Efficient Adsorbent-Desiccant Based on Aluminium Oxide
Abstract
1. Introduction
- -
- Interaction processes between adsorbents and water vapours must be fast. Adsorbents must have high absorption capacity, which will allow the gas to pass through adsorbers at a high rate and use compact adsorption plants for dehydration.
- -
- Adsorbents must have gigh stability after multiple regenerations.
- -
- Adsorbent grains must have high mechanical compression, (crushing) and abrasion strength.
- -
- Adsorbents must be inexpensive and easily regenerated.
- -
- Adsorbents must not react chemically during adsorption and regeneration.
- -
- Large internal pore volume
- -
- Large value of specific surface
- -
- Controlled pore-size distribution, preferably in the micropore range
- -
- Controlled properties of the surface, owing to selected functional groups
- -
- Weak interactions between an adsorbate and an adsorbent (in general, physical adsorption)
2. Methods of Obtainment of Aluminium Oxide Used in Industry
2.1. Methods of Alumina Obtainment
2.1.1. Bauxite Ore Treatment by the Baeyer Method
2.1.2. Sintering Technique
2.2. Methods of AO Obtainment
2.2.1. Aluminate Technology with Alkali Treatment
- (a)
- Dissolution of gibbsite in alkali, accompanied by the formation of sodium aluminate:
- (b)
- Reprecipitation with acid:
2.2.2. Acid Technology
- (a)
- Dissolution of gibbsite in acid:
- (b)
- Precipitation with alkali or ammonia water:
2.2.3. Rapid Calcination of Baeyer Hydrate (Gibbsite)
Method of Thermochemical Activation (TCA)
- -
- Dusty gas emissions
- -
- Probability of contamination of TCA products due to impurities in the fuel and products of its incomplete combustion
- -
- Instability of the operating mode, resulting in poor reproducibility of the physical and chemical properties of the flash product
- -
- Low efficiency of energy use of the heat carrier and, consequently, high specific energy consumption—more than 10 kJ/g of raw materials
Centrifugal Thermal Activation (CTA)
2.2.4. Synthesis of Aluminium Oxide from the Basic Aluminium Salt Al2(OH)5Cl or from Alcoholates Al(OR)3
3. Basic Methods of Moulding of Adsorbent Granules
3.1. Method of Disk Granulation of Adsorbents
3.2. Liquid Moulding Method
3.3. Extrusion Moulding Method
3.4. Comparison of Moulding Methods
4. Industrial Adsorbents-Desiccants and Their Characteristics
5. Physicochemical Properties of the Surface and Sorption Characteristics of Aluminium Oxide Adsorbents
5.1. Aluminium Oxide Surface Characteristics
5.2. Modification of Adsorbents Based on Aluminium Oxide with the Cations of Alkaline Metals
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- White, D.H. Compressed Air and Gas Purification and Fractionation for High Purity Applications by Improved PSA Processes. Sep. Sci. Technol. 2008, 43, 2298–2306. [Google Scholar] [CrossRef]
- Achenbach, K. Dehumidification. Planning Guidelines for Technical Building Services and Specialist Planners. 2016. Available online: https://www.condairgroup.com/m/0/planningbrochure-dehumidification-161202-en.pdf (accessed on 23 December 2020).
- Atlas Copco. White Paper—Compressed Air Drying. 2016. Available online: https://www.atlascopco.com/content/dam/atlas-copco/compressor-technique/oil-free-air/documents/Compressed_air_drying_whitepaper_EN_Antwerp_2937016013.pdf (accessed on 23 December 2020).
- Stewart, M. Dehydration. In Surface Production Operations: Design of Gas-Handling Systems and Facilities; Gulf Professional Publishing: Houston, TX, USA, 2014; Volume 2, pp. 279–373. ISBN 9780123822086. [Google Scholar]
- Shuguang, D. Sorbent Technology. Encyclopedia of Chemical Processing. 2006. Available online: https://www.researchgate.net/publication/267778978_Sorbent_Technology (accessed on 23 December 2020).
- Ciahotný, K.; Hlinčík, T.; Vagenknechtová, A.; Prokeš, O. Adsorbents for the natural gas drying at CNG stations. Acta Montan. Slovaca 2016, 21, 306–313. Available online: https://actamont.tuke.sk/pdf/2016/n4/6ciahotny.pdf (accessed on 23 December 2020).
- Linsen, B. Physical and Chemical Aspects of Adsorbents and Catalysts; Academic Press: London, UK; New York, NY, USA, 1970; ISBN 0124511503. [Google Scholar]
- Desai, R.; Hussain, M.; Ruthven, D.M. Adsorption of water vapour on activated alumina: I—Equilibrium behaviour. Can. J. Chem. Eng. 1992, 70, 699–706. [Google Scholar] [CrossRef]
- Rudisill, E.N.; Hacskaylo, J.J.; LeVan, M.D. Coadsorption of hydrocarbons and water on BPL activated carbon. Ind. Eng. Chem. Res. 1992, 31, 1122–1130. [Google Scholar] [CrossRef]
- Rege, S.U.; Yang, R.T.; Buzanowski, M.A. Sorbents for air prepurification in air separation. Chem. Eng. Sci. 2000, 55, 4827–4838. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, C.-H.; Kim, W.-S.; Lee, J.-S.; Kim, J.-T.; Suh, J.-K.; Lee, J.-M. Adsorption of water vapour on alumina, zeolite 13X and a zeolite X/activated carbon composite. J. Chem. Eng. Data 2003, 48, 137–141. [Google Scholar] [CrossRef]
- Serbezov, A. Adsorption equilibrium of water vapour on F-200 activated alumina. J. Chem. Eng. Data 2003, 48, 421–425. [Google Scholar] [CrossRef]
- CECA. Molecular Sieves. General Technical Information. 2015. Available online: https://www.cecachemicals.com/export/sites/ceca/.content/medias/downloads/products/dtm/brochure-gti-siliporite-2015_bdef.pdf (accessed on 23 December 2020).
