Torrefaction Characteristics of Blended Ratio of Sewage Sludge and Sugarcane Bagasse for Energy Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Solid Fuel Preparation
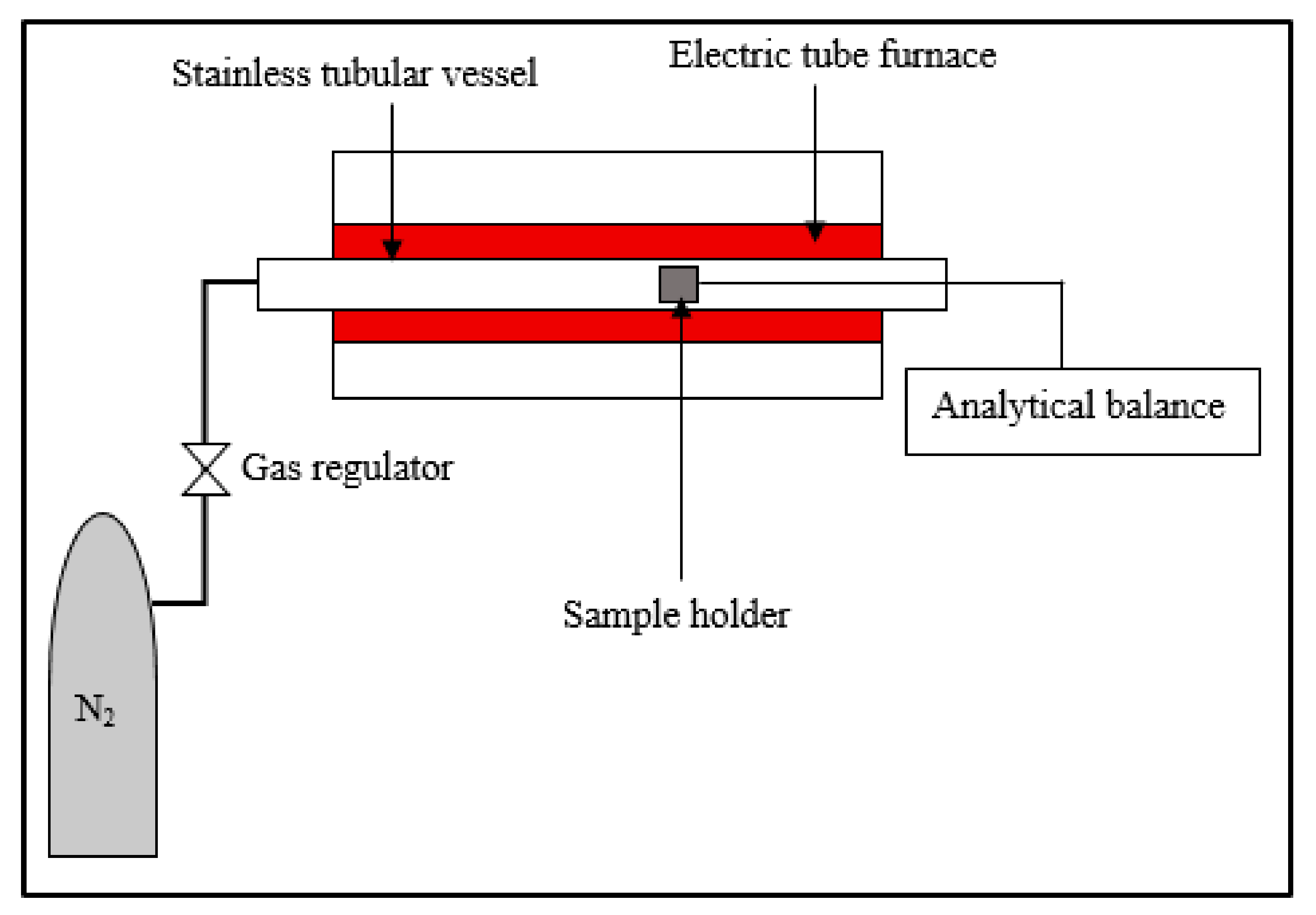
2.2. Torrefaction Process
2.3. Thermogravimetric Analysis (TGA)
2.4. Scanning Electron Microscope (SEM)
2.5. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
3. Results
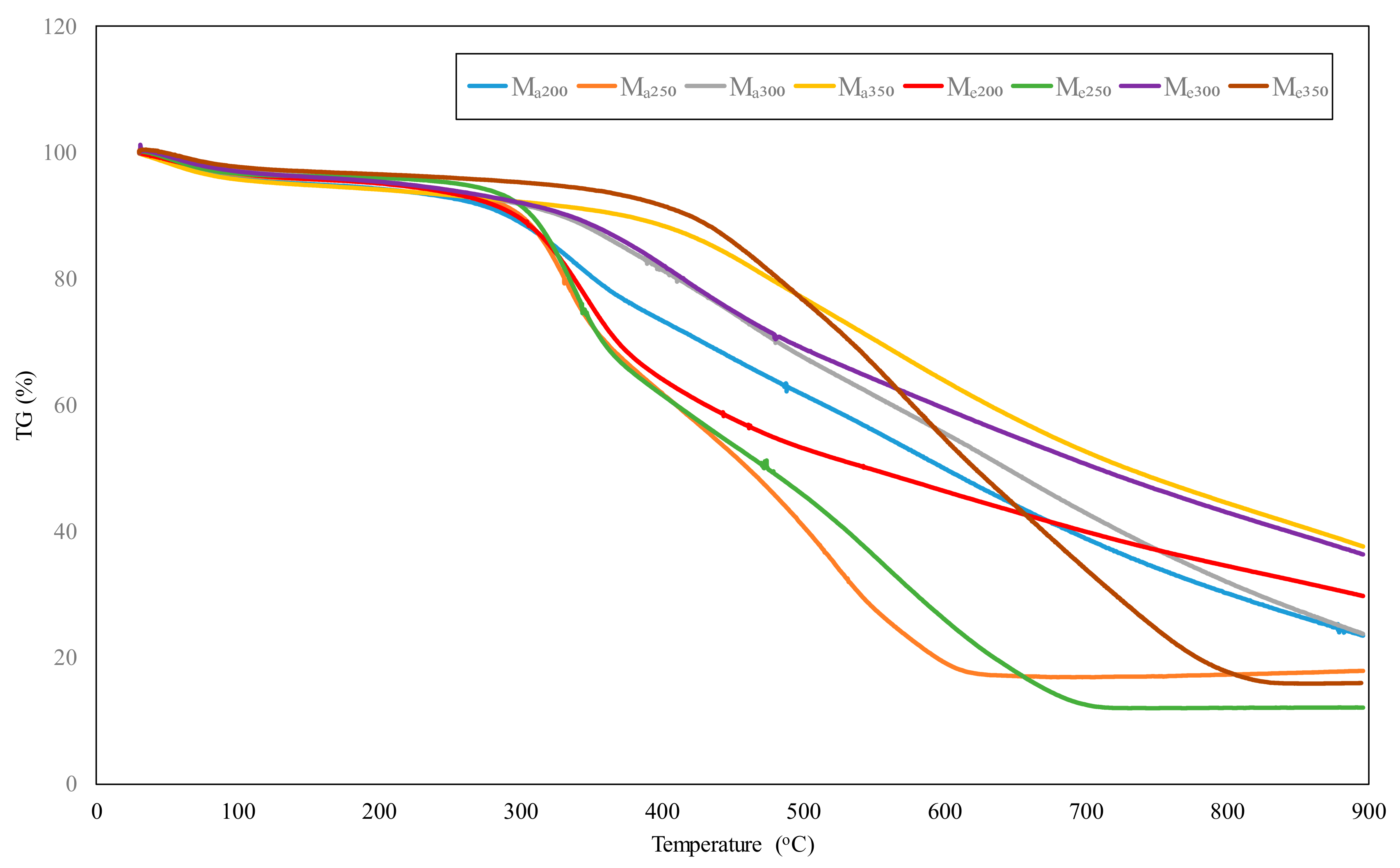
3.1. Thermogravimetric Analysis
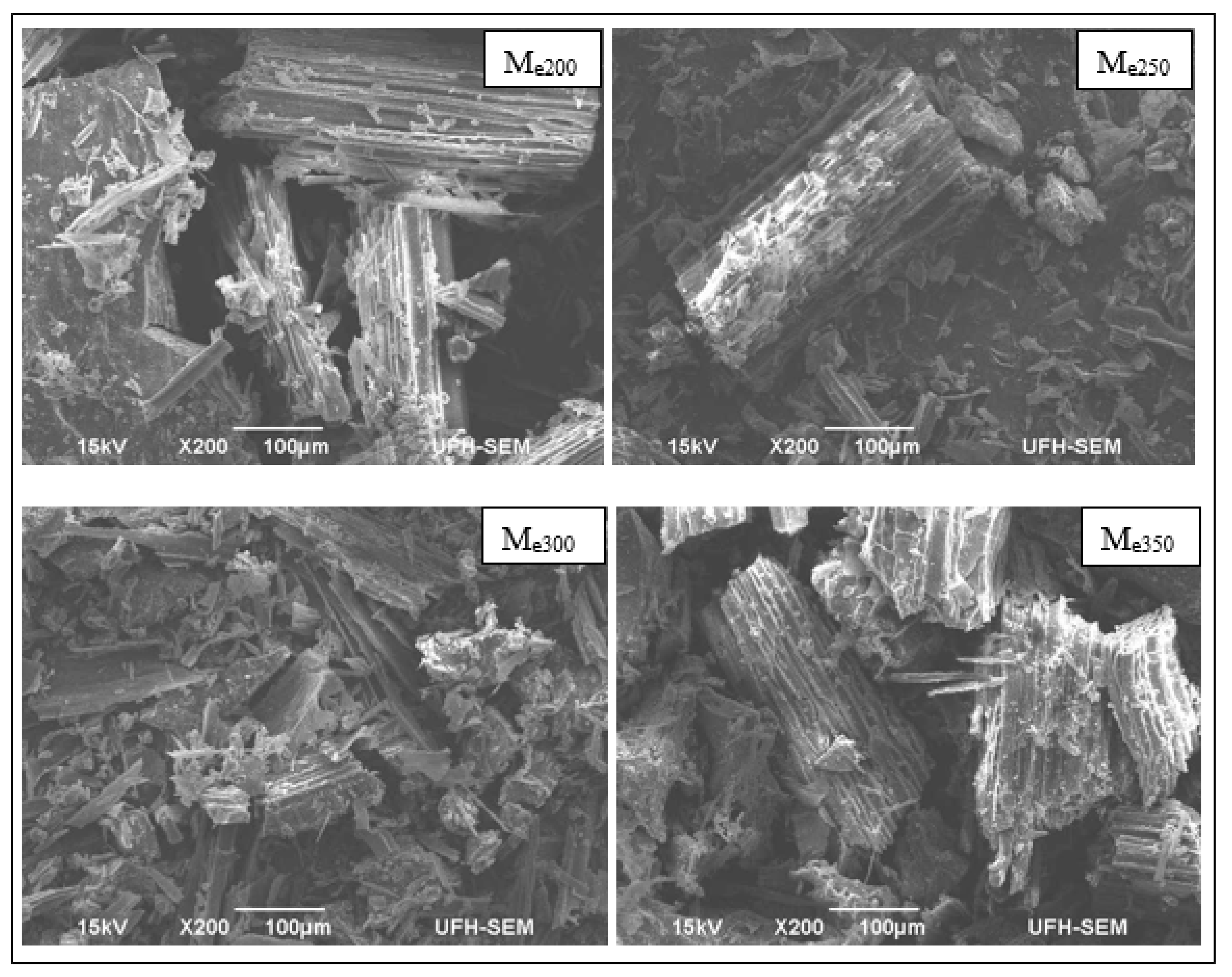
3.2. Scanning Electron Microscopy Analysis
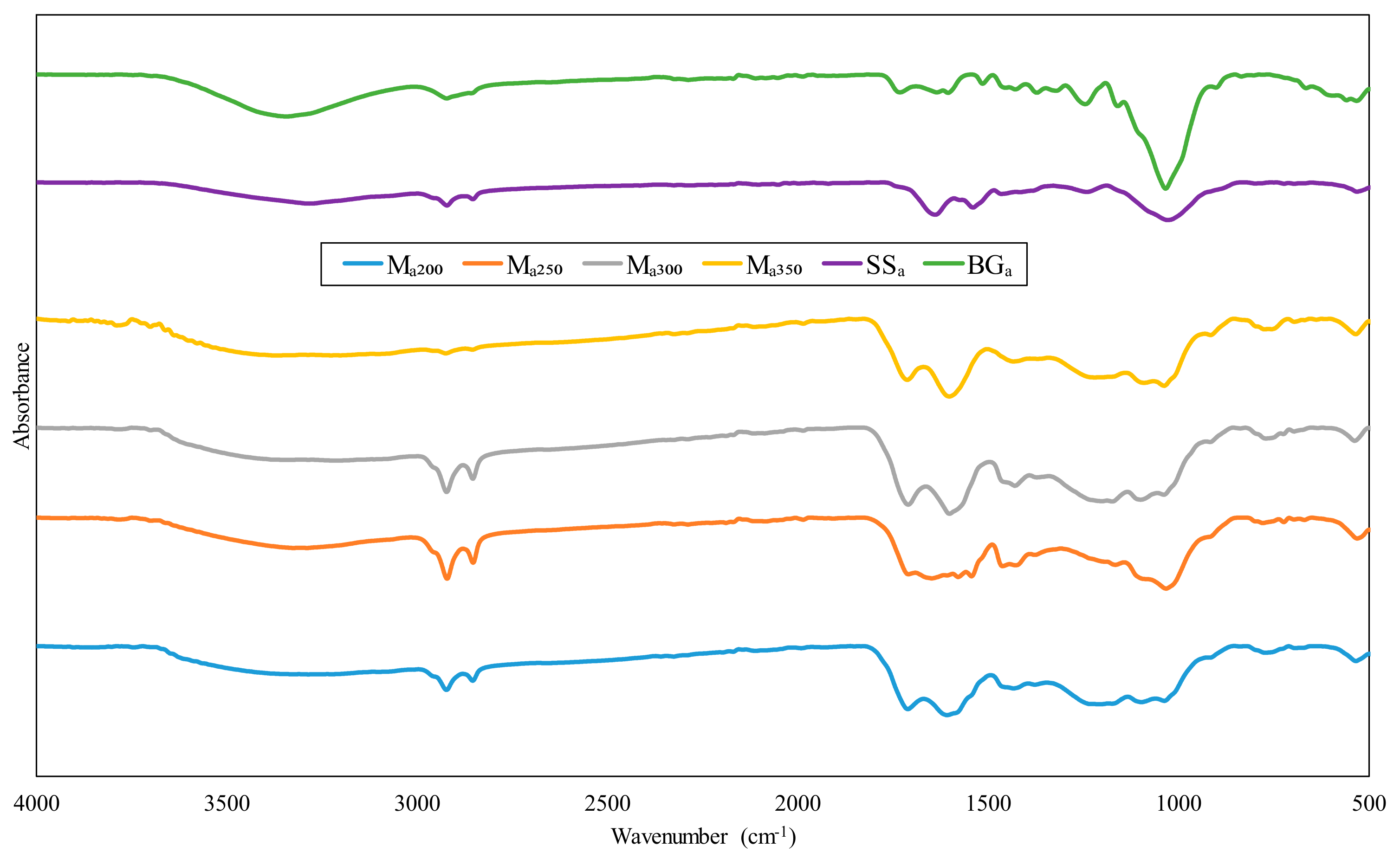
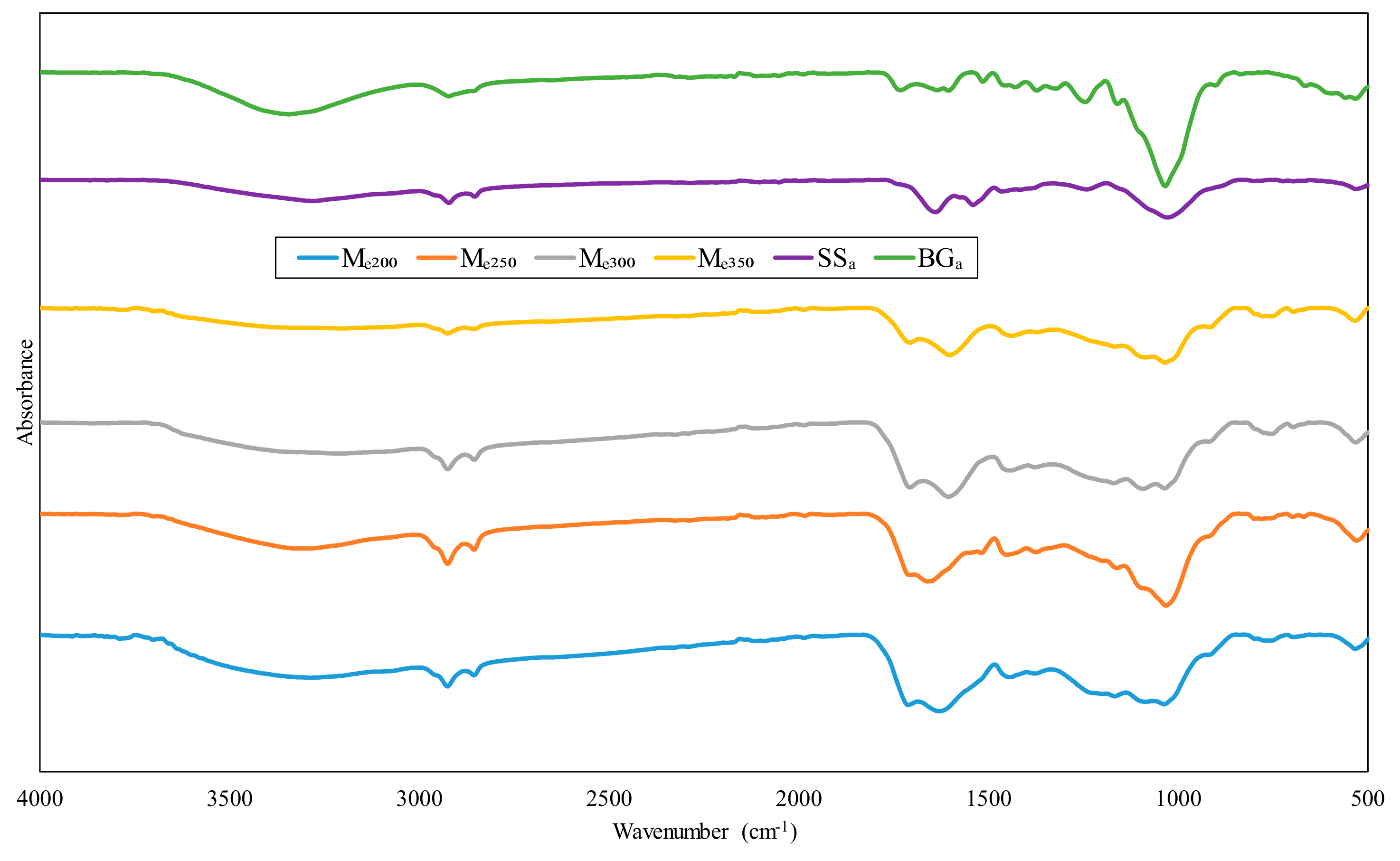
3.3. FTIR Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mamvura, T.; Danha, G. Biomass torrefaction as an emerging technology to aid in energy production. Heliyon 2020, 6, e03531. [Google Scholar] [CrossRef] [PubMed]
- Pulka, J.; Manczarski, P.; Koziel, J.A.; Białowiec, A. Torrefaction of sewage sludge: Kinetics and fuel properties of biochars. Energies 2019, 12, 565. [Google Scholar] [CrossRef]
- Li, H.; Jiang, L.-B.; Li, C.-Z.; Liang, J.; Yuan, X.-Z.; Xiao, Z.-H.; Xiao, Z.-H.; Wang, H. Co-Pelletization of sewage sludge and biomass: The energy input and properties of pellets. Fuel Process. Technol. 2015, 132, 55–61. [Google Scholar] [CrossRef]
- Cahyanti, M.N.; Doddapaneni, T.R.K.C.; Kikas, T. Biomass torrefaction: An overview on process parameters, economic and environmental aspects and recent advancements. Bioresour. Technol. 2020, 301, 122737. [Google Scholar] [CrossRef] [PubMed]
- Acharya, B.; Dutta, A. Fuel property enhancement of lignocellulosic and nonlignocellulosic biomass through torrefaction. Biomass Convers. Biorefin. 2016, 6, 139–149. [Google Scholar] [CrossRef]
