The 2011–2020 Trends of Data-Driven Approaches in Medical Informatics for Active Pharmacovigilance
Abstract
1. Introduction
2. Published Trends
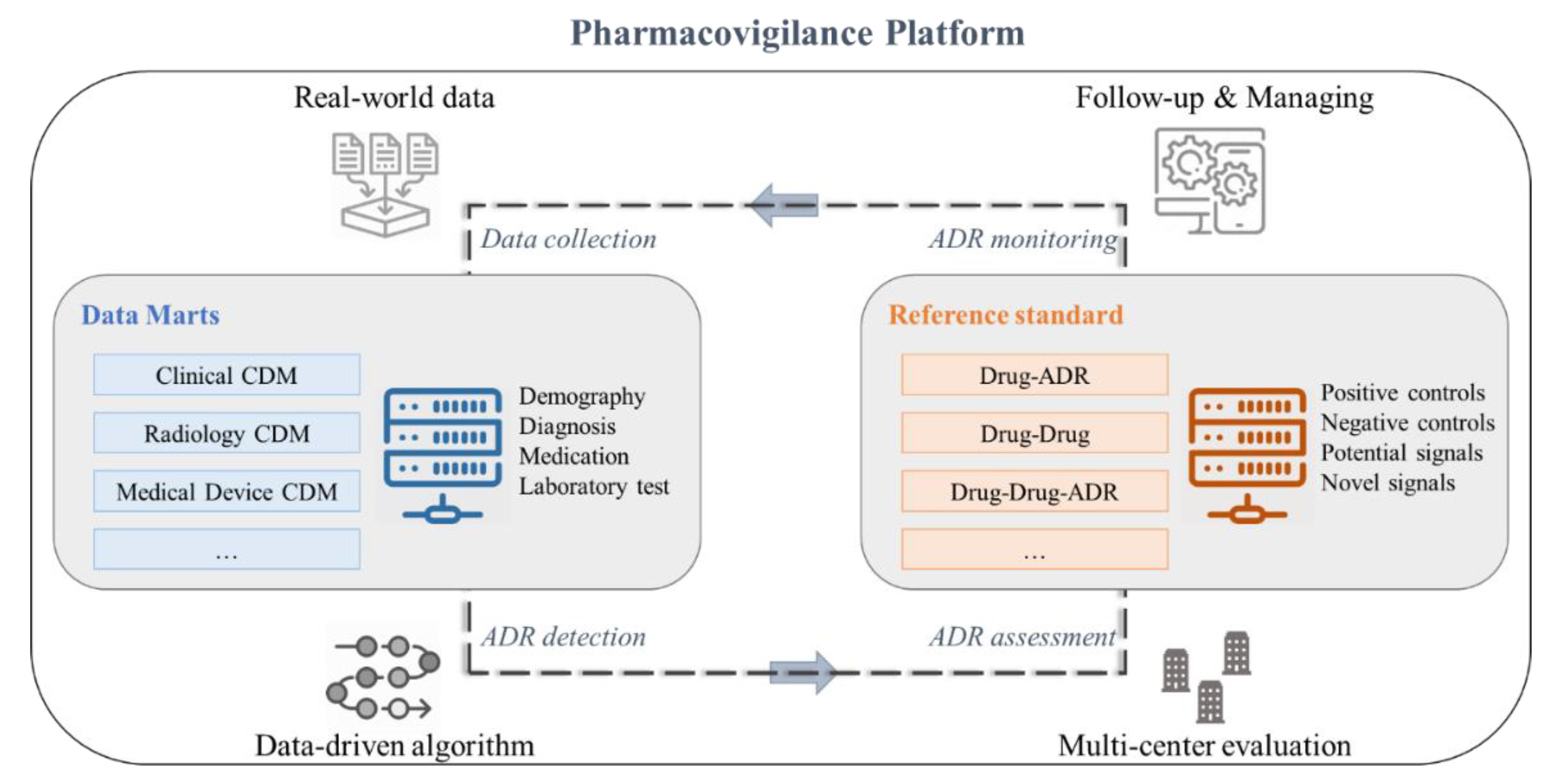
3. Pharmacovigilance Systems
3.1. Data Collection
3.2. ADR Detection for Pharmacovigilance
3.3. Assessment for ADR
3.4. ADR Monitoring for Patient Safety
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Toklu, H.Z.; Mensah, E. Why do we need pharmacists in pharmacovigilance systems? Online J. Public Health Inf. 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Hultgren, K.E. Complementing the us food and drug administration adverse event reporting system with adverse drug reaction reporting from social media: Comparative analysis. JMIR Public Health Surveill. 2020, 6, e19266. [Google Scholar] [CrossRef]
- Ingle, S.S.; Bansod, A.K.; Bashir, M.S.M. Adverse drug reaction profile in Amravati region of India: A pharmacovigilance study. J. Pharm. Bioallied Sci. 2020, 12, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine Committee on Quality of Health Care in America. To Err is Human: Building a Safer Health System; Kohn, L.T., Corrigan, J.M., Donaldson, M.S., Eds.; National Academies Press (US): Washington, DC, USA, 2000. [Google Scholar]
- Preventable Adverse Drug Reactions: A Focus on Drug Interactions. Available online: https://www.fda.gov/drugs/drug-interactions-labeling/preventable-adverse-drug-reactions-focus-drug-interactions (accessed on 24 December 2020).
