Empirical Kinetic Modelling and Mechanisms of Quercetin Thermal Degradation in Aqueous Model Systems: Effect of pH and Addition of Antioxidants
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Model Solutions and Thermal Treatment
2.3. Degradation Kinetics
2.4. Antioxidant Activity
2.5. Liquid Chromatography-Tandem Mass Spectrometry (LC/MS/MS)
2.6. Statistical Analysis
3. Results and Discussion
3.1. The Effect of pH
3.2. The Effect of AsA Addition
3.3. The Effect of CySH Addition
3.4. The Effect of Sulfite Addition
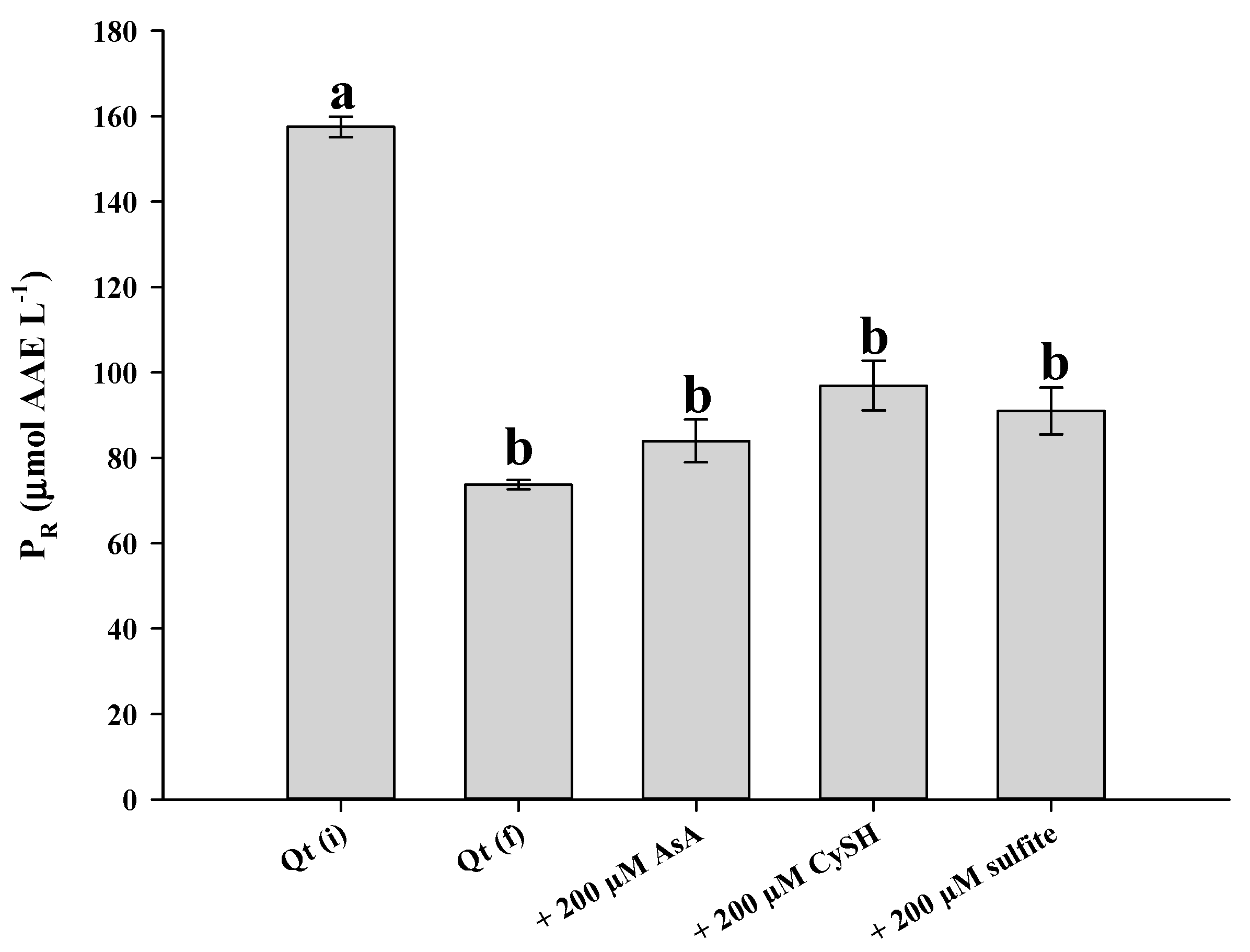
3.5. Impact on Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| AAR | Antiradical activity (mmol TRE L−1) |
| C0 | Initial concentration of quercetin (μmol L−1) |
| Ct | Concentration of quercetin at time t (μmol L−1) |
| k | Quercetin first-order degradation constant (s−1) |
| PR | Ferric-reducing power (μmol AAE L−1) |
| T | Temperature (°C) |
| t | Time (s) |
References
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The role of polyphenols in human health and food systems: A mini-review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Tomas, M.; Capanoglu, E. Dietary Flavonols and O-Glycosides. In Handbook of Dietary Phytochemicals; Springer: Singapore, 2019; pp. 1–40. [Google Scholar]
- Deng, S.; West, B.J.; Jensen, C.J. Thermal degradation of flavonol glycosides in noni leaves during roasting. Adv. J. Food Sci. Technol. 2011, 3, 155–159. [Google Scholar]
- Calani, L.; Dall’Asta, M.; Bruni, R.; Rio, D.D. Flavonoid occurrence, bioavailability, metabolism, and protective effects in humans: Focus on Flavan-3-ols and Flavonols. In Recent Advances in Polyphenol Research; John Wiley & Sons, Ltd.: Chichester, UK, 2014; pp. 239–279. [Google Scholar]
