Additive Manufacturing of Porous Ti6Al4V Alloy: Geometry Analysis and Mechanical Properties Testing
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
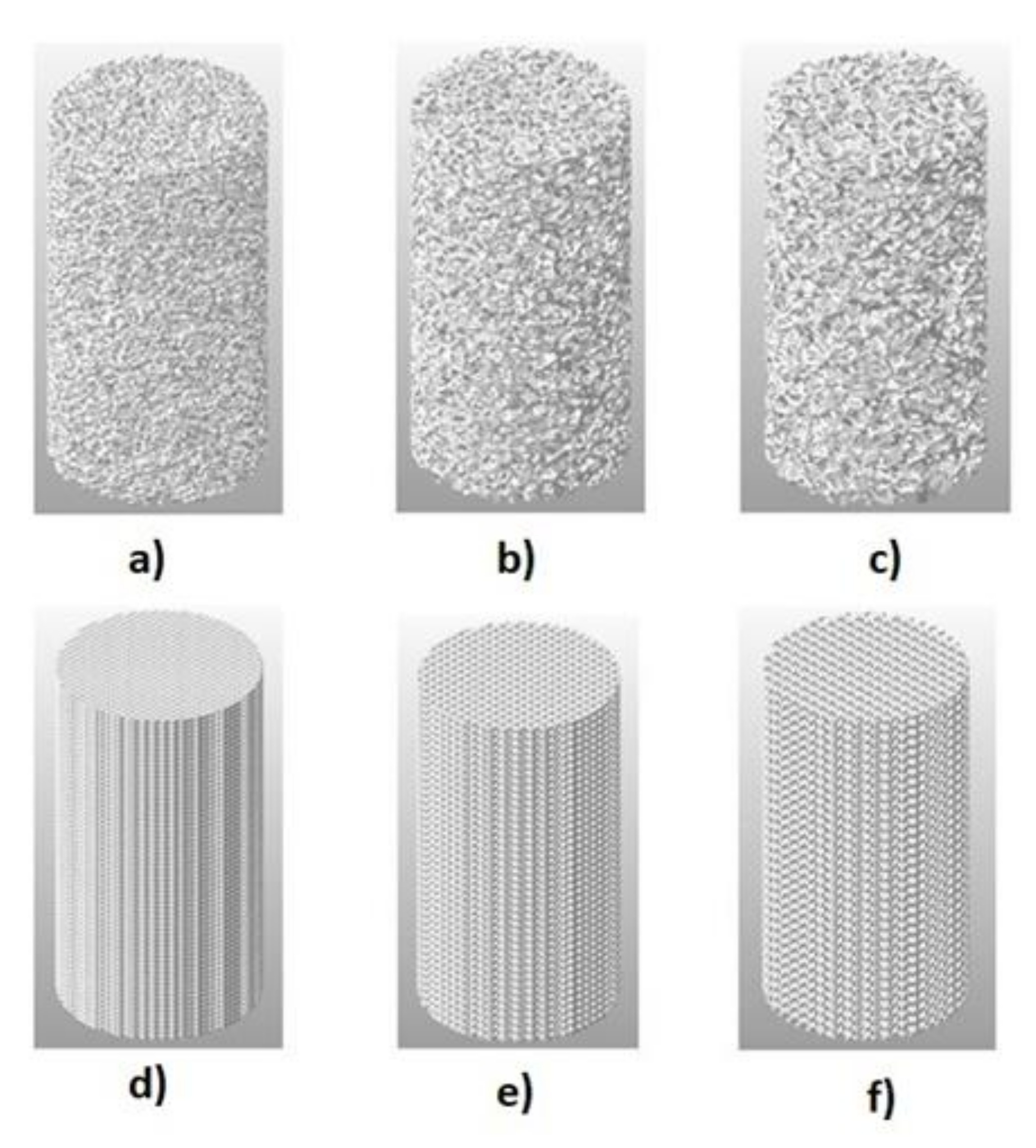
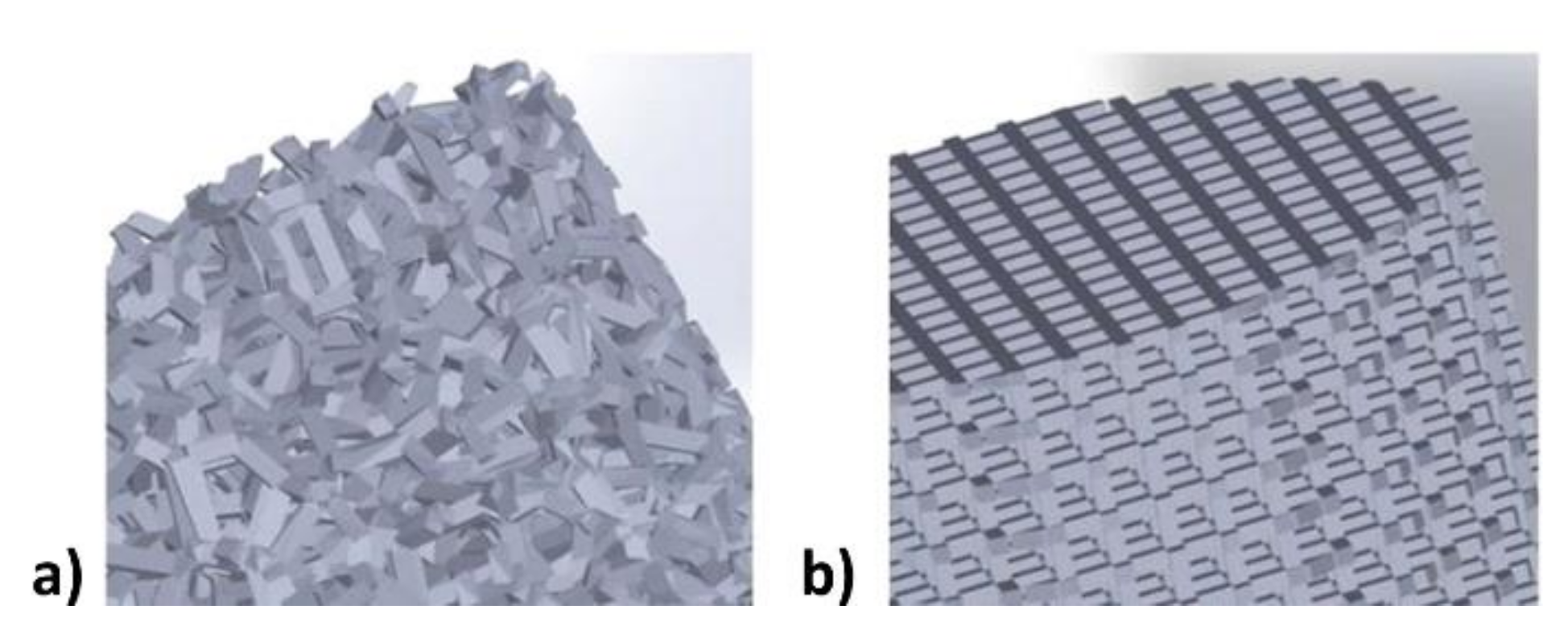
2.2. 3D Models Preparation
2.3. Additive Manufacturing of Ti6Al14V Samples
2.4. Post-Processing of Printed Samples
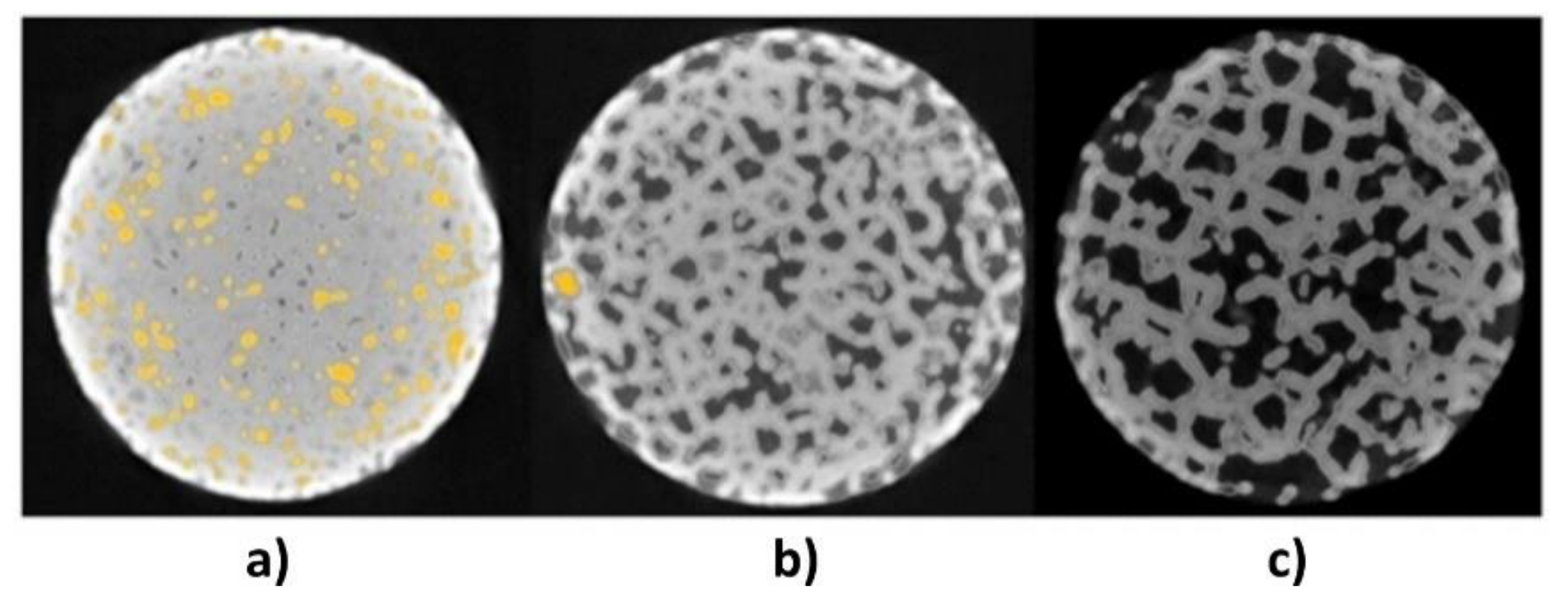
2.5. Computed Tomography
2.6. Mechanical Testing
2.7. Microstructure after Compression
3. Results
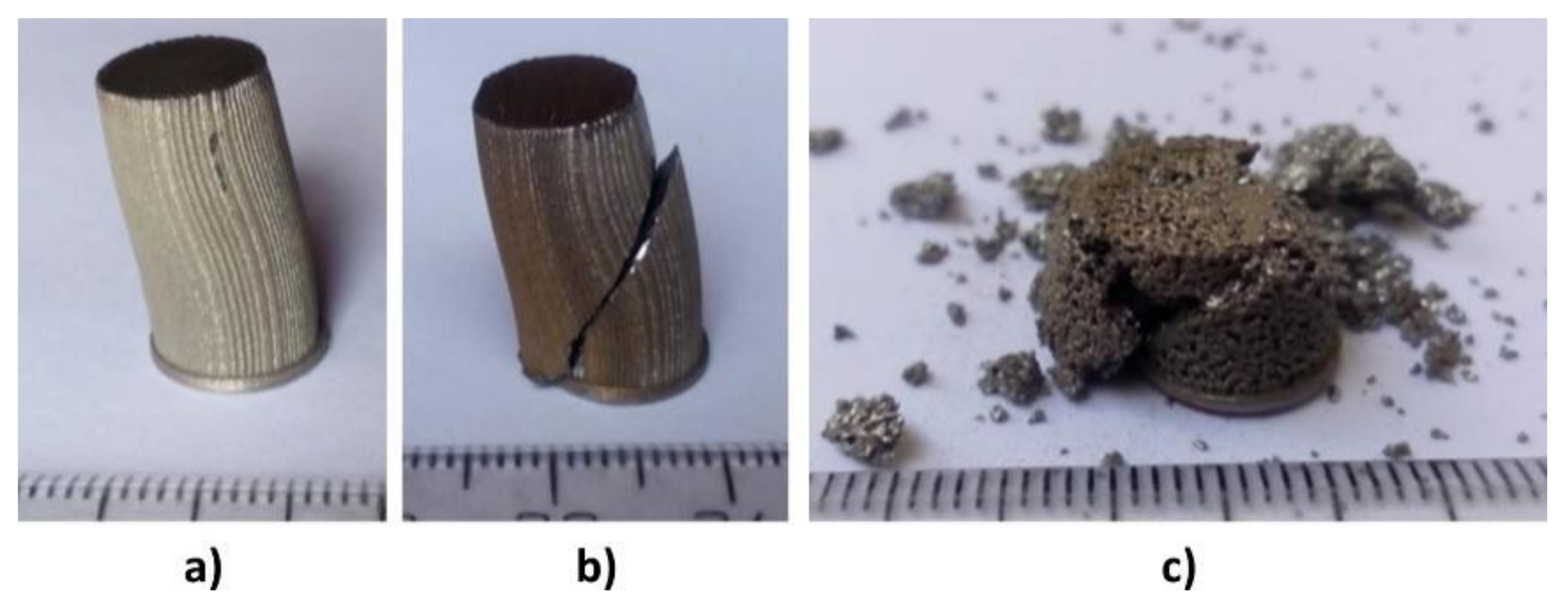
3.1. Prepared Ti6Al4V Samples
3.2. PC—Tomography Scans
3.3. Mechanical Testing
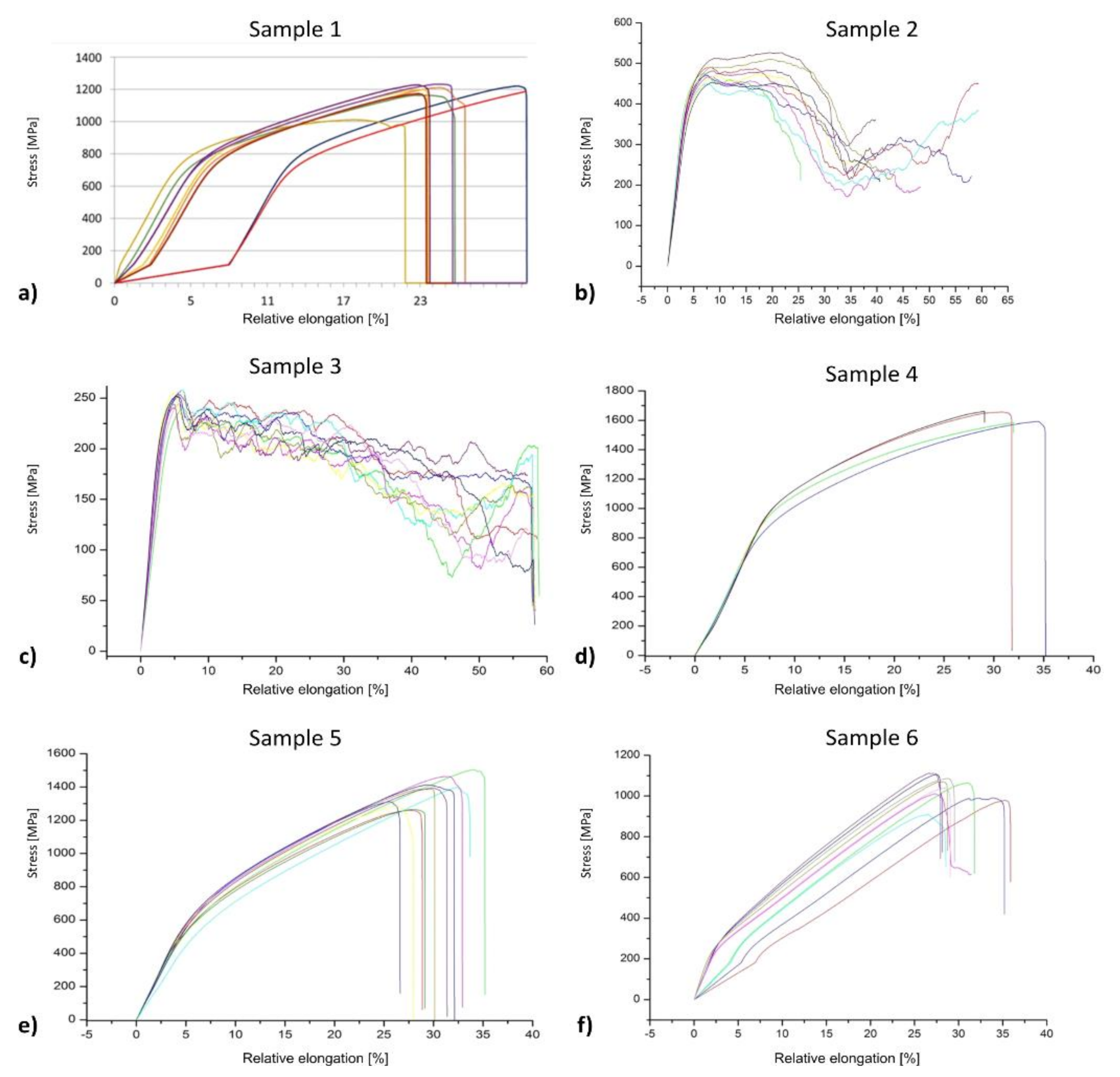
Pressure Testing
- 1—trabecular structure
- 2—cubic structure
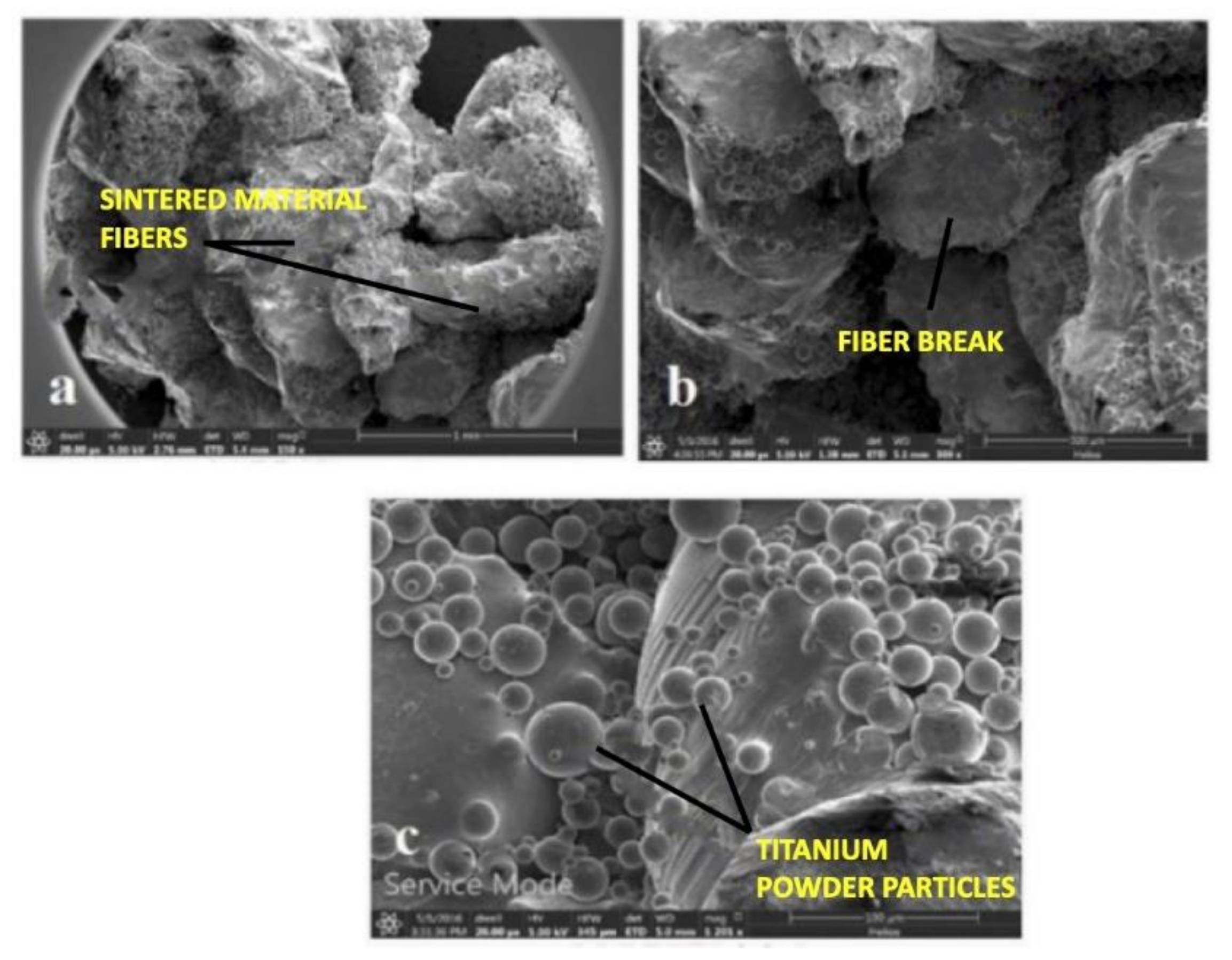
3.4. The Microstructure of Sintered Titanium Powder
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Habashy, S.E.; Eltaher, H.M.; Gaballah, A.; Zaki, E.I.; Mehanna, R.A.; El-Kamel, A.H. Hybrid bioactive hydroxyapatite/polycaprolactone nanoparticles for enhanced osteogenesis. Mater. Sci. Eng. C 2021, 119, 111599. [Google Scholar] [CrossRef] [PubMed]
- Agripa, H.; Botef, I. Modern Production Methods for Titanium Alloys: A Review. Titan. Alloys Nov. Asp. Their Manuf. Process. 2019, 1, 1–15. [Google Scholar] [CrossRef]
- Kuhn, L.T. Biomaterials. In Introduction to Biomedical Engineering; Academic Press: Boston, MA, USA, 2005; pp. 255–312. ISBN 9780122386626. [Google Scholar]
- Chen, F.-M.; Liu, X.; Polym, P.; Author, S. Advancing biomaterials of human origin for tissue engineering HHS Public Access Author manuscript. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef]
