Enzymatic Synthesis of Glucose Fatty Acid Esters Using SCOs as Acyl Group-Donors and Their Biological Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Biological Material and SCO Production
2.3. Enzymatic Synthesis of GEs
2.4. GE Analysis
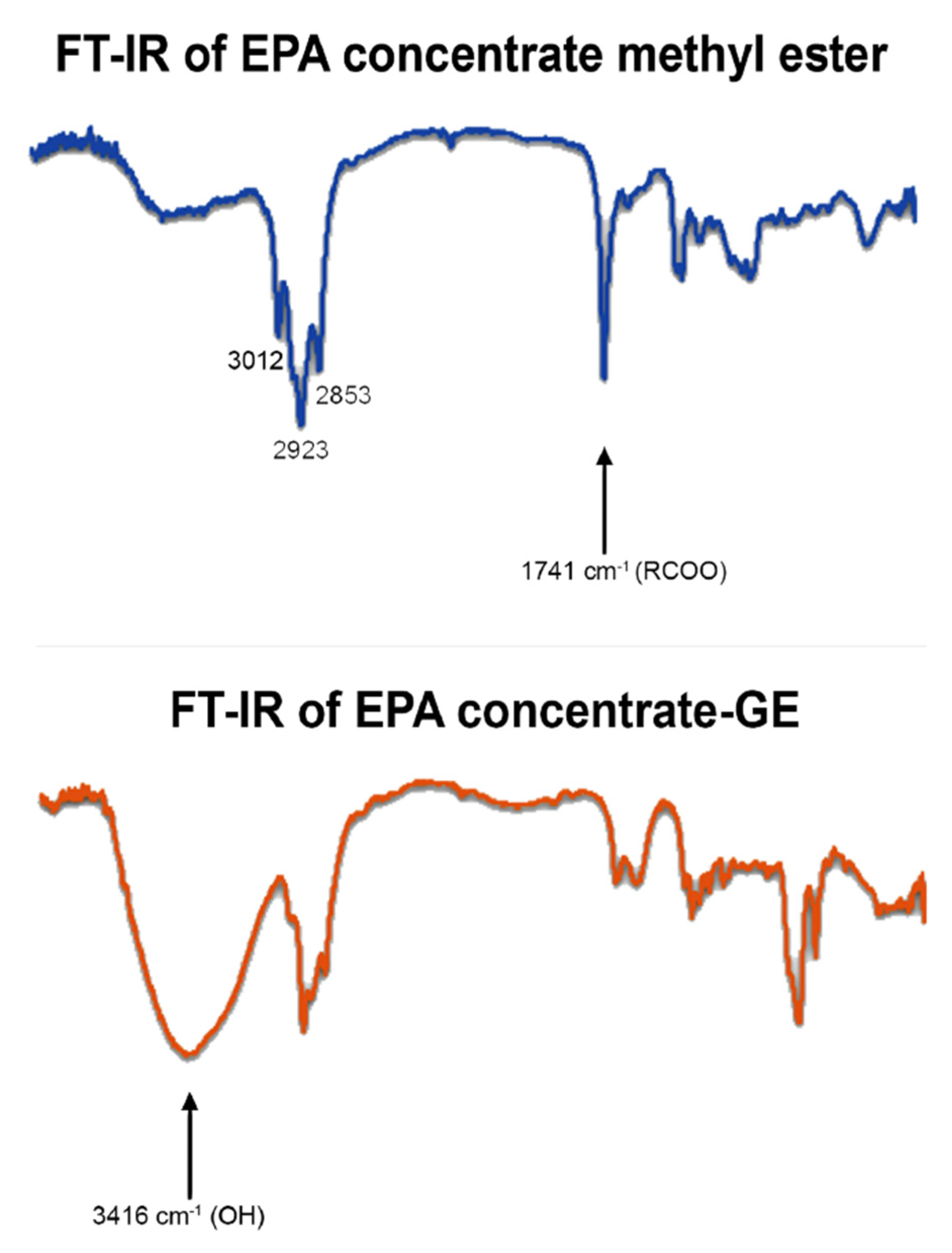
2.4.1. Thin-Layer Chromatography and FTIR
2.4.2. Quantitative Determination of the Enzymatic Conversion through In Situ NMR Monitoring
2.4.3. Reusability of Candida antarctica Lipase
2.5. Biological Activity of GEs
2.6. Statistical Analysis
3. Results and Discussion
3.1. Biomass and SCO Production
3.2. Optimization of the GE Synthesis
3.3. Product Identification and Quantitative Analysis
3.4. GE Synthesis Using FAMEs from Different Origins
3.5. Antimicrobial Activity of GEs
3.6. Insecticidal Activity of GEs
3.7. Quantitative Analysis of Ovarian Cancer Cell Apoptosis Induced by GEs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Furukawa, S.; Akiyoshi, Y.; O’Toole, G.A.; Ogihara, H.; Morinaga, Y. Sugar fatty acid esters inhibit biofilm formation by food-borne pathogenic bacteria. Int. J. Food Microbiol. 2010, 138, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Neta, N.S.; Teixeira, J.A.; Rodrigues, L.R. Sugar ester surfactants: Enzymatic synthesis and applications in food industry. Crit. Rev. Food Sci. Nutr. 2015, 55, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.R.; Rathod, V.K. Enzyme catalyzed synthesis of cosmetic esters and its intensification: A review. Process Biochem. 2015, 50, 1793–1806. [Google Scholar] [CrossRef]
- Li, W.; Das, S.; Ng, K.Y.; Heng, P.W.S. Formulation, biological and pharmacokinetic studies of sucrose ester-stabilized nanosuspensions of oleanolic acid. Pharm. Res. 2011, 28, 2020–2033. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, S.; Fagioli, L.; Campana, R.; Cole, H.; Duranti, A.; Baffone, W.; Vllasaliu, D.; Casettari, L. Unsaturated fatty acids lactose esters: Cytotoxicity, permeability enhancement and antimicrobial activity. Eur. J. Pharm. Biopharm. 2016, 107, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Puterka, G.J.; Farone, W.; Palmer, T.; Barrington, A. Structure-function relationships affecting the insecticidal and miticidal activity of sugar esters. J. Econ. Entomol. 2003, 96, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.H.; Cai, Y.Z.; Lin, X.S.; Xiong, J.; Halling, P.J.; Yang, Z. Enzymatic synthesis of glucose-based fatty acid esters in bisolvent systems containing ionic liquids or deep eutectic solvents. Molecules 2016, 21, 1294. [Google Scholar] [CrossRef] [PubMed]
- De Lima, L.N.; Mendes, A.A.; Fernandez-Lafuente, R.; Tardioli, P.W.; de Lima Camargo Giordano, R. Performance of different immobilized lipases in the syntheses of short- and long-chain carboxylic acid esters by esterification reactions in organic media. Molecules 2018, 23, 766. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.Y.; Chen, Y.; Banwell, M.G.; Wang, Y.; Lan, P. Enzymatic preparation of a homologous series of long-chain 6-O-acylglucose esters and their evaluation as emulsifiers. J. Agric. Food Chem. 2018, 66, 3949–3956. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.S.; Wen, Q.; Huang, Z.L.; Cai, Y.Z.; Halling, P.J.; Yang, Z. Impacts of ionic liquids on enzymatic synthesis of glucose laurate and optimization with superior productivity by response surface methodology. Process Biochem. 2015, 50, 1852–1858. [Google Scholar] [CrossRef]
- Ren, K.; Lamsal, B.P. Synthesis of some glucose-fatty acid esters by lipase from Candida antarctica and their emulsion functions. Food Chem. 2017, 214, 556–563. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wu, J.H.Y. Omega-3 Fatty acids and cardiovascular disease. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef] [PubMed]
- Alakhras, R.; Bellou, S.; Fotaki, G.; Stephanou, G.; Demopoulos, N.A.; Papanikolaou, S.; Aggelis, G. Fatty acid lithium salts from Cunninghamella echinulata have cytotoxic and genotoxic effects on HL-60 human leukemia cells. Eng. Life Sci. 2015, 15, 243–253. [Google Scholar] [CrossRef]
