Silver Nanoparticles Functionalized by Fluorescein Isothiocyanate or Rhodamine B Isothiocyanate: Fluorescent and Plasmonic Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of AgNPs–FITC and AgNPs–RITC
2.3. Characterization of AgNPs–FITC NP and AgNPs–RITC
3. Results and Discussion
3.1. AgNPs–Dye Synthesis
3.2. HR-XPS of AgNPs Dye
3.3. Pump-Probe Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, W.; Goldysa, E.M. Chemical sensing with nanoparticles as optical reporters: From noble metal nanoparticles to quantum dots and upconverting nanoparticles. Analyst 2014, 139, 5321–5334. [Google Scholar] [CrossRef]
- Ma, X.; He, S.; Qiu, B.; Luo, F.; Guo, L.; Lin, Z. Noble Metal Nanoparticle-Based Multicolor Immunoassays: An Approach toward Visual Quantification of the Analytes with the Naked Eye. ACS Sens. 2019, 4, 782–791. [Google Scholar] [CrossRef]
- Prosposito, P.; Buratti, L.; Bellingeri, A.; Protano, G.; Faleri, C.; Corsi, I.; Battocchio, C.; Iucci, G.; Tortora, L.; Secchi, V.; et al. Biofunctionalized silver nanoparticles as Hg2+ plasmonic sensor in water: Synthesis characterizations and ecosafety. Nanomaterials 2019, 9, 1353. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Na, L. Noble Metal Nanoparticles-Based Colorimetric Biosensor for Visual Quantification: A Mini Review. Chemosensors 2019, 7, 53. [Google Scholar]
- Pilla, V.; Gonçalves, A.C.; Dos Santos, A.A.; Lodeiro, C. Lifetime and Fluorescence Quantum Yield of Two Fluorescein-Amino Acid-Based Compounds in Different Organic Solvents and Gold Colloidal Suspensions. Chemosensors 2018, 6, 26. [Google Scholar] [CrossRef] [Green Version]
- Cyprych, K.; Chateau, D.; Désert, A.; Parola, S.; Mysliwiec, J. Plasmonic Nanoparticles Driven Enhanced Light Amplification in a Local 2D and 3D Self-Assembly. Nanomaterials 2018, 8, 1051. [Google Scholar] [CrossRef] [Green Version]
- De Angelis, R.; Venditti, I.; Fratoddi, I.; De Matteis, F.; Prosposito, P.; Cacciotti, I.; D’Amico, L.; Nanni, F.; Yadav, A.; Casalboni, M.; et al. From nanospheres to microribbons: Self-assembled Eosin Y doped PMMA nanoparticles as photonic crystals. J. Colloid Interf. Sci. 2014, 414, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Butburee, T.; Wu, X.; Chen, H.; Liu, G.; Wang, L. Enhanced performance of dye-sensitized solar cells by doping Au nanoparticles into photoanodes: A size effect study. J. Mater. Chem. A 2013, 1, 13524–13531. [Google Scholar] [CrossRef]