- Kalantari, S.M.; Arabi, H.; Mirdamadi, S.; Mirsalehi, S.A. Biocompatibility and compressive properties of Ti-6Al-4V scaffolds having Mg element. J. Mech. Behav. Biomed. Mater. 2015, 48, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Goharian, A.; Abdullah, M.R. Bioinert Metals (Stainless Steel, Titanium, Cobalt Chromium); Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128047583. [Google Scholar]
- Dai, S.J.; Wang, Y.; Chen, F.; Yu, X.Q.; Zhang, Y.F. Design of new biomedical titanium alloy based on d-electron alloy design theory and JMatPro software. Trans. Nonferrous Met. Soc. China 2013, 23, 3027–3032. [Google Scholar] [CrossRef]
- Arifin, A.; Sulong, A.B.; Muhamad, N.; Syarif, J.; Ramli, M.I. Material processing of hydroxyapatite and titanium alloy (HA/Ti) composite as implant materials using powder metallurgy: A review. Mater. Des. 2014, 55, 165–175. [Google Scholar] [CrossRef]
- Takemoto, M.; Fujibayashi, S.; Neo, M.; Suzuki, J.; Kokubo, T.; Nakamura, T. Mechanical properties and osteoconductivity of porous bioactive titanium. Biomaterials 2005, 26, 6014–6023. [Google Scholar] [CrossRef]
- Lewallen, E.A.; Riester, S.M.; Bonin, C.A.; Kremers, H.M.; Dudakovic, A.; Kakar, S.; Cohen, R.C.; Westendorf, J.J.; Lewallen, D.G.; Van Wijnen, A.J. Biological strategies for improved osseointegration and osteoinduction of porous metal orthopedic implants. Tissue Eng. Part B Rev. 2015, 21, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Rifai, A.; Houshyar, S.; Fox, K. Progress towards 3D-printing diamond for medical implants: A review. Ann. 3D Print. Med. 2021, 1, 100002. [Google Scholar] [CrossRef]
