An Adaptive Federated Machine Learning-Based Intelligent System for Skin Disease Detection: A Step toward an Intelligent Dermoscopy Device
Abstract
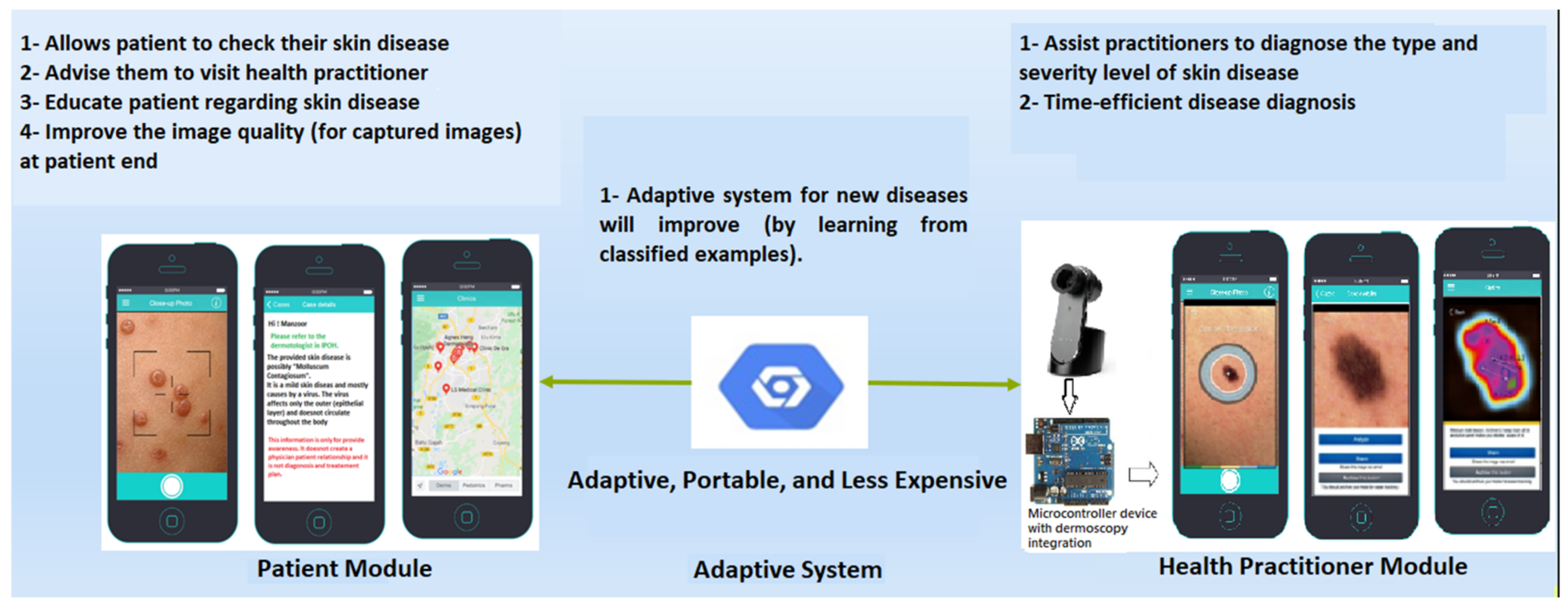
1. Introduction
Contribution
- This study proposes the idea of an adaptive federated machine learning-based skin disease detection system to assist dermatologists.
- This study proposes a federated machine learning-based adaptive framework for skin disease.
- This study validates the proposed model’s classification performance and adaptability at the edge (local device) and cloud (global server) level.
2. Related Work and Theoretical Foundation
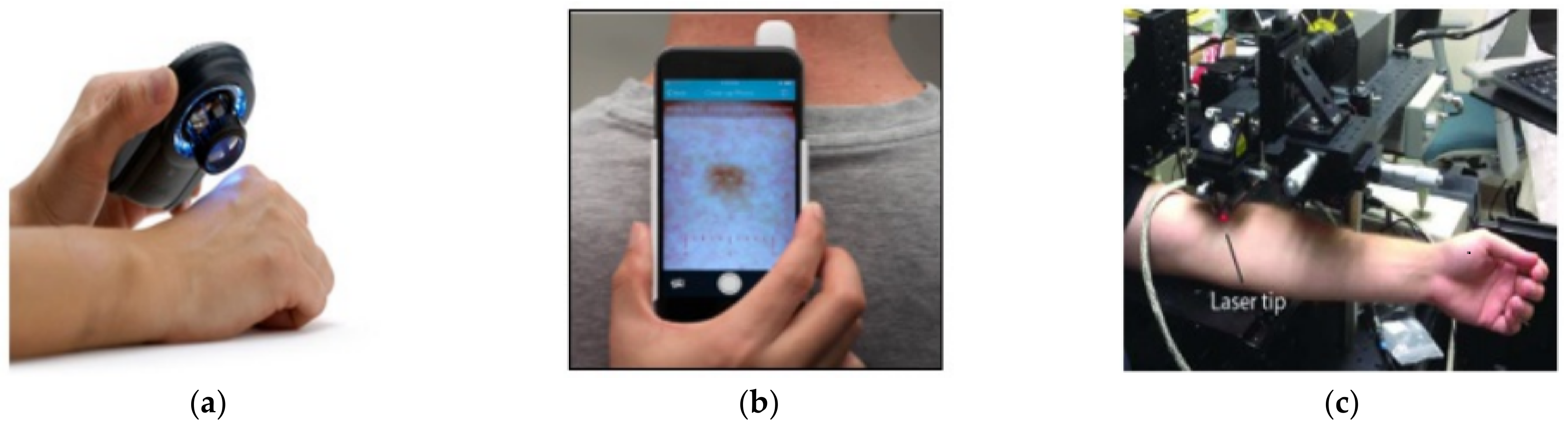
2.1. Existing Devices Used for Skin Disease Analysis
2.2. Current State of Machine Learning in Skin Disease Detection
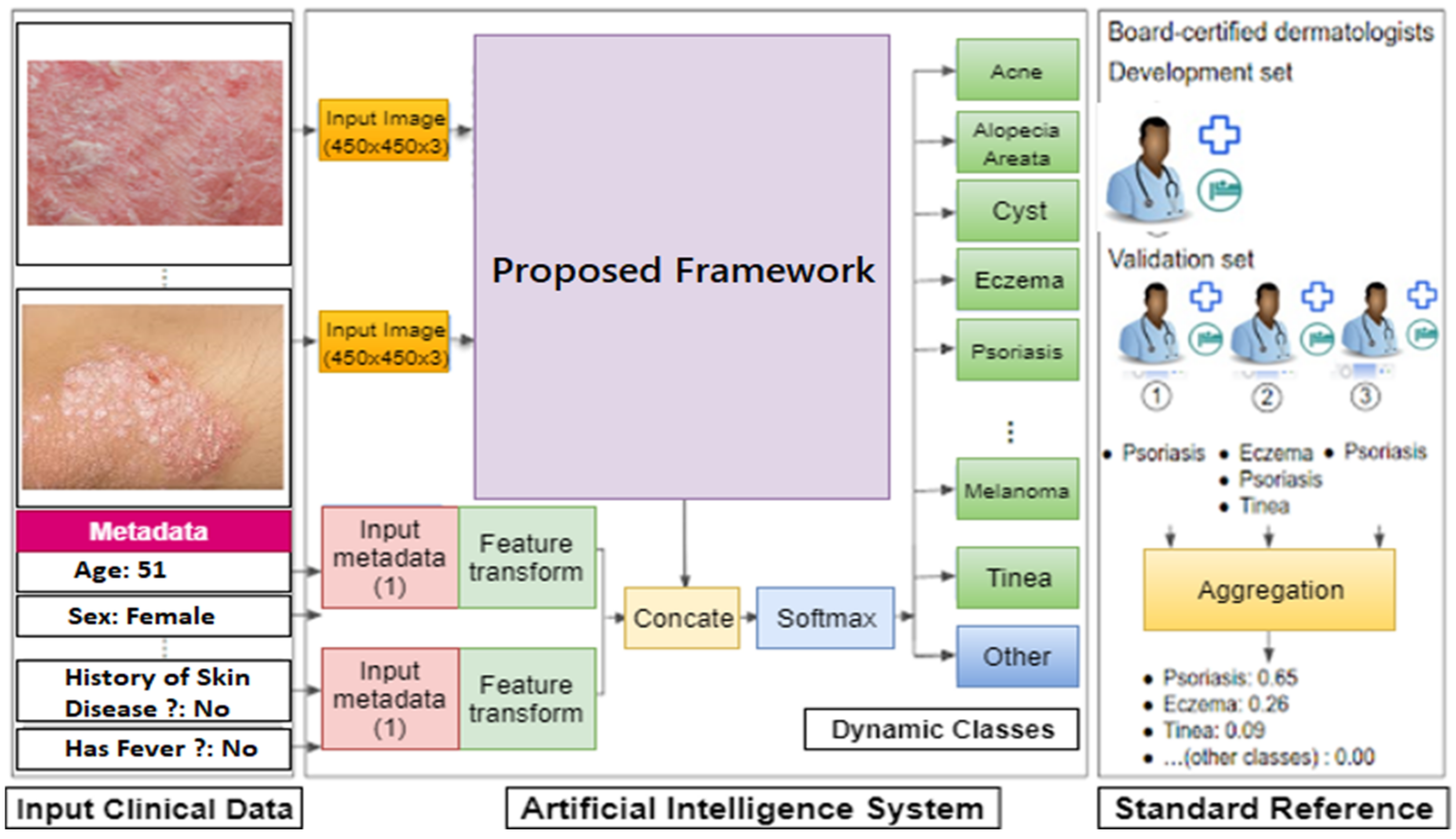
3. Methodology
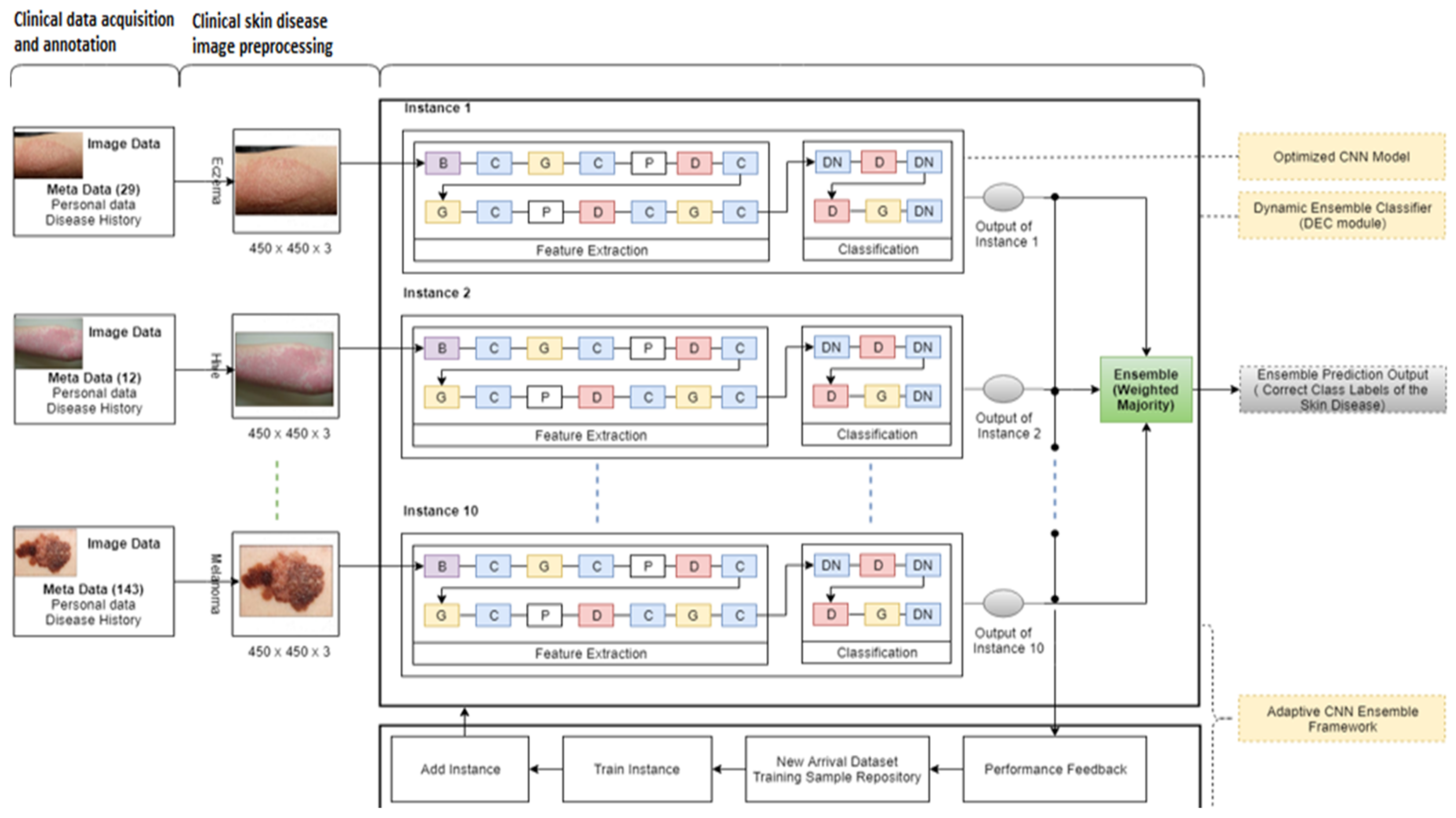
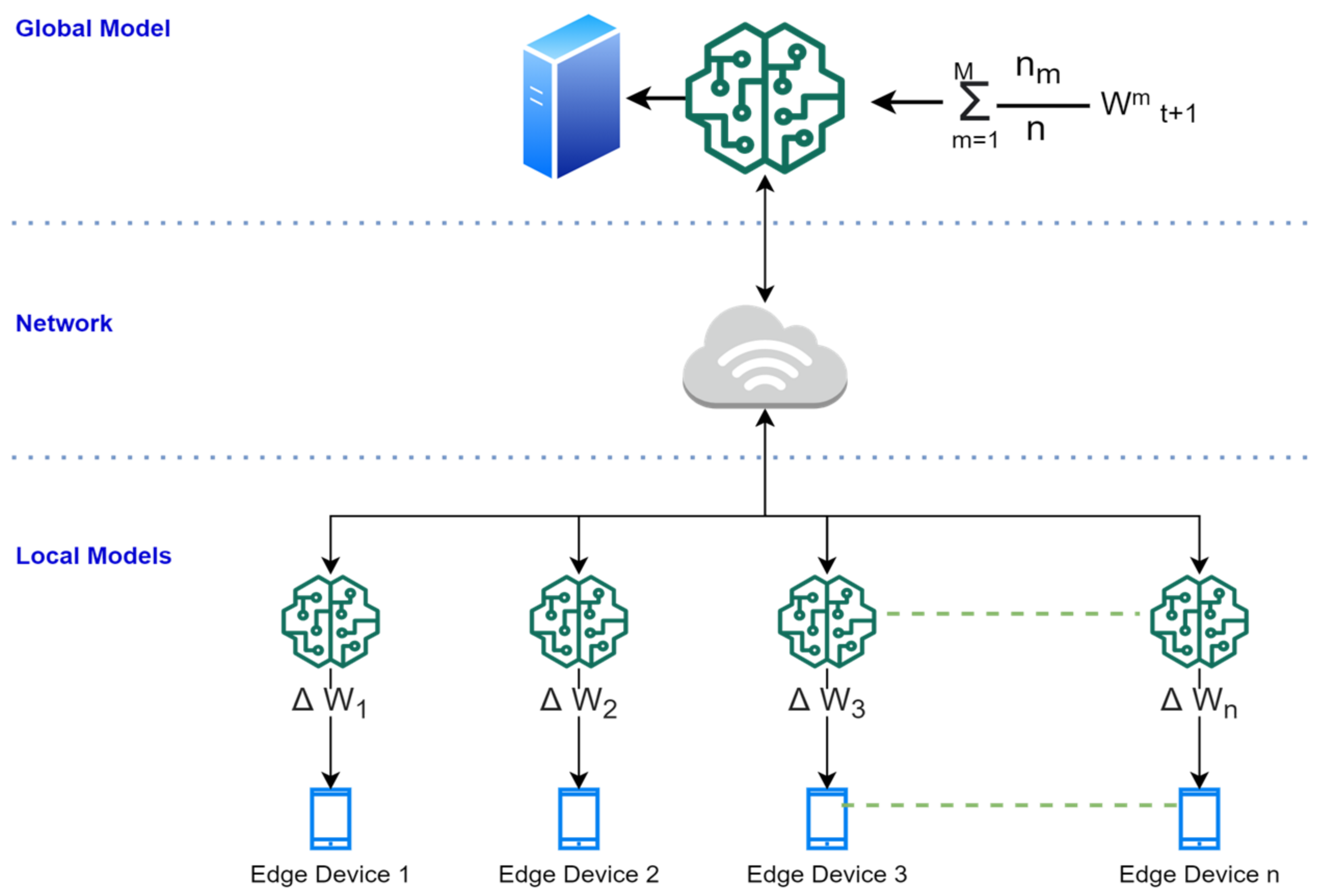
3.1. Federated Machine Learning-Based Algorithm for Cloud Server
| Algorithm 1: Cloud-Based Adaptive Ensemble CNN |