- Naponiello, G.; Venditti, I.; Zardetto, V.; Saccone, D.; Di Carlo, A.; Fratoddi, I.; Barolo, C.; Dini, D. Photoelectrochemical characterization of squaraine-sensitized nickel oxide cathodes deposited via screen-printing for p-type dye-sensitized solar cells. Appl. Surf. Sci. 2015, 356, 911–920. [Google Scholar] [CrossRef]
- Lou, Y.; Yuan, S.; Zhao, Y.; Hu, P.; Wang, Z.; Zhang, M.; Shi, L.; Lic, D. Molecular-scale interface engineering of metal nanoparticles for plasmon-enhanced dye sensitized solar cells. Dalton Trans. 2013, 42, 5330–5337. [Google Scholar] [CrossRef]
- Lee, C.-P.; Li, C.-T.; Ho, K.-C. Use of organic materials in dye-sensitized solar cells. Mater. Today 2017, 20, 267–283. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, Q.; Li, Q.; Song, Q.; Wu, H.; Mao, N. Enhanced Photocatalytic Properties of PET Filaments Coated with Ag-N Co-Doped TiO2 Nanoparticles Sensitized with Disperse Blue Dyes. Nanomaterials 2020, 10, 987. [Google Scholar] [CrossRef]
- Johnson, R.L.; Perras, F.A.; Kobayashi, T.; Schwartz, T.J.; Dumesic, J.A.; Shanksa, B.H.; Pruski, M. Identifying low-coverage surface species on supported noble metal nanoparticle catalysts by DNP-NMR. Chem. Commun. 2016, 52, 1859–1862. [Google Scholar] [CrossRef] [Green Version]
- Carabineiro, S.A.C. Gold Nanoparticles for Catalytic Applications. Appl. Sci. 2019, 9, 2890. [Google Scholar] [CrossRef] [Green Version]
- Kunkel, B.; Seeburg, D.; Peppel, T.; Stier, M.; Wohlrab, S. Combination of Chemo- and Biocatalysis: Conversion of Biomethane to Methanol and Formic Acid. Appl. Sci. 2019, 9, 2798. [Google Scholar] [CrossRef] [Green Version]
- Quek, C.-H.; Leong, K.W. Near-Infrared Fluorescent Nanoprobes for in Vivo Optical Imaging. Nanomaterials 2012, 2, 92–112. [Google Scholar] [CrossRef] [Green Version]
- Venditti, I.; Cartoni, A.; Fontana, L.; Testa, G.; Scaramuzzo, F.A.; Faccini, R.; Mancini Terracciano, C.; Solfaroli Camillocci, E.; Morganti, S.; Giordano, A.; et al. Y3+ embebbed in polymeric nanoparticles: Morphology, dimension and stability of composite colloidal system. Colloid Surf. A 2017, 532, 125–131. [Google Scholar] [CrossRef]
- Zannella, C.; Shinde, S.; Vitiello, M.; Falanga, A.; Galdiero, E.; Fahmi, A.; Santella, B.; Nucci, L.; Gasparro, R.; Galdiero, M.; et al. Antibacterial Activity of Indolicidin-Coated Silver Nanoparticles in Oral Disease. Appl. Sci. 2020, 10, 1837. [Google Scholar] [CrossRef] [Green Version]