- Parthasarathy, J.; Starly, B.; Raman, S.; Christensen, A. Mechanical evaluation of porous titanium (Ti6Al4V) structures with electron beam melting (EBM). J. Mech. Behav. Biomed. Mater. 2010, 3, 249–259. [Google Scholar] [CrossRef]
- Khosravani, M.R.; Reinicke, T. On the Use of X-ray Computed Tomography in Assessment of 3D-Printed Components. J. Nondestruct. Eval. 2020, 39, 1–17. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Z.; Wu, D.; Bai, J. Current status and prospects of polymer powder 3D printing technologies. Materials 2020, 13, 2406. [Google Scholar] [CrossRef]
- Chin, S.Y.; Dikshit, V.; Priyadarshini, B.M.; Zhang, Y. Powder-based 3D printing for the fabrication of device with micro and mesoscale features. Micromachines 2020, 11, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Munir, K.S.; Li, Y.; Wen, C. Metallic scaffolds manufactured by selective laser melting for biomedical applications. In Metallic Foam Bone: Processing, Modification and Characterization and Properties; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–23. ISBN 9780081012901. [Google Scholar]
- Chang Tu, C.; Tsai, P.I.; Chen, S.Y.; Kuo, M.Y.P.; Sun, J.S.; Chang, J.Z.C. 3D laser-printed porous Ti6Al4V dental implants for compromised bone support. J. Formos. Med. Assoc. 2020, 119, 420–429. [Google Scholar] [CrossRef]
- Ahmed, A.; Al-Rasheed, A.; Badwelan, M.; Alghamdi, H.S. Peri-Implant bone response around porous-surface dental implants: A preclinical meta-analysis. Saudi Dent. J. 2021. [Google Scholar] [CrossRef]