- Sayegh, F.; Elazzazy, A.; Bellou, S.; Moustogianni, A.; Elkady, A.I.; Baeshen, M.N.; Aggelis, G. Production of polyunsaturated single cell oils possessing antimicrobial and anticancer properties. Ann. Microbiol. 2016, 66, 937–948. [Google Scholar] [CrossRef]
- Gumel, A.M.; Annuar, M.S.M.; Heidelberg, T.; Chisti, Y. Lipase mediated synthesis of sugar fatty acid esters. Process Biochem. 2011, 46, 2079–2090. [Google Scholar] [CrossRef]
- Plou, F.J.; Cruces, M.A.; Ferrer, M.; Fuentes, G.; Pastor, E.; Bernabé, M.; Christensen, M.; Comelles, F.; Parra, J.L.; Ballesteros, A. Enzymatic acylation of di- and trisaccharides with fatty acids: Choosing the appropriate enzyme, support and solvent. J. Biotechnol. 2002, 96, 55–66. [Google Scholar] [CrossRef]
- Jegannathan, K.R.; Nielsen, P.H. Environmental assessment of enzyme use in industrial production—A literature review. J. Clean. Prod. 2013, 42, 228–240. [Google Scholar] [CrossRef]
- Abdulmalek, E.; Hamidon, N.F.; Abdul Rahman, M.B. Optimization and characterization of lipase catalysed synthesis of xylose caproate ester in organic solvents. J. Mol. Catal. B Enzym. 2016, 132, 1–4. [Google Scholar] [CrossRef]
- Vescovi, V.; dos Santos, J.B.C.; Tardioli, P.W. Porcine pancreatic lipase hydrophobically adsorbed on octyl-silica: A robust biocatalyst for syntheses of xylose fatty acid esters. Biocatal. Biotransform. 2017, 35, 298–305. [Google Scholar] [CrossRef]
- Findrik, Z.; Megyeri, G.; Gubicza, L.; Bélafi-Bakó, K.; Nemestóthy, N.; Sudar, M. Lipase catalyzed synthesis of glucose palmitate in ionic liquid. J. Clean. Prod. 2016, 112, 1106–1111. [Google Scholar] [CrossRef]
- Pöhnlein, M.; Ulrich, J.; Kirschhöfer, F.; Nusser, M.; Muhle-Goll, C.; Kannengiesser, B.; Brenner-Weiß, G.; Luy, B.; Liese, A.; Syldatk, C.; et al. Lipase-catalyzed synthesis of glucose-6-O-hexanoate in deep eutectic solvents. Eur. J. Lipid Sci. Technol. 2015, 117, 161–166. [Google Scholar] [CrossRef]
- Bell, J.G.; Pratoomyot, J.; Strachan, F.; Henderson, R.J.; Fontanillas, R.; Hebard, A.; Guy, D.R.; Hunter, D.; Tocher, D.R. Growth, flesh adiposity and fatty acid composition of Atlantic salmon (Salmo salar) families with contrasting flesh adiposity: Effects of replacement of dietary fish oil with vegetable oils. Aquaculture 2010, 306, 225–232. [Google Scholar] [CrossRef]
- Eroldoğan, T.; Turchini, G.M.; Yılmaz, A.H.; Taşbozan, O.; Engin, K.; Ölçülü, A.; Özşahinoğlu, I.; Mumoğullarında, P. Potential of cottonseed oil as fish oil replacer in European sea bass feed formulation. Turkish J. Fish. Aquat. Sci. 2012, 12, 787–797. [Google Scholar]
- Bellou, S.; Baeshen, M.N.; Elazzazy, A.M.; Aggeli, D.; Sayegh, F.; Aggelis, G. Microalgal lipids biochemistry and biotechnological perspectives. Biotechnol. Adv. 2014, 32, 1476–1493. [Google Scholar] [CrossRef]
- Kothri, M.; Mavromati, M.; Elazazy, A.M.; Baeshen, M.N.; Moussa, T.A.A.; Aggelis, G. Microbial sources of polyunsaturated fatty acids (PUFAs) and the prospect of organic residues and wastes as growth media for PUFA-producing microorganisms. FEMS Microbiol. Lett. 2020, 367, fnaa028. [Google Scholar] [CrossRef] [PubMed]
- Bellou, S.; Triantaphyllidou, I.E.; Aggeli, D.; Elazzazy, A.M.; Baeshen, M.N.; Aggelis, G. Microbial oils as food additives: Recent approaches for improving microbial oil production and its polyunsaturated fatty acid content. Curr. Opin. Biotechnol. 2016, 37, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, S.; Aggelis, G. Sources of microbial oils with emphasis to Mortierella (Umbelopsis) isabellina fungus. World J. Microbiol. Biotechnol. 2019, 35, 63. [Google Scholar] [CrossRef] [PubMed]
- Athenaki, M.; Gardeli, C.; Diamantopoulou, P.; Tchakouteu, S.S.; Sarris, D.; Philippoussis, A.; Papanikolaou, S. Lipids from yeasts and fungi: Physiology, production and analytical considerations. J. Appl. Microbiol. 2018, 124, 336–367. [Google Scholar] [CrossRef] [PubMed]
- Bellou, S.; Aggelis, G. Biochemical activities in Chlorella sp. and Nannochloropsis salina during lipid and sugar synthesis in a lab-scale open pond simulating reactor. J. Biotechnol. 2012, 164, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Dourou, M.; Tsolcha, O.N.; Tekerlekopoulou, A.G.; Bokas, D.; Aggelis, G. Fish farm effluents are suitable growth media for Nannochloropsis gaditana, a polyunsaturated fatty acid producing microalga. Eng. Life Sci. 2018, 18, 851–860. [Google Scholar] [CrossRef]