- Venditti, I. Morphologies and functionalities of polymeric nanocarriers as chemical tools for drug delivery: A review. J. King Saud Univ. Sci. 2019, 31, 398–411. [Google Scholar] [CrossRef]
- Shehata, N.; Samir, E.; Kandas, I. Gold/QDs-Embedded-Ceria Nanoparticles: Optical Fluorescence Enhancement as a Quenching Sensor. Appl. Sci. 2020, 10, 1236. [Google Scholar] [CrossRef] [Green Version]
- Fratoddi, I.; Matassa, R.; Fontana, L.; Venditti, I.; Familiari, G.; Battocchio, C.; Magnano, E.; Nappini, S.; Leahu, G.; Belardini, A.; et al. Electronic Properties of a Functionalized Noble Metal Nanoparticles Covalent Network. J. Phys. Chem. C 2017, 121, 18110–18119. [Google Scholar] [CrossRef]
- Roto, R.; Mellisani, B.; Kuncaka, A.; Mudasir, M.; Suratman, A. Colorimetric Sensing of Pb2+ Ion by Using Ag Nanoparticles in the Presence of Dithizone. Chemosensors 2019, 7, 28. [Google Scholar] [CrossRef] [Green Version]
- Saha, J.; Suklabaidya, S.; Nath, J.; Roy, A.D.; Dey, B.; Dey, D.; Bhattacharjee, D.; Hussain, S.A. Fluorescence- and FRET-based mercury (II) sensor. Int. J. Environ. Anal. Chem. 2020, 100, 789–807. [Google Scholar] [CrossRef] [Green Version]
- Prosposito, P.; Burratti, L.; Venditti, I. Silver nanoparticles as colorimetric sensors for water pollutants. Chemosensors 2020, 8, 26. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Ma, L.; Hou, M.; Li, J.; Xie, Z.; Zhang, Z. Hybridized plasmon modes and near-field enhancement of metallic nanoparticle-dimer on a mirror. Sci. Rep. 2016, 6, 30011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venditti, I. Engineered gold-based nanomaterials: Morphologies and functionalities in biomedical applications. A mini review. Bioengineering 2019, 6, 53. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Li, F.; Liu, Y.; Yao, F.; Xu, L.; Han, X.; Wang, K. Principle and Applications of the Coupling of Surface Plasmons and Excitons. Appl. Sci. 2020, 10, 1774. [Google Scholar] [CrossRef] [Green Version]
- Maccora, D.; Dini, V.; Battocchio, C.; Fratoddi, I.; Cartoni, A.; Rotili, D.; Castagnola, M.; Faccini, R.; Bruno, I.; Scotognella, T.; et al. Gold nanoparticles and nanorods in nuclear medicine: A mini review. Appl. Sci. 2019, 9, 3232. [Google Scholar] [CrossRef] [Green Version]
- Suslov, A.; Lama, P.T.; Dorsinville, R. Fluorescence enhancement of Rhodamine B by monodispersed silver nanoparticles. Opt. Commun. 2015, 345, 116–119. [Google Scholar] [CrossRef]