- Zheng, J.P.; Chen, L.J.; Chen, D.Y.; Shao, C.S.; Yi, M.F.; Zhang, B. Effects of pore size and porosity of surface-modified porous titanium implants on bone tissue ingrowth. Trans. Nonferrous Met. Soc. China 2019, 29, 2534–2545. [Google Scholar] [CrossRef]
- Jakus, A.E.; Geisendorfer, N.R.; Lewis, P.L.; Shah, R.N. 3D-printing porosity: A new approach to creating elevated porosity materials and structures. Acta Biomater. 2018, 72, 94–109. [Google Scholar] [CrossRef]
- Marin, E.; Fusi, S.; Pressacco, M.; Paussa, L.; Fedrizzi, L. Characterization of cellular solids in Ti6Al4V for orthopaedic implant applications: Trabecular titanium. J. Mech. Behav. Biomed. Mater. 2010, 3, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Ran, Q.; Yang, W.; Hu, Y.; Shen, X.; Yu, Y.; Xiang, Y.; Cai, K. Osteogenesis of 3D printed porous Ti6Al4V implants with different pore sizes. J. Mech. Behav. Biomed. Mater. 2018, 84, 1–11. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Espana, F.; Balla, V.K.; Bose, S.; Ohgami, Y.; Davies, N.M. Influence of porosity on mechanical properties and in vivo response of Ti6Al4V implants. Acta Biomater. 2010, 6, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. Mechanical Testing of Metals—Ductility Testing—Compression Test for Porous and Cellular Metals; ISO Standard No. 13314:2011(E); International Organization for Standardization: Geneva, Switzerland, 2011; pp. 1–7. [Google Scholar]
- Concept Laser Titanium Alloy (Powder) Acc. to EN ISO 9693/DIN EN ISO 22674, Type 4. Available online: https://www.dentaurum.de/files/989-800-03.pdf (accessed on 25 February 2021).