- Wang, W.; Sun, C.; Mao, L.; Ma, P.; Liu, F.; Yang, J.; Gao, Y. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- Zhou, A.; Sadik, O.A. Comparative analysis of quercetin oxidation by electrochemical, enzymatic, autoxidation, and free radical generation techniques: A mechanistic study. J. Agric. Food Chem. 2008, 56, 12081–12091. [Google Scholar] [CrossRef] [PubMed]
- Buchner, N.; Krumbein, A.; Rohn, S.; Kroh, L.W. Effect of thermal processing on the flavonols rutin and quercetin. Rapid Commun. Mass Spectrom. 2006, 20, 3229–3235. [Google Scholar] [CrossRef]
- Atala, E.; Fuentes, J.; Wehrhahn, M.J.; Speisky, H. Quercetin and related flavonoids conserve their antioxidant properties despite undergoing chemical or enzymatic oxidation. Food Chem. 2017, 234, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Chaaban, H.; Ioannou, I.; Chebil, L.; Slimane, M.; Gérardin, C.; Paris, C.; Charbonnel, C.; Chekir, L.; Ghoul, M. Effect of heat processing on thermal stability and antioxidant activity of six flavonoids. J. Food Proc. Preserv. 2017, 41, e13203. [Google Scholar] [CrossRef]
- Fuentes, J.; Atala, E.; Pastene, E.; Carrasco-Pozo, C.; Speisky, H.N. Quercetin oxidation paradoxically enhances its antioxidant and cytoprotective properties. J. Agric. Food Chem. 2017, 65, 11002–11010. [Google Scholar] [CrossRef]
- Price, K.R.; Bacon, J.R.; Rhodes, M.J. Effect of storage and domestic processing on the content and composition of flavonol glucosides in onion (Allium cepa). J. Agric. Food Chem. 1997, 45, 938–942. [Google Scholar] [CrossRef]
