Adsorption of Cd2+ Ions from Aqueous Solution Using Biomasses of Theobroma cacao, Zea mays, Manihot esculenta, Dioscorea rotundata and Elaeis guineensis
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Preparation of Biomass
2.2. Characterization of Biomasses
2.3. Adsorption Tests
2.4. Kinetics and Adsorption Isotherms
2.5. Adsorption Thermodynamics
3. Results and Discussion
3.1. Characterization of Bioadsorbents
3.2. Adsorption Capacity of Bioadsorbents
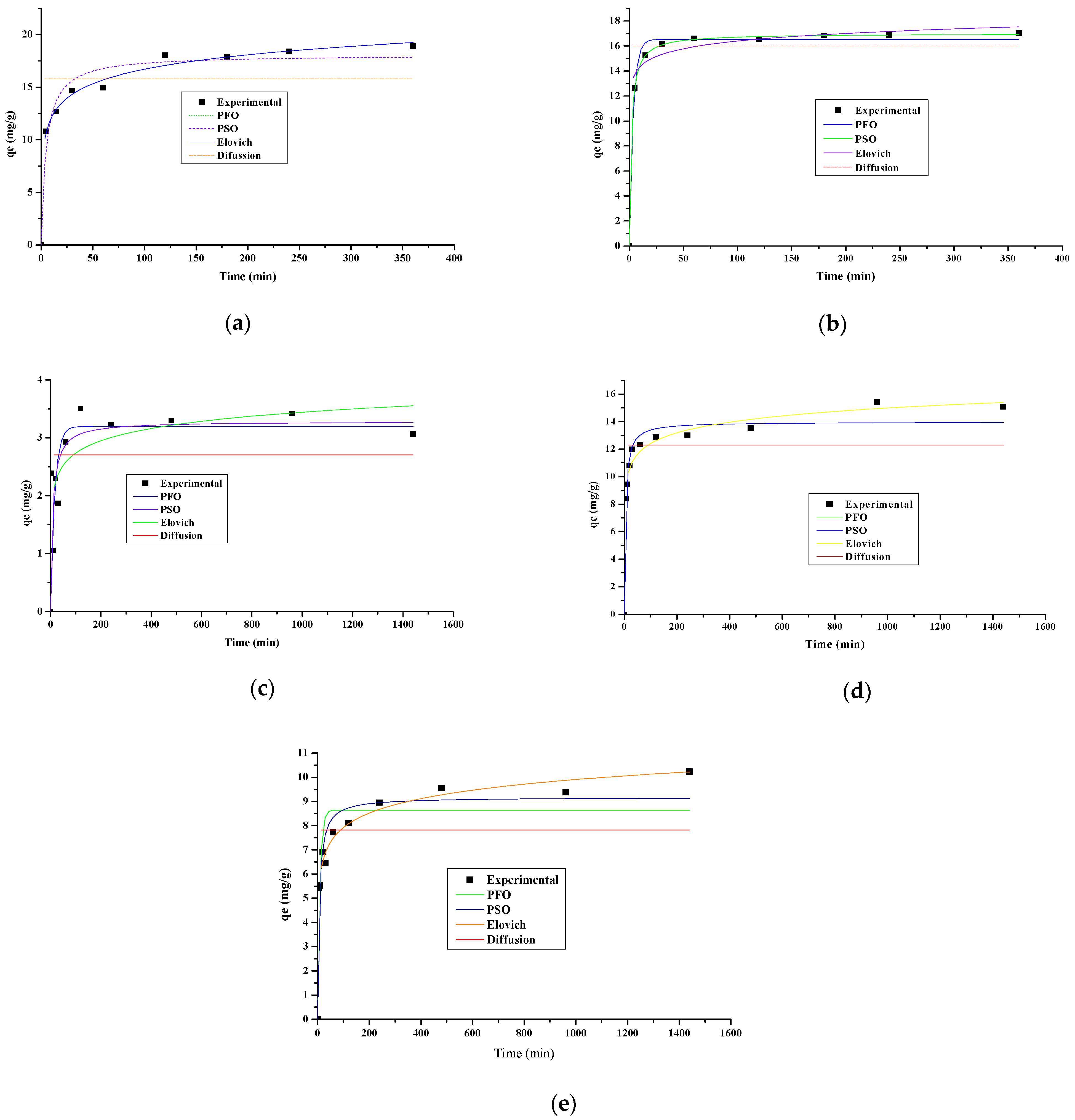
3.3. Adsorption Kinetics
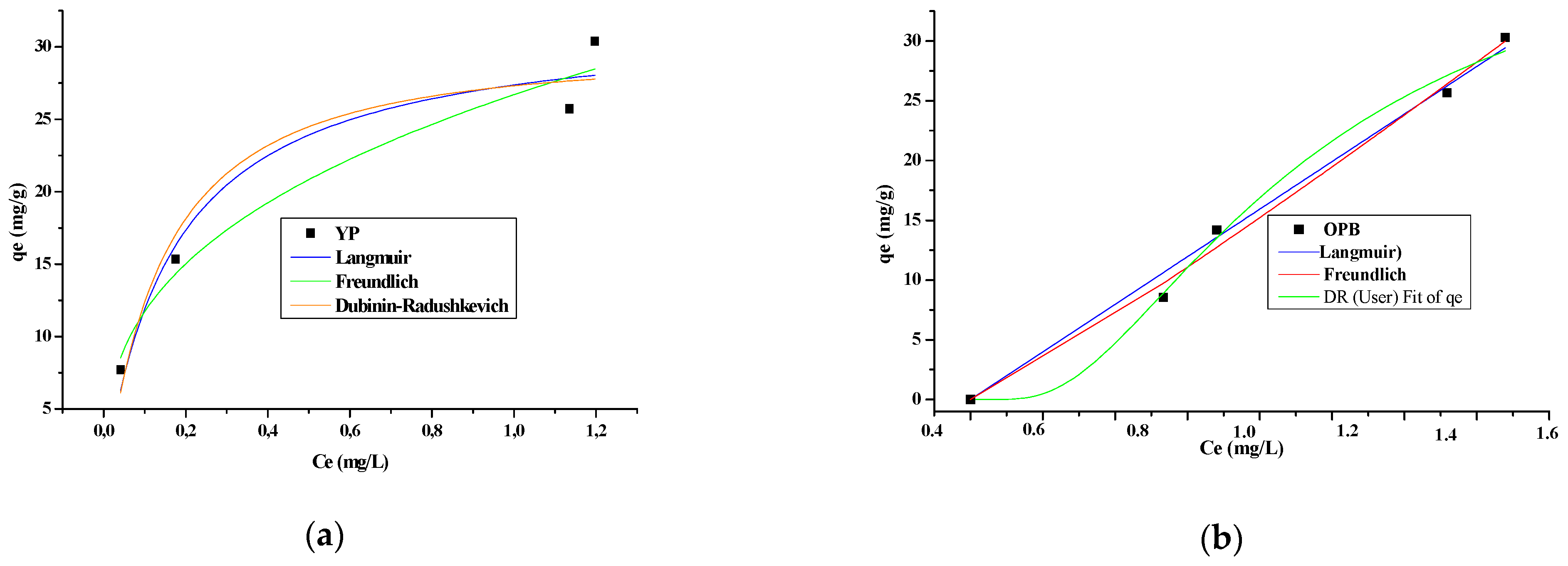
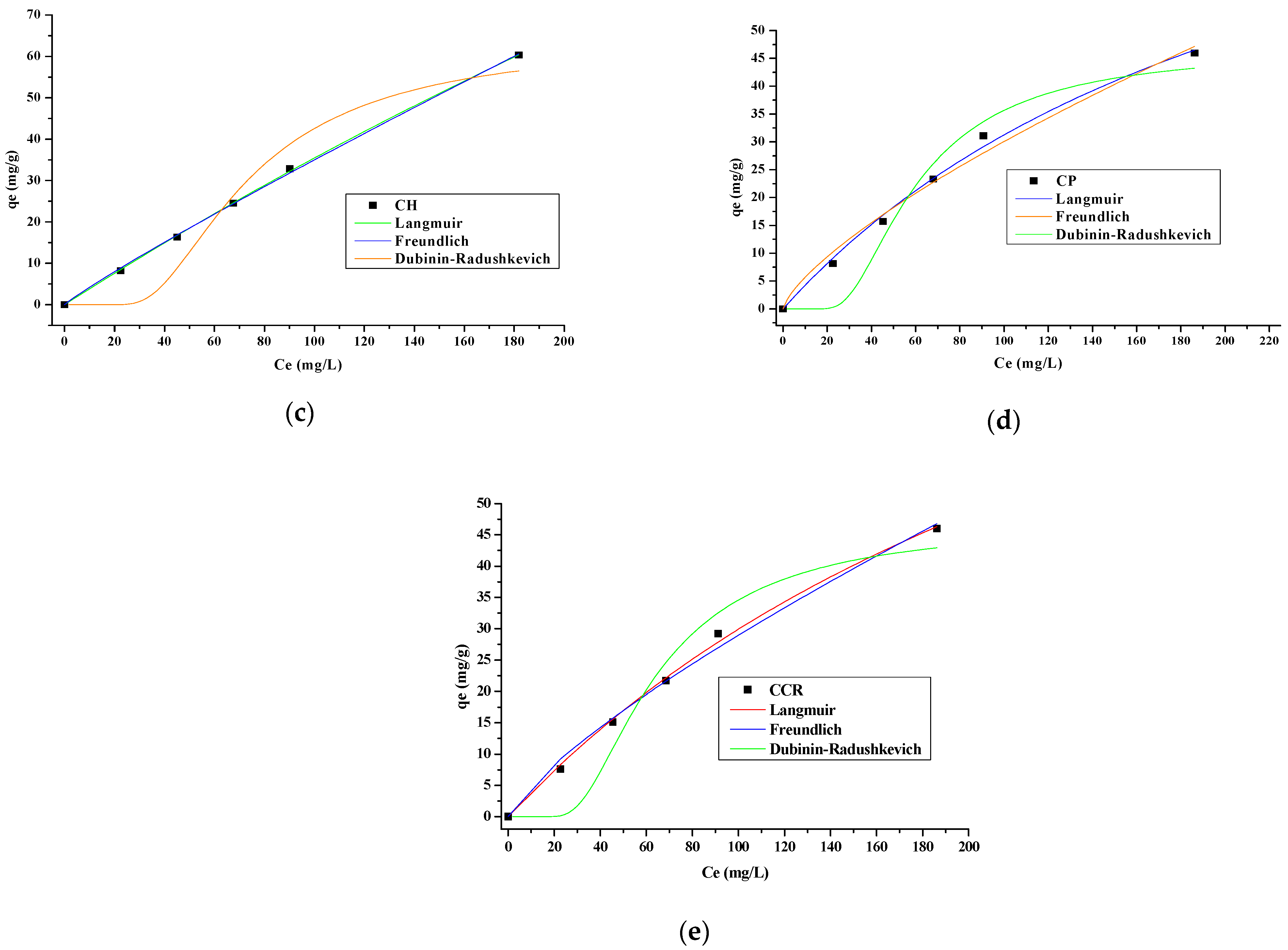
3.4. Adsorption Isotherms
3.5. Thermodynamic Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bazrafshan, E.; Sobhanikia, M.; Mostafapour, F.K.; Kamani, H.; Balarak, D. Chromium biosorption from aqueous environments by mucilaginous seeds of Cydonia oblonga: Kinetic and thermodynamic studies. Glob. Nest J. 2017, 19, 269–277. [Google Scholar]
- Agarwal, S.; Tyagi, I.; Gupta, V.K.; Dehghani, M.; Jaafari, J.; Balarak, D.; Asif, M. Rapid removal of noxious nickel (II) using novel γ-alumina nanoparticles and multiwalled carbon nanotubes: Kinetic and isotherm studies. J. Mol. Liq. 2016, 224, 618–623. [Google Scholar] [CrossRef]
- Yuan, W.; Cheng, J.; Huang, H.; Xiong, S.; Gao, J.; Zhang, J.; Feng, S. Optimization of cadmium biosorption by Shewanella putrefaciens using a Box-Behnken design. Ecotoxicol. Environ. Saf. 2019, 175, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Kumar, P.S.; Carolin, C.F.; Sivanesan, S. Enhanced Adsorption Capacity of Biomass through Ultrasonication for the Removal of Toxic Cadmium Ions from Aquatic System: Temperature Influence on Isotherms and Kinetics. J. Hazardous. Toxic. Radioact. Waste 2017, 21, 04017004. [Google Scholar] [CrossRef]
- Krika, F.; Azzouz, N.; Ncibi, M.C. Adsorptive removal of cadmium from aqueous solution by cork biomass: Equilibrium, dynamic and thermodynamic studies. Arab. J. Chem. 2016, 9, S1077–S1083. [Google Scholar] [CrossRef]
- Liu, X.; Xu, X.; Dong, X.; Park, J. Adsorption characteristics of cadmium ions from aqueous solution onto pine sawdust biomass and biochar. Bioresources 2019, 14, 4270–4283. [Google Scholar] [CrossRef]
- Basu, M.; Guha, A.K.; Ray, L. Adsorption Behavior of Cadmium on Husk of Lentil. Process. Saf. Environ. Prot. 2017, 106, 11–22. [Google Scholar] [CrossRef]
- Azimi, A.; Azari, A.; Rezakazemi, M.; Ansarpour, M. Removal of Heavy Metals from Industrial Wastewaters: A Review. ChemBioEng Rev. 2017, 4, 37–59. [Google Scholar] [CrossRef]
- Bădescu, I.S.; Bulgariu, D.; Ahmad, I.; Bulgariu, L. Valorisation possibilities of exhausted biosorbents loaded with metal ions—A review. J. Environ. Manag. 2018, 224, 288–297. [Google Scholar] [CrossRef]
- Bulgariu, D.; Bulgariu, L. Potential use of alkaline treated algae waste biomass as sustainable biosorbent for clean recovery of cadmium(II) from aqueous media: Batch and column studies. J. Clean. Prod. 2016, 112, 4525–4533. [Google Scholar] [CrossRef]
- Afroze, S.; Sen, T.K. A Review on Heavy Metal Ions and Dye Adsorption from Water by Agricultural Solid Waste Adsorbents. Water Air Soil Pollut. 2018, 229, 225. [Google Scholar] [CrossRef]