- Harpaz, R.; DuMochel, W.; Shah, N. Big Data and Adverse Drug Reaction Detection. Clin. Pharmacol. Ther. 2015, 99, 268–270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wu, H.-Y.; Chiang, C.-W.; Wang, L.; Binkheder, S.; Wang, X.; Zeng, D.; Quinney, S.K.; Donglin, Z. Translational biomedical informatics and pharmacometrics approaches in the drug interactions research. CPT Pharmacomet. Syst. Pharmacol. 2018, 7, 90–102. [Google Scholar] [CrossRef]
- Vilar, S.; Friedman, C.; Hripcsak, G. Detection of drug–drug interactions through data mining studies using clinical sources, scientific literature and social media. Briefings Bioinform. 2018, 19, 863–877. [Google Scholar] [CrossRef] [PubMed]
- Coloma, P.M.; Trifirò, G.; Patadia, V.; Sturkenboom, M. Postmarketing safety surveillance. Drug Saf. 2013, 36, 183–197. [Google Scholar] [CrossRef]
- Mehta, U.; Kalk, E.; Boulle, A.; Nkambule, P.; Gouws, J.; Rees, H.; Cohen, K. Pharmacovigilance: A public health priority for South Africa. S. Afr. Health Rev. 2017, 2017, 125–133. [Google Scholar] [PubMed]
- Weaver, J.; Willy, M.; Avigan, M. Informatic tools and approaches in postmarketing pharmacovigilance used by FDA. AAPS J. 2008, 10, 35–41. [Google Scholar] [CrossRef][Green Version]
- Vohra, S.; Cvijovic, K.; Charrois, T.L.; Arnason, J.T.; Necyk, C.; Ware, M.; Rosychuk, R.J.; Boon, H.; Foster, B.C.; Jaeger, W.; et al. Study of natural health product adverse reactions (Sonar): Active surveillance of adverse events following concurrent natural health product and prescription drug use in community pharmacies. PLoS ONE 2012, 7, e45196. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, L.; Farrell, P.J.; McNair, D.; Krewski, D.; McNair, D. Statistical methods for active pharmacovigilance, with applications to diabetes drugs. J. Biopharm. Stat. 2014, 24, 856–873. [Google Scholar] [CrossRef]
- Naidu, M.V.S.; Sushma, D.S.; Jaiswal, V.; Asha, S.; Pal, T. The role of advanced technologies supplemented with traditional methods in pharmacovigilance sciences. Recent Patents Biotechnol. 2020, 14, 1–13. [Google Scholar] [CrossRef]
- Nikfarjam, A.; Ransohoff, J.D.; Callahan, A.; Jones, E.; Loew, B.; Kwong, B.Y.; Sarin, K.Y.; Shah, N.H. Early detection of adverse drug reactions in social health networks: A natural language processing pipeline for signal detection. JMIR Public Health Surveill. 2019, 5, e11264. [Google Scholar] [CrossRef]
- World Health Organization. The Importance of Pharmacovigilance: Safety Monitoring of Medicinal Products; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Chen, X.; Xie, H.; Cheng, G.; Poon, L.K.M.; Leng, M.; Wang, F.L. Trends and features of the applications of natural language processing techniques for clinical trials text analysis. Appl. Sci. 2020, 10, 2157. [Google Scholar] [CrossRef]
- Beninger, P. Pharmacovigilance: An overview. Clin. Ther. 2018, 40, 1991–2004. [Google Scholar] [CrossRef] [PubMed]
- Olsson, S.; Pal, S.N.; Dodoo, A. Pharmacovigilance in resource-limited countries. Expert Rev. Clin. Pharmacol. 2015, 8, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Hu, Y.; Liu, X.; Yin, Z.; Chen, X.-W.; Liu, M. Improving drug safety: From adverse drug reaction knowledge discovery to clinical implementation. Methods 2016, 110, 14–25. [Google Scholar] [CrossRef]
- Faillie, J.-L.; Montastruc, F.; Montastruc, J.-L.; Pariente, A. Pharmacoepidemiology and its input to pharmacovigilance. Therapies 2016, 71, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Souvignet, J.; Declerck, G.; Asfari, H.; Jaulent, M.-C.; Bousquet, C. OntoADR a semantic resource describing adverse drug reactions to support searching, coding, and information retrieval. J. Biomed. Inform. 2016, 63, 100–107. [Google Scholar] [CrossRef]