- Conceptlaser Mlab Cusing R Metal Laser Melting System. Available online: https://pdf.directindustry.com/pdf/concept-laser/mlab-cusing-r/15662-683559.html (accessed on 25 February 2021).
- Li, X.; Feng, Y.F.; Wang, C.T.; Li, G.C.; Lei, W.; Zhang, Z.Y.; Wang, L. Evaluation of Biological Properties of Electron Beam Melted Ti6Al4V Implant with Biomimetic Coating In Vitro and In Vivo. PLoS ONE 2012, 7, e52049. [Google Scholar] [CrossRef]
- Arabnejad, S.; Burnett Johnston, R.; Pura, J.A.; Singh, B.; Tanzer, M.; Pasini, D. High-strength porous biomaterials for bone replacement: A strategy to assess the interplay between cell morphology, mechanical properties, bone ingrowth and manufacturing constraints. Acta Biomater. 2016, 30, 345–356. [Google Scholar] [CrossRef]
- Weißmann, V.; Bader, R.; Hansmann, H.; Laufer, N. Influence of the structural orientation on the mechanical properties of selective laser melted Ti6Al4V open-porous scaffolds. Mater. Des. 2016, 95, 188–197. [Google Scholar] [CrossRef]
- Guo, M.; Li, X. Development of porous Ti6Al4V/chitosan sponge composite scaffold for orthopedic applications. Mater. Sci. Eng. C 2016, 58, 1177–1181. [Google Scholar] [CrossRef]
- Kadkhodapour, J.; Montazerian, H.; Darabi, A.C.; Anaraki, A.P.; Ahmadi, S.M.; Zadpoor, A.A.; Schmauder, S. Failure mechanisms of additively manufactured porous biomaterials: Effects of porosity and type of unit cell. J. Mech. Behav. Biomed. Mater. 2015, 50, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.M.; Yavari, S.A.; Wauthle, R.; Pouran, B.; Schrooten, J.; Weinans, H.; Zadpoor, A.A. Additively manufactured open-cell porous biomaterials made from six different space-filling unit cells: The mechanical and morphological properties. Materials 2015, 8, 1871–1896. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, S.J.; Hou, W.T.; Hao, Y.L.; Yang, R.; Misra, R.D.K. The influence of cell morphology on the compressive fatigue behavior of Ti-6Al-4V meshes fabricated by electron beam melting. J. Mech. Behav. Biomed. Mater. 2016, 59, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef]
- Itälä, A.I.; Ylänen, H.O.; Ekholm, C.; Karlsson, K.H.; Aro, H.T. Pore diameter of more than 100 μm is not requisite for bone ingrowth in rabbits. J. Biomed. Mater. Res. 2001, 58, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Braem, A.; Chaudhari, A.; Vivan Cardoso, M.; Schrooten, J.; Duyck, J.; Vleugels, J. Peri- and intra-implant bone response to microporous Ti coatings with surface modification. Acta Biomater. 2014, 10, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, N.; Fujibayashi, S.; Takemoto, M.; Sasaki, K.; Otsuki, B.; Nakamura, T.; Matsushita, T.; Kokubo, T.; Matsuda, S. Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: An in vivo experiment. Mater. Sci. Eng. C 2016, 59, 690–701. [Google Scholar] [CrossRef] [PubMed]






| Component | Content (%) |
|---|---|
| Ti | 90 |
| Al | 6 |
| V | 4 |
| Yield Strength (MPa) | Tensile Strength (MPa) | Modulus of Elasticity (MPa) | Melting Rate (°C) | Density (g/cm3) | Type |
|---|---|---|---|---|---|
| 950 | 1005 | 115 | 1604–1655 | 4.5 | 4 |
| Sample type | Structure | Pore Size (µm) | Lamella Size (µm) |
|---|---|---|---|
| 1 | trabecular | 200 | 200 |
| 2 | trabecular | 400 | 200 |
| 3 | trabecular | 600 | 200 |
| 4 | cubic | 200 | 200 |