- Rocha, J.M.S.; Garcia, J.E.C.; Henriques, M.H.F. Growth aspects of the marine microalga Nannochloropsis gaditana. Biomol. Eng. 2003, 20, 237–242. [Google Scholar] [CrossRef]
- Campaña-Torres, A.; Martínez-Córdova, L.R.; Martínez-Porchas, M.; López-Elías, J.A.; Porchas-Cornejo, M.A. Productive response of Nannochloropsis oculata, cultured in different media and their efficiency as food for the rotifer Brachionus rotundiformis. Phyton 2012, 81, 45–50. [Google Scholar]
- Ruan, Z.; Hollinshead, W.; Isaguirre, C.; Tang, Y.J.; Liao, W.; Liu, Y. Effects of inhibitory compounds in lignocellulosic hydrolysates on Mortierella isabellina growth and carbon utilization. Bioresour. Technol. 2015, 183, 18–24. [Google Scholar] [CrossRef]
- Harde, S.M.; Wang, Z.; Horne, M.; Zhu, J.Y.; Pan, X. Microbial lipid production from SPORL-pretreated Douglas fir by Mortierella isabellina. Fuel 2016, 175, 64–74. [Google Scholar] [CrossRef]
- Gardeli, C.; Athenaki, M.; Xenopoulos, E.; Mallouchos, A.; Koutinas, A.A.; Aggelis, G.; Papanikolaou, S. Lipid production and characterization by Mortierella (Umbelopsis) isabellina cultivated on lignocellulosic sugars. J. Appl. Microbiol. 2017, 123, 1461–1477. [Google Scholar] [CrossRef] [PubMed]
- El-Baz, H.A.; Ahmed, M.E.; Saleh, T.S.; Dritsas, P.; Mahyoub, J.A.; Baeshen, M.N.; Madian, H.R.; Alkhaled, M.; Aggelis, G. Singe cell oil (SCO) based bioactive compounds: Enzymatic synthesis of fatty acid amides using microbial lipids as acyl group-donors and their biological activities. Appl. Biochem. Biotechnol. 2020, 193, 822–845. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.; Pauli, M.; Bazerque, P. An antibiotic assay by the agar well diffusion method. Acta Biol. Med. Exp. 1990, 15, 113–115. [Google Scholar]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University Press: Cambridge, UK, 1971. [Google Scholar]
- Ferrer, M.; Soliveri, J.; Plou, F.J.; López-Cortés, N.; Reyes-Duarte, D.; Christensen, M.; Copa-Patiño, J.L.; Ballesteros, A. Synthesis of sugar esters in solvent mixtures by lipases from Thermomyces lanuginosus and Candida antarctica B, and their antimicrobial properties. Enzyme Microb. Technol. 2005, 36, 391–398. [Google Scholar] [CrossRef]
- Ferrer, M.; Plou, F.J.; Fuentes, G.; Cruces, M.A.; Andersen, L.; Kirk, O.; Christensen, M.; Ballesteros, A. Effect of the immobilization method of lipase from Thermomyces lanuginosus on sucrose acylation. Biocatal. Biotransform. 2002, 20, 63–71. [Google Scholar] [CrossRef]
- Habulin, M.; Šabeder, S.; Knez, Ž. Enzymatic synthesis of sugar fatty acid esters in organic solvent and in supercritical carbon dioxide and their antimicrobial activity. J. Supercrit. Fluids 2008, 45, 338–345. [Google Scholar] [CrossRef]
- Ganske, F.; Bornscheuer, U.T. Optimization of lipase-catalyzed glucose fatty acid ester synthesis in a two-phase system containing ionic liquids and t-BuOH. J. Mol. Catal. B Enzym. 2005, 36, 40–42. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, J.; Zhao, A.; Ma, X. Study of glucose ester synthesis by immobilized lipase from Candida sp. Catal. Commun. 2008, 9, 1369–1374. [Google Scholar] [CrossRef]
- Degn, P.; Zimmermann, W. Optimization of carbohydrate fatty acid ester synthesis in organic media by a lipase from Candida antarctica. Biotechnol. Bioeng. 2001, 74, 483–491. [Google Scholar] [CrossRef] [PubMed]
- H-Kittikun, A.; Prasertsan, P.; Zimmermann, W.; Seesuriyachan, P.; Chaiyaso, T. Sugar ester synthesis by thermostable lipase from Streptomyces thermocarboxydus ME168. Appl. Biochem. Biotechnol. 2012, 166, 1969–1982. [Google Scholar] [CrossRef] [PubMed]
- Chaiyaso, T.; H-kittikim, A.; Zimmermann, W. Biocatalytic acylation of carbohydrates with fatty acids from palm fatty acid distillates. J. Ind. Microbiol. Biotechnol. 2006, 33, 338–342. [Google Scholar] [CrossRef]