- Francisco, A.P.; Botequim, D.; Prazeres, D.M.F.; Serra, V.V.; Costa, S.M.B.; Laia, C.A.T.; Paulo, P.M.R. Extreme Enhancement of Single-Molecule Fluorescence from Porphyrins Induced by Gold Nanodimer Antennas. J. Phys. Chem. Lett. 2019, 10, 1542–1549. [Google Scholar] [CrossRef]
- Sarkar, A.; Thankappan, A.; Nampoori, V.P.N. Effect of silver nanoparticles on fluorescence and nonlinear properties of naturally occurring betacyanin dye. Opt. Mater. 2015, 39, 211–217. [Google Scholar] [CrossRef]
- Khatua, S.; Paulo, P.M.R.; Yuan, H.; Gupt, A.; Zijlstra, P.; Orrit, M. Resonant Plasmonic Enhancement of Single-Molecule Fluorescence by Individual Gold Nanorods. ACS Nano 2014, 8, 4440–4449. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.-F.; Li, S.; Xu, Q.-H.; Cao, Y. Aggregation-Induced Plasmon Coupling-Enhanced One- and TwoPhoton Excitation Fluorescence by Silver Nanoparticles. Langmuir 2020, 36, 4721–4727. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Liu, X.; Chen, S.-L.; Gan, W.; Yuan, Q. Surface curvature-dependent adsorption and aggregation of fluorescein isothiocyanate on gold nanoparticles. Phys. Chem. Chem. Phys. 2019, 21, 26598–26605. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Moroz, P.; Jin, Z.; Budkina, D.S.; Sundrani, N.; Porotnikov, D.; Cassidy, J.; Sugiyama, Y.; Tarnovsky, A.N.; Mattoussi, H.; et al. Delayed Photoluminescence in Metal-Conjugated Fluorophores. J. Am. Chem. Soc. 2019, 141, 11286–11297. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Jensen, L.; Sung, J.; Zou, S.; Schatz, G.C.; Van Duyne, R.P. Interaction of Plasmon and Molecular Resonances for Rhodamine 6G Adsorbed on Silver Nanoparticles. J. Am. Chem. Soc. 2007, 129, 7647–7656. [Google Scholar] [CrossRef]
- Zhao, T.; Lia, T.; Liu, Y. Silver nanoparticle plasmonic enhanced förster resonance energy transfer (FRET) imaging of protein-specific sialylation on the cell surface. Nanoscale 2017, 9, 9841–9847. [Google Scholar] [CrossRef] [Green Version]
- Koppala, V.V.; Patil, P.G.; Melavank, R.M.; Kusanur, R.A.; Afi, U.O.; Patil, N.R. Exploring the influence of silver nanoparticles on the mechanism of fluorescence quenching of coumarin dye using FRET. J. Mol. Liq. 2019, 292, 111419. [Google Scholar] [CrossRef]