- Makris, D.P.; Rossiter, J.T. Domestic processing of onion bulbs (Allium cepa) and asparagus spears (Asparagus officinalis): Effect on flavonol content and antioxidant status. J. Agric. Food Chem. 2001, 49, 3216–3222. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.; Brunton, N.; Tiwari, U.; Cummins, E. Human exposure modelling of quercetin in onions (Allium cepa L.) following thermal processing. Food Chem. 2015, 187, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Bolea, C.; Turturică, M.; Stănciuc, N.; Vizireanu, C. Thermal degradation kinetics of bioactive compounds from black rice flour (Oryza sativa L.) extracts. J. Cereal Sci. 2016, 71, 160–166. [Google Scholar] [CrossRef]
- De Paepe, D.; Valkenborg, D.; Coudijzer, K.; Noten, B.; Servaes, K.; De Loose, M.; Voorspoels, S.; Diels, L.; Van Droogenbroeck, B. Thermal degradation of cloudy apple juice phenolic constituents. Food Chem. 2014, 162, 176–185. [Google Scholar] [CrossRef]
- Lombard, K.; Peffley, E.; Geoffriau, E.; Thompson, L.; Herring, A. Quercetin in onion (Allium cepa L.) after heat-treatment simulating home preparation. J. Food Compos. Anal. 2005, 18, 571–581. [Google Scholar] [CrossRef]
- Juániz, I.; Ludwig, I.A.; Huarte, E.; Pereira-Caro, G.; Moreno-Rojas, J.M.; Cid, C.; De Peña, M.-P. Influence of heat treatment on antioxidant capacity and (poly) phenolic compounds of selected vegetables. Food Chem. 2016, 197, 466–473. [Google Scholar] [CrossRef]
- Photiades, A.; Grigorakis, S.; Makris, D.P. Kinetics and modeling of L-cysteine effect on the Cu (II)-induced oxidation of quercetin. Chem. Eng. Commun. 2020, 207, 139–152. [Google Scholar] [CrossRef]