- Davis, R.A.; Lin, C.H.; Gervay-Hague, J. Chemoenzymatic synthesis of cholesteryl-6-O-tetradecanoyl-α-d-glucopyranoside: A product of host cholesterol efflux promoted by Helicobacter pylori. Chem. Commun. 2012, 48, 9083. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, Z.L. Enzymatic synthesis of sugar fatty acid esters in ionic liquids. Catal. Sci. Technol. 2012, 2, 1767–1775. [Google Scholar] [CrossRef]
- Yan, Y.; Bornscheuer, U.T.; Stadler, G.; Lutz-Wahl, S.; Reuss, M.; Schmid, R.D. Production of sugar fatty acid esters by enzymatic esterification in a stirred-tank membrane reactor: Optimization of parameters by response surface methodology. J. Am. Oil Chem. Soc. 2001, 78, 147–153. [Google Scholar] [CrossRef]
- Sebatini, A.M.; Jain, M.; Radha, P.; Kiruthika, S.; Tamilarasan, K. Immobilized lipase catalyzing glucose stearate synthesis and their surfactant properties analysis. 3 Biotech 2016, 6, 184. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, H.; Hao, T.; Li, S. In vitro antibacterial activities and mechanism of sugar fatty acid esters against five food-related bacteria. Food Chem. 2015, 187, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Katayama, S.; Matsubara, M.; Honda, Y.; Kuwahara, M. Antibacterial carbohydrate monoesters suppressing cell growth of Streptococcus mutans in the presence of sucrose. Curr. Microbiol. 2000, 41, 210–213. [Google Scholar] [CrossRef]
- Xiao, D.; Ye, R.; Davidson, P.M.; Hayes, D.G.; Golden, D.A.; Zhong, Q. Sucrose monolaurate improves the efficacy of sodium hypochlorite against Escherichia coli O157: H7 on spinach. Int. J. Food Microbiol. 2011, 145, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Devulapalle, K.S.; Gómez de Segura, A.; Ferrer, M.; Alcalde, M.; Mooser, G.; Plou, F.J. Effect of carbohydrate fatty acid esters on Streptococcus sobrinus and glucosyltransferase activity. Carbohydr. Res. 2004, 339, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Piao, J.; Kawahara-Aoyama, Y.; Inoue, T.; Adachi, S. Bacteriostatic activities of monoacyl sugar alcohols against thermophilic sporeformers. Biosci. Biotechnol. Biochem. 2006, 70, 263–265. [Google Scholar] [CrossRef] [PubMed]
- De Lamo-Castellvi, S.; Ratphitagsanti, W.; Balasubramaniam, V.M.; Yousef, A.E. Inactivation of Bacillus amyloliquefaciens spores by a combination of sucrose laurate and pressure-assisted thermal processing. J. Food Prot. 2010, 73, 2043–2052. [Google Scholar] [CrossRef]
- Shearer, A.E.H.; Dunne, C.P.; Sikes, A.; Hoover, D.G. Bacterial spore inhibition and inactivation in foods by pressure, chemical preservatives, and mild heat. J. Food Prot. 2000, 63, 1503–1510. [Google Scholar] [CrossRef]
- Wagh, A.; Shen, S.; Shen, F.A.; Miller, C.D.; Walsha, M.K. Effect of lactose monolaurate on pathogenic and nonpathogenic bacteria. Appl. Environ. Microbiol. 2012, 78, 3465–3468. [Google Scholar] [CrossRef] [PubMed]
- Karlová, T.; Poláková, L.; Šmidrkal, J.; Filip, V. Antimicrobial effects of fatty acid fructose esters. Czech J. Food Sci. 2010, 28, 146–149. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, W.; Cao, X.; Feng, F. Characterization of enzymatically prepared sugar medium-chain fatty acid monoesters. J. Sci. Food Agric. 2015, 95, 1631–1637. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J. Saccharides: Modifications and applications. In Chemical and Functional Properties of Food Saccharides; CRC Press: Boca Raton, FL, USA, 2004; ISBN 9780203495728. [Google Scholar]
- Powell, J.R. Perspective piece mosquito-borne human viral diseases: Why Aedes aegypti? Am. J. Trop. Med. Hyg. 2018, 98, 1563–1565. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; Sampaio, C. de G.; de Souza, J.S.N.; Campos, A.R.; da Silva, A.B.R.; de Morais, S.M.; Martins, V.E.P. Different susceptibilities of Aedes aegypti and Aedes albopictus larvae to plant-derived products. Rev. Soc. Bras. Med. Trop. 2019, 52, e20180197. [Google Scholar] [CrossRef] [PubMed]
- Chortyk, O.T. Chemically synthesized sugar esters for the control of soft-bodied arthropods. United States Department of Agriculture 2003. U.S. Patent Application No. 6,605,598 B1. Available online: https://patentscope2.wipo.int/search/en/detail.jsf?docId=WO1996001832 (accessed on 12 March 2021).