- Keltsev, N.V. Fundamentals of Adsorption Technology; Chemistry: Moscow, Russia, 1984; p. 595. [Google Scholar]
- Ruthven, D.M.; Farooq, S.; Knaebel, K.S. Pressure Swing Adsorption; VCH: Weinheim, Germany; Cambridge, UK; New York, NY, USA, 1994; p. 352. ISBN 3527895175. [Google Scholar]
- Fleming, H.L. Adsorption on aluminas—Current applications. Stud. Surf. Sci. Catal. 1999, 120, 561–585. [Google Scholar]
- Ivanova, A.S. Aluminium oxide and systems based on it: Properties and applications. Kinet. Catal. 2012, 53, 425–439. [Google Scholar] [CrossRef]
- Zykova, A.; Livanova, A.; Kosova, N.; Godymchuk, A.; Mamontov, G. Aluminium oxide-hydroxides obtained by hydrothermal synthesis: Influence of thermal treatment on phase composition and textural characteristics. IOP Conf. Ser. Mater. Sci. Eng. 2015, 98, 012032. [Google Scholar] [CrossRef]
- Shackelford, J.F.; Doremus, R.H. (Eds.) Ceramic and Glass Materials; Springer Science and Business Media, LLC: New York, NY, USA, 2008; p. 201. ISBN 978-0-387-73362-3. [Google Scholar]
- Ingram-Jones, V.J.; Slade, R.C.T.; Davies, T.W.; Southem, J.C.; Salvador, S. Dehydroxylation sequences of gibbsite and boelumite: Study of differences between soak and flash calcination and of particle-size effects. J. Mater. Chem. 1996, 6, 73–79. [Google Scholar] [CrossRef]
- Wefers, K.; Misra, C. Oxides and Hydroxides of Aluminium; Alcoa Laboratories: Pittsburgh, PA, USA, 1987; Volume 19, p. 92. [Google Scholar]
- Yamada, K.; Harato, T.; Hamano, S.; Horinouchi, K. Dehydration products of gibbsite by rotary kiln and stational calciner. Light Met. 1984, 157–171. [Google Scholar] [CrossRef]
- Neymark, I.E. Synthetic Mineral Adsorbents and Catalyst Supports; Naukova Dumka: Kiev, Ukraine, 1982; p. 216. [Google Scholar]
- Stiles, A.B. Catalyst Supports and Supported Catalysts: Theoretical and Applied Concepts; Butterworth-Heinemann: Boston, MA, USA, 1987; p. 270. ISBN 9780409951486. [Google Scholar]
- Bhabhor, K.K.; Jani, D.B. Progressive development in solid desiccant cooling: A review. Int. J. Ambient. Energy 2019, 1–65. [Google Scholar] [CrossRef]
- Vivekh, P.; Kumja, M.; Bui, D.T.; Chua, K.J. Recent developments in solid desiccant coated heat exchangers—A review. Appl. Energy 2018, 229, 778–803. [Google Scholar] [CrossRef]
- Rabia, A.R.; Ibrahim, A.H.; Zulkepli, N.N. Activated alumina preparation and characterisation: The review on recent advancement. E3S Web Conf. 2018, 34. [Google Scholar] [CrossRef]
- Wang, L.; Shi, C.; Wang, L.; Pan, L.; Zhang, X.; Zou, J. Rational design, synthesis, adsorption principles and applications of metal oxide adsorbents: A review. Nanoscale 2020, 12, 4790–4815. [Google Scholar] [CrossRef]
- Hami, H.K.; Abbas, R.F.; Eltayef, E.M.; Mahdi, N.I. Applications of aluminium oxide and nano aluminium oxide as adsorbents: Review. Samarra J. Pure Appl. Sci. 2020, 2, 19–32. [Google Scholar]
- Abuzov, A.M. Aluminium Oxide and Alumina Ceramics (review). Part 1. Properties of Al2O3 and Commercial Production of Dispersed Al2O3. Refract. Ind. Ceram. 2019, 60, 24–32. [Google Scholar] [CrossRef]
- Wasserman, I.M. Chemical Deposition from Solutions; Chemistry: Leningrad, Russia, 1980; p. 208. [Google Scholar]
- Schüth, F.; Sing, K.S.W.; Weitkamp, J. Handbook of Porous Solids; Wiley-Vch: Weinheim, Germany, 2002; pp. 1591–1677. ISBN 9783527302468. [Google Scholar]
- Senyuta, A.; Panov, A.; Suss, A.; Layner, Y. Innovative technology for alumina production from low-grade raw materials. In Light Metals 2013; Springer: Cham, Switzerland, 2016; pp. 203–208. [Google Scholar] [CrossRef]
- Zolotovsky, B.P.; Bukhtiyarova, G.A.; Buyanov, R.A.; Murin, V.I.; Grunvald, V.R.; Efremova, L.V. Method for Obtaining Spherical Aluminium Oxide. R.U. Patent RU2096325C1, 20 November 1997. [Google Scholar]
- Icaev, B.N.; Kuyazev, V.M.; Moroz, E.M.; Murin, V.I.; Grunvald, V.R.; Efremova, L. Method of Preparing Granulated Activated Alumina. G.B. Patent GB1599374A, 30 September 1981. [Google Scholar]
- Lakhmostov, V.S.; Tanashev, Y.Y.; Sokolov, D.N.; Danilevich, V.V.; Zolotarskij, I.A.; Parmon, V.N. Method and Device for Pulse Heat Treatment of Loose Materials. R.U. Patent RU2264589C1, 20 November 2005. [Google Scholar]
- Lakhmostov, V.S.; Tanashev, Y.Y.; Sokolov, D.N.; Danilevich, V.V.; Zolotarskij, I.A.; Parmon, V.N. Device for Pulsed Heat Treatment of Bulk Materials. R.U. Patent RU2360196C2, 27 June 2009. [Google Scholar]