- Parlayici, Ş.; Pehlivan, E. Comparative study of Cr(VI) removal by bio-waste adsorbents: Equilibrium, kinetics, and thermodynamic. J. Anal. Sci. Technol. 2019, 10, 15. [Google Scholar] [CrossRef]
- Vera-Cabezas, L.; Bermejo, D.; Uguña Rosas, M.; Garcia Alvear, N.; Flores Zamora, M.; Brazales, D. Biosorción de Cd (II) y Pb (II) en columna de lecho fijo con cáscara de cacao. Afinidad 2018, 75, 16–22. [Google Scholar]
- Lawal, O.S.; Ayanda, O.S.; Rabiu, O.O.; Adebowale, K.O. Application of black walnut (Juglans nigra) husk for the removal of lead (II) ion from aqueous solution. Water Sci. Technol. 2017, 75, 2454–2464. [Google Scholar] [CrossRef]
- Asuquo, E.D.; Martin, A.D. Sorption of cadmium (II) ion from aqueous solution onto sweet potato (Ipomoea batatas L.) peel adsorbent: Characterisation, kinetic and isotherm studies. J. Environ. Chem. Eng. 2016, 4, 4207–4228. [Google Scholar] [CrossRef]
- Dai, Y.; Sun, Q.; Wang, W.; Lu, L.; Liu, M.; Li, J.; Yang, S.; Sun, Y.; Zhang, K.; Xu, J.; et al. Utilizations of agricultural waste as adsorbent for the removal of contaminants: A review. Chemosphere 2018, 211, 235–253. [Google Scholar] [CrossRef]
- Angulo-Padilla, J.; La Ossa, L.L.-D.; Gonzalez-Delgado, A.; Sanchez-Tuiran, E.; Ojeda-Delgado, K. Effect of alkaline pretreatment on biogas production from corn (Zea mays) crop residues biomass. Contemp. Eng. Sci. 2018, 11, 973–981. [Google Scholar] [CrossRef]
- Nitta, S.; Akagi, M.; Iwamoto, H. A porous chitosan nanofiber-poly(ethylene glycol) diacrylate hydrogel for metal adsorption from aqueous solutions. Polym. J. 2019, 51, 501–509. [Google Scholar] [CrossRef]
- Zhang, M.; Yin, Q.; Ji, X.; Wang, F.; Gao, X.; Zhao, M. High and fast adsorption of Cd(II) and Pb(II) ions from aqueous solutions by a waste biomass based hydrogel. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Çelebi, H.; Gök, G.; Gök, O. Adsorption capability of brewed tea waste in waters containing toxic lead(II), cadmium (II), nickel (II), and zinc(II) heavy metal ions. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Zhao, J.; Shen, X.-J.; Domene, X.; Alcañiz, J.-M.; Liao, X.; Palet, C. Comparison of biochars derived from different types of feedstock and their potential for heavy metal removal in multiple-metal solutions. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.-X.; Xu, J.; Deng, N.-F.; Dong, X.-W.; Tang, H.; Liang, Y.; Fan, X.-W.; Li, Y.-Z. A novel approach of utilization of the fungal conidia biomass to remove heavy metals from the aqueous solution through immobilization. Sci. Rep. 2016, 6, 36546. [Google Scholar] [CrossRef] [PubMed]
- Pennesi, C.; Amato, A.; Occhialini, S.; Critchley, A.T.; Totti, C.; Giorgini, E.; Conti, C.; Beolchini, F. Adsorption of indium by waste biomass of brown alga Ascophyllum nodosum. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Mosoarca, G.; Vancea, C.; Popa, S.; Gheju, M.; Boran, S. Syringa vulgaris leaves powder a novel low-cost adsorbent for methylene blue removal: Isotherms, kinetics, thermodynamic and optimization by Taguchi method. