. [Google Scholar] [CrossRef]
- Mansbach, C.M., 2nd; Gorelick, F. Development and physiological regulation of intestinal lipid absorption. II. Dietary lipid absorption, complex lipid synthesis, and the intracellular packaging and secretion of chylomicrons. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G645–G650. [Google Scholar] [CrossRef]
- Lammert, F.; Wang, D.Q. New insights into the genetic regulation of intestinal cholesterol absorption. Gastroenterology 2005, 129, 718–734. [Google Scholar] [CrossRef]
- Groen, A.K.; Bloks, V.W.; Verkade, H.; Kuipers, F. Cross-talk between liver and intestine in control of cholesterol and energy homeostasis. Mol. Asp. Med. 2014, 37, 77–88. [Google Scholar] [CrossRef] [PubMed]
- van der Velde, A.E.; Brufau, G.; Groen, A.K. Transintestinal cholesterol efflux. Curr. Opin. Lipidol. 2010, 21, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Tietge, U.J.; Groen, A.K. Role the TICE? Advancing the concept of transintestinal cholesterol excretion. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1452–1453. [Google Scholar] [CrossRef][Green Version]
- van der Velde, A.E.; Vrins, C.L.; van den Oever, K.; Seemann, I.; Oude Elferink, R.P.; van Eck, M.; Kuipers, F.; Groen, A.K. Regulation of direct transintestinal cholesterol excretion in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G203–G208. [Google Scholar] [CrossRef]
- Vrins, C.L. From blood to gut: Direct secretion of cholesterol via transintestinal cholesterol efflux. World J. Gastroenterol. 2010, 16, 5953–5957. [Google Scholar] [CrossRef] [PubMed]
- Wegner, C.J.; Kim, B.; Lee, J. Trust your gut: Galvanizing nutritional interest in intestinal cholesterol metabolism for protection against cardiovascular diseases. Nutrients 2013, 5, 208–222. [Google Scholar] [CrossRef]
- Wang, D.Q.H.; Portincasa, P.; Tso, P. Transintestinal Cholesterol Excretion: A Secondary, Nonbiliary Pathway Contributing to Reverse Cholesterol Transport. Hepatology 2017, 66, 1337–1340. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Wang, D.Q.; Garruti, G.; Wang, H.H.; Grattagliano, I.; de Bari, O.; Portincasa, P. Therapeutic reflections in cholesterol homeostasis and gallstone disease: A review. Curr. Med. Chem. 2014, 21, 1435–1447. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, R.; Zhang, L.L.; Rocchetti, G.; Lucini, L.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, J.M. Elderberry (Sambucus nigra L.) as potential source of antioxidants. Characterization, optimization of extraction parameters and bioactive properties. Food Chem. 2020, 330, 127266. [Google Scholar] [CrossRef]
- Mlynarczyk, K.; Walkowiak-Tomczak, D.; Lysiak, G.P. Bioactive properties of Sambucus nigra L. as a functional ingredient for food and pharmaceutical industry. J. Funct. Foods 2018, 40, 377–390. [Google Scholar] [CrossRef]
- Farrell, N.J.; Norris, G.H.; Ryan, J.; Porter, C.M.; Jiang, C.; Blesso, C.N. Black elderberry extract attenuates inflammation and metabolic dysfunction in diet-induced obese mice. Brit. J. Nutr. 2015, 114, 1123–1131. [Google Scholar] [CrossRef]
- Farrell, N.; Norris, G.; Lee, S.G.; Chun, O.K.; Blesso, C.N. Anthocyanin-rich black elderberry extract improves markers of HDL function and reduces aortic cholesterol in hyperlipidemic mice. Food Funct. 2015, 6, 1278–1287. [Google Scholar] [CrossRef]