- Pinakov, V.I.; Stoyanovsky, O.I.; Tanashev, Y.Y.; Pikarevsky, A.A.; Grinberg, B.E.; Dryab, V.N.; Kulik, K.V.; Danilevich, V.V.; Kuznetsov, D.V.; Parmon, V.N. TSEFLARTM—the centrifugal flash reactor for rapid thermal treatment of powdered materials. Chem. Eng. J. 2005, 107, 157–161. [Google Scholar] [CrossRef]
- Kul’ko, E.V.; Ivanova, A.S.; Kruglyakov, V.Y.; Moroz, E.M.; Shefer, K.I.; Litvak, G.S.; Kryukova, G.N.; Tanashev, Y.Y.; Parmon, V.N. Synthesis of aluminium oxides from the products of the rapid thermal decomposition of hydrargillite in a centrifugal flash reactor: II. Structural and textural properties of aluminium hydroxide and oxide obtained from the product of the centrifugal thermal activation of hydrargillite (CTA product). Kinet. Catal. 2007, 48, 316–326. [Google Scholar]
- Mista, W.; Wrzyszcz, J. Rehydration of transition alumina obtained by flash calcination of gibbsite. Thermochim. Acta 1999, 331, 67–72. [Google Scholar] [CrossRef]
- Kharina, I.V.; Isupova, L.A.; Litvak, G.S.; Moroz, E.M.; Kryukova, G.N.; Rudina, N.A.; Tanashev, Y.Y.; Parmon, V.N. Synthesis of aluminium oxides from the products of the rapid thermal decomposition of hydrargillite in a centrifugal flash reactor: III. Properties of aluminium hydroxides and oxides obtained via the mild rehydration of the products of the centrifugal thermal activation of hydrargillite. Kinet. Catal. 2007, 48, 327–335. [Google Scholar] [CrossRef]
- Bollmann, U.; Becker, K.; Berger, H.-J.; Birke, P.; Engels, S.; Gruhn, G.; Jancke, K.; Kraak, P.; Lange, R.; Steinike, U. On preparation and reactivity of partial-crystalline aluminas. Cryst. Res. Technol. 1988, 23, 1303–1313. [Google Scholar] [CrossRef]
- Shkrabina, R.A.; Moroz, E.M.; Kambarova, T.D.; Khomyakova, L.G.; Bychkova, T.G.; Levitskii, E.A. Synthesis of various forms of aluminium hydroxide catalyst components from the thermal dispersion products of gibbsite. Kinet. Catal. 1981, 22, 1281–1285. [Google Scholar]
- Isupova, L.A.; Tanashev, Y.Y.; Kharina, I.V.; Moroz, E.M.; Litvak, G.S.; Boldyreva, N.N.; Paukshtis, E.A.; Burgina, E.B.; Budneva, A.A.; Shmakov, A.N.; et al. Physico-chemical properties of TSEFLARTM-treated gibbsite and its reactivity in rehydration process under mild conditions. Chem. Eng. J. 2005, 107, 163–169. [Google Scholar] [CrossRef]
- Isupova, L.A.; Kharina, I.V.; Parmon, V.N. Granulated Active Aluminium Oxide and Preparation Method Thereof. R.U. Patent RU2390495C2, 27 May 2010. [Google Scholar]
- Danilevich, V.V.; Isupova, L.A.; Kagyrmanova, A.P.; Kharina, I.V.; Zyuzin, D.A.; Noskov, A.S. Highly Effective Water Adsorbents Based on Aluminium Oxide. Kinet. Catal. 2012, 53, 632–639. [Google Scholar] [CrossRef]
- Ziegler, K. Linked polymerization of ethylene and its homologs. Brennstoff Chem. 1954, 35, 321–325. [Google Scholar]
- Poisson, R.; Brunelle, J.-P.; Nortier, P. Alumina. In Catalysts Supports Supported Catalysts; Stiles, A.B., Ed.; Butterworth-Heinemann: Boston, MA, USA, 1987; pp. 11–55. [Google Scholar]
- Jacques, R.; Poisson, R. Production of Spheroidal Alumina Shaped Articles. U.S. Patent US4273735A, 16 June 1981. [Google Scholar]
- Banta, F. Demetalation, Desulfurization, and Decarbonization of Petroleum Oils by Hydrotreatment in a Dual Bed System Prior to Cracking. U.S. Patent US4447314A, 8 May 1984. [Google Scholar]
- Gibson, K.R. Large Pore Shaped Hydroprocessing Catalysts. U.S. Patent US4489173, 18 December 1984. [Google Scholar]
- Jovanovic, N.; Novakovic, T.; Janacovich, J.; Terlecki-Baricevic, A. Properties of activated alumina obtained by flash calcination of gibbsite. J. Colloid Interface Sci. 1992, 150, 36–42. [Google Scholar] [CrossRef]
- Khoyakova, L.G.; Vorobev, Y.K.; Shkrabina, R.A.; Levutskij, E.A. Method of Producing Active Granulated Aluminium Oxide. S.U. Patent SU615645A1, 23 December 1986. [Google Scholar]
- Zolotovskii, B.P.; Buyanov, R.A.; Bukhtiyarova, G.A.; Taraban, E.A.; Murin, V.I.; Grunval’d, V.R.; Demin, V.V.; Saifullin, R.A. Production and properties of spherical alumina supports, adsorbents, and catalysts. Russ. J. Appl. Chem. 1997, 70, 285–291. [Google Scholar]
- Activated Alumina F200 Data Sheet—PSB Industries Inc. Available online: http://www.psbindustries.com/pdf/Activated%20Alumina%20F200%20Data%20Sheet.pdf (accessed on 23 December 2020).