- Krugliak, M.; Deharo, E.; Shalmiev, G.; Sauvain, M.; Moretti, C.; Ginsburg, H. Antimalarial effects of C18 fatty acids on Plasmodium falciparum in culture and on Plasmodium vinckei petteri and Plasmodium yoelii nigeriensis in vivo. Exp. Parasitol. 1995, 81, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Morin, C.; Rousseau, É.; Fortin, S. Anti-proliferative effects of a new docosapentaenoic acid monoacylglyceride in colorectal carcinoma cells. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Siena, L.; Cipollina, C.; Di Vincenzo, S.; Ferraro, M.; Bruno, A.; Gjomarkaj, M.; Pace, E. Electrophilic derivatives of omega-3 fatty acids counteract lung cancer cell growth. Cancer Chemother. Pharmacol. 2018, 81, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Campana, R.; Merli, A.; Verboni, M.; Biondo, F.; Favi, G.; Duranti, A.; Lucarini, S. Synthesis and evaluation of saccharide-based aliphatic and aromatic esters as antimicrobial and antibiofilm agents. Pharmaceuticals 2019, 12, 186. [Google Scholar] [CrossRef] [PubMed]
- Petrova, K.T.; Barros, M.T.; Calhelha, R.C.; Soković, M.; Ferreira, I.C.F.R. Antimicrobial and cytotoxic activities of short carbon chain unsaturated sucrose esters. Med. Chem. Res. 2018, 27, 980–988. [Google Scholar] [CrossRef]
- An, D.; Feng, D. Enzymic synthesis, physicochemical, and cell activity of glucosyl ester derivatives based on N-fatty acyl amino acid. Chem. Pap. 2019, 73, 653–662. [Google Scholar] [CrossRef]
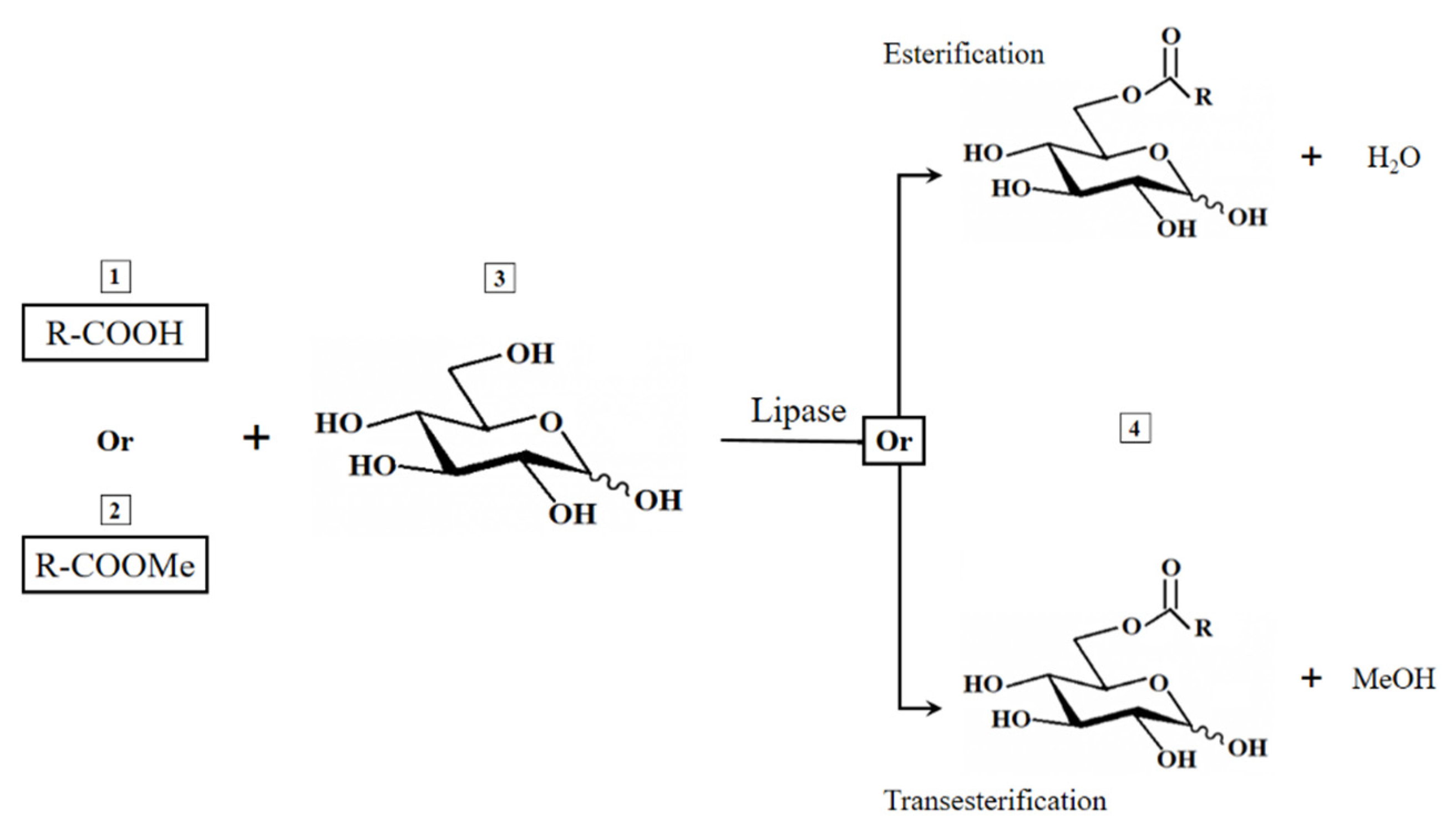
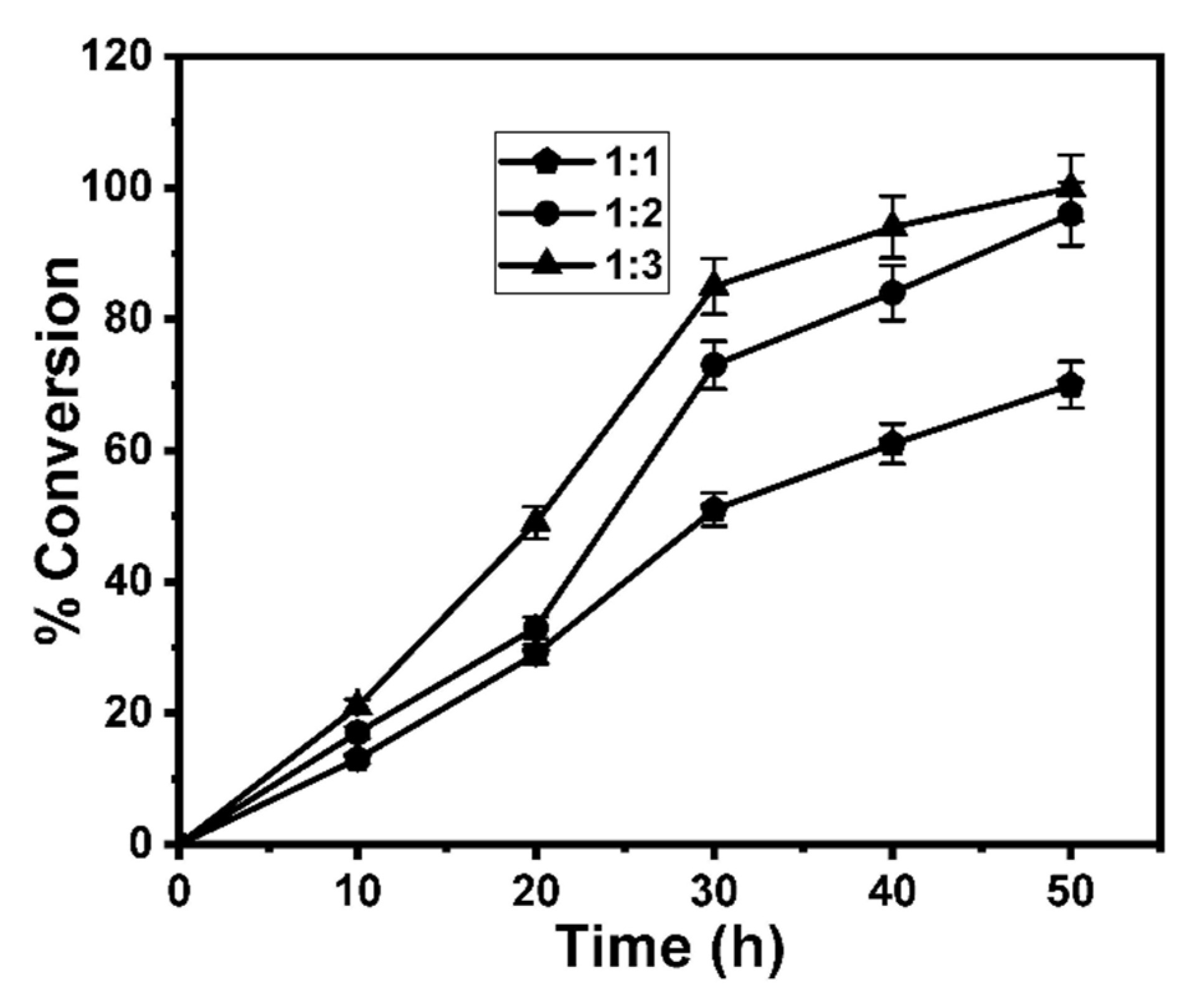