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Alammar, A.; Park, S.-H.; Ibrahim, I.; Arun, D.; Holtzl, T.; Dumée, L.F.; Lim, H.N.; Szekely, G. Architecting neonicotinoid-scavenging nanocomposite hydrogels for environmental remediation. Appl. Mater. Today 2020, 21, 100878. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, S.; Guan, Y. Excellent Adsorption–Desorption of Ammonium by a Poly(acrylic acid)-Grafted Chitosan and Biochar Composite for Sustainable Agricultural Development. ACS Sustain. Chem. Eng. 2020, 8, 16451–16462. [Google Scholar] [CrossRef]
- Tejada-Tovar, R.E.; Tejeda-Benítez, L.P.; Tejada-Tovar, C.; Villabona-Ortíz, A.; Granados-Conde, C. Aprovechamiento de ñame espino post-cosecha (Dioscorea rotundata P.) en la extracción de ácido láctico. Rev. Prospect. 2018, 16, 76–82. [Google Scholar] [CrossRef]
- Zhu, C.; Jiang, W.; Hu, J.; Sun, P.; Li, A.-M.; Zhang, Q. Polylactic Acid Nonwoven Fabric Surface Modified with Stereocomplex Crystals for Recyclable Use in Oil/Water Separation. ACS Appl. Polym. Mater. 2020, 2, 2509–2516. [Google Scholar] [CrossRef]
- Torres-Caban, R.; Vega-Olivencia, C.A.; Mina-Camilde, N. Adsorption of Ni2+ and Cd2+ from Water by Calcium Alginate/Spent Coffee Grounds Composite Beads. Appl. Sci. 2019, 9, 4531. [Google Scholar] [CrossRef]
- Suganthi, N.; Srinivasan, K. Phosphorylated tamarind nut carbon for the removal of cadmium ions from aqueous solutions. Indian J. Eng. Mater. Sci. 2010, 17, 382–388. [Google Scholar]
- Jia, Y.; Shi, S.; Liu, J.; Su, S.; Liang, Q.; Zeng, X.; Li, T. Study of the Effect of Pyrolysis Temperature on the Cd2+ Adsorption Characteristics of Biochar. Appl. Sci. 2018, 8, 1019. [Google Scholar] [CrossRef]
- Villabona-Ortiz, Á.; Tejada-Tovar, C.; González-Delgado, Á.D.; Herrera-Barros, A.; Silvera-Charris, R. Removal of Cr(VI) ions from aqueous solution using orange peel residual biomass: Thermodynamic and sorption-desorption study. Desalination Water Treat. 2020, 203, 309–314. [Google Scholar] [CrossRef]
- Villabona-Ortiz, Á.; Tejada-Tovar, C.; Gonzalez-Delgado, Á.; Herrera-Barros, A.; Cantillo-Arroyo, G. Immobilization of Lead and Nickel Ions from Polluted Yam Peels Biomass Using Cement-Based Solidification/Stabilization Technique. Int. J. Chem. Eng. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Tejada-Tovar, C.; Gallo-Mercado, J.; Moscote, J.; Villabona-Ortíz, A.; Acevedo-Correra, D. Adsorción competitiva de plomo y niquel sobre cáscara de ñame y bagazo de palma en sistema continuo Competitive adsorption of lead and nickel ont yam husk and palm bagasse in continuous system Adsorção competitiva de líquido e níquel em yam husk e palm ba. Rev. Biotecnol. en el Sect. Agropecu. y Agroind. 2018, 16, 52–61. [Google Scholar] [CrossRef]
- Moncaleano-Niño, Á.M. Biomarcadores Bioquímicos de Exposición en Bivalvos Como Herramientas Para la Evaluación de la Salud Ambiental Frente a la Contaminación de Plaguicidas y Metales Pesados, en Ecosistemas Marino-Costeros del Caribe Colombiano. Thesis; Published by Jorge Tadeo Lozano University. 2018. Available online: https://expeditiorepositorio.utadeo.edu.co/handle/20.500.12010/8170 (accessed on 18 November 2020).