- Arnous, A.; Makris, D.P.; Kefalas, P. Correlation of pigment and flavanol content with antioxidant properties in selected aged regional wines from Greece. J. Food Compos. Anal. 2002, 15, 655–665. [Google Scholar] [CrossRef]
- Hajji, H.E.; Nkhili, E.; Tomao, V.; Dangles, O. Interactions of quercetin with iron and copper ions: Complexation and autoxidation. Free Radic. Res. 2006, 40, 303–320. [Google Scholar] [CrossRef]
- Dangles, O.; Fargeix, G.; Dufour, C. One-electron oxidation of quercetin and quercetin derivatives in protic and non protic media. J. Chem. Soc. Perkin Trans. 2 1999, 1, 1387–1396. [Google Scholar] [CrossRef]
- Makris, D.P.; Rossiter, J.T. Comparison of quercetin and a non-orthohydroxy flavonol as antioxidants by competing in vitro oxidation reactions. J. Agric. Food Chem. 2001, 49, 3370–3377. [Google Scholar] [CrossRef]
- Makris, D.P.; Rossiter, J.T. Heat-induced, metal-catalyzed oxidative degradation of quercetin and rutin (quercetin 3-O-rhamnosylglucoside) in aqueous model systems. J. Agric. Food Chem. 2000, 48, 3830–3838. [Google Scholar] [CrossRef] [PubMed]
- Mocek, M.; Richardson, P.J. Kinetics and mechanism of quercetin oxidation. J. Inst. Brew. 1972, 78, 459–465. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.-H. Degradation kinetics of fisetin and quercetin in solutions as effected by pH, temperature and coexisted proteins. J. Serb. Chem. Soc. 2016, 81, 243–253. [Google Scholar] [CrossRef]