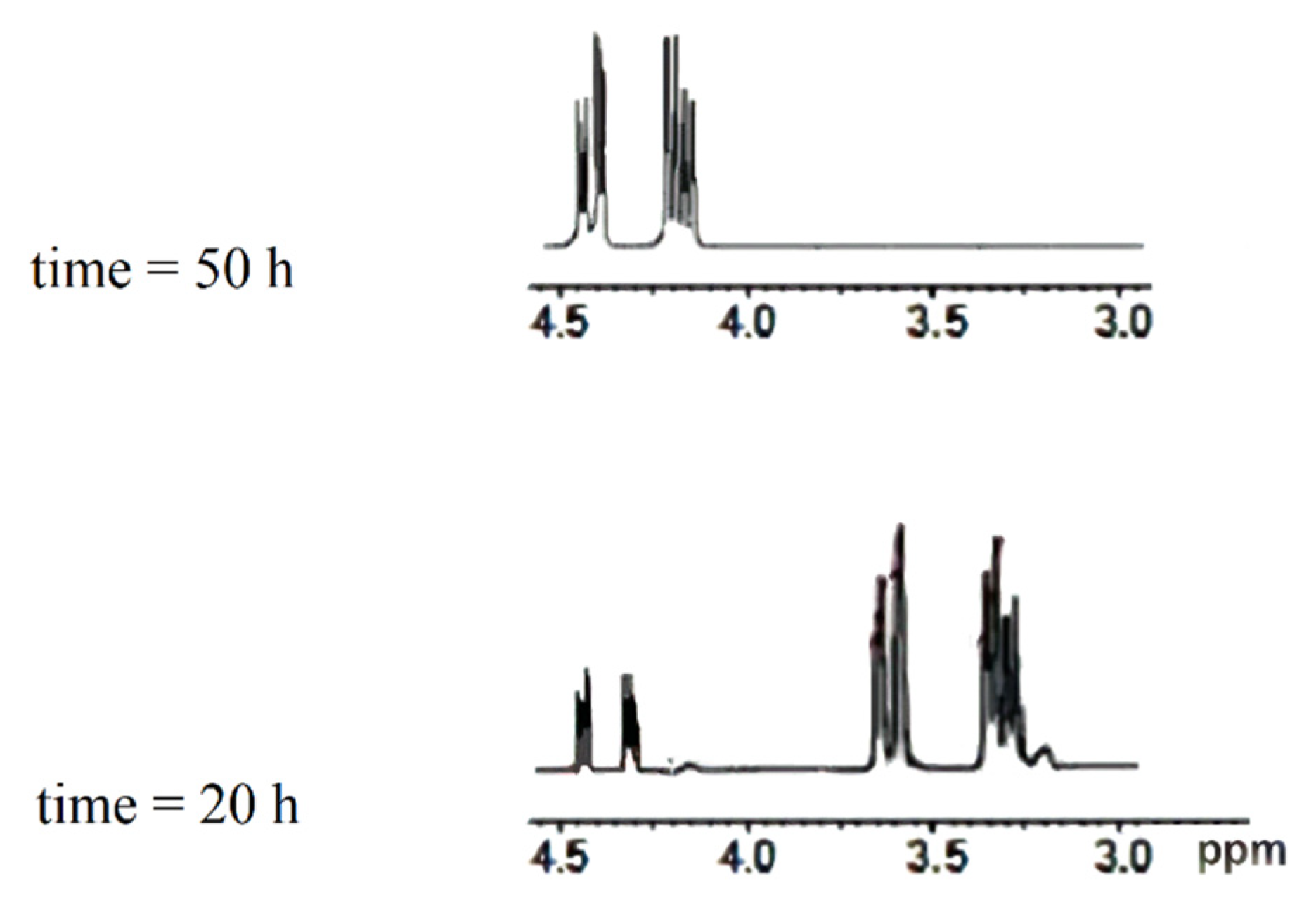

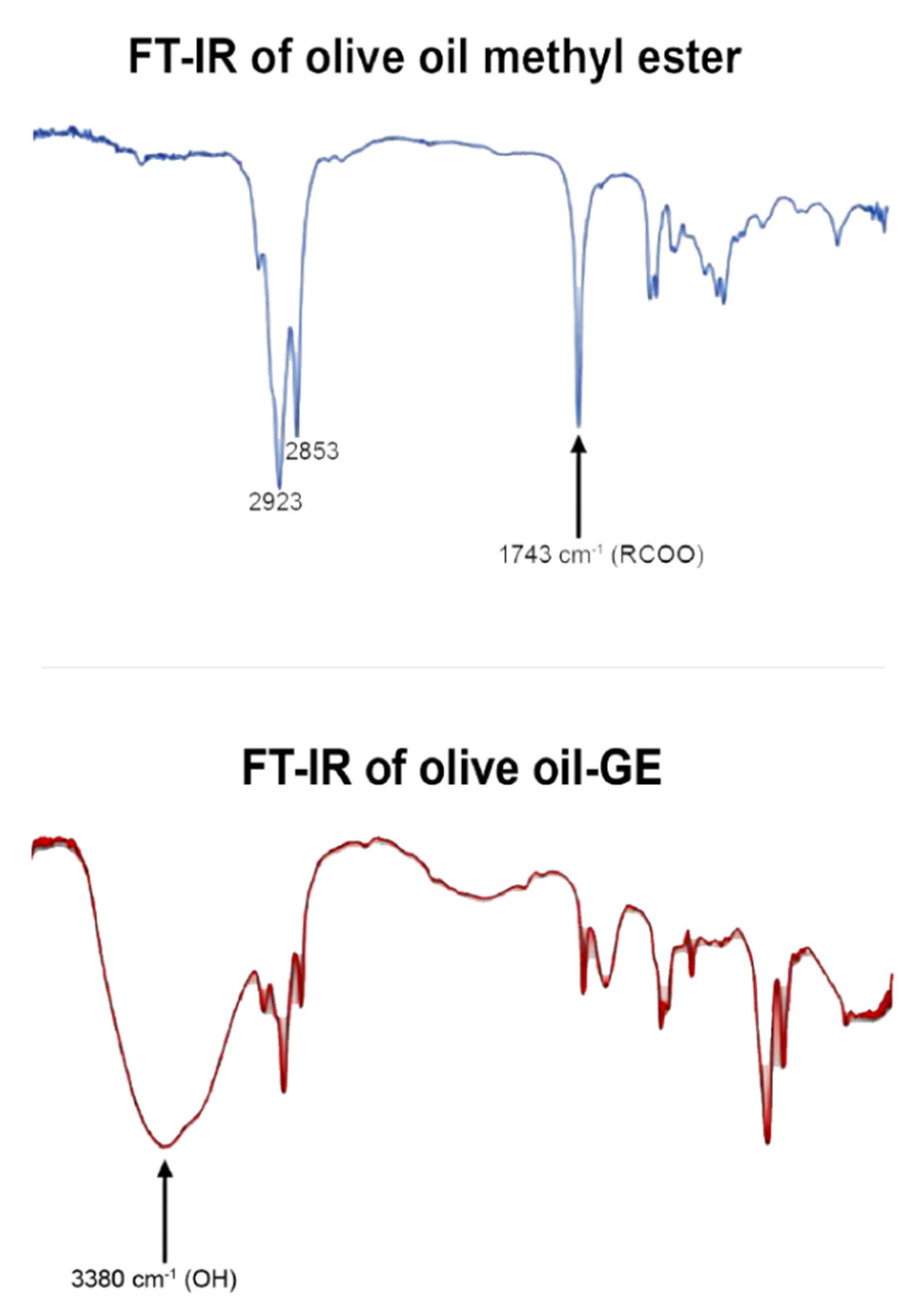



| Entry | Substrate | Immobilized Enzyme | Olive Oil: Glucose Ratio | Product | Conversion (%) |
|---|---|---|---|---|---|
| 1 | FFAs | Lipase CA | 1:1 | Still contains FFAs | 50.5 ± 2.5 |
| 2 | 1:2 | Still contains FFAs | 70.7 ± 3.5 | ||
| 3 | 1:3 | Almost 100% ester | 95.2 ± 4.8 | ||
| 4 | FAMEs | 1:1 | Still contains FAMEs | 70.1 ± 3.5 | |
| 5 | 1:2 | Still contains FAMEs | 96.1 ± 3.5 | ||
| 6 | 1:3 | 100% ester | 100.0 ± 5.0 | ||
| 7 | FFAs | Lipase CR | 1:1 | Still contains FFAs | 30 ± 1.5 |
| 8 | 1:2 | Still contains FFAs | 63 ± 3.2 | ||
| 9 | 1:3 | Still contains FFAs | 89 ± 4.5 | ||
| 10 | FAMEs | 1:1 | Still contains FAMEs | 64 ± 3.2 | |
| 11 | 1:2 | Still contains FAMEs | 87 ± 4.4 | ||
| 12 | 1:3 | Still contains FAMEs | 92 ± 4.6 |
| Entry | CA Lipase (g/25 mL) | Conversion (%) |
|---|---|---|
| 1 | 0.10 | 80.3 ± 4.0 |
| 2 | 0.15 | 92.1 ± 4.6 |
| 3 | 0.20 | 96.3 ± 4.8 |
| 4 | 0.25 | 100.3 ± 5.0 |
| 5 | 0.30 | 100.2 ± 5.0 |
| Entry | Source of FAMEs | Conversion (%) |
|---|---|---|
| 1 | Cunninghamella echinulata | 80.3 ± 4.0 |
| 2 | Umbelopsis isabellina | 85.1 ± 4.3 |
| 3 | Nannochloropsis gaditana | 86.3 ± 4.3 |
| 4 | EPA concentrate | 99.1 ± 5.0 |
| GEs Synthesized Using FAMEs (at 40 μg/mL) Derived From: | |||||
|---|---|---|---|---|---|
| C. echinulata | U. isabellina | N. gaditana | Olive Oil | EPA Concentrate | |
| Inhibition Zone (mm) | |||||
| Escherichia coli (ATCC 25922) | 9.4 ± 0.3 | 0.0 | 10.0 ± 0.4 | 11.5 ± 0.4 | 11.1 ± 0.0 |