- Banerjee, S.; Preeyanka, N.; Dey, H.; Seth, S.; Rahaman, A.; Sarkar, M. Highly Efficient Energy Transfer from Fluorescent Gold Nanoclusters to Organic J-Aggregates. J. Phys. Chem. C 2020, 124, 5009–5020. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.; Lim, S.C.; Lim, K.; Lee, E.S.; Oh, K.T.; Choi, H.-G.; Youn, Y.S. Gold nanocluster-loaded hybrid albumin nanoparticles with fluorescence-based optical visualization and photothermal conversion for tumor detection/ablation. J. Control. Release 2019, 304, 7–18. [Google Scholar] [CrossRef]
- Dong, W.; Wang, R.; Gong, X.; Liang, W.; Dong, C. A far-red FRET fluorescent probe for ratiometric detection of L-cysteine based on carbon dots and N-acetylL-cysteine-capped gold nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 213, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Dubertret, B.; Calame, M.; Libchaber, A.J. Single-mismatch detection using gold-quenched fluorescent oligonucleotides. Nat. Biotechnol. 2001, 19, 365–370. [Google Scholar]
- Ma, H.; Li, Z.; Xue, N.; Cheng, Z.; Miao, X. A gold nanoparticle based fluorescent probe for simultaneous recognition of single-stranded DNA and double-stranded DNA. Microchim. Acta 2018, 185, 93. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C.L. Application of Nanomaterials in Biomedical Imaging and Cancer Therapy. Nanomaterials 2020, 10, 1700. [Google Scholar] [CrossRef]
- Kravets, V.; Almemar, Z.; Jiang, K.; Culhane, K.; Machado, R.; Hagen, G.; Kotko, A.; Dmytruk, I.; Spendier, K.; Pinchuk, A. Imaging of Biological Cells Using Luminescent Silver Nanoparticles. Nanoscale Res. Lett. 2016, 11, 30. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, D.A.; Fernandes, D.D.; Li, Y.; Wang, Y.; Zhang, Z.; Rousseau, D.R.; Gradinaru, C.C.; Kolios, M.C. Synthesis of stable multifunctional perfluorocarbon nanoemulsions for cancer therapy and imaging. Langmuir 2016, 32, 10870–10880. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, D.A.; Kolios, M.C. Perfluorocarbon bubbles as photoacoustic signal amplifiers for cancer theranostics. Opt. Mater. Express 2019, 9, 4532–4544. [Google Scholar] [CrossRef]
- Yan, N.; Tang, B.Z.; Wang, W.-X. In Vivo Bioimaging of Silver Nanoparticle Dissolution in the Gut Environment of Zooplankton. ACS Nano 2018, 12, 12212–12223. [Google Scholar] [CrossRef]