- Malec, S.A.; Wei, P.; Xu, H.; Bernstam, E.V.; Myneni, S.; Cohen, T. Literature-based discovery of confounding in observational clinical data. AMIA Annu. Symp. Proc. 2017, 2016, 1920–1929. [Google Scholar] [PubMed]
- Trifirò, G.; Sultana, J.; Bate, A. From big data to smart data for pharmacovigilance: The role of healthcare databases and other emerging sources. Drug Saf. 2017, 41, 143–149. [Google Scholar] [CrossRef]
- The Knowledge Base workgroup of the Observational Health Data Sciences and Informatics (OHDSI) Collaborative; Boyce, R.D. Large-scale adverse effects related to treatment evidence standardization (LAERTES): An open scalable system for linking pharmacovigilance evidence sources with clinical data. J. Biomed. Semant. 2017, 8, 11:1–11:15. [Google Scholar] [CrossRef]
- Fornasier, G.; Francescon, S.; Leone, R.; Baldo, P. An historical overview over Pharmacovigilance. Int. J. Clin. Pharm. 2018, 40, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Han, C.Y.; Kim, K.S.; Kim, S.G. Future directions of pharmacovigilance studies using electronic medical recording and human genetic databases. Toxicol. Res. 2019, 35, 319–330. [Google Scholar] [CrossRef] [PubMed]
- AlOmar, M.; Palaian, S.; Al-Tabakha, M.M. Pharmacovigilance in perspective: Drug withdrawals, data mining and policy implications. F1000Research 2019, 8, 2109. [Google Scholar] [CrossRef] [PubMed]
- Bihan, K.; Lebrun-Vignes, B.; Funck-Brentano, C.; Salem, J.-E. Uses of pharmacovigilance databases: An overview. Therapies 2020, 75, 591–598. [Google Scholar] [CrossRef]
- Lindquist, M. VigiBase, the WHO global ICSR database system: Basic facts. Ther. Innov. Regul. Sci. 2008, 42, 409–419. [Google Scholar] [CrossRef]
- Blake, K.V.; Zaccaria, C.; Domergue, F.; La Mache, E.; Saint-Raymond, A.; Hidalgo-Simon, A. Comparison between paediatric and adult suspected adverse drug reactions reported to the European medicines agency: Implications for pharmacovigilance. Pediatr. Drugs 2014, 16, 309–319. [Google Scholar] [CrossRef]
- Xu, R.; Wang, Q. Large-scale combining signals from both biomedical literature and the FDA Adverse Event Reporting System (FAERS) to improve post-marketing drug safety signal detection. BMC Bioinform. 2014, 15, 17. [Google Scholar] [CrossRef]
- Montastruc, G.; Favreliere, S.; Sommet, A.; Pathak, A.; Lapeyre-Mestre, M.; Perault-Pochat, M.-C.; Montastruc, J.-L. French Association of Regional PharmacoVigilance Centres Drugs and dilated cardiomyopathies: A case/noncase study in the French PharmacoVigilance Database. Br. J. Clin. Pharmacol. 2010, 69, 287–294. [Google Scholar] [CrossRef]
- Platt, R.; Wilson, M.; Chan, K.A.; Benner, J.S.; Marchibroda, J.; McClellan, M. The new sentinel network—improving the evidence of medical-product safety. N. Engl. J. Med. 2009, 361, 645–647. [Google Scholar] [CrossRef] [PubMed]
- Trifiro, G.; Fourrier-Reglat, A.; Sturkenboom, M.C.J.M.; D’iacuteaz, A.C.; Van Der Lei, J. The EU-ADR project: Preliminary results and perspective. SHTI 2009, 148, 43–49. [Google Scholar] [CrossRef]
- Dandala, B.; Joopudi, V.; Tsou, C.-H.; Liang, J.J.; Suryanarayanan, P. Extraction of information related to drug safety surveillance from electronic health record notes: Joint modeling of entities and relations using knowledge-aware neural attentive models. JMIR Med. Inform. 2020, 8, e18417. [Google Scholar] [CrossRef]
- Davazdahemami, B.; Delen, D. A chronological pharmacovigilance network analytics approach for predicting adverse drug events. J. Am. Med. Inform. Assoc. 2018, 25, 1311–1321. [Google Scholar] [CrossRef]
- Winnenburg, R.; Sorbello, A.; Ripple, A.; Harpaz, R.; Tonning, J.; Szarfman, A.; Francis, H.; Bodenreider, O. Leveraging MEDLINE indexing for pharmacovigilance – Inherent limitations and mitigation strategies. J. Biomed. Inform. 2015, 57, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Yang, H. Mining heterogeneous networks with topological features constructed from patient-contributed content for pharmacovigilance. Artif. Intell. Med. 2018, 90, 42–52. [Google Scholar] [CrossRef]