- Jurasekova, Z.; Torreggiani, A.; Tamba, M.; Sanchez-Cortes, S.; Garcia-Ramos, J. Raman and surface-enhanced Raman scattering (SERS) investigation of the quercetin interaction with metals: Evidence of structural changing processes in aqueous solution and on metal nanoparticles. J. Mol. Struct. 2009, 918, 129–137. [Google Scholar] [CrossRef]
- Jurasekova, Z.; Domingo, C.; García-Ramos, J.V.; Sánchez-Cortés, S. Effect of pH on the chemical modification of quercetin and structurally related flavonoids characterized by optical (UV-visible and Raman) spectroscopy. Phys. Chem. Chem. Phys. 2014, 16, 12802–12811. [Google Scholar] [CrossRef] [PubMed]
- Zenkevich, I.; Pushkareva, T. Systematization of the results of the chromatography–mass spectrometry identification of the products of quercetin oxidation by atmospheric oxygen in aqueous solutions. J. Anal. Chem. 2017, 72, 1061–1075. [Google Scholar] [CrossRef]
- Osman, A.; Makris, D.P.; Kefalas, P. Investigation on biocatalytic properties of a peroxidase-active homogenate from onion solid wastes: An insight into quercetin oxidation mechanism. Process Biochem. 2008, 43, 861–867. [Google Scholar] [CrossRef]
- Makris, D.P.; Rossiter, J.T. Hydroxyl free radical-mediated oxidative degradation of quercetin and morin: A preliminary investigation. J. Food Compos. Anal. 2002, 15, 103–113. [Google Scholar] [CrossRef]
- Barnes, J.S.; Schug, K.A. Oxidative degradation of quercetin with hydrogen peroxide using continuous-flow kinetic electrospray–ion trap–time-of-flight mass spectrometry. J. Agric. Food Chem. 2014, 62, 4322–4331. [Google Scholar] [CrossRef]