- Poon, C.K.; Tang, O.; Chen, X.M.; Kim, B.; Hartlieb, M.; Pollock, C.A.; Hawkett, B.S.; Perrier, S. Fluorescent Labeling and Biodistribution of Latex Nanoparticles Formed by Surfactant-Free RAFT Emulsion Polymerization. Macromol. Biosci. 2017, 17, 1600366. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Wang, C.; Wang, X.; Li, L. Facile Synthesis of Biocompatible Fluorescent Nanoparticles for Cellular Imaging and Targeted Detection of Cancer Cells. ACS Appl. Mater. Interfaces 2015, 7, 25077–25083. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, Y.; Huang, H.; Zhang, Y.; Du, B.; Zheng, Q.; He, X.; Wang, K.; Chen, F. Conjugated polyelectrolyte-stabilized silver nanoparticles coupled with pyrene derivative for ultrasensitive fluorescent detection of iodide. Talanta 2015, 131, 678–683. [Google Scholar] [CrossRef]
- Swanner, J.; Singh, R. Synthesis, Purification, Characterization, and Imaging of Cy3Functionalized Fluorescent Silver Nanoparticles in 2D and 3D Tumor Models. Methods Mol. Biol. 2018, 1790, 209–218. [Google Scholar] [PubMed]
- Aslan, K.; Gryczynski, I.; Malicka, J.; Matveeva, E.; Lakowicz, J.R.; Geddes, C.D. Metal-enhanced fluorescence: An emerging tool in biotechnology. Curr. Opin. Biotechnol. 2005, 16, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Chaganti, L.K.; Venkatakrishnan, N.; Bose, K. An efficient method for FITC labelling of proteins using tandem affinity purification. Biosci. Rep. 2018, 38, BSR20181764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tira, D.S.; Focsan, M.; Ulinici, S.; Maniu, D.; Astilean, S. Rhodamine B-Coated Gold Nanoparticles as Effective “Turn-on” Fluorescent Sensors for Detection of Zinc II Ions in Water. Spectrosc. Lett. 2013, 47, 153–159. [Google Scholar] [CrossRef]
- Petri, L.; Szijj, P.A.; Kelemen, A.; Imre, T.; Gomory, A.; Lee, M.T.W.; Hegedus, K.; Abranyi-Balogh, P.; Chudasama, V.; Miklos Keser, G. Cysteine specific bioconjugation with benzyl isothiocyanates. RSC Adv. 2020, 10, 14928–14936. [Google Scholar] [CrossRef]
- Fratoddi, I.; Cartoni, A.; Venditti, I.; Catone, D.; O’Keeffe, P.; Paladini, A.; Toschi, F.; Turchini, S.; Sciubba, F.; Testa, G.; et al. Gold nanoparticles functionalized by rhodamine B isothiocyanate to tune plasmonic effects. J. Colloid Interf. Sci. 2018, 513, 10–19. [Google Scholar] [CrossRef]
- Rossi, S.; Donadio, S.; Fontana, L.; Porcaro, F.; Battocchio, C.; Venditti, I.; Bracci, L.; Fratoddi, I. Negatively charged gold nanoparticles as dexamethasone carrier: Stability and citotoxic activity. RCS Adv. 2016, 6, 99016–99022. [Google Scholar]
- Catone, D.; Di Mario, L.; Martelli, F.; O’Keeffe, P.; Paladini, A.; Jacopo Stefano Pelli Cresi, J.S.; Sivan, A.K.; Tian, L.; Toschi, F.; Turchini, S. Ultrafast optical spectroscopy of semiconducting and plasmonic nanostructures and their hybrids. Nanotechnology. 2020, 32, 025703. [Google Scholar] [CrossRef]