- McCarren, M.; Qiu, H.; Ziyadeh, N.; Jiang, R.; Wang, Y.; McAfee, A.T. Follow-up study of a pharmacovigilance signal. J. Clin. Psychopharmacol. 2012, 32, 743–749. [Google Scholar] [CrossRef]
- Sun, A.P.; Kirby, B.; Black, C.; Helms, P.J.; Bennie, M.; McLay, J.S. Unplanned medication discontinuation as a potential pharmacovigilance signal: A nested young person cohort study. BMC Pharmacol. Toxicol. 2014, 15, 11. [Google Scholar] [CrossRef]
- Dupuch, M.; Grabar, N. Semantic distance-based creation of clusters of pharmacovigilance terms and their evaluation. J. Biomed. Inform. 2015, 54, 174–185. [Google Scholar] [CrossRef]
- Pacurariu, A.C.; Straus, S.M.; Arlett, P.; Van Der Lei, J.; Sturkenboom, M.C.; Coloma, P.M.; Trifirò, G.; Schuemie, M.J.; Gini, R.; Herings, R.; et al. Useful interplay between spontaneous ADR reports and electronic healthcare records in signal detection. Drug Saf. 2015, 38, 1201–1210. [Google Scholar] [CrossRef]
- Usui, M.; Aramaki, E.; Iwao, T.; Wakamiya, S.; Sakamoto, T.; Mochizuki, M.; Mayer, M.A.; Aripin, K.N.B.N. Extraction and standardization of patient complaints from electronic medication histories for pharmacovigilance: Natural language processing analysis in japanese. JMIR Med. Inform. 2018, 6, e11021. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.; Daikou, S.; Ueno, K.; Batista-Navarro, R.; Tsujii, J.; Ananiadou, S. Annotation and detection of drug effects in text for pharmacovigilance. J. Chemin 2018, 10, 37–37:33. [Google Scholar] [CrossRef] [PubMed]
- Kiguba, R.; Ndagije, H.B.; Nambasa, V.; Bird, S.M. Adverse drug reaction onsets in Uganda’s Vigibase®: Delayed international visibility, data quality and illustrative signal detection analyses. Pharm. Med. 2018, 32, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Big data and pharmacovigilance: Data mining for adverse drug events and interactions. P T 2018, 43, 340–351. [Google Scholar] [PubMed]
- Wang, C.-S.; Lin, P.-J.; Cheng, C.-L.; Tai, S.-H.; Yang, Y.-H.K.; Chiang, J.-H. Detecting potential adverse drug reactions using a deep neural network model. J. Med. Internet Res. 2019, 21, e11016. [Google Scholar] [CrossRef]
- Richesson, R.; Hume, S.; Tsuji, S.; Huang, M.; Liu, H.; Shah, N.; Jiang, G.; Tingay, K.; Lin, C.-H.; Kijsanayotin, B.; et al. Detecting and filtering immune-related adverse events signal based on text mining and observational health data sciences and informatics common data model: Framework development study. JMIR Med. Inform. 2020, 8, e17353. [Google Scholar] [CrossRef]
- Wang, L.; Rastegar-Mojarad, M.; Liu, H.; Ji, Z.; Liu, S.; Liu, K.; Moon, S.; Shen, F.; Wang, Y.; Yao, L.; et al. Detecting pharmacovigilance signals combining electronic medical records with spontaneous reports: A case study of conventional disease-modifying antirheumatic drugs for rheumatoid arthritis. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef]
- Rho, M.J.; Kim, S.R.; Park, S.H.; Jang, K.S.; Park, B.J.; Hong, J.Y.; Choi, I.Y. Common data model for decision support system of adverse drug reaction to extract knowledge from multi-center database. Inf. Technol. Manag. 2015, 17, 57–66. [Google Scholar] [CrossRef]
- Vilar, S.; Harpaz, R.; Santana, L.; Uriarte, E.; Friedman, C. Enhancing adverse drug event detection in electronic health records using molecular structure similarity: Application to pancreatitis. PLoS ONE 2012, 7, e41471. [Google Scholar] [CrossRef]
- Coloma, P.M.; Avillach, P.; Sturkenboom, M.; Trifirò, G.; Salvo, F.; Schuemie, M.J.; Ferrajolo, C.; Pariente, A.; Fourrier-Réglat, A.; Molokhia, M.; et al. A reference standard for evaluation of methods for drug safety signal detection using electronic healthcare record databases. Drug Saf. 2013, 36, 13–23. [Google Scholar] [CrossRef]