| Klebsiella pneumoniae (ATCC 700603) | 10.5 ± 0.5 | 0.0 | 11.1 ± 0.4 | 10.3 ± 0.6 | 14.2 ± 0.1 |
| Salmonella typhimurium (ATCC 14028) | 5.6 ± 0.5 | 5.8 ± 0.7 | 6.1 ± 0.3 | 10.1 ± 0.7 | 15.0 ± 0.0 |
| Pseudomonas aeruginosa (ATCC 15442) | 6.8 ± 0.8 | 5.5 ± 0.8 | 10.5 ± 0.5 | 10.1 ± 0.4 | 13.2 ± 0.0 |
| Bacillus subtilis (ATCC 6633) | 14.1 ± 0.5 | 0.0 | 9.0 ± 0.0 | 9.8 ± 0.7 | 17.0 ± 0.5 |
| MRSA Staphylococcus aureus (ATCC 4330) | 11.2 ± 0.1 | 5.8 ± 1.0 | 8.5 ± 0.5 | 10.1 ± 0.2 | 16.0 ± 0.1 |
| Staphylococcus aureus (ATCC 25923) | 12.3 ± 0.2 | 0.0 | 8.6 ± 0.3 | 12.0 ± 0.6 | 17.0 ± 0.2 |
| Candida albicans (ATCC 10231) | 14.3 ± 0.0 | 13.3 ± 0.0 | 14.0 ± 0.0 | 15.0 ± 0.3 | 20.0 ± 0.1 |
| Source of FAMEs | ||||||||
|---|---|---|---|---|---|---|---|---|
| Test organisms | C. echinulata | N. gaditana | Olive Oil | EPA Concentrate | ||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Klebsiella pneumoniae (ATCC 700603) | 25.0 ± 0.0 | 100.0 ± 0.0 | 50.0 ± 0.0 | 100.0 ± 0.0 | 66.7 ± 28.8 | 83.3 ± 28.8 | 33.3 ± 14.4 | 100.0 ± 0.0 |
| Pseudomonas aeruginosa (ATCC 15442) | 41.7 ± 14.4 | 100.0 ± 0.0 | 50.0 ± 0.0 | 100.0 ± 0.0 | 25.0 ± 0.0 | 83.3 ± 28.8 | 20.8 ± 7.2 | 41.7 ± 14.4 |
| Bacillus subtilis (ATCC 6633) | 16.7 ± 7.3 | 50.0 ± 0.0 | 20.8 ± 7.3 | 100.0 ± 0.0 | 33.3 ± 14.4 | 100.0 ± 0.0 | 10.4 ± 3.6 | 41.7 ± 14.4 |
| Staphylococcus aureus (ATCC 25923) | 12.5 ± 0.0 | 50.0 ± 0.0 | 50.0 ± 0.0 | 83.3 ± 28.8 | 83.3 ± 28.7 | 100.0 ± 0.0 | 8.3 ± 3.6 | 33.3 ± 14.4 |
| Source of GEs | LC50 (mg/L) | Lower Limit | Upper Limit | RR |
|---|---|---|---|---|