- Sinigaglia, G.; Magro, M.; Miotto, G.; Cardillo, S.; Agostinelli, E.; Zboril, R.; Bidollari, E.; Vianello, F. Catalytically active bovine serum amine oxidase bound to fluorescent and magnetically drivable nanoparticles. Int. J. Nanomed. 2012, 7, 2249–2259. [Google Scholar]
- Bardsley, K.; Wimpenny, I.; Yang, Y.; El Haj, A.J. Fluorescent, online monitoring of PLGA degradation for regenerative medicine applications. RSC Adv. 2016, 6, 44364–44370. [Google Scholar] [CrossRef]
- Dou, X.; Zhang, L.; Liu, C.; Li, Q.; Luo, J.; Yang, M. Fluorometric competitive immunoassay for chlorpyrifos using rhodamine-modified gold nanoparticles as a label. Microchim. Acta 2018, 185, 41. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Rel. 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Eaton, P.; Quaresma, P.; Soares, C.; Neves, C.; de Almeida, M.P.; Pereira, E.; West, P. A direct comparison of experimental methods to measure dimensions of synthetic nanoparticles. Ultramicroscopy 2017, 182, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.K.; Yeap, S.P.; Che, H.X.; Low, S.C. Characterization of magnetic nanoparticle by dynamic light scattering. Nanoscale Res. Lett. 2013, 8, 381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swift, P.; Shuttleworth, D.; Seah, M.P. Practical Surface Analysis by Auger and X-ray Photoelectron Spectroscopy; Briggs, D., Seah, M.P., Eds.; J. Wiley & Sons: Chichester, UK, 1983; Chapter 4. [Google Scholar]
- Schiesaro, I.; Burratti, L.; Meneghini, C.; Fratoddi, I.; Prosposito, P.; Lim, J.; Scheu, C.; Venditti, I.; Iucci, G.; Battocchio, C. Hydrophilic Silver Nanoparticles for Hg(II) Detection in Water: Direct evidence for Mercury-Silver Interaction. J. Phys. Chem. C 2020, 124, 25975–25983. [Google Scholar] [CrossRef]
- Linstorm, P. NIST Chemistry webbook, NIST standard reference database number 69. J. Phys. Chem. Ref. Data Monogr. 1998, 9, 1–1951. [Google Scholar]
- Loscutoff, P.W.; Wong, K.T.; Bent, S.F. Reaction of phenyl isocyanate and phenyl isothiocyanate with the Ge(100)-2x1 surface J. Phys. Chem. C 2010, 114, 14193–14201. [Google Scholar] [CrossRef]
- Smirl, A.L.; Clark, J.B.; Van Stryland, E.W.; Russell, B.R. Population and rotational kinetics of the rhodamine B monomer and dimer: Picosecond transient spectrometry. J. Chem. Phys. 1982, 77, 631–640. [Google Scholar] [CrossRef] [Green Version]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A Review on Surface-Enhanced Raman Scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef] [Green Version]