| Input: The proposed model contains the instances I = (I1, I2, …, In), which are trained initial classes of skin disease, contains classes such as Cntrain: (cn1, cn2, …, cni), and classifies the input sample from dermoscopy device samples from edge DS. DS can have multiple samples (dermoscopy images), whereby S = (s1, s2, …, sn) is related to classes such as Cn (cn1, cn2, …, cni, cni + j,) at time interval (t + 1). Samples from cni + j are novel image samples or the same classes with additional complex features. Initialization: Threshold value for performance (Th) = 50 1: Counter (c) = 1 2: While data source > null //validate the input data source 3: Classify (S) using a single instance module (optimized CNN network) [34] 4: Identify the misclassified images using the activate performance feedback module 5: Determine the ensemble accuracies using the majority voting mechanism 6: if (percentage of) % accuracy for S ≥ Th //correctly classify 7: Repeat algorithm steps 3, 4, and 5 8: if % accuracy for S < Th //wrongly classify 9: Save S //save samples 10: Counter++ 11: Repeat algorithm steps 3, 4, and 5 12: if the counter is equal to 100 //number of wrongly classified instances reaches 100 13: Identify possible new classes using Algorithm 2 [36]. 14: Repeat step 3 15: Send the updated model to the edge node 16: End while Output: Module with (in+1) instances and classification using Cni + j. |