- Millar, C.L.; Norris, G.H.; Jiang, C.; Kry, J.; Vitols, A.; Garcia, C.; Park, Y.K.; Lee, J.Y.; Blesso, C.N. Long-Term Supplementation of Black Elderberries Promotes Hyperlipidemia, but Reduces Liver Inflammation and Improves HDL Function and Atherosclerotic Plaque Stability in Apolipoprotein E-Knockout Mice. Mol. Nutr. Food Res. 2018, 62, e1800404. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, J.; Baker, C.; Cherry, L.; Dunne, E. Black elderberry (Sambucus nigra) supplementation effectively treats upper respiratory symptoms: A meta-analysis of randomized, controlled clinical trials. Complement. Ther. Med. 2019, 42, 361–365. [Google Scholar] [CrossRef]
- David, L.; Moldovan, B.; Vulcu, A.; Olenic, L.; Perde-Schrepler, M.; Fischer-Fodor, E.; Florea, A.; Crisan, M.; Chiorean, I.; Clichici, S.; et al. Green synthesis, characterization and anti-inflammatory activity of silver nanoparticles using European black elderberry fruits extract. Colloids Surf. B 2014, 122, 767–777. [Google Scholar] [CrossRef]
- Barak, V.; Halperin, T.; Kalickman, I. The effect of Sambucol, a black elderberry-based, natural product, on the production of human cytokines: I. Inflammatory cytokines. Eur. Cytokine Netw. 2001, 12, 290–296. [Google Scholar] [PubMed]
- Strugala, P.; Loi, S.; Bazanow, B.; Kuropka, P.; Kucharska, A.Z.; Wloch, A.; Gabrielska, J. A Comprehensive Study on the Biological Activity of Elderberry Extract and Cyanidin 3-O-Glucoside and Their Interactions with Membranes and Human Serum Albumin. Molecules 2018, 23, 2566. [Google Scholar] [CrossRef] [PubMed]
- Zielinska-Wasielica, J.; Olejnik, A.; Kowalska, K.; Olkowicz, M.; Dembczynski, R. Elderberry (Sambucus nigra L.) Fruit Extract Alleviates Oxidative Stress, Insulin Resistance, and Inflammation in Hypertrophied 3T3-L1 Adipocytes and Activated RAW 264.7 Macrophages. Foods 2019, 8, 326. [Google Scholar] [CrossRef] [PubMed]
- Waldbauer, K.; Seiringer, G.; Sykora, C.; Dirsch, V.M.; Zehl, M.; Kopp, B. Evaluation of Apricot, Bilberry, and Elderberry Pomace Constituents and Their Potential To Enhance the Endothelial Nitric Oxide Synthase (eNOS) Activity. ACS Omega 2018, 3, 10545–10553. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xie, J.; Zhang, H.; Pang, J.; Li, Q.; Wang, X.; Xu, H.; Sun, X.; Zhao, H.; Yang, Y.; et al. Anthocyanin supplementation at different doses improves cholesterol efflux capacity in subjects with dyslipidemia-a randomized controlled trial. Eur. J. Clin. Nutr. 2021, 75, 345–354. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, X.; Zhang, Y.; Wang, Y.; Liu, Y.; Sun, R.; Xia, M. Anthocyanin supplementation improves HDL-associated paraoxonase 1 activity and enhances cholesterol efflux capacity in subjects with hypercholesterolemia. J. Clin. Endocrinol. Metab. 2014, 99, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Serraino, I.; Dugo, L.; Dugo, P.; Mondello, L.; Mazzon, E.; Dugo, G.; Caputi, A.P.; Cuzzocrea, S. Protective effects of cyanidin-3-O-glucoside from blackberry extract against peroxynitrite-induced endothelial dysfunction and vascular failure. Life Sci. 2003, 73, 1097–1114. [Google Scholar] [CrossRef]