- Yue, Y.; Singh, H.; Singh, B.; Mani, S. Torrefaction of sorghum biomass to improve fuel properties. Bioresour. Technol. 2017, 232, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Phanphanich, M.; Mani, S. Impact of torrefaction on the grindability and fuel characteristics of forest biomass. Bioresour. Technol. 2011, 102, 1246–1253. [Google Scholar] [CrossRef]
- Granados, D.; Ruiz, R.; Vega, L.; Chejne, F. Study of reactivity reduction in sugarcane bagasse as consequence of a torrefaction process. Energy 2017, 139, 818–827. [Google Scholar] [CrossRef]
- Anukam, A.; Mamphweli, S.; Okoh, O.; Reddy, P. Influence of torrefaction on the conversion efficiency of the gasification process of sugarcane bagasse. Bioengineering 2017, 4, 22. [Google Scholar] [CrossRef]
- Atienza-Martínez, M.; Mastral, J.F.; Ábrego, J.; Ceamanos, J.S.; Gea, G. Sewage sludge torrefaction in an auger reactor. Energy Fuels 2015, 29, 160–170. [Google Scholar] [CrossRef]
- Poudel, J.; Ohm, T.-I.; Lee, S.-H.; Oh, S.C. A study on torrefaction of sewage sludge to enhance solid fuel qualities. Waste Manag. 2015, 40, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Pawlak-Kruczek, H.; Wnukowski, M.; Niedzwiecki, L.; Czerep, M.; Kowal, M.; Krochmalny, K.; Zgóra, J.; Ostrycharczyk, M.; Baranowski, M.; Tic, W.J. Torrefaction as a valorization method used prior to the gasification of sewage sludge. Energies 2019, 12, 175. [Google Scholar] [CrossRef]
- Arias, B.; Pevida, C.; Fermoso, J.; Plaza, M.; Rubiera, F.; Pis, J. Influence of torrefaction on the grindability and reactivity of woody biomass. Fuel Process. Technol. 2008, 89, 169–175. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, J.; Liu, B.; Mei, Q.; Luo, Z. Influence of torrefaction pretreatment on biomass gasification technology. Chin. Sci. Bull. 2011, 56, 1449–1456. [Google Scholar] [CrossRef]