| 5 | cubic | 400 | 200 |
| 6 | cubic | 600 | 200 |
| 7 | solid | – | – |
| Basic Parameters | Value |
|---|---|
| Layer width (µm) | 25 |
| Production speed (cm3/h) | 1–5 |
| Laser system (W) | Fiber laser 100 |
| Maximum scanning rate (m/s) | 7 |
| Operation conditions (°C) | 15 to 30 |
| Material | rematitan CL—Ti6Al4V ELI |
| Type | Pore size (µm) | Topology | Weight (g) | Porosity (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Nominal | Actual | Difference | Nominal | Actual | Difference | |||
| 1 | 200 | trabecular | 7.08 | 6.14 | −0.94 | 24.6 | 16.3 | −8.3 |
| 2 | 400 | trabecular | 4.63 | 4.27 | −0.36 | 35.9 | 35.6 | −0.3 |
| 3 | 600 | trabecular | 2.88 | 3.12 | +0.24 | 60.9 | 54.8 | −6.3 |
| 4 | 200 */160 ** | cubic | 4.7 | 6.5 | +1.8 | 36.1 | 5.5 | 30.6 |
| 5 | 400 */270 ** | cubic | 2.9 | 5.3 | +2.4 | 60.2 | 22.6 | −3.6 |
| 6 | 600 */410 ** | cubic | 2.0 | 3.8 | +1.8 | 73.0 | 43.6 | −2.4 |
| 7 | - | solid | 7.3 | ρ = 0.0043 g/mm3 | ||||
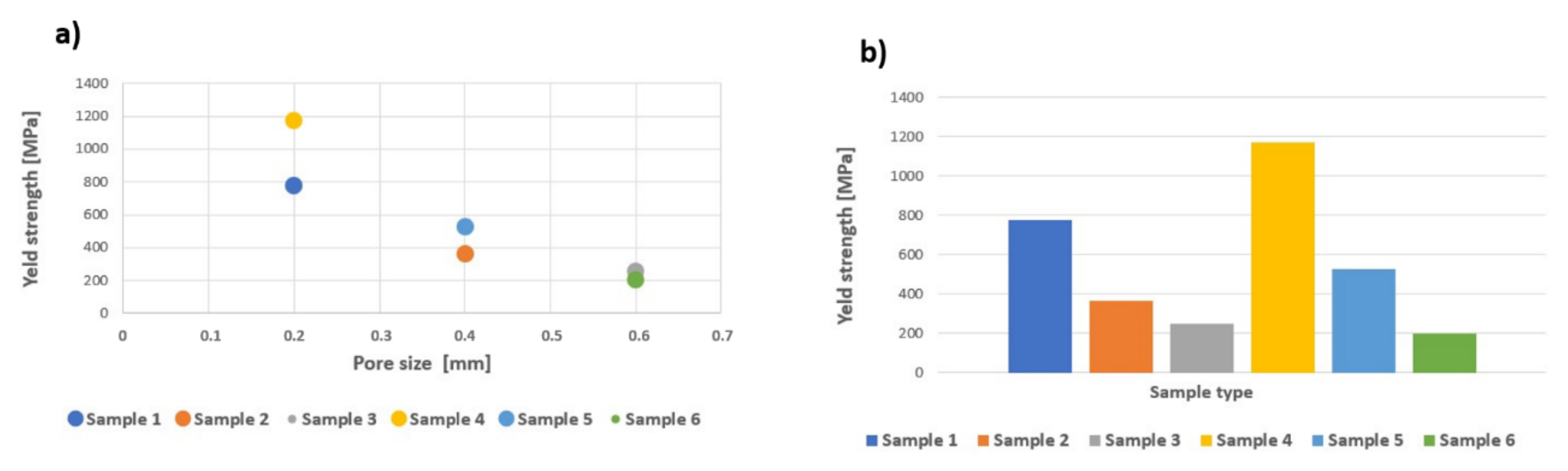
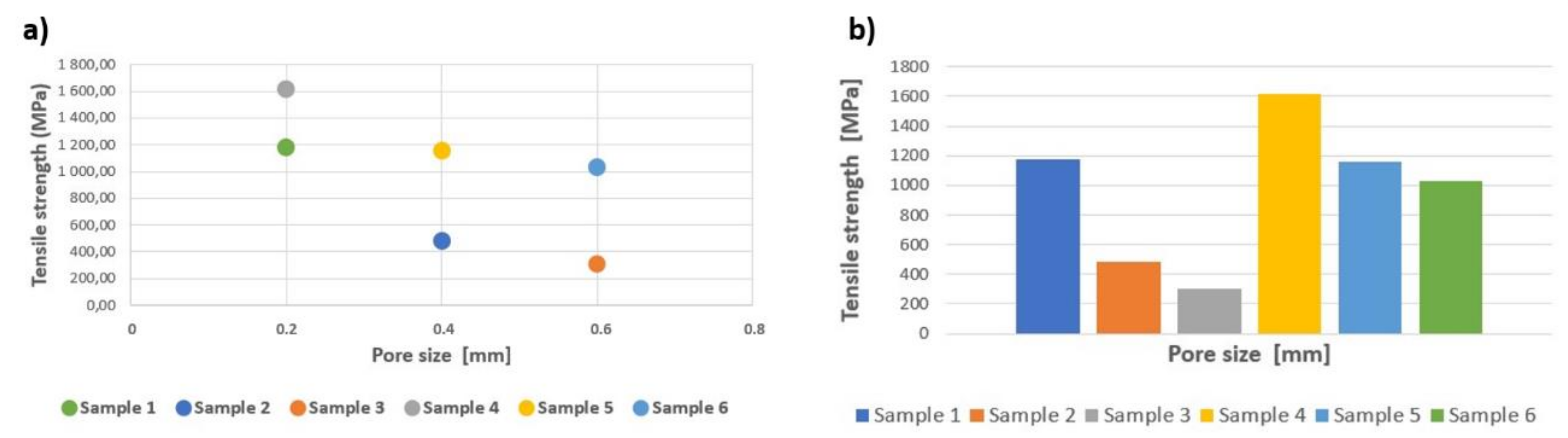
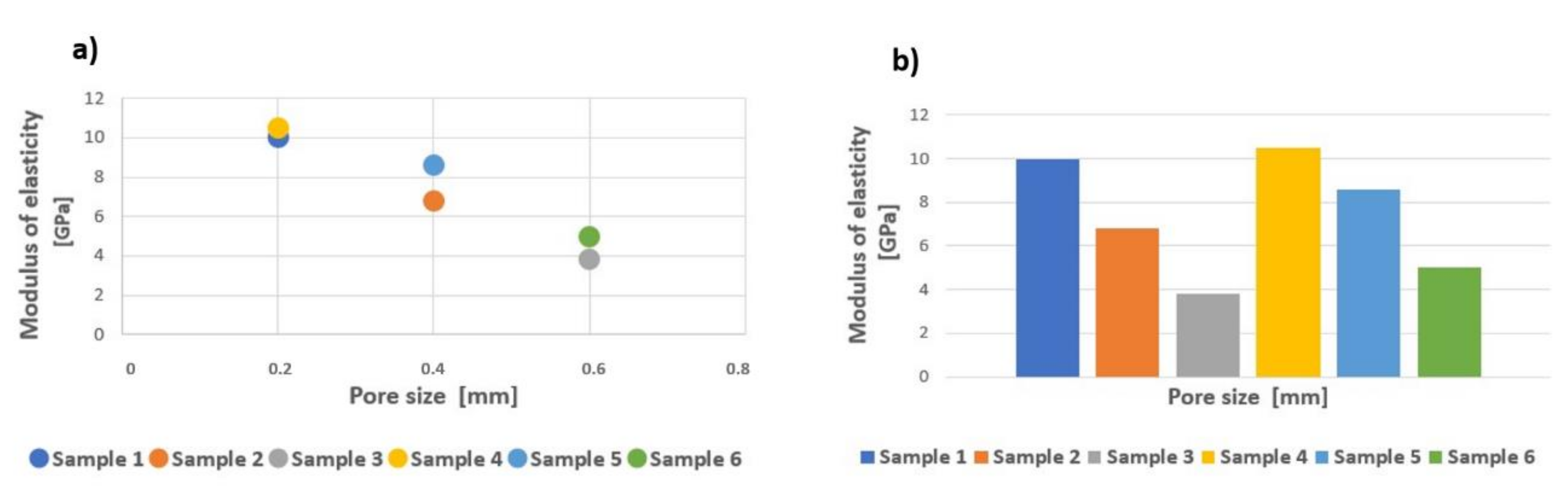
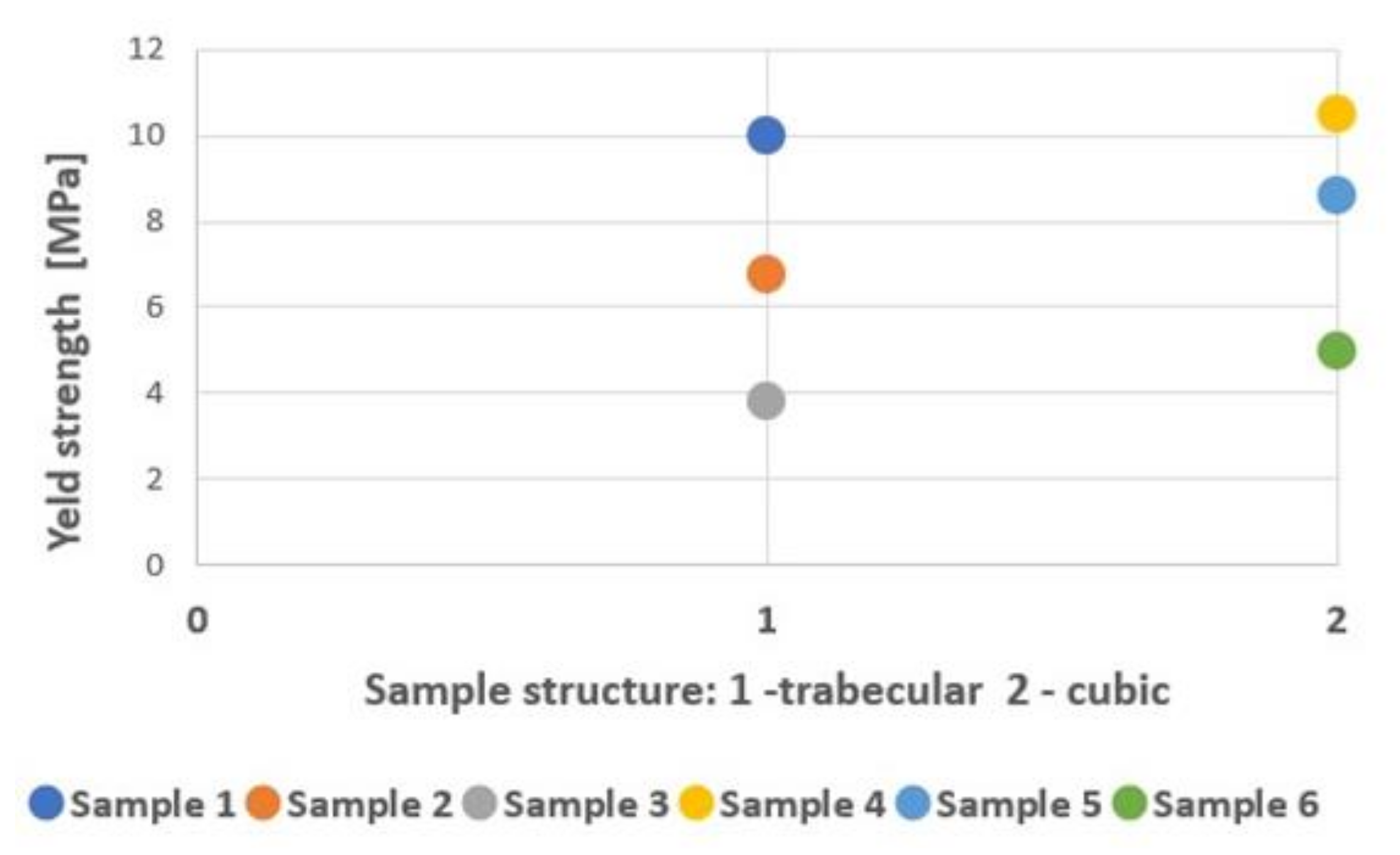
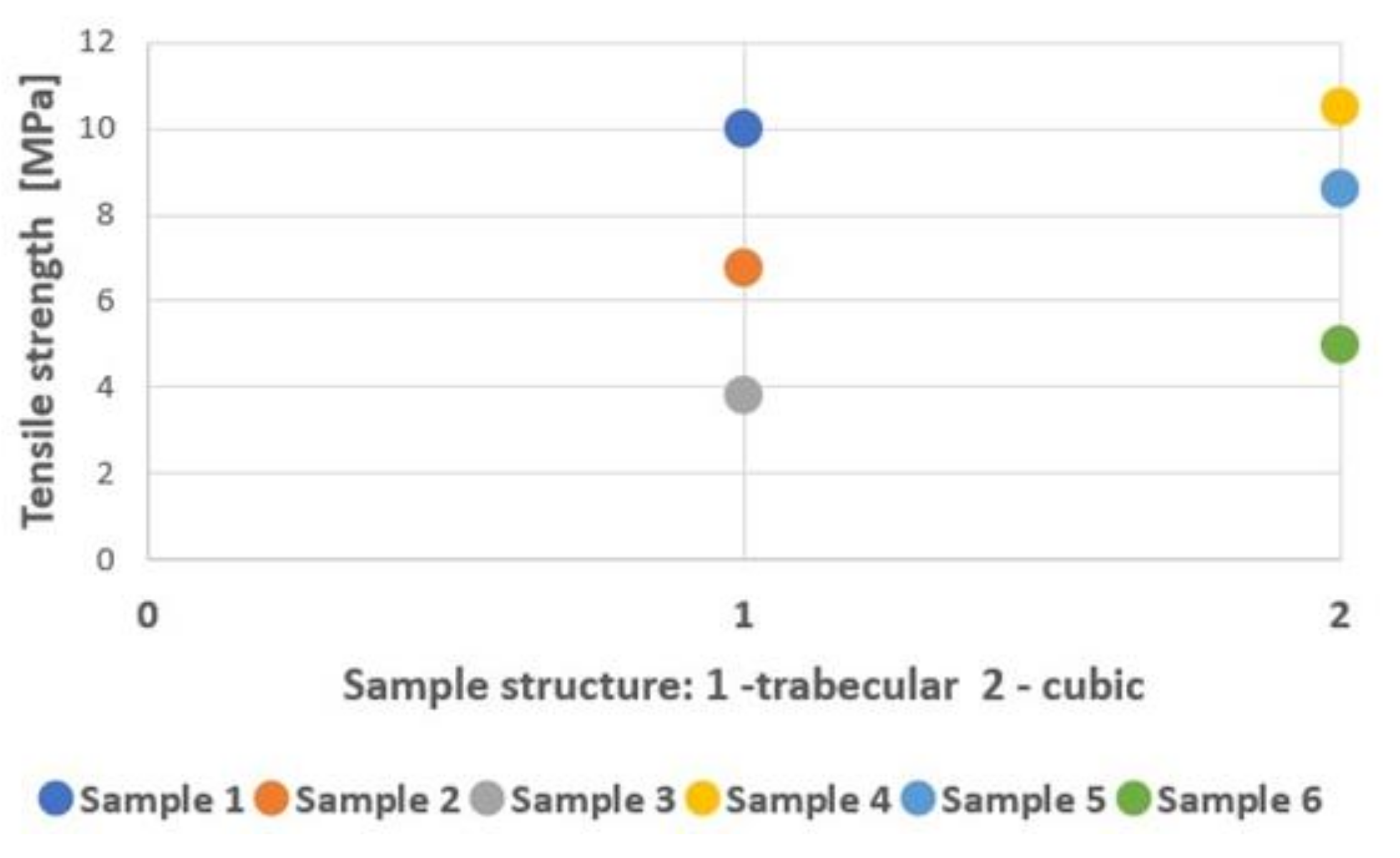
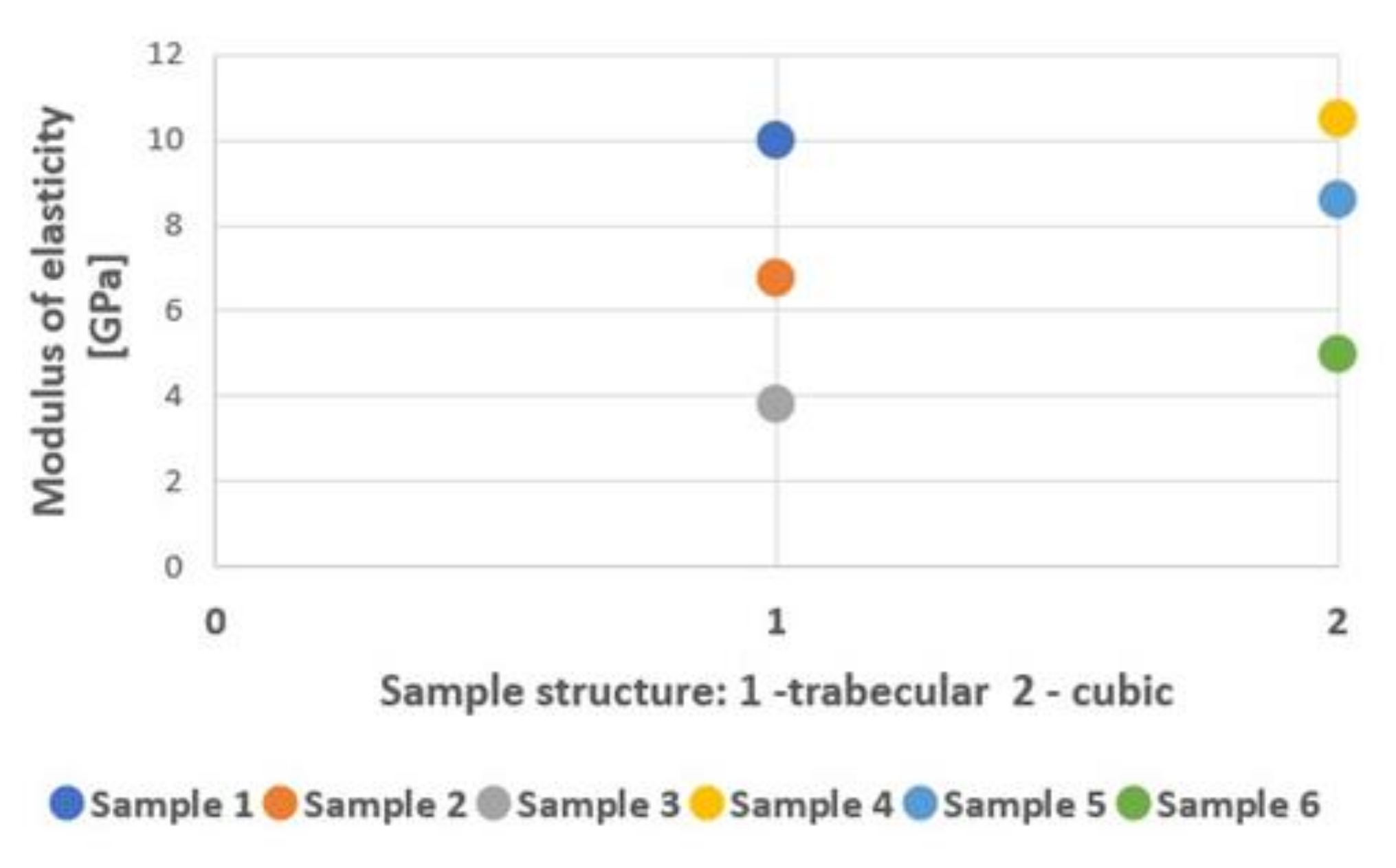
| Sample Type | Max. Force (kN) | Yield Strength (MPa) | Tensile Strength (MPa) | Modulus of Elasticity (GPa) | Energy Absorption (kJ/mm3) |
|---|---|---|---|---|---|
| 1 | 82.08 ± 4.49 | 775.88 ± 36.33 | 1179.99 ± 64.49 | 10.40 ± 0.84 | 22 032.80 ± 2790.74 |
| 2 | 23.12 ± 1.16 | 363.65 ± 8.99 | 481.67 ± 24.25 | 6.80 ± 0.42 | 16 412.20 ± 3137.35 |
| 3 | 10.57 ± 0.28 | 251.77 ± 6.94 | 302.00 ± 8.09 | 3.80 ± 0.42 | 10 637.60 ± 521.71 |