- Ducreux, O.; Lavigne, C.; Nedez, C. Air and Gas Drying with Activated Aluminas. Available online: http://www.cabestisrl.com.ar/AirGas%20Drying%20Brochure.pdf (accessed on 23 December 2020).
- Sircar, S.; Rao, M.B.; Golden, T.C. Drying of gases and liquids by activated alumina. Stud. Surf. Sci. Catal. 1996, 99, 629–646. [Google Scholar] [CrossRef]
- Katalizator, J.S.C. Dryers and Supports. Available online: https://www.katcom.ru/upload/buklet_eng.pdf (accessed on 23 December 2020).
- Zotov, R.A.; Glazyrin, A.A.; Danilevich, V.V.; Kharina, I.V.; Zyuzin, D.A.; Volodin, A.M.; Isupova, L.A. Influence of the Temperature of Calcination of Bayerite-Containing Aluminium Hydroxide Pellets on the Water Vapour Adsorption Capacity and Acid-Base Properties of Alumina. Kinet. Catal. 2012, 53, 570–576. [Google Scholar] [CrossRef]
- Zolotarskii, I.A.; Voennov, L.I.; Zudilina, L.Y.; Isupova, L.A.; Zotov, R.A.; Medvedev, D.A.; Stepanov, D.A.; Livanova, A.V.; Meshcheryakov, E.P.; Kurzina, I.A. Theoretical Optimization of the Shape and Size of Adsorbent Grains for Associated Petroleum Gas Drying. Catal. Ind. 2018, 10, 49–56. [Google Scholar] [CrossRef]
- Tanabe, K.; Misono, M.; Hattori, H.; Ono, Y. New Solid Acids and Bases: Their Catalytic Properties; Elsevier Science: Tokyo, Japan, 1990; Volume 51, p. 364. [Google Scholar]
- Paukshtis, E.A. Infrared Spectroscopy in Heterogeneous: Acid Catalysis; Nauka: Novosibirsk, Russia, 1992; p. 255. ISBN 5-02-029281-8. [Google Scholar]
- Volodin, A.M.; Bolshov, V.A.; Konovalova, T.A. Photostimulated Formation of Radicals on Oxide Surfaces. Molec. Eng. 1994, 4, 201–206. [Google Scholar] [CrossRef]
- Bedilo, A.F.; Volodin, A.M. Radical Cations of Aromatic Molecules with High Ionization Potentials on the Surfaces of Oxide Catalysts: Formation, Properties, and Reactivity. Kinet. Catal. 2009, 50, 314–324. [Google Scholar] [CrossRef]