- Zafra-Stone, S.; Yasmin, T.; Bagchi, M.; Chatterjee, A.; Vinson, J.A.; Bagchi, D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol. Nutr. Food Res. 2007, 51, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, R.D.; Carvalho, N.C.; Martin-Moreno, J.M.; Pimenta, A.M.; Lopes, A.C.S.; Gea, A.; Martinez-Gonzalez, M.A.; Bes-Rastrollo, M. Total polyphenol intake, polyphenol subtypes and incidence of cardiovascular disease: The SUN cohort study. Nutr. Metab. Cardiovas 2019, 29, 69–78. [Google Scholar] [CrossRef]
- Rienks, J.; Barbaresko, J.; Nothlings, U. Review Association of Polyphenol Biomarkers with Cardiovascular Disease and Mortality Risk: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2017, 9, 415. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Park, Y.; Wegner, C.J.; Bolling, B.W.; Lee, J.Y. Polyphenol-rich black chokeberry (Aronia melanocarpa) extract regulates the expression of genes critical for intestinal cholesterol flux in Caco-2 cells. J. Nutr. Biochem. 2013, 24, 1564–1570. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Bae, M.; Park, Y.K.; Ma, H.; Yuan, T.; Seeram, N.P.; Lee, J.Y. Blackcurrant anthocyanins stimulated cholesterol transport via post-transcriptional induction of LDL receptor in Caco-2 cells. Eur. J. Nutr. 2018, 57, 405–415. [Google Scholar] [CrossRef]
- Nelson, R.H. Hyperlipidemia as a Risk Factor for Cardiovascular Disease. Prim. Care 2013, 40, 195–211. [Google Scholar] [CrossRef]
- de Boer, J.F.; Schonewille, M.; Boesjes, M.; Wolters, H.; Bloks, V.W.; Bos, T.; van Dijk, T.H.; Jurdzinski, A.; Boverhof, R.; Wolters, J.C.; et al. Intestinal Farnesoid X Receptor Controls Transintestinal Cholesterol Excretion in Mice. Gastroenterology 2017, 152, 1126–1138.e6. [Google Scholar] [CrossRef]
- Bura, K.S.; Lord, C.; Marshall, S.; McDaniel, A.; Thomas, G.; Warrier, M.; Zhang, J.; Davis, M.A.; Sawyer, J.K.; Shah, R.; et al. Intestinal SR-BI does not impact cholesterol absorption or transintestinal cholesterol efflux in mice. J. Lipid Res. 2013, 54, 1567–1577. [Google Scholar] [CrossRef]
- Farahnak, Z.; Chapados, N.; Lavoie, J.M. Exercise training increased gene expression of LDL-R and PCSK9 in intestine: Link to transintestinal cholesterol excretion. Gen. Physiol. Biophys. 2018, 37, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Jakulj, L.; van Dijk, T.H.; de Boer, J.F.; Kootte, R.S.; Schonewille, M.; Paalvast, Y.; Boer, T.; Bloks, V.W.; Boverhof, R.; Nieuwdorp, M.; et al. Transintestinal Cholesterol Transport Is Active in Mice and Humans and Controls Ezetimibe-Induced Fecal Neutral Sterol Excretion. Cell Metab. 2016, 24, 783–794. [Google Scholar] [CrossRef]
- Vrins, C.; van der Velde, A.; van den Oever, K.; Kunne, C.; van Eck, M.; Rensen, P.; Groen, A. Trans Intestinal Cholesterol Efflux Pathway Is Reduced in Apoe Knockout Mice. Atheroscler. Suppl. 2008, 9, 29. [Google Scholar] [CrossRef]
- Reeskamp, L.F.; Meessen, E.C.E.; Groen, A.K. Transintestinal cholesterol excretion in humans. Curr. Opin. Lipidol. 2018, 29, 10–17. [Google Scholar] [CrossRef]
- Moreau, F.; Blanchard, C.; Perret, C.; Flet, L.; Douane, F.; Frampas, E.; Mirallie, E.; Croyal, M.; Aguesse, A.; Krempf, M.; et al. In vivo evidence for transintestinal cholesterol efflux in patients with complete common bile duct obstruction. J. Clin. Lipidol. 2019, 13, 213–217.e211. [Google Scholar] [CrossRef]