- Chen, W.-H.; Du, S.-W.; Tsai, C.-H.; Wang, Z.-Y. Torrefied biomasses in a drop tube furnace to evaluate their utility in blast furnaces. Bioresour. Technol. 2012, 111, 433–438. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lu, K.-M.; Tsai, C.-M. An experimental analysis on property and structure variations of agricultural wastes undergoing torrefaction. Appl. Energy 2012, 100, 318–325. [Google Scholar] [CrossRef]
- Wu, K.-T.; Tsai, C.-J.; Chen, C.-S.; Chen, H.-W. The characteristics of torrefied microalgae. Appl. Energy 2012, 100, 52–57. [Google Scholar] [CrossRef]
- Wen, J.-L.; Sun, S.-L.; Yuan, T.-Q.; Xu, F.; Sun, R.-C. Understanding the chemical and structural transformations of lignin macromolecule during torrefaction. Appl. Energy 2014, 121, 1–9. [Google Scholar] [CrossRef]
- Asadullah, M.; Adi, A.M.; Suhada, N.; Malek, N.H.; Saringat, M.I.; Azdarpour, A. Optimization of palm kernel shell torrefaction to produce energy densified bio-coal. Energy Convers. Manag. 2014, 88, 1086–1093. [Google Scholar] [CrossRef]
- Bridgeman, T.; Jones, J.; Shield, I.; Williams, P. Torrefaction of reed canary grass, wheat straw and willow to enhance solid fuel qualities and combustion properties. Fuel 2008, 87, 844–856. [Google Scholar] [CrossRef]
- Álvarez-Álvarez, P.; Pizarro, C.; Barrio-Anta, M.; Cámara-Obregón, A.; Bueno, J.L.M.; Álvarez, A.; Gutiérrez, I.; Burslem, D.F. Evaluation of tree species for biomass energy production in Northwest Spain. Forests 2018, 9, 160. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Sung, H.-T.; Chiueh, P.-T.; Lo, S.-L. Co-Torrefaction of sewage sludge and leucaena by using microwave heating. Energy 2016, 116, 1–7. [Google Scholar] [CrossRef]