- Crepin, S.; Godet, B.; Carrier, P.; Villeneuve, C.; Merle, L.; Laroche, M.-L. Probable drug-induced liver injury associated with aliskiren: Case report and review of adverse event reports from pharmacovigilance databases. Am. J. Health Pharm. 2014, 71, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Benkirane, R.; Soulaymani-Bencheikh, R.; Khattabi, A.; Benabdallah, G.; Alj, L.; Sefiani, H.; Hedna, K.; Ouammi, L.; Olsson, S.; Pal, S.N. Assessment of a new instrument for detecting preventable adverse drug reactions. Drug Saf. 2014, 38, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Winnenburg, R.; Shah, N.H. Generalized enrichment analysis improves the detection of adverse drug events from the biomedical literature. BMC Bioinform. 2016, 17, 250. [Google Scholar] [CrossRef] [PubMed]
- Batel-Marques, F.; Penedones, A.; Mendes, D.; Alves, C. A systematic review of observational studies evaluating costs of adverse drug reactions. Clin. Outcomes Res. 2016, 8, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Scalfaro, E.; Streefkerk, H.J.; Merz, M.; Meier, C.; Lewis, D. Preliminary results of a novel algorithmic method aiming to support initial causality assessment of routine pharmacovigilance case reports for medication-induced liver injury: The PV-RUCAM. Drug Saf. 2017, 40, 715–727. [Google Scholar] [CrossRef]
- Caster, O.; Dietrich, J.; Kürzinger, M.-L.; Lerch, M.; Maskell, S.; Norén, G.N.; Tcherny-Lessenot, S.; Vroman, B.; Wisniewski, A.; Van Stekelenborg, J. Assessment of the utility of social media for broad-ranging statistical signal detection in pharmacovigilance: Results from the WEB-RADR project. Drug Saf. 2018, 41, 1355–1369. [Google Scholar] [CrossRef]
- Oosterhuis, I.; Zweers, P.; Rümke, H.; Muller-Hansma, A.; Van Puijenbroek, E.P. A tailor-made approach for causality assessment for ADR reports on drugs and vaccines. Pharmacoepidemiol. Drug Saf. 2019, 28, 544–550. [Google Scholar] [CrossRef]
- Lee, S.; Han, J.; Park, R.W.; Kim, G.J.; Rim, J.H.; Cho, J.; Lee, K.H.; Lee, J.; Kim, S.; Kim, J.H. Development of a controlled vocabulary-based adverse drug reaction signal dictionary for multicenter electronic health record-based pharmacovigilance. Drug Saf. 2019, 42, 657–670. [Google Scholar] [CrossRef]
- Blake, K.V.; Prilla, S.; Accadebled, S.; Guimier, M.; Biscaro, M.; Persson, I.; Arlett, P.; Blackburn, S.; Fitt, H. European Medicines Agency review of post-authorisation studies with implications for the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance. Pharmacoepidemiol. Drug Saf. 2011, 20, 1021–1029. [Google Scholar] [CrossRef]
- Ruggiero, S.; Rafaniello, C.; Rossi, F.; Capuano, A.; Bravaccio, C.; Grimaldi, G.; Granato, R.; Pascotto, A.; Sportiello, L.; Parretta, E.; et al. Safety of attention-deficit/Hyperactivity disorder medications in children: An intensive pharmacosurveillance monitoring study. J. Child Adolesc. Psychopharmacol. 2012, 22, 415–422. [Google Scholar] [CrossRef]
- Härmark, L.; Alberts, S.; Van Puijenbroek, E.; Denig, P.; Van Grootheest, K. Representativeness of diabetes patients participating in a web-based adverse drug reaction monitoring system. Pharmacoepidemiol. Drug Saf. 2012, 22, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Hinz, E.R.M.; Matheny, M.E.; Denny, J.C.; Schildcrout, J.S.; A. Miller, R.; Xu, H. Comparative analysis of pharmacovigilance methods in the detection of adverse drug reactions using electronic medical records. J. Am. Med. Inform. Assoc. 2013, 20, 420–426. [Google Scholar] [CrossRef]
- Pal, S.N.; Olsson, S.; Brown, E.G. The monitoring medicines project: A multinational pharmacovigilance and public health project. Drug Saf. 2015, 38, 319–328. [Google Scholar] [CrossRef]