- Bedilo, A.F.; Ivanova, A.S.; Pahomov, N.A.; Volodin, A.M. Development of an ESR Technique for Testing Sulfated Zirconia Catalysts. J. Mol. Catal. A. 2000, 158, 409–412. [Google Scholar] [CrossRef]
- Sung, D.M.; Kim, Y.H.; Park, E.D. Correlation between acidity and catalytic activity for the methanol dehydration over various aluminium oxides. Res. Chem. Intermed. 2010, 36, 653–660. [Google Scholar] [CrossRef]
- Kulko, E.V.; Ivanova, A.S.; Budneva, A.A.; Paukshtis, E.A. Acid-base properties of alumina prepared from a hydrated product of centrifugal thermal activation of hydrargillite (CTA-product). React. Kinet. Catal. Lett. 2006, 88, 381–390. [Google Scholar] [CrossRef]
- Danilevich, V.V.; Isupova, L.A.; Danilova, I.G.; Zotov, R.A.; Ushakov, V.A. Characteristics optimization of activated alumina desiccants based on product of a centrifugal thermal activation of gibbsite. Russ. J. Appl. Chem. 2016, 89, 343–353. [Google Scholar] [CrossRef]
- Tanabe, K. Solid Acids and Bases—Their Catalytic Properties; Academic Press: New York, NY, USA; London, UK, 1970; p. 184. ISBN 9780323160582. [Google Scholar]
- Krylov, O.V. Heterogeneous Catalysis; IKC Academkniga: Moscow, Russia, 2004. [Google Scholar]
- Burova, M.V. Donor-Acceptor and Catalytic Properties of Systems Based on Aluminium and Zirconium Oxides. Ph.D. Thesis, Lomonosov Moscow State University, Moscow, Russia, 2007. [Google Scholar]
- Fionov, A.V.; Burova, M.V.; Tveritinova, E.A.; Krylova, I.V. Donor-acceptor properties, catalytic activity, and negative charge exoemission from the alumina surface modified with lithium cations. Russ. Chem. Bull. 2006, 55, 639–644. [Google Scholar] [CrossRef]
- Morterra, C.; Bolis, V.; Magnacca, G. IR spectroscopic and microcalorimetric characterisation of lewis acid sites on (transition phase) AI2O3 using adsorbed CO. Langmuir 1994, 10, 1812–1824. [Google Scholar] [CrossRef]
- Cortez, G.G.; Miguel, S.R.; Scelza, O.A.; Castro, A.A. Study of the Poisoning of y-Al2O3 by alkali metals addition. J. Chem. Technol. Biotechnol. 1992, 53, 177–180. [Google Scholar] [CrossRef]