- Demirel, M.; Kayan, B. Application of response surface methodology and central composite design for the optimization of textile dye degradation by wet air oxidation. Int. J. Ind. Chem. 2012, 3, 24. [Google Scholar] [CrossRef]
- Basu, M.; Guha, A.K.; Ray, L. Adsorption of Lead on Cucumber Peel. J. Clean. Prod. 2017, 151, 603–615. [Google Scholar] [CrossRef]
- Rodiguez, M.H.; Yperman, J.; Carleer, R.; Maggen, J.; Dadi, D.; Gryglewicz, G.; Van der Bruggen, B.; Hernández, J.F.; Calvis, A.O. Adsorption of Ni(II) on spent coffee and coffee husk based activated carbon. J. Environ. Chem. Eng. 2018, 6, 1161–1170. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Chao, H.-P. Thermodynamic parameters of cadmium adsorption onto orange peel calculated from various methods: A comparison study. J. Environ. Chem. Eng. 2016, 4, 2671–2682. [Google Scholar] [CrossRef]
- Warui, S.; Wachira, J.; Kawira, M.; Murithi, G.; Mwiti, J. Characterization of composite material from the copolymerized polyphenolic matrix with treated cassava peels starch. Heliyon 2020, 6, e04574. [Google Scholar]
- Deng, Y.; Huang, S.; Laird, D.A.; Wang, X.; Dong, C. Quantitative mechanisms of cadmium adsorption on rice straw- and swine manure-derived biochars. Environ. Sci. Pollut. Res. 2018, 25, 32418–32432. [Google Scholar] [CrossRef]
- Xia, L.; Xu, X.; Zhu, W.; Huang, Q.; Chen, W. A Comparative Study on the Biosorption of Cd2+ onto Paecilomyces lilacinus XLA and Mucoromycote sp. XLC. Int. J. Mol. Sci. 2015, 16, 15670–15687. [Google Scholar] [CrossRef] [PubMed]
- Herrera, A.; Tejada-Tovar, C.; González-Delgado, Á.D. Enhancement of Cadmium Adsorption Capacities of Agricultural Residues and Industrial Fruit Byproducts by the Incorporation of Al2O3 Nanoparticles. ACS Omega 2020, 5, 23645–23653. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Barros, A.; Tejada-Tovar, C.; Villabona-Ortíz, A.; González-Delgado, A.; Benitez-Monroy, J. Cd (II) and Ni (II) uptake by novel biosorbent prepared from oil palm residual biomass and Al2O3 nanoparticles. Sustain. Chem. Pharm. 2020, 15, 100216. [Google Scholar] [CrossRef]
- Lara, J.; Tejada, C.; Villabona, A.; Arrieta, A. Adsorción de plomo y cadmio en sistema continuo de lecho fijo sobre residuos de cacao. Revista ION 2016, 29, 111–122. [Google Scholar] [CrossRef]
- Rinaldi, R.; Yasdi, Y.; Hutagalung, W.L.C. Removal of Ni (II) and Cu (II) ions from aqueous solution using rambutan fruit peels (Nephelium lappaceum L.) as adsorbent. Fourth Huntsville Gamma-Ray Burst Symp. 2018, 2026, 020098. [Google Scholar] [CrossRef]
- Huang, X.; Chen, T.; Zou, X.; Zhu, M.; Chen, D.; Pan, M. The Adsorption of Cd(II) on Manganese Oxide Investigated by Batch and Modeling Techniques. Int. J. Environ. Res. Public Health 2017, 14, 1145. [Google Scholar] [CrossRef]
- Mahdi, Z.; Yu, Q.J.; El Hanandeh, A. Investigation of the kinetics and mechanisms of nickel and copper ions adsorption from aqueous solutions by date seed derived biochar. J. Environ. Chem. Eng. 2018, 6, 1171–1181. [Google Scholar] [CrossRef]
- Tovar, C.T.; Ortiz, Á.V.; Jaraba, L.E.G. Kinetics of Adsorption in Mercury Removal Using Cassava (Manhiot esculenta) and Lemon (Citrus limonum) Wastes Modified with Citric Acid. Ing. Univ. 2015, 19, 283–298. [Google Scholar]
- Tejada, C.; Villabona, A.; Ruiz, E. Adsorción de NI (ii) por cáscaras de ñame (Discorea Rotundata) y Bazago de Palma (Elaeis guineensis) Pretratadas. Luna Azul 2016, 42, 30–43. [Google Scholar]