- Layton, D.; Shakir, S.A.W. Specialist cohort event monitoring studies: A new study method for risk management in pharmacovigilance. Drug Saf. 2015, 38, 153–163. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bahk, C.Y.; Goshgarian, M.; Donahue, K.; Freifeld, C.C.; Menone, C.M.; Pierce, C.E.; Rodriguez, H.; Brownstein, J.S.; Furberg, R.; Dasgupta, N. Increasing patient engagement in pharmacovigilance through online community outreach and mobile reporting applications: An analysis of adverse event reporting for the essure device in the US. Pharm. Med. 2015, 29, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Sarker, A.; Ginn, R.; Nikfarjam, A.; O’Connor, K.; Smith, K.; Jayaraman, S.; Upadhaya, T.; Gonzalez, G. Utilizing social media data for pharmacovigilance: A review. J. Biomed. Inform. 2015, 54, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Pierce, C.E.; Bouri, K.; Dasgupta, N.; Pamer, C.; Proestel, S.; Rodriguez, H.W.; Van Le, H.; Freifeld, C.C.; Brownstein, J.S.; Walderhaug, M.; et al. Evaluation of facebook and Twitter monitoring to detect safety signals for medical products: An analysis of recent FDA safety alerts. Drug Saf. 2017, 40, 317–331. [Google Scholar] [CrossRef]
- Irving, E.; Bor, R.V.D.; Welsing, P.; Walsh, V.; Alfonso-Cristancho, R.; Harvey, C.; Garman, N.; Grobbee, D.E. Series: Pragmatic trials and real world evidence: Paper 7. Safety, quality and monitoring. J. Clin. Epidemiol. 2017, 91, 6–12. [Google Scholar] [CrossRef]
- Simbrich, A.; for the REGIMS Investigators; Thibaut, J.; Khil, L.; Maximov, S.; Wiendl, H.; Berger, K. Chances and challenges of registry-based pharmacovigilance in multiple sclerosis: Lessons learnt from the implementation of the multicenter regims registry. Drug Saf. 2021, 44, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Moinuddin, K.; Al-Aqqad, A.Q.; Salem, S.O.; Al-Dossari, M.A.; Ananzeh, A.M.; Bin Baqar, J. Knowledge and attitude of health-care professionals toward adverse drug reactions reporting at King Saud Medical City. J. Pharm. Bioallied Sci. 2018, 10, 29. [Google Scholar] [CrossRef]
- Arulappen, A.L.; Danial, M.; Sulaiman, S.A.S. Evaluation of reported adverse drug reactions in antibiotic usage: A retrospective study from a tertiary care hospital, Malaysia. Front. Pharmacol. 2018, 9, 809. [Google Scholar] [CrossRef] [PubMed]


| “Pharmacovigilance” [MeSH] OR “Pharmacovigilance” [All Fields] OR “PV” [All Fields] |
| AND |
| “Drug-Related Side Effects and Adverse Reactions” [MeSH] OR “ADR” [All Fields] OR “ADRs” [All Fields] OR “ADE” [All Fields] OR “ADEs” [All Fields] OR “AE” [All Fields] OR “AEs” [All Fields] OR “Drug-induced” [All Fields] |
| AND |
| “Collection” [All Fields] OR “Detection” [All Fields] OR “Assessment” [All Fields] OR “Monitoring” [All Fields] |
| Author(s) | Data | Objective | Method(s) |
|---|---|---|---|
| Olsson et al. [19] | 1. SRSs | Legislation and regulatory framework, as well as financial support to build pharmacovigilance systems are needed | - |
| Tan et al. [20] | 1. SRSs 2. Drug information databases 3. ADE databases 4. Genetics and biochemical databases 5. Bibliographic databases | Presenting the current status of the clinical decision support system (CDSS) | 1. Text mining 2. NLP 3. Machine learning 4. Deep learning 5. Statistical analysis |
| Faillie et al. [21] | 1. SRSs 2. Drug information databases 3. Genetics and biochemical databases | Discuss the contribution of pharmacoepidemiology to pharmacovigilance | 1. Statistical analysis |
| Souvignet et al. [22] | 1. Drug information databases | Build a semantic resource based on formal description logic to aid the generation of on-demand custom groupings by appropriately selecting terms: OntoADR | 1. Statistical analysis |
| Malec et al. [23] | 1. EHRs 2. Drug information databases 3. Bibliographic databases | Presenting methods to discover confounding variables based on scalable literature | 1. NLP 2. Statistical analysis |