- Romanova, R.G.; Petrova, E.V. Acid-base surface properties of aluminium oxides. Bull. Kazan Technol. Univ. 2006, 6, 73–90. [Google Scholar]
- Danilevich, V.V.; Isupova, L.A.; Paukshtis, E.A.; Ushakov, V.A. Effect of modifying desiccants with sulfuric acid on their physiochemical properties. Kinet. Catal. 2014, 55, 372–379. [Google Scholar] [CrossRef]
- Zotov, R.; Meshcheryakov, E.; Livanova, A.; Minakova, T.; Magaev, O.; Isupova, L.; Kurzina, I. Influence of the Composition, Structure, and Physical and Chemical Properties of Aluminium-Oxide-Based Sorbents on Water Adsorption Ability. Materials 2018, 11, 132. [Google Scholar] [CrossRef]
- Reshetnikov, S.I.; Livanova, A.V.; Meshcheryakov, E.P.; Kurzina, I.A.; Isupova, L.A. Kinetic Aspects of the Adsorption on Aluminium Oxide Drying Agents Doped with Alkali Metal Ions. Russ. J. Appl. Chem. 2017, 90, 1760–1765. [Google Scholar] [CrossRef]
- Zotov, R.A.; Isupova, L.A.; Danilevich, V.V.; Babina, A.A.; Sinelnikov, A.N.; Meshcheryakov, E.P.; Kurzina, I.A. Dynamic capacity dryers based on modified alumina at elevated pressures. Catal. Ind. 2017, 9, 91–98. [Google Scholar] [CrossRef]
- Isupova, L.A.; Danilova, I.G.; Danilevich, V.V.; Ushakov, V.A. Enhancement of the Sorption Ability of Aluminium Oxide Desiccants by Alkaline Modification. Russ. J. Appl. Chem. 2017, 90, 1810–1818. [Google Scholar] [CrossRef]
- Reshetnikov, S.; Kurzina, I.; Livanova, A.; Meshcheryakov, E.; Isupova, L. Effect of Li, Na and K Modification of Alumina on its Physical and Chemical Properties and Water Adsorption Ability. Materials 2019, 12, 4212. [Google Scholar] [CrossRef] [PubMed]
- Kruglyakov, V.Y.; Glazyrin, A.V.; Mescheryakov, E.P.; Kurzina, I.A.; Isupova, L.A. A High-Performance Alumina Desiccant. Catal. Ind. 2020, 12, 169–175. [Google Scholar] [CrossRef]