| 4 | 120.70 ± 3.07 | 1055.03 ± 135.90 | 1631.08 ± 41.49 | 10.50 ± 0.58 | 37 251.00 ± 2815.18 |
| 5 | 82.78 ± 5.08 | 525.07 ± 44.48 | 1160.59 ± 74.42 | 8.60 ± 0.70 | 28 658.90 ± 3862.16 |
| 6 | 44.92 ± 2.79 | 198.45 ± 83.29 | 1032.64 ± 64.18 | 4.70 ± 1.95 | 19 042.40 ± 1354.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hudák, R.; Schnitzer, M.; Králová, Z.O.; Gorejová, R.; Mitrík, L.; Rajťúková, V.; Tóth, T.; Kovačević, M.; Riznič, M.; Oriňaková, R.; et al. Additive Manufacturing of Porous Ti6Al4V Alloy: Geometry Analysis and Mechanical Properties Testing. Appl. Sci. 2021, 11, 2611. https://doi.org/10.3390/app11062611
Hudák R, Schnitzer M, Králová ZO, Gorejová R, Mitrík L, Rajťúková V, Tóth T, Kovačević M, Riznič M, Oriňaková R, et al. Additive Manufacturing of Porous Ti6Al4V Alloy: Geometry Analysis and Mechanical Properties Testing. Applied Sciences. 2021; 11(6):2611. https://doi.org/10.3390/app11062611
Chicago/Turabian StyleHudák, Radovan, Marek Schnitzer, Zuzana Orságová Králová, Radka Gorejová, Lukáš Mitrík, Viktória Rajťúková, Teodor Tóth, Mila Kovačević, Marcel Riznič, Renáta Oriňaková, and et al. 2021. "Additive Manufacturing of Porous Ti6Al4V Alloy: Geometry Analysis and Mechanical Properties Testing" Applied Sciences 11, no. 6: 2611. https://doi.org/10.3390/app11062611
APA StyleHudák, R., Schnitzer, M., Králová, Z. O., Gorejová, R., Mitrík, L., Rajťúková, V., Tóth, T., Kovačević, M., Riznič, M., Oriňaková, R., & Živčák, J. (2021). Additive Manufacturing of Porous Ti6Al4V Alloy: Geometry Analysis and Mechanical Properties Testing. Applied Sciences, 11(6), 2611. https://doi.org/10.3390/app11062611