3.2. Federated Machine Learning-Based Algorithm for Edges
| Algorithm 2: Edge-Based Adaptive Ensemble CNN |
| Input: Edge receives the computed gradient (model M), ∑W, and computes the new gradient ∆W. Initialization: The edge model downloads the global model 1: Receive the sample data to perform classification//Initial model is received from the server 2: If sample data belong to existing classes, then 3: Perform the classification//regular operation 4: Perform training within the edge device//to compute the updated gradients 5: Update gradient weight to update the global model 6: Send the global updates to all local models 7: If sample data do not belong to existing classes, then 8: Create and train and update the new instance//using Algorithm 1 [36] 9: Update gradient weight to update the global model 10: Send the global updates to all local models Output: The edges send the updated ∆W to the cloud model. |
4. Experimental Results
4.1. Data Preparation and Transformation
Skin Disease Data Stream Pipeline Preparation to Simulate Concept Drift (CD)
4.2. Experimental Criteria and Performance Measures
4.2.1. Environment and Libraries
- Python version (Python 3.6.3), installed using PyPI;
- Virtual environment from Anaconda;
- TensorFlow (1.13), Theano, and Keras (as backend) for complicated deep learning classification.
- TensorFlow federated;
- Federated core API;
- Scikit-learn library to perform basic machine learning tasks;
- OpenCV to perform image processing tasks;
- NumPy and Pandas for data manipulation and processing;
- Seaborn and Matplotlib for visualization of the results.
4.2.2. Hyperparameter Optimization and Performance Measures
4.3. Experimental Results and Discussion
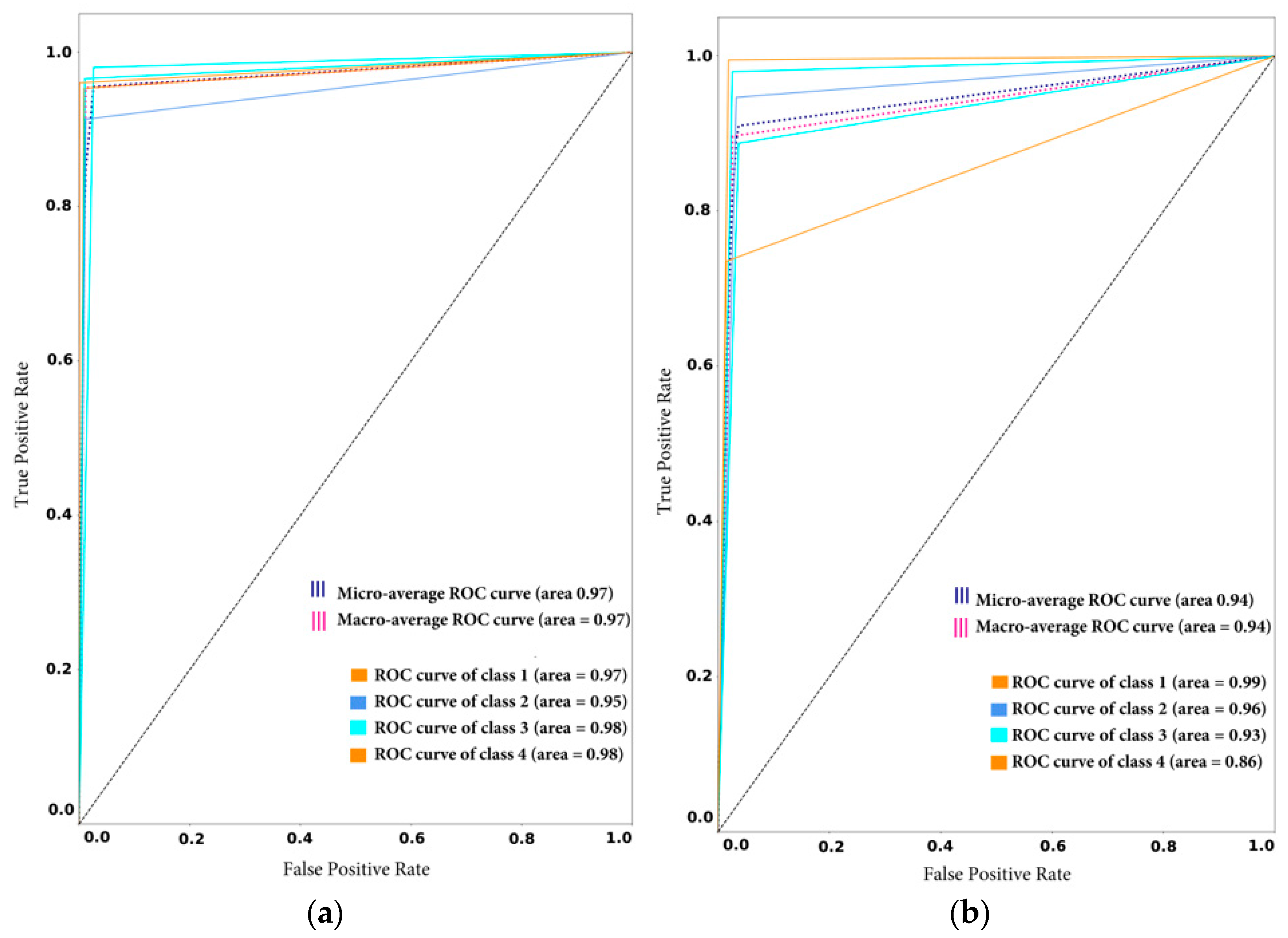
4.3.1. Experiment 1: Validation of the Global and Local Models by Measuring the Classification Evaluation Measures before (Case 1) and after (Case 2) Observing New Data Samples
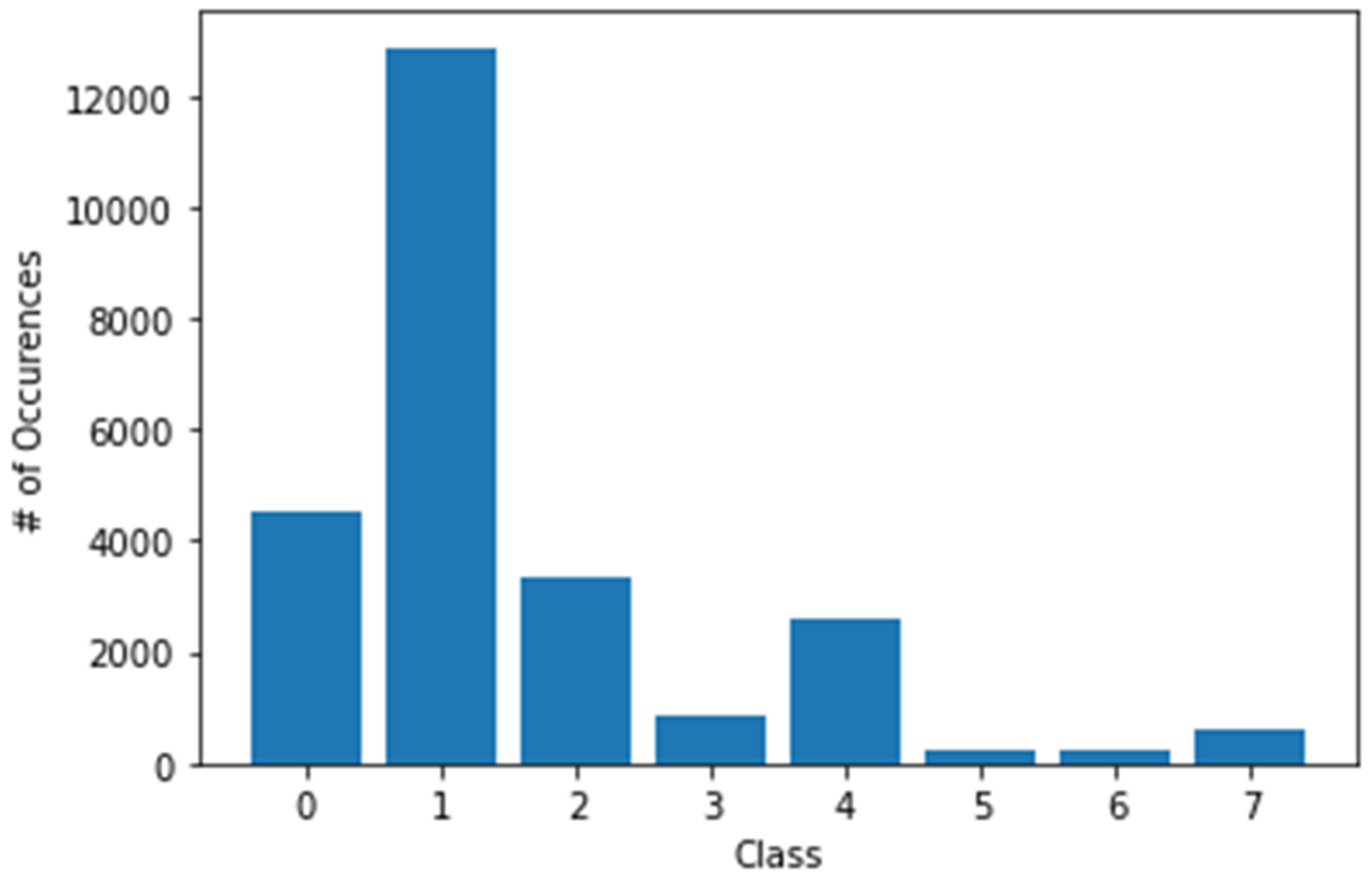
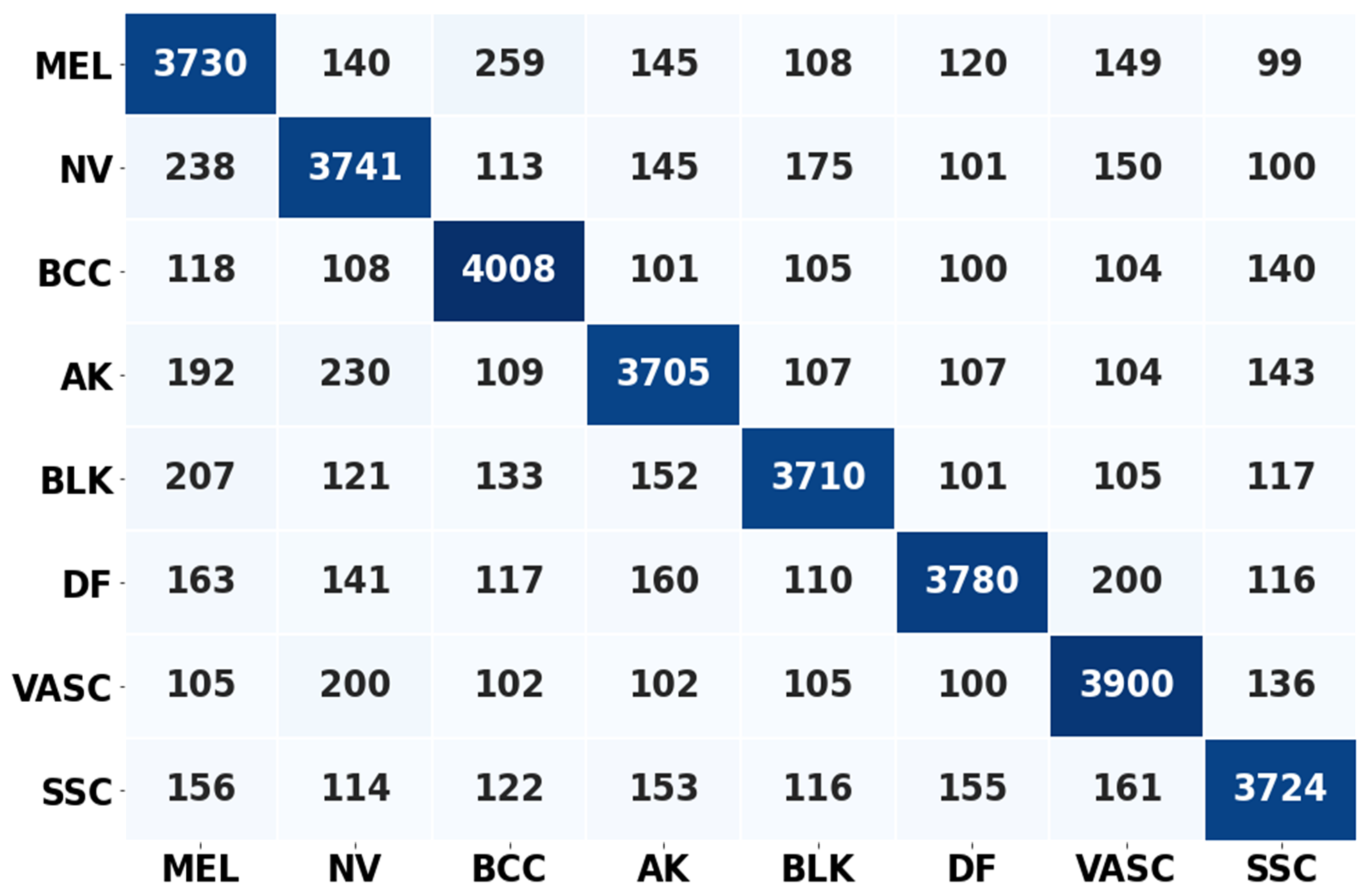
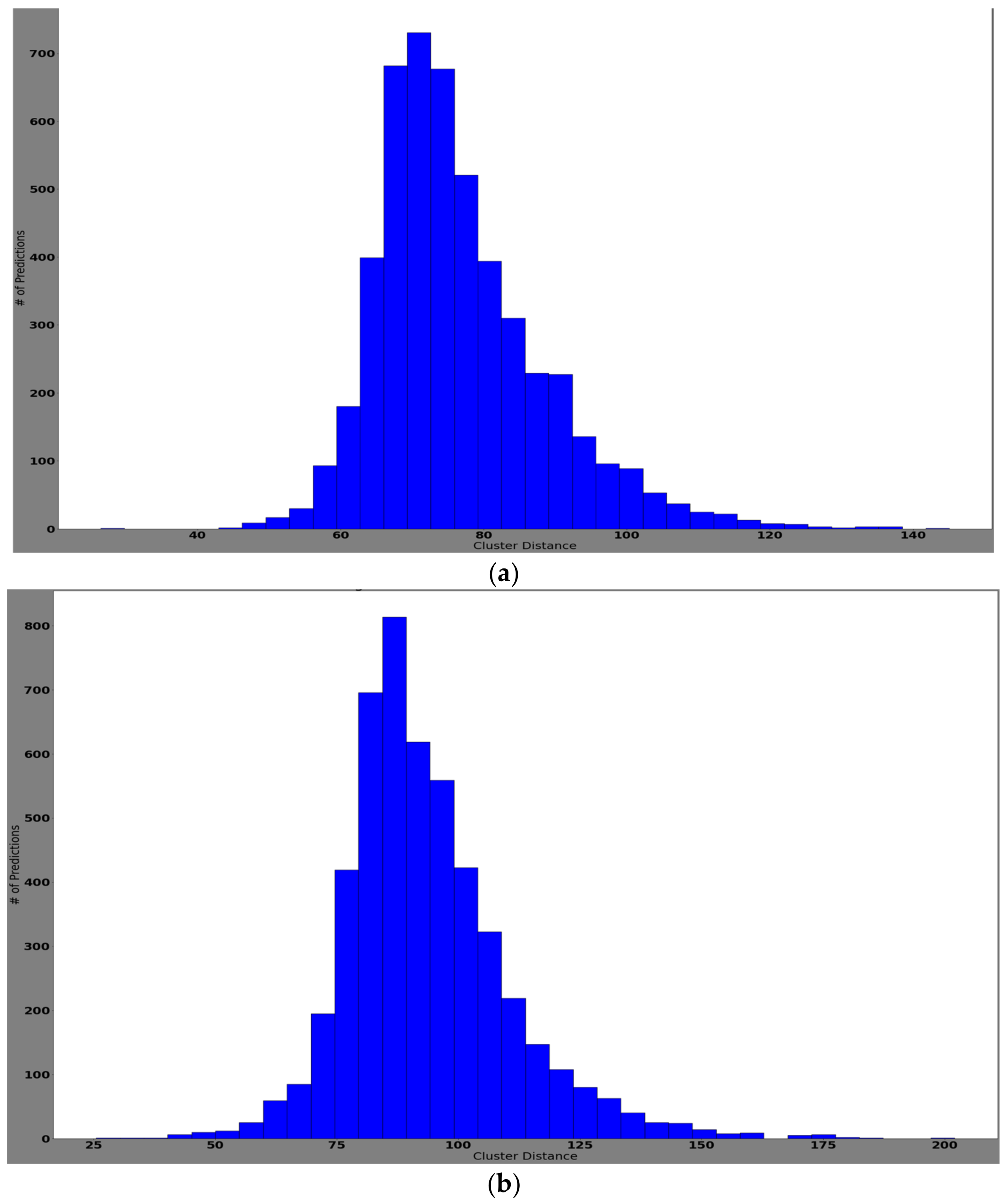
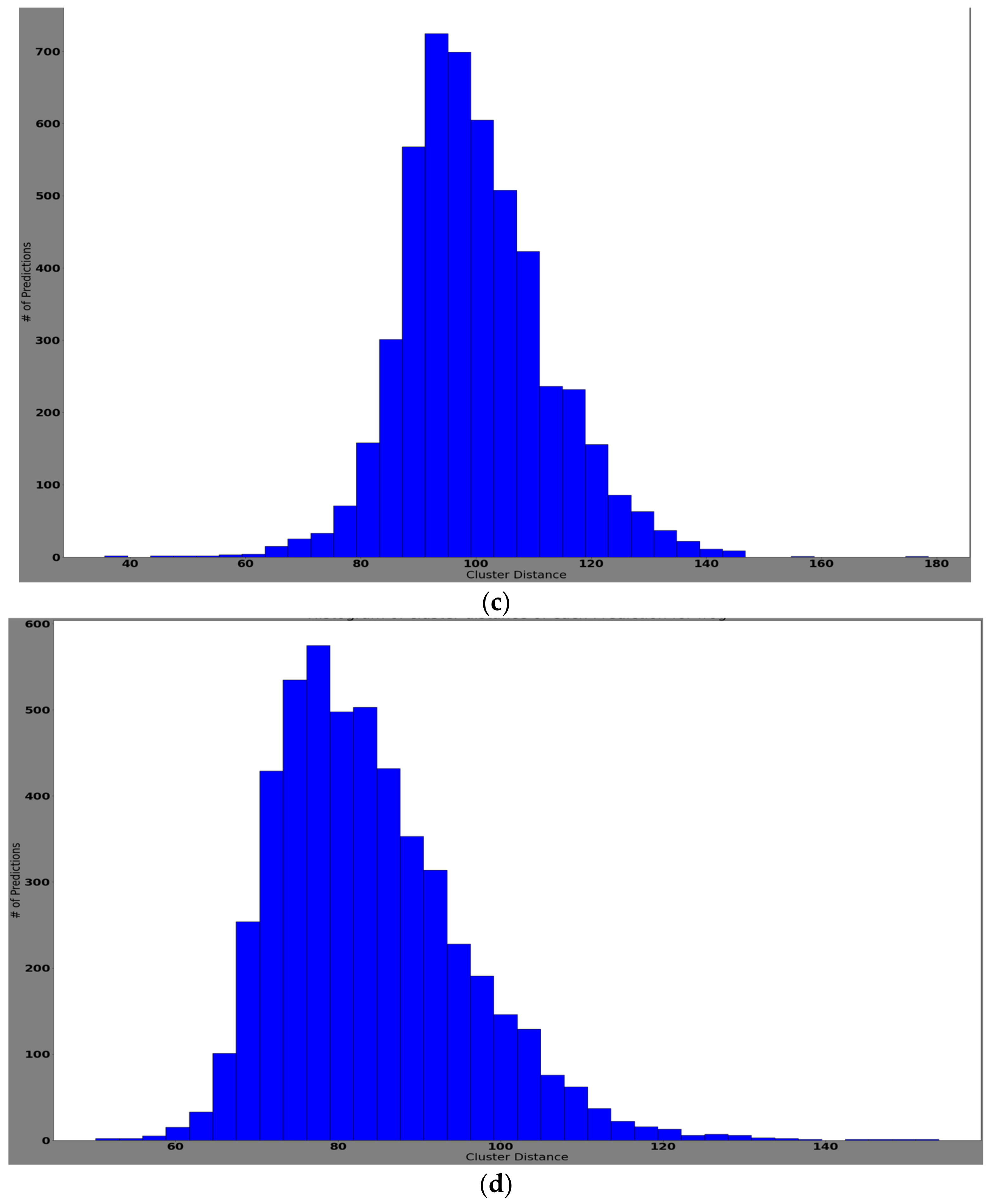
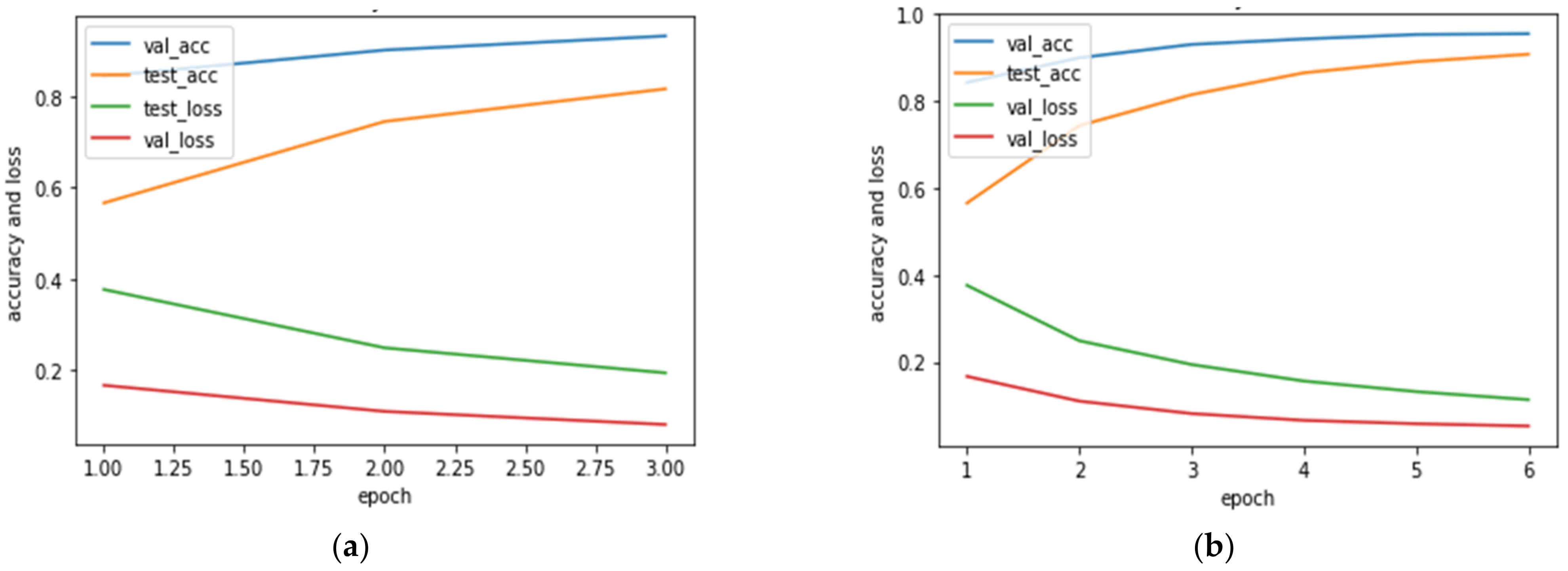
4.3.2. Experiment 2: Local Model Overall Classification Accuracy Performance, the Histogram of Clustering Distance during Edge Training and Testing, and Validation of Performance with New Samples