- Núñez-Zarur, J.; Tejada-Tovar, C.; Villabona-Ortíz, A.; Acevedo, D.; Tejada-Tovar, R. Thermodynamics, Kinetics and Equilibrium Adsorption of Cr (VI) and Hg (II) in Aqueous Solution on corn cob (Zea mays). Int. J. ChemTech Res. 2018, 11, 265–280. [Google Scholar]
- Tejada-Tovar, C.; Villabona-Ortiz, A.; Jiménez-Villadiego, M. Remoción de Cromo hexavalente sobre residuos de cacao pretratados químicamente. Rev. U.D.C.A Act. Div. Cient. 2017, 20, 139–147. [Google Scholar] [CrossRef][Green Version]
- Naseem, K.; Huma, R.; Shahbaz, A.; Jamal, J.; Rehman, M.Z.U.; Sharif, A.; Ahmed, E.; Begum, R.; Irfan, A.; Al-Sehemi, A.G.; et al. Extraction of Heavy Metals from Aqueous Medium by Husk Biomass: Adsorption Isotherm, Kinetic and Thermodynamic study. Z. Phys. Chem. 2018, 233, 201–223. [Google Scholar] [CrossRef]
- Tejada-Tovar, C.N.; Villabona-Ortíz, A.; Toro, R.O. Cr(VI) biosorption: Effect of temperature, particle size and bed height. Rev. Fac. Ing. Univ. Antioq. 2020, 96, 78–86. [Google Scholar] [CrossRef]
- Cherik, D.; Louhab, K. A kinetics, isotherms, and thermodynamic study of diclofenac adsorption using activated carbon prepared from olive stones. J. Dispers. Sci. Technol. 2017, 39, 814–825. [Google Scholar] [CrossRef]
- Johari, K.; Saman, N.; Song, S.T.; Chin, C.S.; Kong, H.; Mat, H. Adsorption enhancement of elemental mercury by various surface modified coconut husk as eco-friendly low-cost adsorbents. Int. Biodeterior. Biodegrad. 2016, 109, 45–52. [Google Scholar] [CrossRef]
- Amro, A.N.; Abhary, M.K.; Shaikh, M.M.; Ali, S. Removal of Lead and Cadmium Ions from Aqueous Solution by Adsorption on a Low-Cost Phragmites Biomass. Processes 2019, 7, 406. [Google Scholar] [CrossRef]
- Al-Shannag, M.; Al-Qodah, Z.; Nawasreh, M.; Al-Hamamreh, Z.; Bani-Melhem, K.; Alkasrawi, M. On the performance of Ballota undulata biomass for the removal of cadmium(II) ions from water. Desalination Water Treat. 2017, 67, 223–230. [Google Scholar] [CrossRef]
- Peng, S.-H.; Wang, R.; Yang, L.-Z.; He, L.; He, X.; Liu, X. Biosorption of copper, zinc, cadmium and chromium ions from aqueous solution by natural foxtail millet shell. Ecotoxicol. Environ. Saf. 2018, 165, 61–69. [Google Scholar] [CrossRef]
- Labied, R.; Benturki, O.; Hamitouche, A.Y.E.; Donnot, A. Adsorption of hexavalent chromium by activated carbon obtained from a waste lignocellulosic material (Ziziphus jujuba cores): Kinetic, equilibrium, and thermodynamic study. Adsorpt. Sci. Technol. 2018, 36, 1066–1099. [Google Scholar] [CrossRef]
- Tejada-Tovar, C.N.; Villabona-Ortiz, Á.; Alvarez-Bajaire, G.; Attin-Torres, L.; Granados-Conde, C. Influencia de la altura del lecho sobre el comportamiento dinámico de columna de lecho fijo en la biosorción de mercurio. TecnoLógicas 2017, 20, 71–81. [Google Scholar] [CrossRef][Green Version]
- Tejada-Tovar, C.; Montiel-Rodríguez, Z.P.; Nieto-Muñoz, Y. Estudio de la Adsorción Competitiva de Pb+2 y Ni+2 Usando Cáscara de yuca (Manihot esculenta) Modificada Con Ácido Cítrico; Instituto Tecnológico Metropolitano: Medellín, Colombia, 2014. [Google Scholar]
- Tejada-Tovar, C.; Villabona-Ortíz, A.; González-Delgado, Á.D.; Granados-Conde, C.; Jiménez-Villadiego, M. Kinetics of Mercury and Nickel adsorption using chemically pretrated cocoa (Theobroma cacao) husk. Trans. ASABE 2019, 62, 461–466. [Google Scholar] [CrossRef]
- Balarak, D.; Azarpira, H.; Kord, F. Thermodynamics of removal of cadmium by adsorption on Barleyhusk biomass. Der Pharma Chem. 2016, 8, 243–247. [Google Scholar]
- Azarpira, H.; Mahdavi, Y.; Blarak, D. Removal of Cd(II) by adsorption on agricultural waste biomass. Der Pharma Chem. 2016, 8, 61–67. [Google Scholar]