- Temel, R.E.; Brown, J.M. A new model of reverse cholesterol transport: EnTICEing strategies to stimulate intestinal cholesterol excretion. Trends Pharmacol. Sci. 2015, 36, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Alphonse, P.A.S.; Jones, P.J.H. Revisiting Human Cholesterol Synthesis and Absorption: The Reciprocity Paradigm and its Key Regulators. Lipids 2016, 51, 519–536. [Google Scholar] [CrossRef]
- Vaklavas, C.; Chatzizisis, Y.S.; Ziakas, A.; Zamboulis, C.; Giannoglouc, G.D. Molecular basis of statin-associated myopathy. Atherosclerosis 2009, 202, 18–28. [Google Scholar] [CrossRef]
- Sato, R. Sterol metabolism and SREBP activation. Arch. Biochem. Biophys. 2010, 501, 177–181. [Google Scholar] [CrossRef]
- Hussain, M.M. Intestinal lipid absorption and lipoprotein formation. Curr. Opin. Lipidol. 2014, 25, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Betters, J.L.; Yu, L.Q. Niemann-Pick C1-Like 1 (NPC1L1) Protein in Intestinal and Hepatic Cholesterol Transport. Annu. Rev. Physiol. 2011, 73, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Kidambi, S.; Patel, S.B. Cholesterol and non-cholesterol sterol transporters: ABCG5, ABCG8 and NPC1L1: A review. Xenobiotica 2008, 38, 1119–1139. [Google Scholar] [CrossRef]
- Chang, T.Y.; Chang, C. Ezetimibe blocks internalization of the NPC1L1/cholesterol complex. Cell Metab. 2008, 7, 469–471. [Google Scholar] [CrossRef][Green Version]
- Garcia-Calvo, M.; Lisnock, J.M.; Bull, H.G.; Hawes, B.E.; Burnett, D.A.; Braun, M.P.; Crona, J.H.; Davis, H.R.; Dean, D.C.; Detmers, P.A.; et al. The target of ezetimibe is Niemann-Pick Cl-Like 1 (NPC1L1). Proc. Natl. Acad. Sci. USA 2005, 102, 8132–8137. [Google Scholar] [CrossRef] [PubMed]
- Field, F.J.; Watt, K.; Mathur, S.N. Origins of intestinal ABCA1-mediated HDL-cholesterol. J. Lipid Res. 2008, 49, 2605–2619. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Sawyer, J.K.; Kelley, K.L.; Davis, M.A.; Rudel, L.L. Cholesterol esterification by ACAT2 is essential for efficient intestinal cholesterol absorption: Evidence from thoracic lymph duct cannulation. J. Lipid Res. 2012, 53, 95–104. [Google Scholar] [CrossRef]
- Vidal, R.; Hernandez-Vallejo, S.; Pauquai, T.; Texier, O.; Rousset, M.; Chambaz, J.; Demignot, S.; Lacorte, J.M. Apple procyanidins decrease cholesterol esterification and lipoprotein secretion in Caco-2/TC7 enterocytes. J. Lipid Res. 2005, 46, 258–268. [Google Scholar] [CrossRef]
- Hui, D.Y.; Labonte, E.D.; Howles, P.N. Development and physiological regulation of intestinal lipid absorption. III. Intestinal transporters and cholesterol absorption. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G839–G843. [Google Scholar] [CrossRef]
- Le May, C.; Berger, J.M.; Lespine, A.; Pillot, B.; Prieur, X.; Letessier, E.; Hussain, M.M.; Collet, X.; Cariou, B.; Costet, P. Transintestinal cholesterol excretion is an active metabolic process modulated by PCSK9 and statin involving ABCB1. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
- Houtkooper, R.H.; Pirinen, E.; Auwerx, J. Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Biol. 2012, 13, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Sygitowicz, G.; Sitkiewicz, D. Sirtuins and their role as physiological modulators of metabolism. Postep. Hig. Med. Dosw. 2020, 74, 489–496. [Google Scholar] [CrossRef]
- Ye, X.; Li, M.T.; Hou, T.Y.; Gao, T.; Zhu, W.G.; Yang, Y. Sirtuins in glucose and lipid metabolism. Oncotarget 2017, 8, 1845–1859. [Google Scholar] [CrossRef]