- Kanwal, S.; Chaudhry, N.; Munir, S.; Sana, H. Effect of torrefaction conditions on the physicochemical characterization of agricultural waste (sugarcane bagasse). Waste Manag. 2019, 88, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Standard, A. ASTM standards-D5142-04 standard test methods for proximate analysis of the analysis sample of coal and coke by instrumental procedures. In Annual Book of ASTM Standards, Section; ASTM International: West Conshohocken, PA, USA, 2002; Volume 5. [Google Scholar]
- Hernandez, A.-B.; Ferrasse, J.-H.; Akkache, S.; Roche, N. Thermochemical conversion of sewage sludge by TGA-FTIR analysis: Influence of mineral matter added. Dry. Technol. 2015, 33, 1318–1326. [Google Scholar] [CrossRef]
- Wilk, M.; Magdziarz, A.; Kalemba, I. Characterisation of renewable fuels’ torrefaction process with different instrumental techniques. Energy 2015, 87, 259–269. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction: Practical Design and Theory; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Manatura, K. Inert torrefaction of sugarcane bagasse to improve its fuel properties. Case Stud. Therm. Eng. 2020, 100623. [Google Scholar] [CrossRef]
- Świechowski, K.; Hnat, M.; Stępień, P.; Stegenta-Dąbrowska, S.; Kugler, S.; Koziel, J.A.; Białowiec, A. Waste to energy: Solid fuel production from biogas plant digestate and sewage sludge by torrefaction-process kinetics, fuel properties, and energy balance. Energies 2020, 13, 3161. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lin, Y.-Y.; Liu, H.-C.; Chen, T.-C.; Hung, H.-C.; Chen, C.-H. Analysis of physicochemical properties of liquefaction bio-oil from food waste. Energy Procedia 2019, 158, 61–66. [Google Scholar] [CrossRef]