- Abou Samra, M.; Chedea, V.S.; Economou, A.; Calokerinos, A.; Kefalas, P. Antioxidant/prooxidant properties of model phenolic compounds: Part I. Studies on equimolar mixtures by chemiluminescence and cyclic voltammetry. Food Chem. 2011, 125, 622–629. [Google Scholar] [CrossRef]
- Choueiri, L.; Chedea, V.S.; Calokerinos, A.; Kefalas, P. Antioxidant/pro-oxidant properties of model phenolic compounds. Part II: Studies on mixtures of polyphenols at different molar ratios by chemiluminescence and LC–MS. Food Chem. 2012, 133, 1039–1044. [Google Scholar] [CrossRef]
- Makris, D.P.; Rossiter, J.T. Effect of natural antioxidants on heat-induced, copper (II)-catalysed, oxidative degradation of quercetin and rutin (quercetin 3-O-rutinoside) in aqueous model systems. J. Sci. Food Agric. 2002, 82, 1147–1153. [Google Scholar] [CrossRef]
- Barberá, J.J.; Metzger, A.; Wolf, M. Sulfites, thiosulfates, and dithionites. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co: Weinheim, Germany, 2000. [Google Scholar]
- Makhotkina, O.; Kilmartin, P.A. Electrochemical oxidation of wine polyphenols in the presence of sulfur dioxide. J. Agric. Food Chem. 2013, 61, 5573–5581. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Z.; Cao, X.; Wei, Y.; Zhao, M.; Duan, J.-A. Pharmacokinetic study of rutin and quercetin in rats after oral administration of total flavones of mulberry leaf extract. Rev. Bras. Farm. 2013, 23, 776–782. [Google Scholar] [CrossRef]
- Kleinenkuhnen, N.; Büchel, F.; Gerlich, S.C.; Kopriva, S.; Metzger, S. A novel method for identification and quantification of sulfated flavonoids in plants by neutral loss scan mass spectrometry. Front. Plant Sci. 2019, 10, 885. [Google Scholar] [CrossRef]