Overall Classification Performance of the Cloud Models
Histogram of Clustering Distance during Edge Model Training for New Samples
Training Performance of Edge Models with New Samples
5. Conclusions and Future Work
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pezzolo, E.; Naldi, L. Epidemiology of major chronic inflammatory immune-related skin diseases in 2019. Expert Rev. Clin. Immunol. 2020, 16, 155–166. [Google Scholar] [CrossRef]
- Tizek, L.; Schielein, M.; Seifert, F.; Biedermann, T.; Böhner, A.; Zink, A. Skin diseases are more common than we think: Screening results of an unreferred population at the Munich Oktoberfest. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1421–1428. [Google Scholar] [CrossRef]
- Han, W.H.; Yong, S.S.; Tan, L.L.; Toh, Y.F.; Chew, M.F.; Pailoor, J.; Kwan, Z. Characteristics of skin cancers among adult patients in an urban Malaysian population. Australas. J. Dermatol. 2019, 60, e327–e329. [Google Scholar] [CrossRef] [PubMed]
- Hay, R.J.; Johns, N.E.; Williams, H.C.; Bolliger, I.W.; Dellavalle, R.P.; Margolis, D.J.; Marks, R.; Naldi, L.; Weinstock, M.A.; Wulf, S.K.; et al. The global burden of skin disease in 2010: An analysis of skin conditions’ prevalence and impact. J. Investig. Dermatol. 2014, 134, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, C.J.P.; Jiang, B.; Chen, J.; Song, J.; Liu, Z.; He, Z.; Krittanawong, C.; Fang, P.-H.; Ming, W.-K. Artificial intelligence versus clinicians in disease diagnosis: Systematic review. JMIR Med. Inform. 2019, 7, e10010. [Google Scholar] [CrossRef] [PubMed]
- Mizeva, I.; Makovik, I.; Dunaev, A.; Krupatkin, A.; Meglinski, I. Analysis of skin blood microflow oscillations in patients with rheumatic diseases. J. Biomed. Opt. 2017, 22, 070501. [Google Scholar] [CrossRef] [PubMed]
- Zharkikh, E.; Dremin, V.; Zherebtsov, E.; Dunaev, A.; Meglinski, I. Biophotonics methods for functional monitoring of complications of diabetes mellitus. J. Biophotonics 2020, 13, e202000203. [Google Scholar] [CrossRef] [PubMed]
- Dremin, V.; Zherebtsov, E.; Bykov, A.; Popov, A.; Doronin, A.; Meglinski, I. Influence of blood pulsation on diagnostic volume in pulse oximetry and photoplethysmography measurements. Appl. Opt. 2019, 58, 9398–9405. [Google Scholar] [CrossRef]