- Meshcheryakov, E.; Kozlov, M.; Reshetnikov, S.; Isupova, L.; Livanova, A.; Kurzina, I. Studies of Water-Vapour Adsorption Dynamics of High-Efficiency Desiccant Based on Aluminium Oxide and NaX Zeolite. Appl. Sci. 2020, 10, 5320. [Google Scholar] [CrossRef]
- Desai, R.; Hussain, M.; Ruthven, D.M. Adsorption on Activated Alumina: I1—Kinetic Behaviour. Can. J. Chem. Eng. 1992, 70, 707–715. [Google Scholar] [CrossRef]
- Marcussen, L. The influence of temperature on effective diffusivity and adsorption kinetics for humid air-porous alumina. Chem. Eng. Sci. 1974, 29, 2061–2069. [Google Scholar] [CrossRef]
- Reshetnikov, S.I.; Kurzina, I.A. Investigation of adsorption of water vapour on porous aluminium oxide material. IOP Conf. Ser. Mater. Sci. Eng. 2019, 597, 1–6. [Google Scholar] [CrossRef]

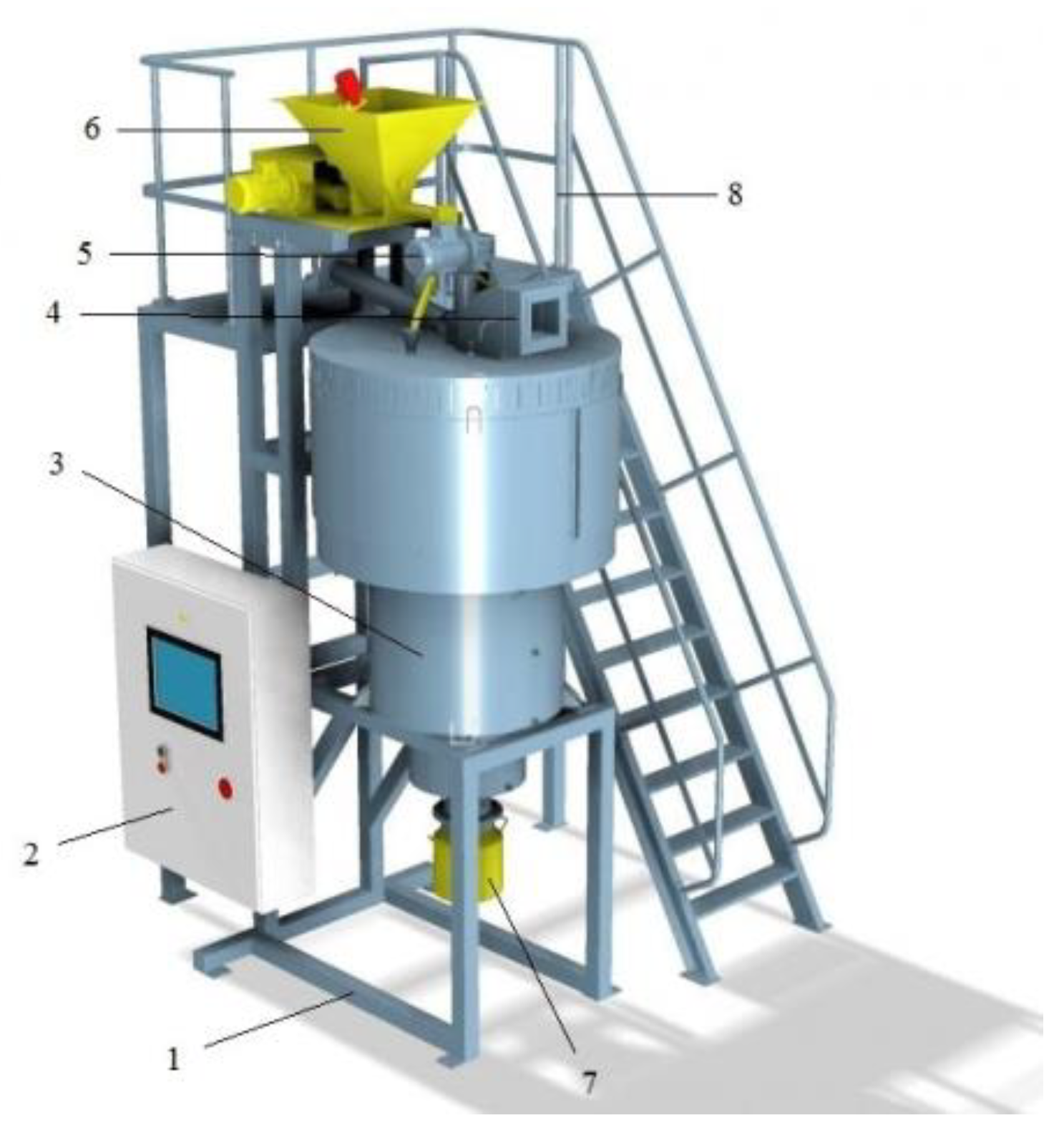

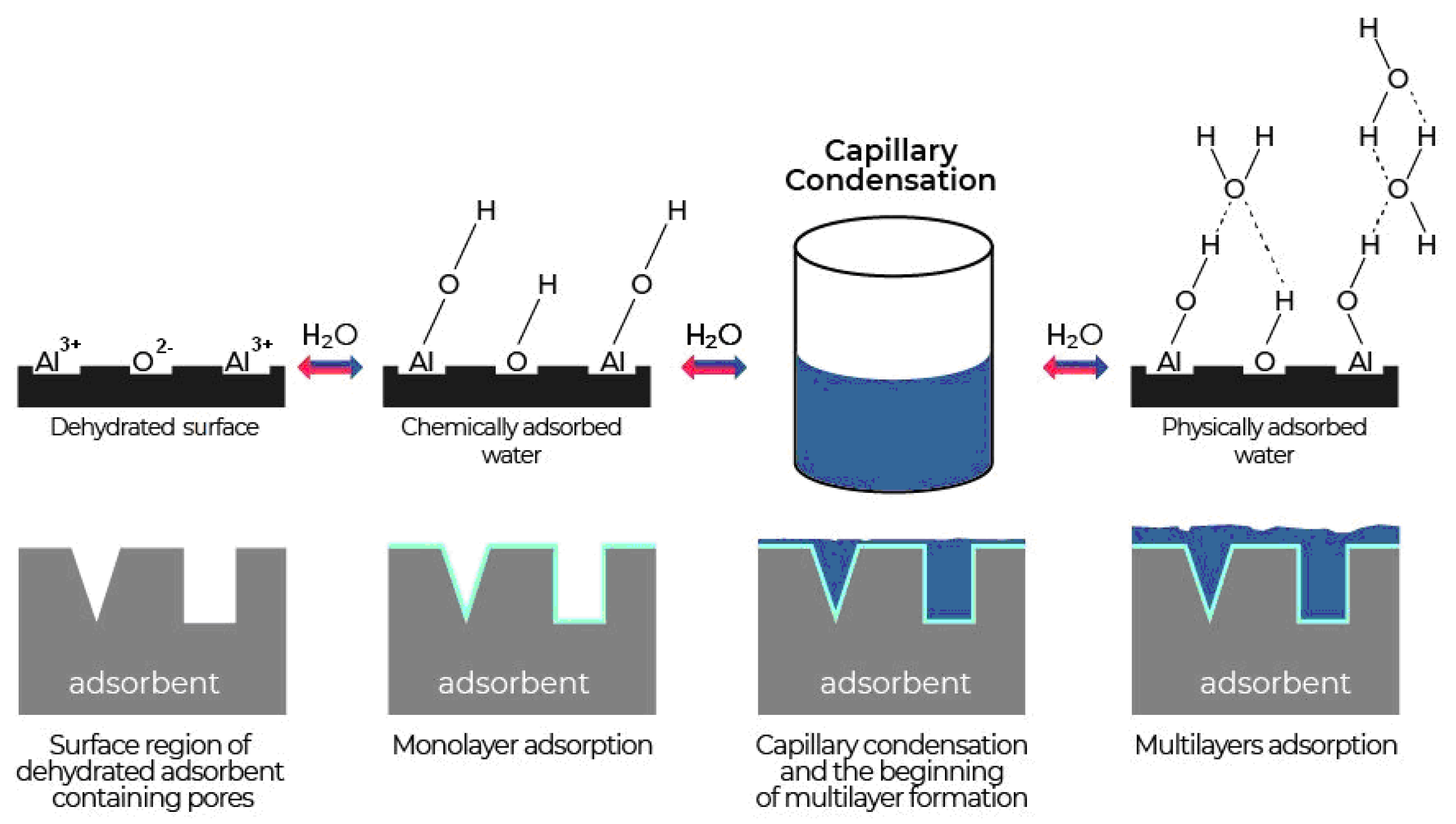
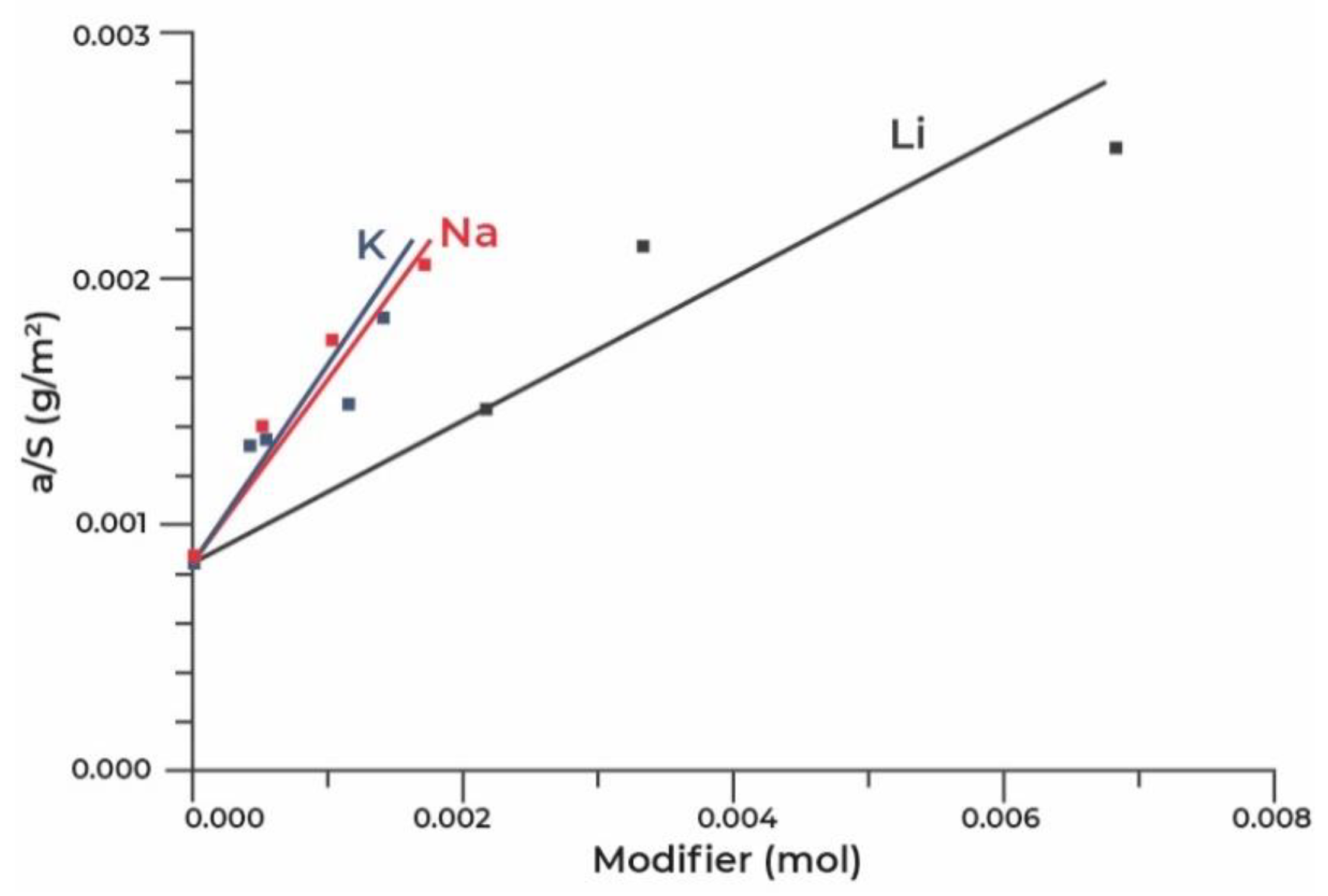
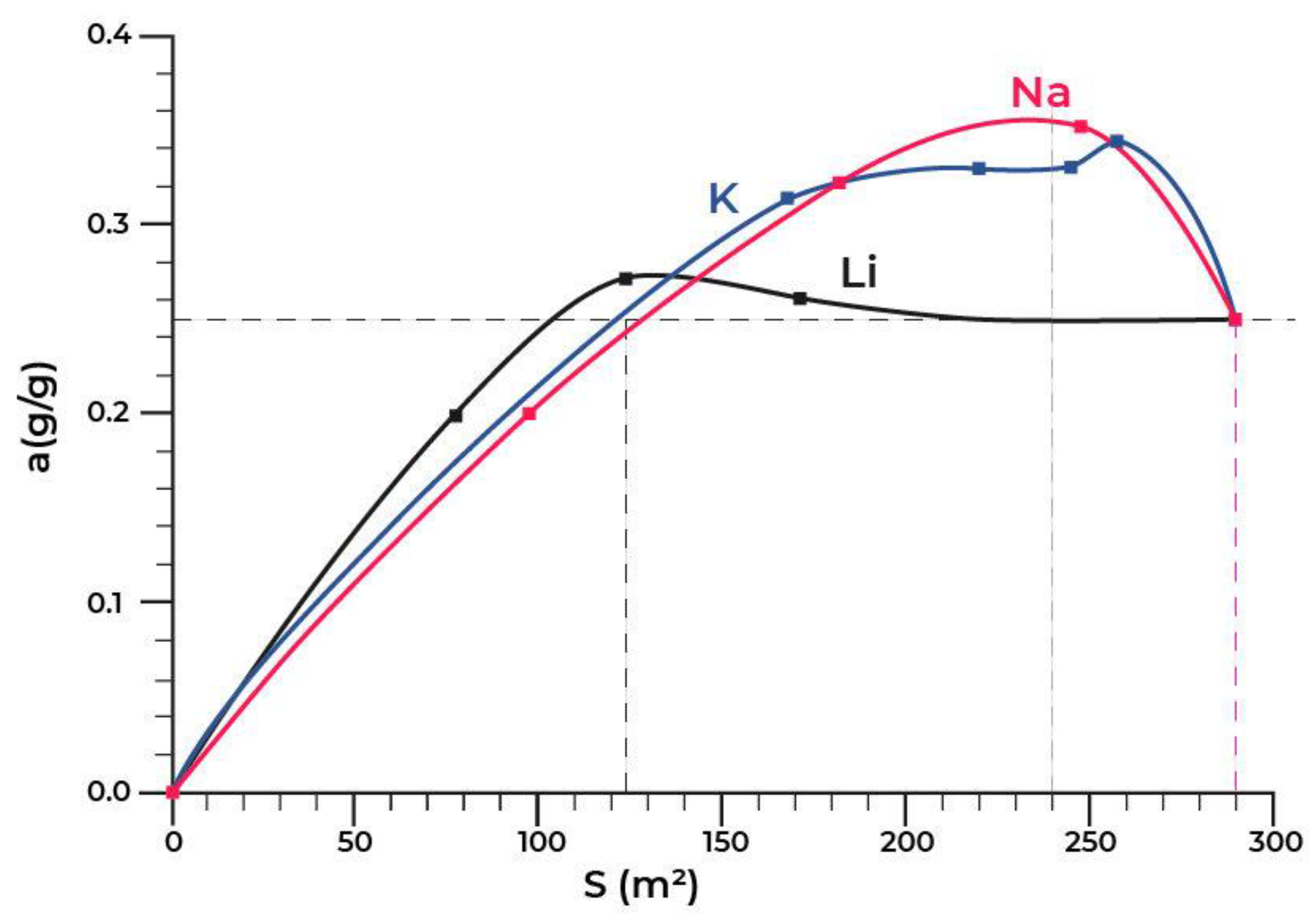
| Title | Scope of the Review | Year of Publication | Ref. |
|---|---|---|---|
| Progressive development in solid desiccant cooling: A review | In this paper review, the solid and liquid desiccant cooling systems were discussed in terms of their progressive development and working principle. | 2019 | [25] |
| Recent developments in solid desiccant coated heat exchangers | In this paper, the advantages of desiccant coated heat exchangers (DCHEs) over other dehumidifiers are established, and a comprehensive review of various desiccant materials and binders used in DCHEs was carried out. | 2018 | [26] |
| Activated alumina preparation and characterisation: The review on recent advancement | Activated alumina particles (AAP), synthesised electrochemically from aluminium, are an inexpensive material that can be used for water filtration due to its active surface. | 2018 | [27] |
| Rational design, synthesis, adsorption principles and applications of metal oxide adsorbents: a review | This review discussed the adsorption properties of several typical metal oxides and key parameters affecting adsorption performance and their applications for removing various inorganic and organic contaminants. | 2020 | [28] |