- Anukam, A.; Mamphweli, S.; Reddy, P.; Okoh, O.; Meyer, E. An investigation into the impact of reaction temperature on various parameters during torrefaction of sugarcane bagasse relevant to gasification. J. Chem. 2015, 2015. [Google Scholar] [CrossRef]
- Caillat, S.; Vakkilainen, E. Large-Scale biomass combustion plants: An overview. In Biomass Combustion Science, Technology and Engineering; Elsevier: Amsterdam, The Netherlands, 2013; pp. 189–224. [Google Scholar]
- Kumar, R.M.D.; Anand, R. Production of biofuel from biomass downdraft gasification and its applications. In Advanced Biofuels; Elsevier: Amsterdam, The Netherlands, 2019; pp. 129–151. [Google Scholar]
- Devi, L.; Ptasinski, K.J.; Janssen, F.J. A review of the primary measures for tar elimination in biomass gasification processes. Biomass Bioenergy 2003, 24, 125–140. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lin, B.-J.; Colin, B.; Chang, J.-S.; Pétrissans, A.; Bi, X.; Pétrissans, M. Hygroscopic transformation of woody biomass torrefaction for carbon storage. Appl. Energy 2018, 231, 768–776. [Google Scholar] [CrossRef]
- Aytenew, G.; Nigus, G.; Bedewi, B. Improvement of the energy density of rice husk using dry and chemical treated torrefaction. J. Adv. Chem. Eng. 2018, 8, 1000185. [Google Scholar]
- Karki, S.; Poudel, J.; Oh, S.C. Thermal pre-treatment of sewage sludge in a lab-scale fluidized bed for enhancing its solid fuel properties. Appl. Sci. 2018, 8, 183. [Google Scholar] [CrossRef]