- Smith, T.; Irwanto, M.; Haines, D.J.; Ghiggino, K.P.; Millar, D.P. Time-resolved fluorescence anisotropy measurements of the adsorption of Rhodamine-B and a labelled polyelectrolyte onto colloidal silica. Colloid Polym. Sci. 1998, 276, 1032–1037. [Google Scholar] [CrossRef]
- Olejnik, M.; Bujak, L.; Mackowski, S. Plasmonic molecular nanohybrids-spectral dependence of fluorescence quenching. Int. J. Mol. Sci. 2012, 13, 1018–1028. [Google Scholar] [CrossRef] [PubMed]

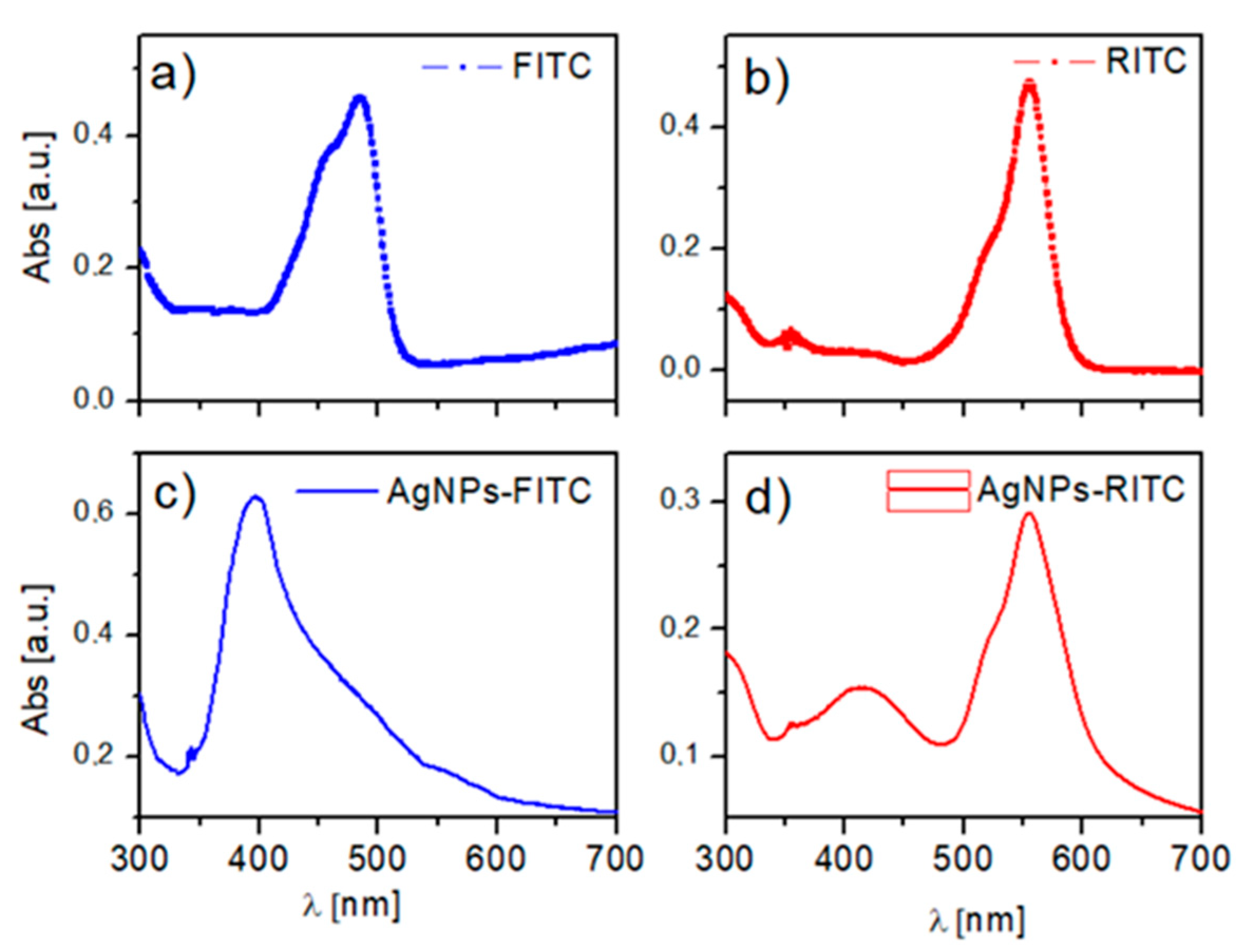
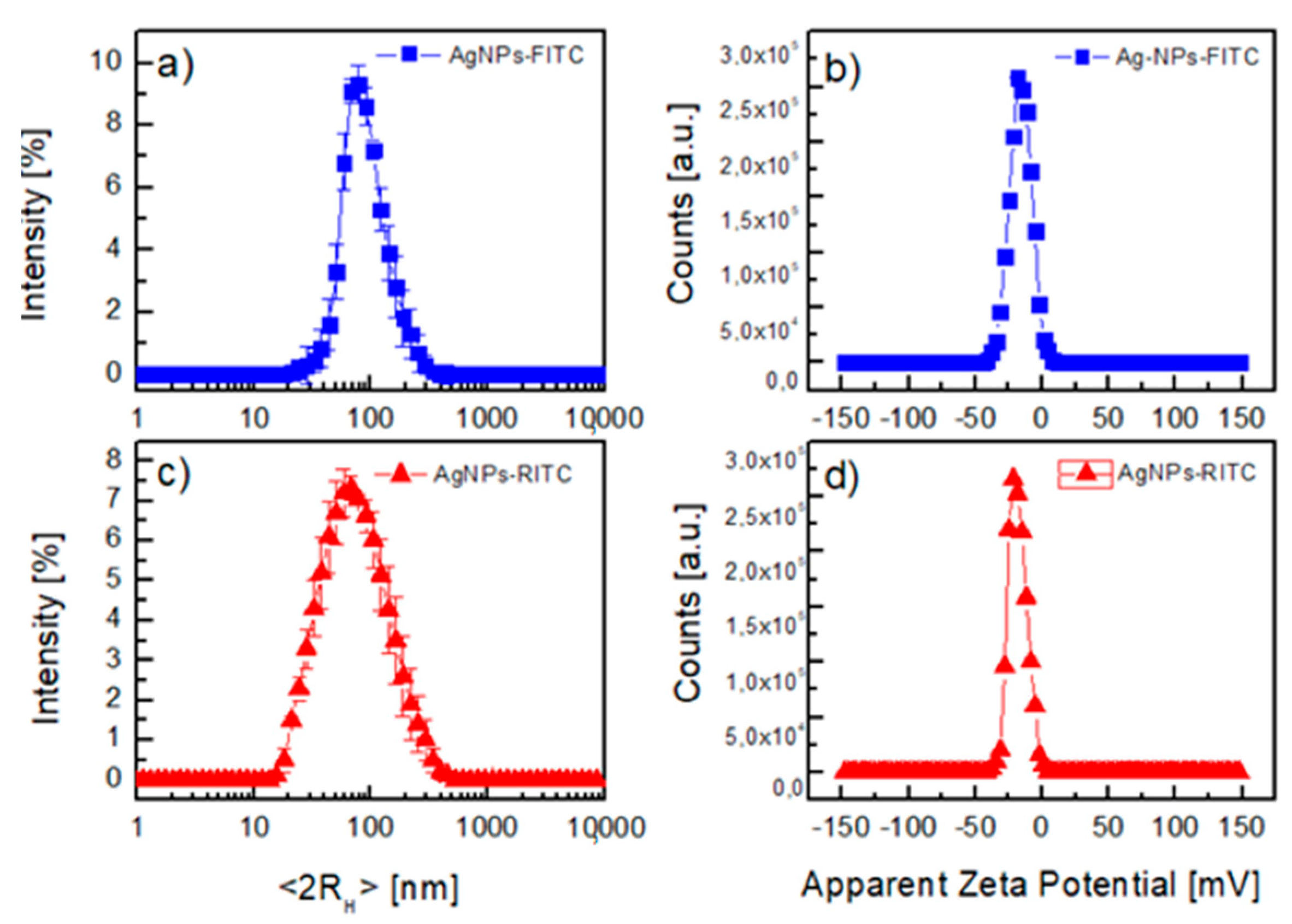

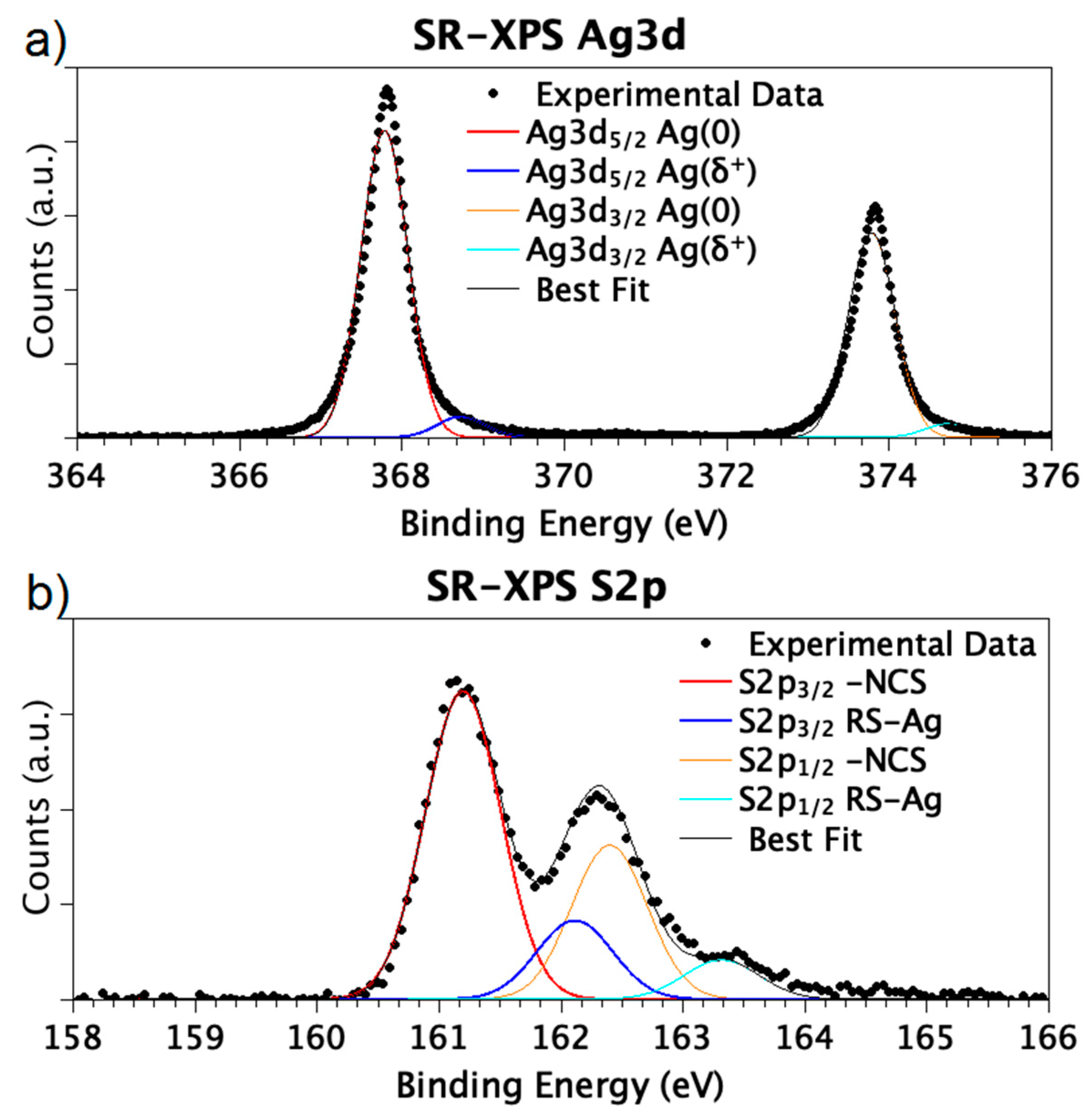
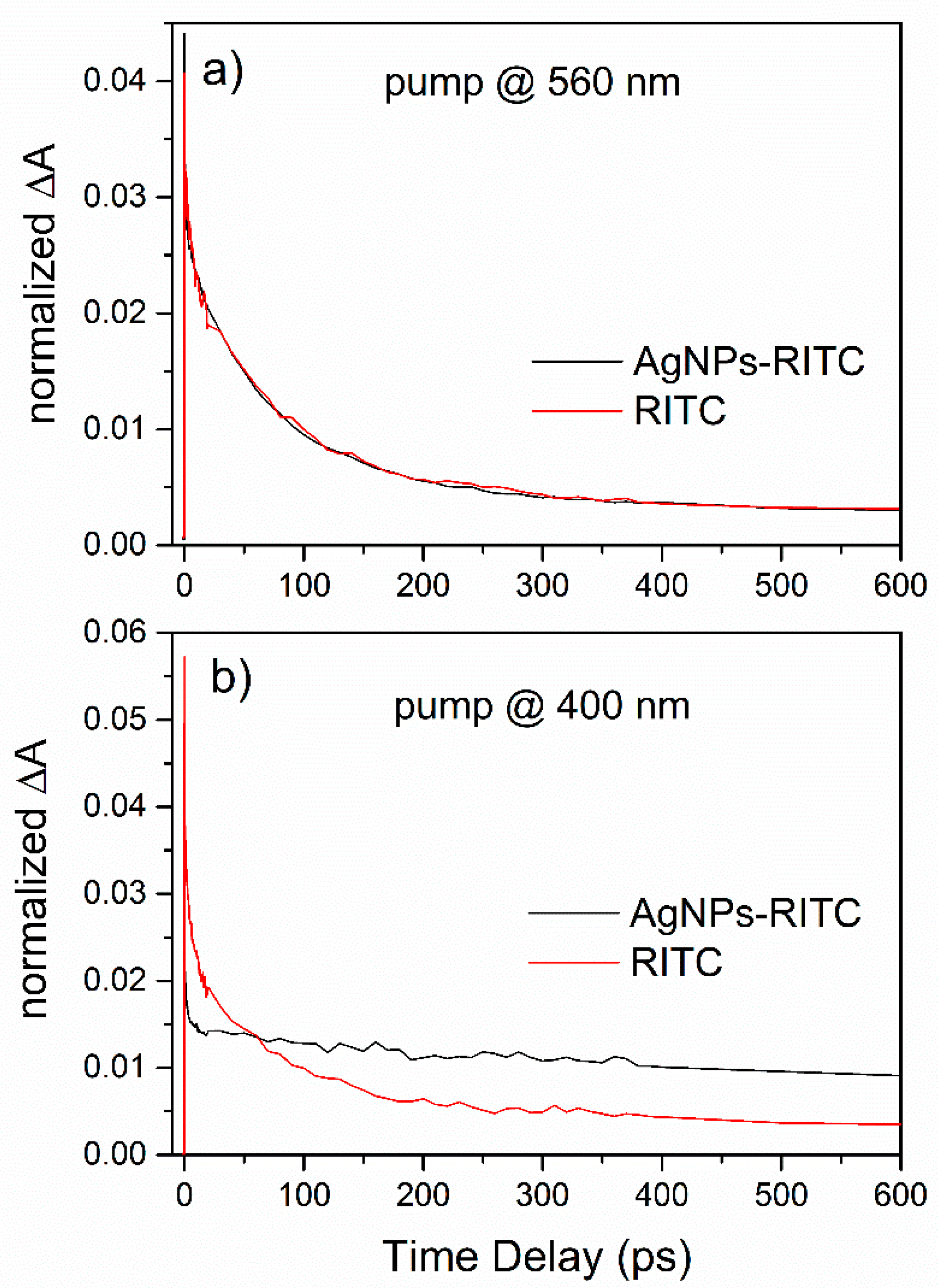
| Sample | BE (eV) | FWHM (eV) | Exp. Atomic Ratio | * Theor. Atomic Ratio | Assignment |
|---|---|---|---|---|---|
| AgNPs–FITC | 284.7 | 1.11 | 7.5 | 7.5 | C–C |
| 285.84 | 1.11 | 1 | 1 | C–N, SCN | |
| 286.43 | 1.11 | 1.2 | 1 | C–O | |
| 287.66 | 1.11 | 0.5 | 1.5 | C=O | |
| 288.84 | 1.11 | - | - | COOH (impurities) | |
| AgNPs–RITC | 284.7 | 1.31 | 20.9 | 18 | C–C |
| 285.8 | 1.31 | 8.1 | 7 | C–N, SCN | |
| 286.78 | 1.31 | 3.4 | 2 | C–O | |
| 287.85 | 1.31 | 2 | 2 | C=O | |
| 288.92 | 1.31 | 1 | 1 | COOH |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fratoddi, I.; Battocchio, C.; Iucci, G.; Catone, D.; Cartoni, A.; Paladini, A.; O’Keeffe, P.; Nappini, S.; Cerra, S.; Venditti, I. Silver Nanoparticles Functionalized by Fluorescein Isothiocyanate or Rhodamine B Isothiocyanate: Fluorescent and Plasmonic Materials. Appl. Sci. 2021, 11, 2472. https://doi.org/10.3390/app11062472
Fratoddi I, Battocchio C, Iucci G, Catone D, Cartoni A, Paladini A, O’Keeffe P, Nappini S, Cerra S, Venditti I. Silver Nanoparticles Functionalized by Fluorescein Isothiocyanate or Rhodamine B Isothiocyanate: Fluorescent and Plasmonic Materials. Applied Sciences. 2021; 11(6):2472. https://doi.org/10.3390/app11062472
Chicago/Turabian StyleFratoddi, Ilaria, Chiara Battocchio, Giovanna Iucci, Daniele Catone, Antonella Cartoni, Alessandra Paladini, Patrick O’Keeffe, Silvia Nappini, Sara Cerra, and Iole Venditti. 2021. "Silver Nanoparticles Functionalized by Fluorescein Isothiocyanate or Rhodamine B Isothiocyanate: Fluorescent and Plasmonic Materials" Applied Sciences 11, no. 6: 2472. https://doi.org/10.3390/app11062472