- Popov, A.P.; Bykov, A.V.; Meglinski, I.V. Influence of probe pressure on diffuse reflectance spectra of human skin measured in vivo. J. Biomed. Opt. 2017, 22, 110504. [Google Scholar] [CrossRef]
- Ahmed, I.; Halder, S.; Bykov, A.; Popov, A.; Meglinski, I.V.; Katz, M. In-body Communications Exploiting Light: A Proof-of-concept Study using ex vivo Tissue Samples. IEEE Access 2020, 8, 190378–190389. [Google Scholar] [CrossRef]
- Spigulis, J.; Rupenheits, Z.; Matulenko, M.; Oshina, I.; Rubins, U. A snapshot multi-wavelengths imaging device for in-vivo skin diagnostics. In Multimodal Biomedical Imaging XV; International Society for Optics and Photonics: Bellingham, WA, USA, 2020; Volume 11232, p. 112320I. [Google Scholar]
- Zherebtsov, E.A.; Zherebtsova, A.I.; Doronin, A.; Dunaev, A.V.; Podmasteryev, K.V.; Bykov, A.; Meglinski, I. Combined use of laser Doppler flowmetry and skin thermometry for functional diagnostics of intradermal finger vessels. J. Biomed. Opt. 2017, 22, 040502. [Google Scholar] [CrossRef][Green Version]
- Zherebtsov, E.; Dremin, V.; Popov, A.; Doronin, A.; Kurakina, D.; Kirillin, M.; Meglinski, I.; Bykov, A. Hyperspectral imaging of human skin aided by artificial neural networks. Biomed. Opt. Exp. 2019, 10, 3545–3559. [Google Scholar] [CrossRef] [PubMed]