- Aich, S.; Behera, D.; Nandi, B.K.; Bhattacharya, S. Relationship between proximate analysis parameters and combustion behaviour of high ash Indian coal. Int. J. Coal Sci. Technol. 2020, 1–12. [Google Scholar] [CrossRef]
- Parikh, J.; Channiwala, S.; Ghosal, G. A correlation for calculating HHV from proximate analysis of solid fuels. Fuel 2005, 84, 487–494. [Google Scholar] [CrossRef]
- Demirbaş, A. Calculation of higher heating values of biomass fuels. Fuel 1997, 76, 431–434. [Google Scholar] [CrossRef]
- Kizuka, R.; Ishii, K.; Sato, M.; Fujiyama, A. Characteristics of wood pellets mixed with torrefied rice straw as a biomass fuel. Int. J. Energy Environ. Eng. 2019, 10, 357–365. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lu, K.-M.; Lee, W.-J.; Liu, S.-H.; Lin, T.-C. Non-Oxidative and oxidative torrefaction characterization and SEM observations of fibrous and ligneous biomass. Appl. Energy 2014, 114, 104–113. [Google Scholar] [CrossRef]
- He, C.; Tang, C.; Liu, W.; Dai, L.; Qiu, R. Co-Pyrolysis of sewage sludge and hydrochar with coals: Pyrolytic behaviors and kinetics analysis using TG-FTIR and a discrete distributed activation energy model. Energy Convers. Manag. 2020, 203, 112226. [Google Scholar] [CrossRef]
- Pawar, P.M.-A.; Koutaniemi, S.; Tenkanen, M.; Mellerowicz, E.J. Acetylation of woody lignocellulose: Significance and regulation. Front. Plant Sci. 2013, 4, 118. [Google Scholar] [CrossRef]
- Hoi, L.W.S.; Martincigh, B.S. Sugar cane plant fibres: Separation and characterisation. Ind. Crop. Prod. 2013, 47, 1–12. [Google Scholar]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]