| Cunninghamellaechinulata | 0.54 | 0.47 | 0.64 | 1.00 |
| Umbelopsis isabellina | 39.62 | 33.75 | 46.10 | 73.23 |
| Nannochloropsis gaditana | 16.92 | 12.60 | 25.82 | 31.27 |
| Olive oil | 12.88 | 10.08 | 17.54 | 23.81 |
| EPA concentrate | 10.24 | 7.84 | 14.00 | 18.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Baz, H.A.; Elazzazy, A.M.; Saleh, T.S.; Dourou, M.; Mahyoub, J.A.; Baeshen, M.N.; Madian, H.R.; Aggelis, G. Enzymatic Synthesis of Glucose Fatty Acid Esters Using SCOs as Acyl Group-Donors and Their Biological Activities. Appl. Sci. 2021, 11, 2700. https://doi.org/10.3390/app11062700
El-Baz HA, Elazzazy AM, Saleh TS, Dourou M, Mahyoub JA, Baeshen MN, Madian HR, Aggelis G. Enzymatic Synthesis of Glucose Fatty Acid Esters Using SCOs as Acyl Group-Donors and Their Biological Activities. Applied Sciences. 2021; 11(6):2700. https://doi.org/10.3390/app11062700
Chicago/Turabian StyleEl-Baz, Hatim A., Ahmed M. Elazzazy, Tamer S. Saleh, Marianna Dourou, Jazem A. Mahyoub, Mohammed N. Baeshen, Hekmat R. Madian, and George Aggelis. 2021. "Enzymatic Synthesis of Glucose Fatty Acid Esters Using SCOs as Acyl Group-Donors and Their Biological Activities" Applied Sciences 11, no. 6: 2700. https://doi.org/10.3390/app11062700
APA StyleEl-Baz, H. A., Elazzazy, A. M., Saleh, T. S., Dourou, M., Mahyoub, J. A., Baeshen, M. N., Madian, H. R., & Aggelis, G. (2021). Enzymatic Synthesis of Glucose Fatty Acid Esters Using SCOs as Acyl Group-Donors and Their Biological Activities. Applied Sciences, 11(6), 2700. https://doi.org/10.3390/app11062700