- Amorati, R.; Baschieri, A.; Cowden, A.; Valgimigli, L. The antioxidant activity of quercetin in water solution. Biomimetics 2017, 2, 9. [Google Scholar] [CrossRef] [PubMed]

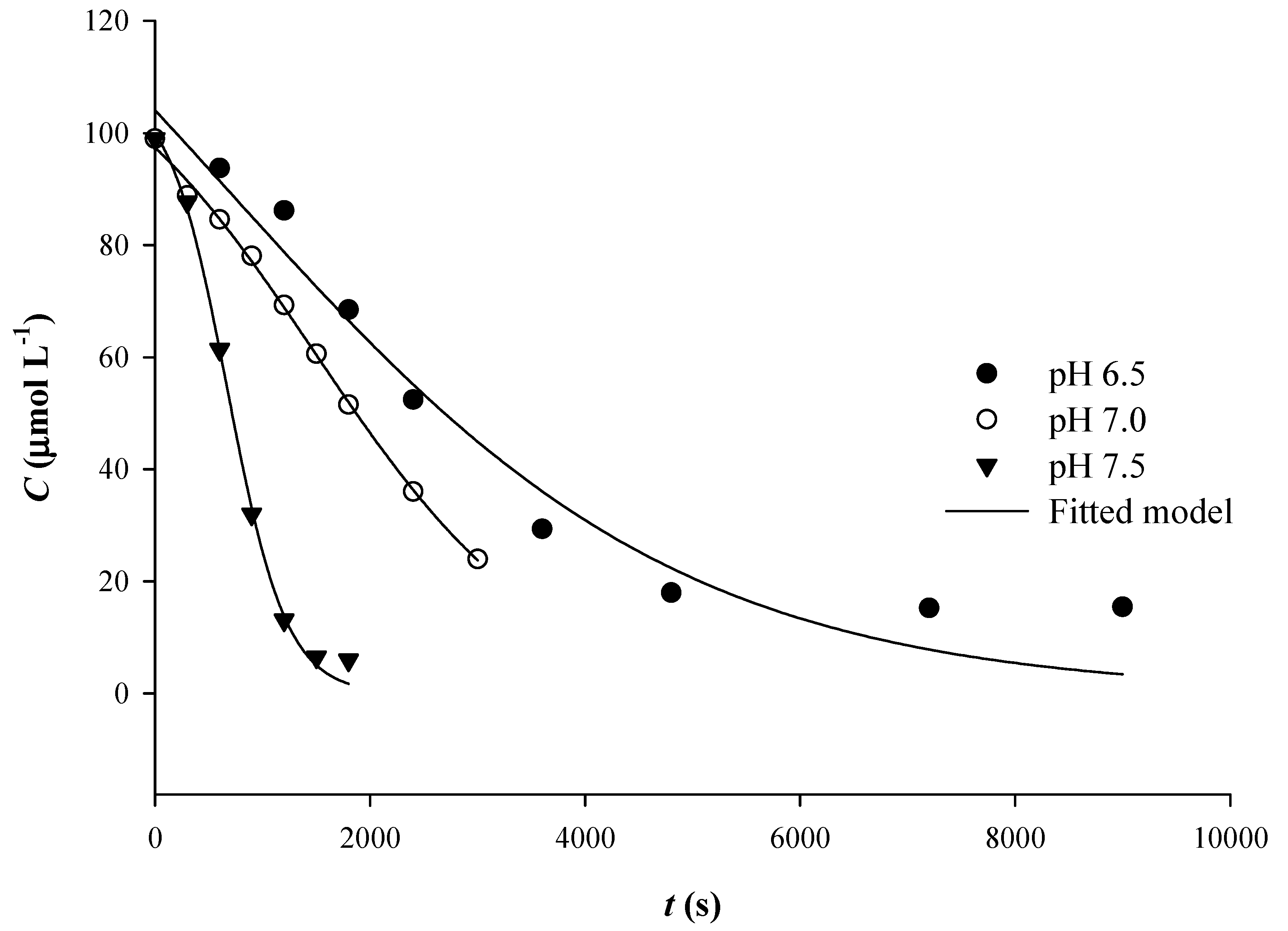


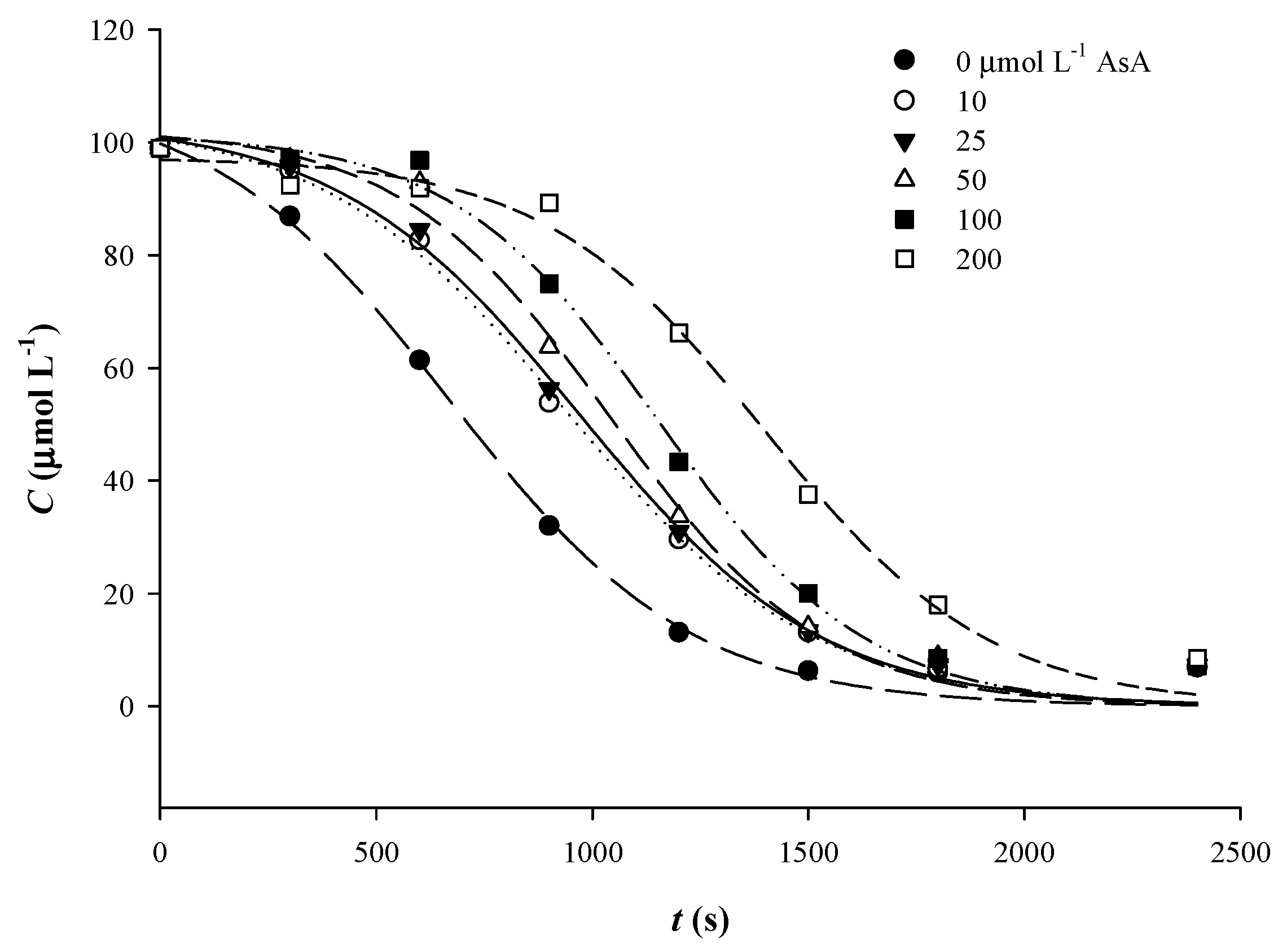

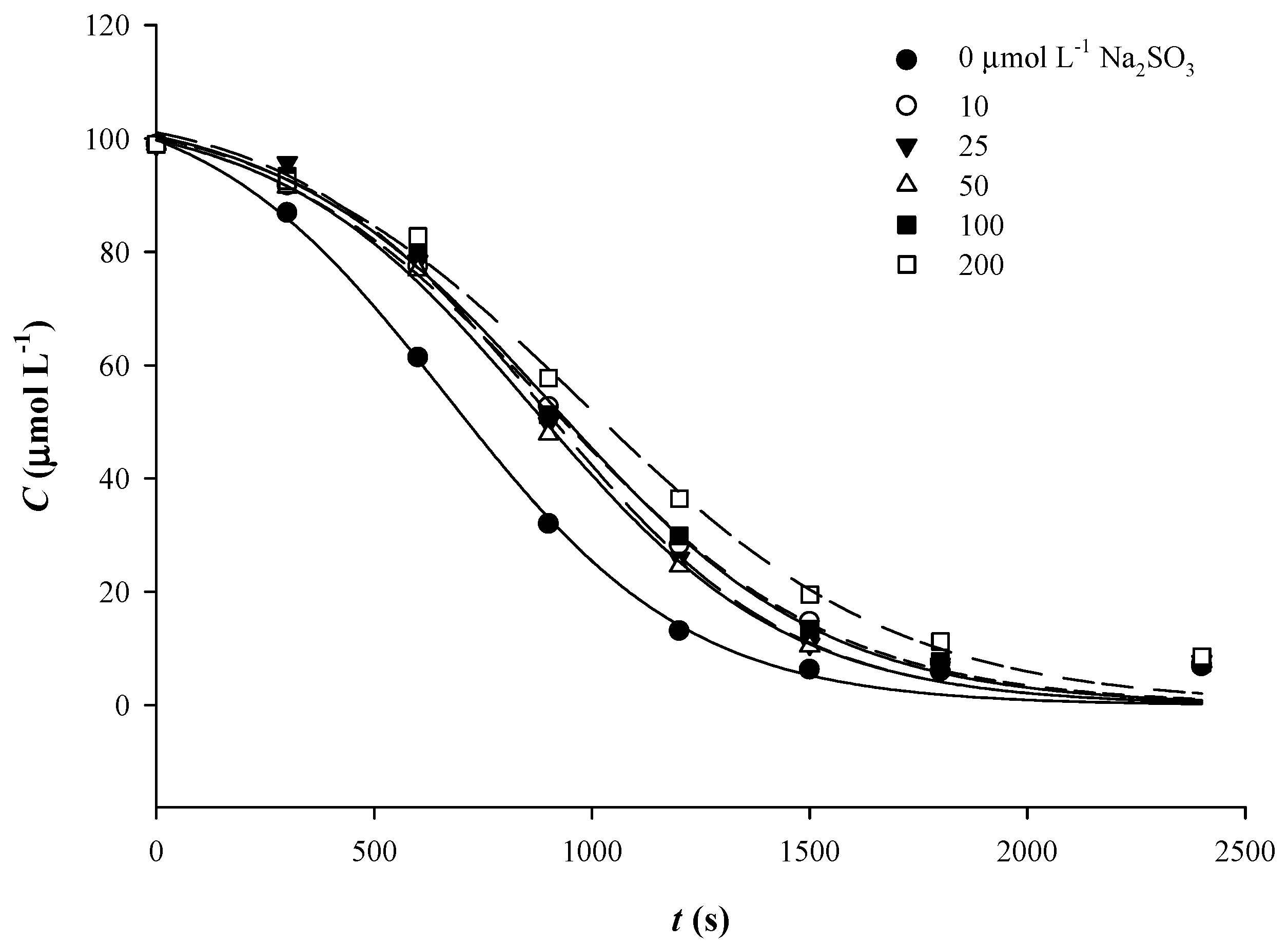

| pH | First-Order Model | Sigmoidal Model | ||||
|---|---|---|---|---|---|---|
| k (s−1) (×10−3) | t½ (s) | R2 | k (s−1) (×10−3) | t½ (s) | R2 | |
| 6.5 | 0.30 | 2310 | 0.94 | 0.76 | 2189 | 0.99 |
| 7.0 | 0.40 | 1733 | 0.97 | 0.92 | 1470 | 0.99 |
| 7.5 | 1.60 | 433 | 0.93 | 3.65 | 673 | 1.00 |
| Peak # | Rt (min) | Molecular Ion (m/z) | Other Fragments (m/z) | Tentative Identity | Formula | Qt a | Qt + AsA b | Qt + CySH c | Qt + Sulfite d |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.90 | 169 | 151 (-H2O) | Phloroglucinol carboxylic acid (2,4,6,-trihydroxybenzoic acid) | C7H5O5 | + | + | + | + |
| 2 | 5.16 | 153 | 109 (-CO2) | Protocatechuic acid (2,4-dihydroxybenzoic acid) | C7H5O4 | + | + | + | + |
| 3 | 12.10 | 179 | 119 | 3,4-dione-2-oxo-phenylacetic acid | C8H3O5 | - | + | - | - |
| 4 | 12.41 | 381 | - | Quercetin 3-sulfate | C15H13O7S | - | - | - | + |
| 5 | 14.22 | 379 | - | Quercetin 3-sulfate quinone | C15H11O7S | - | - | - | + |
| 6 | 20.27 | 301 | - | Quercetin | C15H10O7 | - | + | + | + |
| 7 | 21.66 | 299 | - | Quercetin quinone | C15H8O7 | + | + | + | + |
| Antioxidant Added (μmol L−1) | Kinetic Constants * | ||
|---|---|---|---|
| k (s−1) (×10−3) | t½ (s) | R2 | |
| No Addition | 3.65 | 673 | 1.00 |
| AsA | |||
| 10 | 3.51 | 942 | 0.99 |