- Vachharajani, V.T.; Liu, T.F.; Wang, X.F.; Hoth, J.J.; Yoza, B.K.; McCall, C.E. Sirtuins Link Inflammation and Metabolism. J. Immunol. Res. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.H.; Li, Y.S.; Liu, H.F.; Xiao, S.S.; Li, L.J.; Tian, J.; Cheng, C.; Zhang, G.; Zhang, F.J. The role of sirtuin 1 and its activator, resveratrol in osteoarthritis. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [PubMed]
- Roggerio, A.; Strunz, C.M.C.; Pacanaro, A.P.; Leal, D.P.; Takada, J.Y.; Avakian, S.D.; Mansur, A.D. Gene Expression of Sirtuin-1 and Endogenous Secretory Receptor for Advanced Glycation End Products in Healthy and Slightly Overweight Subjects after Caloric Restriction and Resveratrol Administration. Nutrients 2018, 10, 937. [Google Scholar] [CrossRef]



| Genes | Forward | Reverse |
|---|---|---|
| HMGR | 5′–CCCAGTTGTGCGTCTTCCA–3′ | 5′–TTCGAGCCAGGCTTTCACTT–3′ |
| SREBP2 | 5′–TCCGCCTGTTCCGATGTAC–3′ | 5′–TGCACATTCAGCCAGGTTCA–3′ |
| NPC1L1 | 5′–CACTGGATCACTCGAGGTGTTG–3′ | 5′–CCAGTCCCACGCTGATGTG–3′ |
| SR-B1 | 5′–AGAATAAGCCCATGACCCTGAA–3′ | 5′–CGCCGAGGGTGGTGAA–3′ |
| ABCA1 | 5′–TTTCTCAGACAACACTTGACCAAGTA–3′ | 5′–GGTTTTTGTGTAATGAGAGGTCTTTTAA–3′ |
| MTTP | 5′–TCCCCGTTCGGCATCTAC–3′ | 5′–CTTAGAATGCCAGAACCCGAGTA–3′ |
| ACAT2 | 5′–TGGGCCACCCTCTTGGA–3′ | 5′–CCAGTGTGTGTAACAGGGTCACA–3′ |
| LDLR | 5–ACTGGGTTGACTCCAAACTTCAC–3′ | 5–GGTTGCCCCCGTTGACA–3′ |
| PCSK9 | 5′–TTCCTGGTGAAGATGAGT–3′ | 5′–TTCCTGGTGAAGATGAGT–3′ |
| ABCG5 | 5′–GCGTAGGTCTCCTTTACCAGTTTG–3′ | 5′–GGAAACAGATTCACAGCGTTCA–3′ |
| ABCG8 | 5′–GCCGCCCTCTTGTTCATG–3′ | 5′–TAACATTTGGAGATGACATCCAGAA–3′ |
| ABCB1 | 5′–CTATAATGCGACAGGAGA–3′ | 5′–TTAATCTTGGAGACATCATC–3′ |
| FAS | 5′–CGCTCGGCATGGCTATCT–3′ | 5′–CTCGTTGAAGAACGCATCCA–3′ |
| SCD-1 | 5′–CCGACGTGGCTTTTTCTTCT–3′ | 5′–TGGGTGTTTGCGCACAAG–3′ |
| SREBP1c | 5′–TCAGCGAGGCGGCTTTGGAGCAG–3′ | 5′–CATGTCTTCGATGTCGGTCAG–3′ |
| CPT1 | 5′–TTATCGCCAAGGATGGCTCTA–3′ | 5′–CCACACCATCACCCCAAGA–3′ |
| ACOX | 5′–CTTGCTTCACCAGGCAACTG–3′ | 5′–TTCCAGGCGGGCATGA–3′ |
| SIRT1 | 5′–TAGTTCTTGTGGCAGTAA–3′ | 5′–CATCAGGCTCATCTTCTA–3′ |
| SIRT2 | 5′–AACCATCTGTCACTACTT–3′ | 5′–TATCTATGTTCTGCGTGTA–3′ |
| SIRT3 | 5′–GCTCCCAGTTTCTTCTTT–3′ | 5′–CCACTTCCAACAACACTT–3′ |
| SIRT4 | 5′–CTTCATCACCCTTTCCAA–3′ | 5′–ACCTGTAGTCTGGTATCC–3′ |
| SIRT5 | 5′–AAGCACATAGTCATCATCT–3′ | 5′–TTCTCCAATAACCTCCAG–3′ |
| SIRT6 | 5′–AGGGACAAACTGGCAGAG–3′ | 5′–TGTGTCTCGGACGTACTG–3′ |
| SIRT7 | 5′–AATACTTGGTCGTCTACAC–3′ | 5′–TGTCCACACTCCATTAGG–3′ |
| GAPDH | 5′–GGTGGTCTCCTCTGACTTCAACA–3′ | 5′–GTTGCTGTAGCCAAATTCGTTGT–3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, S.; Kim, M.; Kim, B. Polyphenol-Rich Black Elderberry Extract Stimulates Transintestinal Cholesterol Excretion. Appl. Sci. 2021, 11, 2790. https://doi.org/10.3390/app11062790
Jeon S, Kim M, Kim B. Polyphenol-Rich Black Elderberry Extract Stimulates Transintestinal Cholesterol Excretion. Applied Sciences. 2021; 11(6):2790. https://doi.org/10.3390/app11062790
Chicago/Turabian StyleJeon, Sohyeon, Minji Kim, and Bohkyung Kim. 2021. "Polyphenol-Rich Black Elderberry Extract Stimulates Transintestinal Cholesterol Excretion" Applied Sciences 11, no. 6: 2790. https://doi.org/10.3390/app11062790
APA StyleJeon, S., Kim, M., & Kim, B. (2021). Polyphenol-Rich Black Elderberry Extract Stimulates Transintestinal Cholesterol Excretion. Applied Sciences, 11(6), 2790. https://doi.org/10.3390/app11062790