- Kumar, P.S.; Ramakrishnan, K.; Kirupha, S.D.; Sivanesan, S. Thermodynamic and kinetic studies of cadmium adsorption from aqueous solution onto rice husk. Braz. J. Chem. Eng. 2010, 27, 347–355. [Google Scholar] [CrossRef]
- Sellaoui, L.; Guedidi, H.; Knani, S.; Reinert, L.; Duclaux, L.; Ben Lamine, A. Application of statistical physics formalism to the modeling of adsorption isotherms of ibuprofen on activated carbon. Fluid Phase Equilibria 2015, 387, 103–110. [Google Scholar] [CrossRef]
- Bibaj, E.; Lysigaki, K.; Nolan, J.W.; Seyedsalehi, M.; Deliyanni, E.A.; Mitropoulos, A.C.; Kyzas, G.Z. Activated carbons from banana peels for the removal of nickel ions. Int. J. Environ. Sci. Technol. 2018, 16, 667–680. [Google Scholar] [CrossRef]
- Ibisi, N.; Asoluka, C. Use of agro-waste (Musa paradisiaca peels) as a sustainable biosorbent for toxic metal ions removal from contaminated water. Chem. Int. 2018, 4, 52–59. [Google Scholar]
- Villabona-Ortíz, A.; De Cartagena, U.; Tejada-Tovar, C.N.; Ortega-Toro, R. Modelling of the adsorption kinetics of chromium (VI) using waste biomaterials. Rev. Mex. Ing. Química 2019, 19, 401–408. [Google Scholar] [CrossRef]
- Obike, A.; Igwe, J.; Emeruwa, C.; Uwakwe, K. Equilibrium and kinetic studies of Cu (II), Cd (II), Pb (II) and Fe (II) adsorption from aqueous solution using cocoa (Theobroma cacao) pod husk. J. Appl. Sci. Environ. Manag. 2018, 22, 182. [Google Scholar] [CrossRef]
- Castro, L.; Blázquez, M.L.; González, F.; Muñoz, J.A.; Ballester, A. Heavy metal adsorption using biogenic iron compounds. Hydrometallurgy 2018, 179, 44–51. [Google Scholar] [CrossRef]
- Madala, S.; Nadavala, S.K.; Vudagandla, S.; Boddu, V.M.; Abburi, K. Equilibrium, kinetics and thermodynamics of Cadmium (II) biosorption on to composite chitosan biosorbent. Arab. J. Chem. 2017, 10, S1883–S1893. [Google Scholar] [CrossRef]
- El Ass, K. Adsorption of cadmium and copper onto natural clay: Isotherm, kinetic and thermodynamic studies. Glob. Nest J. 2018, 20, 198–207. [Google Scholar]
- Li, X.; Wang, C.; Tian, J.; Liu, J.; Chen, G. Comparison of adsorption properties for cadmium removal from aqueous solution by Enteromorpha prolifera biochar modified with different chemical reagents. Environ. Res. 2020, 186, 109502. [Google Scholar] [CrossRef] [PubMed]
- Leizou, E.K.; Ashraf, M.A.; Chowdhury, J.A.; Rashid, H. Adsorption studies of Pb2+ and Mn2+ ions on low-cost ddsorbent: Unripe Plantain (Musa Paradisiaca) peel biomass. Acta Chem. Malays. 2018, 2, 11–15. [Google Scholar]
- Batool, F.; Akbar, J.; Iqbal, S.; Noreen, S.; Bukhari, S.N.A. Study of Isothermal, Kinetic, and Thermodynamic Parameters for Adsorption of Cadmium: An Overview of Linear and Nonlinear Approach and Error Analysis. Bioinorg. Chem. Appl. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Meneguin, J.G.; Moisés, M.P.; Karchiyappan, T.; Faria, S.H.B.; Gimenes, M.L.; de Barros, M.A.S.; Venkatachalam, S. Preparation and characterization of calcium treated bentonite clay and its application for the removal of lead and cadmium ions: Adsorption and thermodynamic modeling. Process. Saf. Environ. Prot. 2017, 111, 244–252. [Google Scholar] [CrossRef]
- Wang, H.; Chu, Y.; Fang, C.; Huang, F.; Song, Y.; Xue, X. Sorption of tetracycline on biochar derived from rice straw under different temperatures. PLoS ONE 2017, 12, e0182776. [Google Scholar] [CrossRef]
- Hadavifar, M.; Bahramifar, N.; Younesi, H.; Li, Q. Adsorption of mercury ions from synthetic and real wastewater aqueous solution by functionalized multi-walled carbon nanotube with both amino and thiolated groups. Chem. Eng. J. 2014, 237, 217–228. [Google Scholar] [CrossRef]