| Sample | MC (wt %) | VM (wt %) | FC (wt %) | AC (wt %) | FR |
|---|---|---|---|---|---|
| Ma200 | 5.05 | 44.90 | 26.54 | 23.51 | 0.59 |
| Ma250 | 4.16 | 76.89 | 1.15 | 17.80 | 0.014 |
| Ma300 | 3.56 | 40.94 | 31.77 | 23.73 | 0.77 |
| Ma350 | 5.00 | 31.11 | 26.50 | 37.39 | 0.85 |
| Me200 | 4.05 | 49.6 | 16.76 | 29.59 | 0.33 |
| Me250 | 3.63 | 70.62 | 13.87 | 11.88 | 0.19 |
| Me300 | 3.65 | 36.89 | 23.18 | 36.28 | 0.62 |
| Me350 | 2.95 | 29.88 | 51.37 | 15.8 | 1.71 |
| Correlation | Sample | HHV1 (MJ/kg) | HHV2 (MJ/kg) |
|---|---|---|---|
| HHV1 = 0.3536 FC + 0.1559 VM − 0.0078 AC HHV2 = 0.1846 VM + 0.3525 FC | Ma200 | 16.20 | 17.64 |
| Ma250 | 12.25 | 14.59 | |
| Ma300 | 17.41 | 18.75 | |
| Ma350 | 13.93 | 15.08 | |
| Me200 | 13.43 | 15.06 | |
| Me250 | 15.82 | 17.92 | |
| Me300 | 13.66 | 14.98 | |
| Me350 | 22.70 | 23.62 |
| Ma200 | Ma250 | Ma300 | Ma350 | Me200 | Me250 | Me300 | Me350 | SSa | BGa | Assignment | H | C | L |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3283.27 | 3287.20 | 3213.11 | - | 3285.20 | 3285.98 | 3210.38 | 3196.89 | 3278.21 | 3342.36 | OH stretching vibration (H-bonded) | x | ||
| 2921.21 | 2919.62 | 2920.79 | 2923.13 | 2923.85 | 2923.44 | 2923.15 | 2923.59 | 2920.00 | 2920.46 | C-H stretching and aliphatic alkanes vibration | x | ||
| 2852.4 | 2851.32 | 2852.02 | - | 2853.96 | 2853.95 | 2853.20 | 2853.52 | 2851.98 | - | C-H stretching and aliphatic alkanes vibration | x | ||
| 1709.66 | 1707.85 | 1708.69 | 1711.34 | 1709.49 | - | 1704.85 | 1705.27 | 1730.17 | - | C=O stretching (carbonyl/carboxyl group) | x | x | |
| 1606.57 | 1646.05 | 1600.19 | 1600.58 | 1628.44 | 1659.52 | 1603.43 | 1600.90 | 1638.08 | 1633.33 | C=C stretching | x | ||