| 25 | 3.58 | 969 | 0.99 |
| 50 | 4.09 | 1042 | 0.99 |
| 100 | 4.18 | 1151 | 0.99 |
| 200 | 3.86 | 1402 | 0.99 |
| CySH | |||
| 10 | 3.36 | 709 | 1.00 |
| 25 | 2.84 | 749 | 0.99 |
| 50 | 2.37 | 880 | 0.99 |
| 100 | 2.11 | 875 | 1.00 |
| 200 | 2.10 | 855 | 0.99 |
| Na2SO3 | |||
| 10 | 3.09 | 901 | 0.99 |
| 25 | 3.51 | 885 | 0.99 |
| 50 | 3.42 | 865 | 0.99 |
| 100 | 3.21 | 910 | 0.99 |
| 200 | 2.78 | 980 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kellil, A.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Empirical Kinetic Modelling and Mechanisms of Quercetin Thermal Degradation in Aqueous Model Systems: Effect of pH and Addition of Antioxidants. Appl. Sci. 2021, 11, 2579. https://doi.org/10.3390/app11062579
Kellil A, Grigorakis S, Loupassaki S, Makris DP. Empirical Kinetic Modelling and Mechanisms of Quercetin Thermal Degradation in Aqueous Model Systems: Effect of pH and Addition of Antioxidants. Applied Sciences. 2021; 11(6):2579. https://doi.org/10.3390/app11062579
Chicago/Turabian StyleKellil, Abdessamie, Spyros Grigorakis, Sofia Loupassaki, and Dimitris P. Makris. 2021. "Empirical Kinetic Modelling and Mechanisms of Quercetin Thermal Degradation in Aqueous Model Systems: Effect of pH and Addition of Antioxidants" Applied Sciences 11, no. 6: 2579. https://doi.org/10.3390/app11062579
APA StyleKellil, A., Grigorakis, S., Loupassaki, S., & Makris, D. P. (2021). Empirical Kinetic Modelling and Mechanisms of Quercetin Thermal Degradation in Aqueous Model Systems: Effect of pH and Addition of Antioxidants. Applied Sciences, 11(6), 2579. https://doi.org/10.3390/app11062579