- Haroon, H.; Ashfaq, T.; Gardazi, S.M.H.; Sherazi, T.A.; Ali, M.; Rashid, N.; Bilal, M. Equilibrium kinetic and thermodynamic studies of Cr(VI) adsorption onto a novel adsorbent of Eucalyptus camaldulensis waste: Batch and column reactors. Korean J. Chem. Eng. 2016, 33, 2898–2907. [Google Scholar] [CrossRef]
- Xin, Y.; Li, C.; Liu, J.; Liu, J.; Liu, Y.; He, W.; Gao, Y. Adsorption of heavy metal with modified eggshell membrane and the in situ synthesis of Cu–Ag/modified eggshell membrane composites. R. Soc. Open Sci. 2018, 5, 180532. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’Ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Bereza-Malcolm, L.; Aracic, S.; Kannan, R.; Mann, G.; Franks, A.E. Functional characterization of Gram-negative bacteria from different genera as multiplex cadmium biosensors. Biosens. Bioelectron. 2017, 94, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Pan, H.; Gonzalez-Vila, A.; Guo, T.; Gillan, D.C.; Wattiez, R.; Caucheteur, C. Selective detection of cadmium ions using plasmonic optical fiber gratings functionalized with bacteria. Opt. Express 2020, 28, 19740–19749. [Google Scholar] [CrossRef] [PubMed]
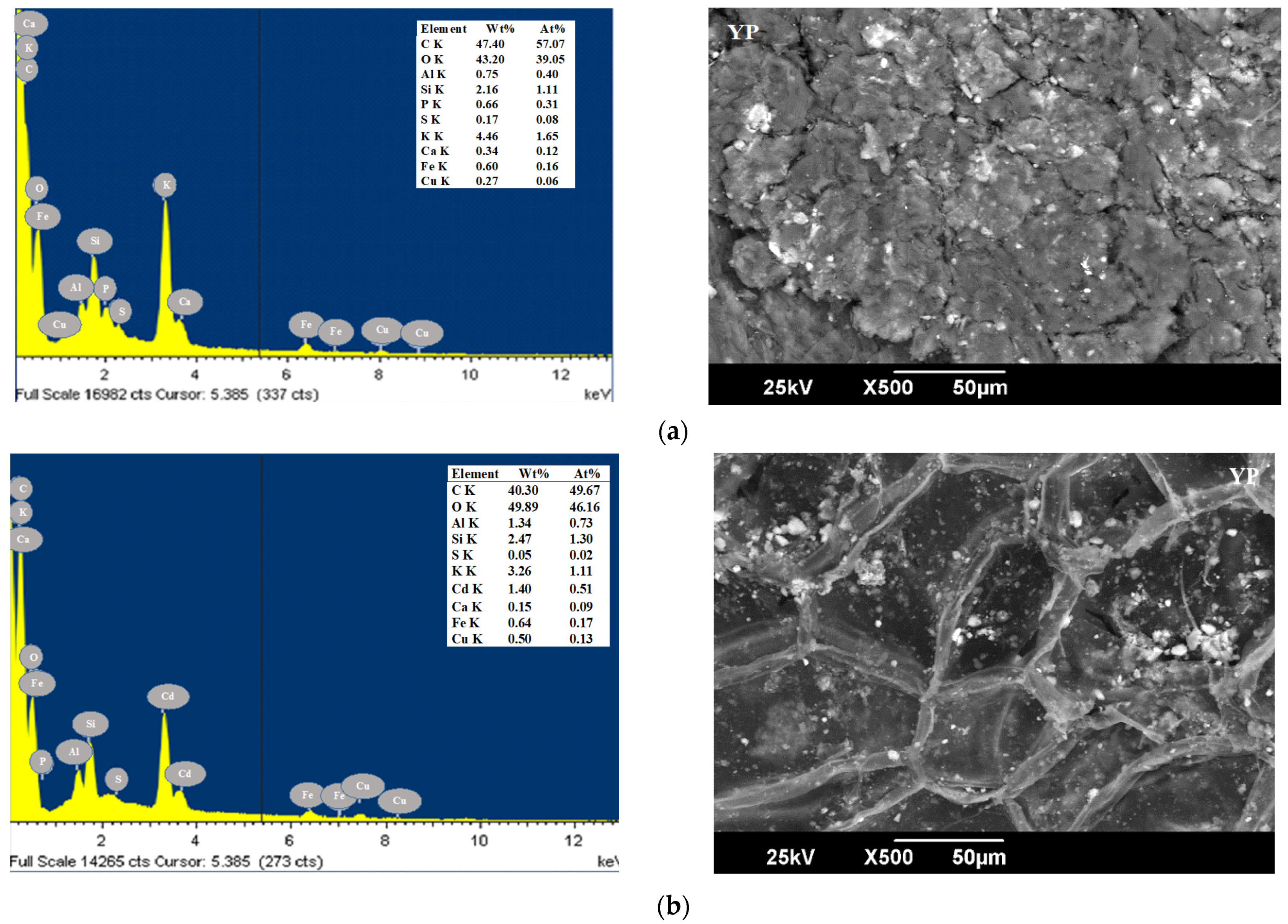
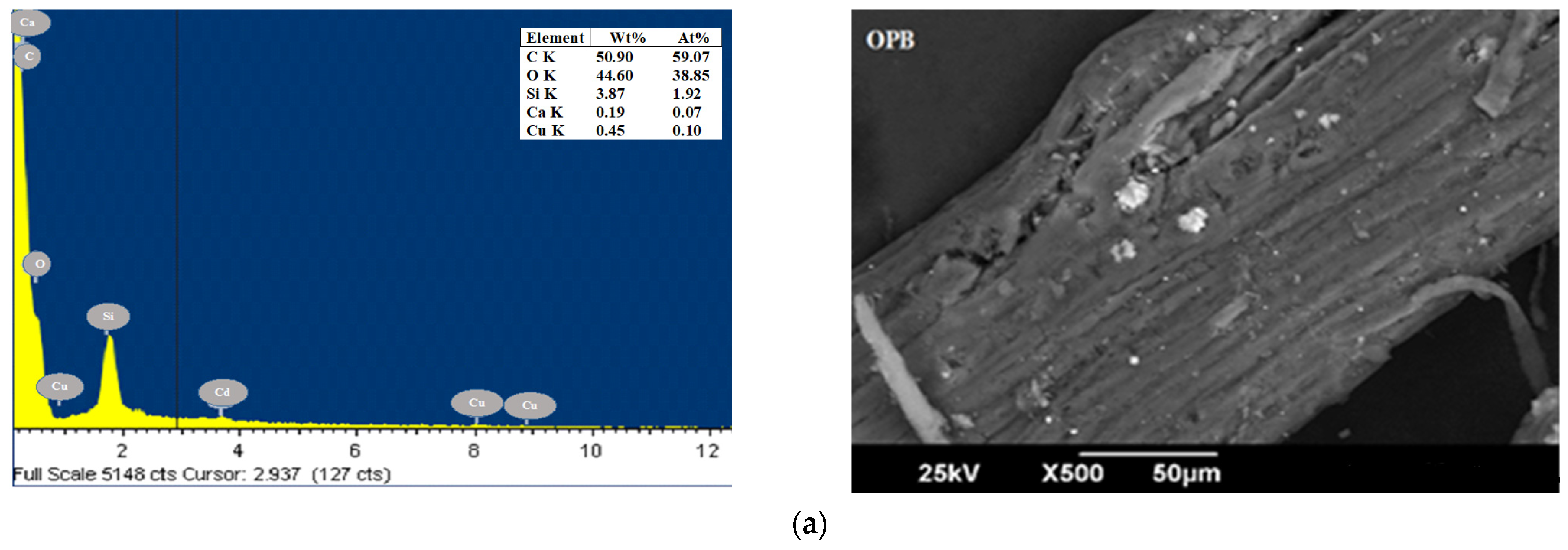
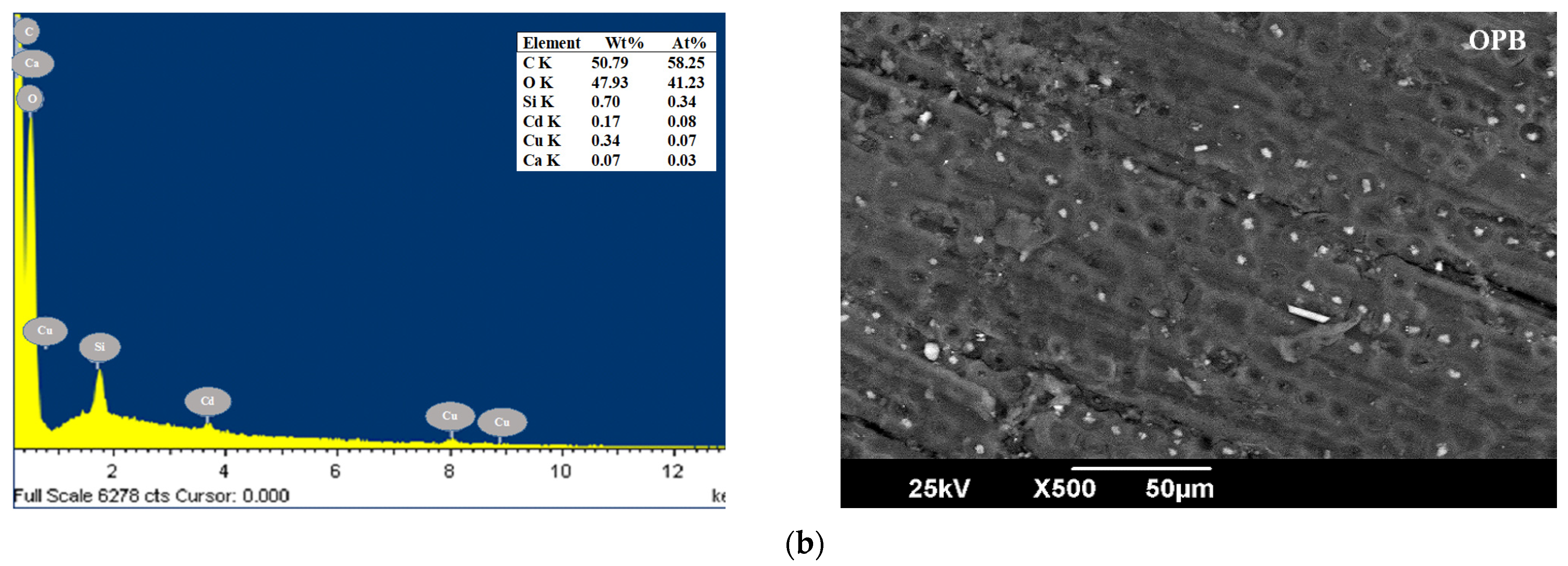
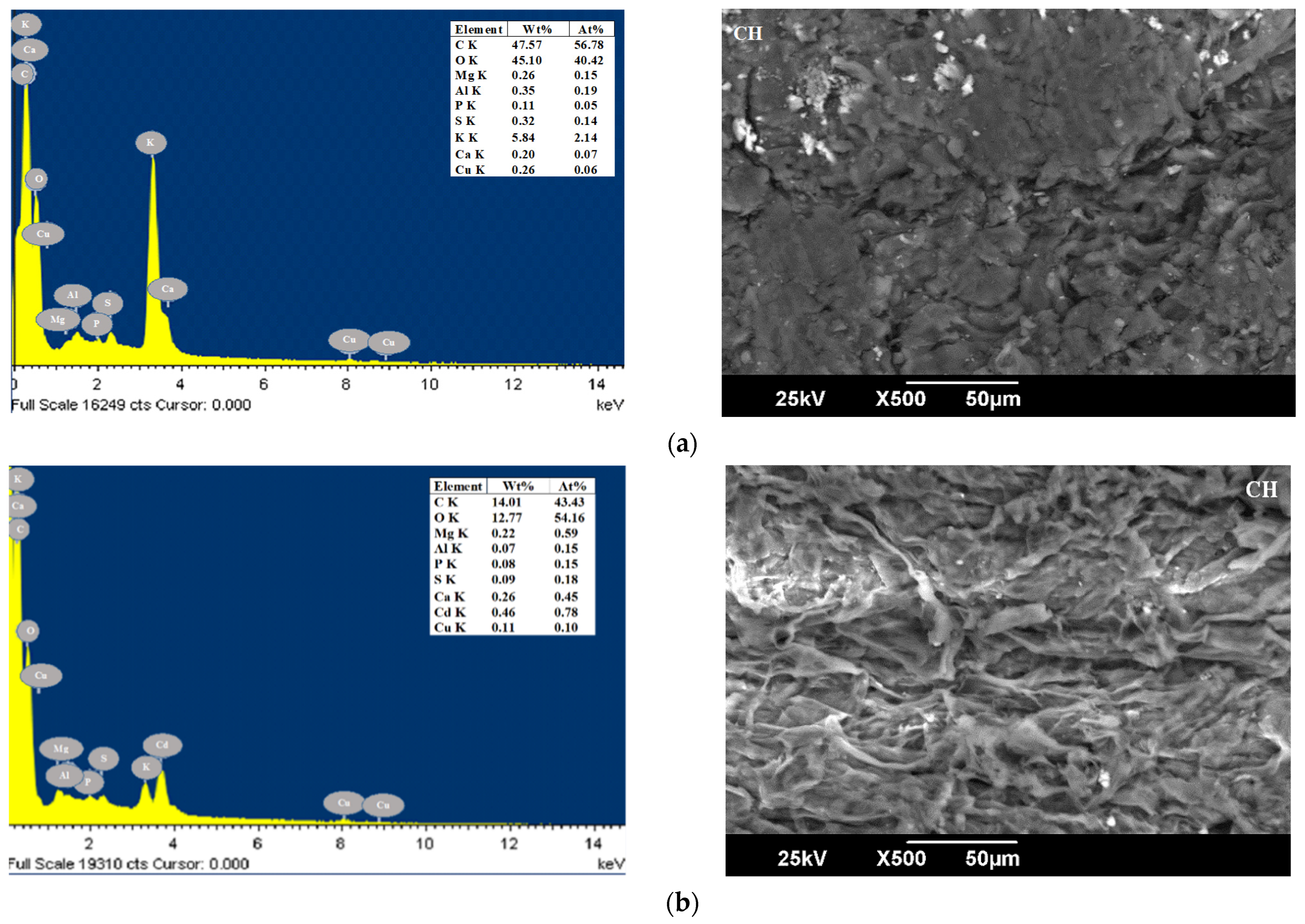

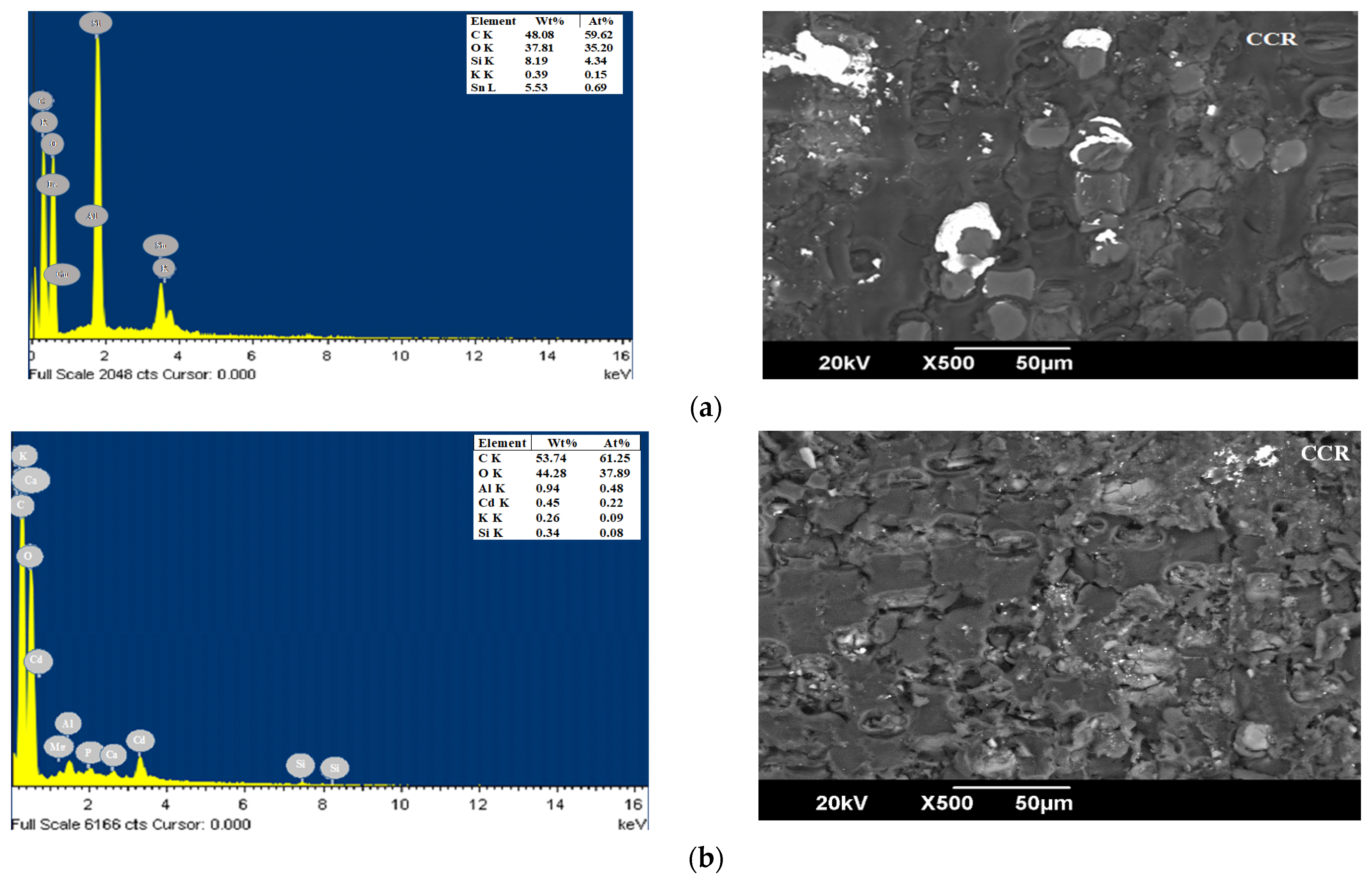
| Parameters (%) | CP [49] | YP [50] | OPB [50] | CCR [51] | CH [52] | Method |
|---|---|---|---|---|---|---|
| Cellulose | 18.47 | 13.08 | 19.90 | 13.08 | 19.82 | Digestion-thermogravimetry |
| Hemicellulose | 6.01 | 6.47 | 7.00 | 6.47 | 9.45 | Digestion-thermogravimetry |
| Lignin | 2.20 | 27.73 | 17.11 | 6.51 | 12.66 | Photocolorimetry |
| Pectin | 2.84 | 10.98 | 4.88 | 7.98 | 9.54 | Acid digestion-thermogravimetry |
| Carbon | 39.96 | 48.14 | 38.27 | 39.89 | 50.35 | AOAC 949.14 |
| Hydrogen | 3.98 | 5.44 | 4.71 | 3.28 | 5.08 | AOAC 949.14 |
| Nitrogen | 0.26 | 0.18 | 0.18 | 0.46 | 1.28 | AOAC 949.13-Kjeldahl |
| Temperature (°C) | Particle Size (mm) | Adsorbent Dose (g) | Adsorption Capacity (mg/g) | ||||
|---|---|---|---|---|---|---|---|
| YP | OPB | CH | CP | CCR | |||
| 40 | 1.00 | 0.15 | 57.46 | 42.10 | 33.31 | 29.88 | 23.89 |
| 70 | 1.00 | 0.15 | 58.33 | 47.50 | 33.16 | 28.07 | 22.20 |
| 80 | 0.50 | 0.33 | 18.26 | 23.12 | 13.15 | 14.24 | 11.49 |
| 29.8 | 0.50 | 0.33 | 22.33 | 13.93 | 14.33 | 13.77 | 11.83 |
| 55 | 0.50 | 0.03 | 123.66 | 197.95 | 160.83 | 76.86 | 105.40 |
| 55 | 1.22 | 0.33 | 29.80 | 9.26 | 13.00 | 12.57 | 11.60 |
| 70 | 1.00 | 0.50 | 10.71 | 19.93 | 9.95 | 8.16 | 8.05 |
| 55 | 0.14 | 0.33 | 14.57 | 25.71 | 15.29 | 12.33 | 12.25 |
| 70 | 0.36 | 0.15 | 56.21 | 47.76 | 33.08 | 26.02 | 21.66 |
| 40 | 0.36 | 0.15 | 40.07 | 29.54 | 33.31 | 29.98 | 24.55 |
| 40 | 0.36 | 0.50 | 18.92 | 14.93 | 9.99 | 8.99 | 8.07 |
| 55 | 0.50 | 0.62 | 15.12 | 14.08 | 8.04 | 7.03 | 7.14 |
| 55 | 0.50 | 0.33 | 22.34 | 24.99 | 15.42 | 12.03 | 12.29 |
| 40 | 1.00 | 0.50 | 18.92 | 18.64 | 9.99 | 8.98 | 8.59 |
| 70 | 0.36 | 0.50 | 18.64 | 19.00 | 9.95 | 8.60 | 8.24 |
| Adsorbent | Adsorption Capacity (mg/g) | Conditions | Reference |
|---|---|---|---|
| Barley husk biomass | 41.25 | pH = 7, dose = 3 g/L and temp = 30 ± 2 °C | Balarak et al. [64] |
| Canola biomass | 25.16 | pH = 7, Co = 100 mg/L | Azarpira et al. [65] |
| Oil palm residual biomass | 17.40 | pH = 6, dose = 0.5 g, temp = 28 °C | Herrera-Barros et al. [44] |
| Cork biomass | 14.77 | pH = 6, temp = 40° | Krika et al. [5] |