| Applications of aluminium oxide and nano aluminium oxide as adsorbents: review | The aim of this review was to clarify the role of aluminium oxide and nano aluminium oxide in removing some chemicals contaminants that influence human health, such as dyes, antibiotics and heavy metals. | 2020 | [29] |
| Typical Properties | F 200 “BASF” [55] | Axsorb 2–5 D “Axens” [56] | F-1 “Alcoa” [57] | Alusorb 675 Salavatski Catalytic Factory | AO Novomichurinski Catalytic Factory | |
|---|---|---|---|---|---|---|
| Grain size, mm | 4.7 | 2.0–5.0 | 4.0–8.0 | 7–12 | 2.0–5.0 | 2.8–8.0 |
| Specific surface area, m2/g | 340 | 335 | 330 | 260 | 387 | 294 |
| Pore volume, mL/g | 0.50 | 0.44 | 0.44 | 0.40 | 0.60 | 0.54 |
| Statistic capacity with 60% of relative humidity, % | 21.0 | 21.5 | 21.0 | - | 21.0 | 11.0 |
| Packed bulk density, kg/L | 0.80 | 0.82 | 0.79 | 0.85 | 0.70 | 0.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meshcheryakov, E.P.; Reshetnikov, S.I.; Sandu, M.P.; Knyazev, A.S.; Kurzina, I.A. Efficient Adsorbent-Desiccant Based on Aluminium Oxide. Appl. Sci. 2021, 11, 2457. https://doi.org/10.3390/app11062457
Meshcheryakov EP, Reshetnikov SI, Sandu MP, Knyazev AS, Kurzina IA. Efficient Adsorbent-Desiccant Based on Aluminium Oxide. Applied Sciences. 2021; 11(6):2457. https://doi.org/10.3390/app11062457
Chicago/Turabian StyleMeshcheryakov, Eugene P., Sergey I. Reshetnikov, Mariya P. Sandu, Alexey S. Knyazev, and Irina A. Kurzina. 2021. "Efficient Adsorbent-Desiccant Based on Aluminium Oxide" Applied Sciences 11, no. 6: 2457. https://doi.org/10.3390/app11062457
APA StyleMeshcheryakov, E. P., Reshetnikov, S. I., Sandu, M. P., Knyazev, A. S., & Kurzina, I. A. (2021). Efficient Adsorbent-Desiccant Based on Aluminium Oxide. Applied Sciences, 11(6), 2457. https://doi.org/10.3390/app11062457