| 1431.50 | 1460.50 | 1428.10 | 1432.22 | 1440.97 | 1449.80 | 1439.18 | 1436.06 | 1426.75 | 1463.25 | C-H bond deformation and deflection | x | x | x |
| 1206.63 | 1164.95 | - | 1218.50 | 1165.07 | 1160.02 | - | - | 1243.49 | 1239.39 | C-O stretching of aromatic ring | x | ||
| 1035.77 | 1031.20 | 1037.18 | 1036.27 | 1035.05 | 1030.47 | 1033.61 | 1033.08 | 1032.47 | 1026.17 | C-O, C=C and C–C-O stretching | x | x | x |
| 692.18 | 667.40 | 768.78 | 772.38 | 531.45 | 528.58 | 695.13 | 694.18 | 663.49 | 529.92 | Out-of-plane O-H bending | x | x |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nwabunwanne, N.; Vuyokazi, T.; Olagoke, A.; Mike, O.; Patrick, M.; Anthony, O. Torrefaction Characteristics of Blended Ratio of Sewage Sludge and Sugarcane Bagasse for Energy Production. Appl. Sci. 2021, 11, 2654. https://doi.org/10.3390/app11062654
Nwabunwanne N, Vuyokazi T, Olagoke A, Mike O, Patrick M, Anthony O. Torrefaction Characteristics of Blended Ratio of Sewage Sludge and Sugarcane Bagasse for Energy Production. Applied Sciences. 2021; 11(6):2654. https://doi.org/10.3390/app11062654
Chicago/Turabian StyleNwabunwanne, Nwokolo, Tonga Vuyokazi, Adeniji Olagoke, Ojemaye Mike, Mukumba Patrick, and Okoh Anthony. 2021. "Torrefaction Characteristics of Blended Ratio of Sewage Sludge and Sugarcane Bagasse for Energy Production" Applied Sciences 11, no. 6: 2654. https://doi.org/10.3390/app11062654
APA StyleNwabunwanne, N., Vuyokazi, T., Olagoke, A., Mike, O., Patrick, M., & Anthony, O. (2021). Torrefaction Characteristics of Blended Ratio of Sewage Sludge and Sugarcane Bagasse for Energy Production. Applied Sciences, 11(6), 2654. https://doi.org/10.3390/app11062654