| Rice husk | 21.28 | pH = 6, temp = 25 °C, Co = 100 mg/L | Kumar et al. [66] |
| Kinetic Model | Parameters | Values | ||||
|---|---|---|---|---|---|---|
| YP | OPB | CH | CP | CCR | ||
| PFO | qe1 | 17.00 | 16.52 | 3.51 | 13.31 | 10.09 |
| k1 | 0.14 | 0.28 | 0.04 | 0.14 | 0.06 | |
| SS | 10.43 | 0.86 | 1.92 | 9.17 | 5.08 | |
| R2 | 0.90 | 0.99 | 0.76 | 0.90 | 0.67 | |
| PSO | k2 | 1.2 × 103 | 2.8 × 103 | 3.58 | 13.98 | 10.58 |
| qe2 | 18.087 | 16.99 | 4.27 | 617.52 | 136.38 | |
| SS | 4.70 | 0.06 | 1.92 | 9.17 | 5.07 | |
| R2 | 0.97 | 0.99 | 0.80 | 0.96 | 0.85 | |
| Elovich | β | 0.51 | 1.13 | 2.94 | 0.89 | 0.93 |
| α | 90.81 | 1.2 × 104 | 16.69 | 702.92 | 36.71 | |
| SS | 1.27 | 1.74 | 2.82 | 10.52 | 6.89 | |
| R2 | 0.99 | 0.99 | 0.86 | 0.98 | 0.75 | |
| Intraparticle Diffusion | k3 | 15.79 | 15.98 | 2.70 | 12.28 | 7.82 |
| SS | 5.04 | 3.52 | 6.58 | 2.03 | 4.25 | |
| R2 | 0.78 | 0.94 | 0.54 | 0.75 | 0.68 | |
| Isothermal Model | Parameters | YP | OBP | CH | CP | CCR |
|---|---|---|---|---|---|---|
| Langmuir | qmax | 32.02 | 178.18 | 426.28 | 107.01 | 126.93 |
| B | 5.90 | 5.38 | 0.44 | 0.19 | 0.08 | |
| R2 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | |
| SS | 0.36 | 0.36 | 69.93 | 18.24 | 6.02 | |
| Freundlich | Kf | 26.70 | 19.47 | 20.62 | 13.72 | 6.28 |
| 1/n | 0.36 | 2.04 | 0.37 | 0.30 | 0.42 | |
| B | 2.79 | 0.49 | 2.70 | 3.33 | 2.38 | |
| R2 | 0.98 | 0.98 | 0.99 | 0.98 | 0.99 | |
| SS | 4.91 | 4.91 | 1.68 | 6.68 | 2.31 | |
| Dubinin-Radushkevich | qDR | 29.37 | 42.36 | 63.93 | 46.77 | 46.89 |
| KDR | 2.0 × 10−8 | 1.9 × 10−7 | 5.5 × 10−4 | 3.7 × 10−4 | 4.1 × 10−4 | |
| E | 4.77 | 1.63 | 3.01 | 3.69 | 3.48 | |
| SS | 2.96 | 2.83 | 12.45 | 11.56 | 13.78 | |
| R2 | 0.92 | 0.99 | 0.90 | 0.92 | 0.91 |
| Biomaterial | Temperature (K) | ΔH° (KJ*mol−1*K−1) | ΔS° (KJ/mol) | ΔG° (KJ/mol) |
|---|---|---|---|---|
| YP | 302.92 | 4.34 | −2.3 × 10−2 | 5.01 |
| 328.15 | 5.59 | |||
| 353.37 | 6.16 | |||
| OPB | 302.92 | −11.29 | −7.0 × 10−2 | −9.20 |
| 328.15 | −7.43 | |||
| 353.37 | −5.66 | |||
| CH | 302.92 | −14.73 | 5.4 × 10−2 | 1.84 |
| 328.15 | 3.22 | |||
| 353.37 | 4.60 | |||
| CP | 302.92 | 5.72 | 59.95 | −3.08 |
| 328.15 | −2.78 | |||
| 353.37 | −2.48 | |||
| CCR | 302.92 | −2.07 | −2.4 × 10−2 | 5.44 |
| 328.15 | 6.06 | |||
| 353.37 | 6.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villabona-Ortíz, Á.; Tejada-Tovar, C.; Gonzalez-Delgado, Á.D. Adsorption of Cd2+ Ions from Aqueous Solution Using Biomasses of Theobroma cacao, Zea mays, Manihot esculenta, Dioscorea rotundata and Elaeis guineensis. Appl. Sci. 2021, 11, 2657. https://doi.org/10.3390/app11062657
Villabona-Ortíz Á, Tejada-Tovar C, Gonzalez-Delgado ÁD. Adsorption of Cd2+ Ions from Aqueous Solution Using Biomasses of Theobroma cacao, Zea mays, Manihot esculenta, Dioscorea rotundata and Elaeis guineensis. Applied Sciences. 2021; 11(6):2657. https://doi.org/10.3390/app11062657
Chicago/Turabian StyleVillabona-Ortíz, Ángel, Candelaria Tejada-Tovar, and Ángel Darío Gonzalez-Delgado. 2021. "Adsorption of Cd2+ Ions from Aqueous Solution Using Biomasses of Theobroma cacao, Zea mays, Manihot esculenta, Dioscorea rotundata and Elaeis guineensis" Applied Sciences 11, no. 6: 2657. https://doi.org/10.3390/app11062657
APA StyleVillabona-Ortíz, Á., Tejada-Tovar, C., & Gonzalez-Delgado, Á. D. (2021). Adsorption of Cd2+ Ions from Aqueous Solution Using Biomasses of Theobroma cacao, Zea mays, Manihot esculenta, Dioscorea rotundata and Elaeis guineensis. Applied Sciences, 11(6), 2657. https://doi.org/10.3390/app11062657