| Trifirò et al. [24] | 1. SRSs 2. Drug information databases 3. ADE databases | Discussion on the use of big data after drug safety evaluation | 1. NLP 2. Machine learning |
| The Knowledge Base workgroup of the Observational Health Data Sciences and Informatics (OHDSI) collaborative. [25] | 1. EHRs 2. SRSs 3. SPLs 4. Genetics and biochemical databases 5. Bibliographic databases | Introduce the structure and functionality of the Largescale Adverse Effects Related to Treatment Evidence Standardization (LAERTES) | 1. NLP 2. Machine learning 3. Statistical analysis |
| Fornasier et al. [26] | 1. SRSs 2. ADE databases | Understand the important role of pharmacovigilance as a historical phase | - |
| Choi et al. [27] | 1. EHRs 2. Genetics and biochemical databases | Future personalized therapy considering ADEs | 1. Statistical analysis |
| Alomar et al. [28] | 1. SRSs | The importance of policy framework in relation to pharmacovigilance is discussed in detail | 1. Machine learning 2. Statistical analysis |
| Author | Data | Objective | Methods |
|---|---|---|---|
| McCarren et al. [40] | 1. EHRs 2. Claims databases | Detailed study on antipsychotics prescribed to evaluate the effects of risperidone | 1. Statistical analysis |
| Sun et al. [41] | 1. EHRs | Assess the utility of unplanned medication discontinuation as a signal for possible ADEs in children and young people | 1. Statistical analysis |
| Dupuch and Grabar [42] | 1. SRSs 2. Drug information databases | Propose an automatic method to assist in the creation of Standardized MedDRA Queries (SMQs) using the clustering of terms | 1. Statistical analysis |
| Pacurariu et al. [43] | 1. SRSs 2. ADE databases 3. Bibliographic databases | Investigate the potential of EHRs to be used alongside SRSs, to improve signal detection | 1. Statistical analysis |
| Usui et al. [44] | 1. EHRs | Develop a method to extract and standardize patient complaints from electronic medication history data (EMHD) | 1. NLP 2. Machine learning |
| Thompson et al. [45] | 1. Bibliographic databases | Describe the development process of Pharmacovigilance Entity Drug Annotation (PHAEDRA) with annotation | 1. Text mining 2. Machine learning |
| Kiguba et al. [46] | 1. SRSs | Characterize the reported ADE onsets registered with VigiBase and describe an analytical approach for patients receiving antiretroviral therapy | 1. Statistical analysis |
| Ventola [47] | 1. EHRs 2. SRSs 3. ADE databases 4. Bibliographic databases 5. Social media | Discuss data mining for big data and pharmacovigilance | 1. Text mining 2. NLP 3. Machine learning 4. Statistical analysis |
| Wang et al. [48] | 1. ADE databases 2. Bibliographic databases | Identify a method to detect potential ADEs of drugs automatically using a deep neural network (DNN) | 1. Deep learning 2. Statistical analysis |
| Yu et al. [49] | 1. ADE databases 2. Bibliographic databases | Develop a new irAE signal detection and filtering framework concerning six FDA-approved immune checkpoint inhibitor drugs | 1. Text mining 2. Statistical analysis |
| Author | Data | Objective | Methods |
|---|---|---|---|
| Vilar et al. [52] | 1. EHRs | Develop molecular fingerprint-based models (MFBMs) to strengthen ADE signals generated from EHR data | 1. NLP 2. Statistical analysis |
| Coloma et al. [53] | 1. EHRs 2. SRSs 3. ADE databases 4. Bibliographic databases | Develop and evaluate methods in the EU-ADR project | 1. Statistical analysis |
| Crepin et al. [54] | 1. SRSs 2. Bibliographic databases 3. ADE databases | Review similar reports in pharmacovigilance databases | 1. Statistical analysis |
| Benkirane et al. [55] | 1. SRSs | Present P method (PM) and evaluate its inter-rater reliability | 1. Statistical analysis |
| Winnenburg and Shah [56] | 1. Bibliographic databases | Evaluate how to improve detection of side effects in MeSH | 1. NLP 2. Statistical analysis |
| Batel-Marques et al. [57] | 1. Bibliographic databases | Assess ADEs and their associated costs | 1. Statistical analysis |
| Scalfaro et al. [58] | 1. EHRs 2. SRSs | Evaluate the performance of Pharmacovigilance—Roussel Uclaf Causality Assessment Method (PV-RUCAM) | 1. Statistical analysis |
| Caster et al. [59] | 1. SRSs 2. Social media | Assess the performance of statistical signal detection algorithms established in social media data | 1. Statistical analysis |
| Oosterhuis et al. [60] | 1. Drug information databases 2. ADE databases | Test the validity and reliability of the causality documentation (CausDoc) tool | 1. Statistical analysis |
| Lee et al. [61] | 1. EHRs 2. ADE databases | Development for pharmacovigilance to detect and evaluate ADE signals based on multicenter EHR | 1. Statistical analysis |
| Author | Data | Objective | Methods |
|---|---|---|---|
| Blake et al. [62] | 1. Drug information databases 2. ADE databases | Provide a broad estimate of the need for pharmacoepidemiologic resources in the European Union (EU) | 1. Statistical analysis |
| Ruggiero et al. [63] | 1. SRSs | Systematic collection of more thorough data on the safety of atomoxetine and methylphenidate in pediatric settings | 1. Statistical analysis |
| Härmark et al. [64] | 1. Genetics and biochemical databases | Compare the LIM diabetes population with an external diabetes reference population on characteristics that may influence the patient’s susceptibility for ADEs | 1. Statistical analysis |
| Liu et al. [65] | 1. EHRs | Examine the use of retrospective medication orders and inpatient laboratory results documented in the EHR | 1. Statistical analysis |
| Pal et al. [66] | 1. SRSs | Support and strengthen consumer reporting of ADEs and develop methods to complement spontaneous reporting | 1. Statistical analysis |
| Layton and Shakir. [67] | 1. EHRs | Monitor, research, and add missing information before drug marketing | 1. Statistical analysis |
| Bahk et al. [68] | 1. SRSs 2. ADE databases | Evaluate the quality of data collected through a MedWatcher | 1. Statistical analysis |
| Sarker et al. [69] | 1. Bibliographic databases | Perform a methodical review to characterize the different approaches to ADEs detection/extraction from social media | 1. Text mining 2. NLP 3. Machine learning 4. Statistical analysis |
| Pierce et al. [70] | 1. SRSs 2. Social media | Examine whether specific product–adverse event pairs were reported via social media before being reported to FAERS | 1. Machine learning 2. Statistical analysis |
| Irving et al. [71] | 1. EHRs 2. SRSs | Describe the impact of design choices on the practical implementation of pragmatic trials | 1. Statistical analysis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, H.; Cha, J.; Lee, C.; Song, H.; Jeong, H.; Kim, J.-Y.; Lee, S. The 2011–2020 Trends of Data-Driven Approaches in Medical Informatics for Active Pharmacovigilance. Appl. Sci. 2021, 11, 2249. https://doi.org/10.3390/app11052249
Shin H, Cha J, Lee C, Song H, Jeong H, Kim J-Y, Lee S. The 2011–2020 Trends of Data-Driven Approaches in Medical Informatics for Active Pharmacovigilance. Applied Sciences. 2021; 11(5):2249. https://doi.org/10.3390/app11052249
Chicago/Turabian StyleShin, Hyunah, Jaehun Cha, Chungchun Lee, Hyejin Song, Hyuntae Jeong, Jong-Yeup Kim, and Suehyun Lee. 2021. "The 2011–2020 Trends of Data-Driven Approaches in Medical Informatics for Active Pharmacovigilance" Applied Sciences 11, no. 5: 2249. https://doi.org/10.3390/app11052249
APA StyleShin, H., Cha, J., Lee, C., Song, H., Jeong, H., Kim, J.-Y., & Lee, S. (2021). The 2011–2020 Trends of Data-Driven Approaches in Medical Informatics for Active Pharmacovigilance. Applied Sciences, 11(5), 2249. https://doi.org/10.3390/app11052249