- Dremin, V.; Marcinkevics, Z.; Zherebtsov, E.; Popov, A.; Grabovskis, A.; Kronberga, H.; Geldnere, K.; Doronin, A.; Meglinski, I.; Bykov, A. Skin complications of diabetes mellitus revealed by polarized hyperspectral imaging and machine learning. IEEE Trans. Med. Imaging 2021. [Google Scholar] [CrossRef] [PubMed]
- Liao, H. A Deep Learning Approach to Universal Skin Disease Classification. Available online: https://www.cs.rochester.edu/u/hliao6/projects/other/skinprojectreport.pdf (accessed on 2 August 2020).
- Masood, A.; Al-Jumaily, A.A. Computer-aided diagnostic support system for skin cancer: A review of techniques and algorithms. Int. J. Biomed. Imaging 2013, 2013, 323268. [Google Scholar] [CrossRef]
- Zakhem, G.A.; Fakhoury, J.W.; Motosko, C.C.; Ho, R.S. Characterizing the role of dermatologists in developing artificial intelligence for assessment of skin cancer: A systematic review. J. Am. Acad. Dermatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Binder, M.; Kittler, H.; Seeber, A.; Steiner, A.; Pehamberger, H.; Wolff, K. Epiluminescence microscopy-based classification of pigmented skin lesions using computerized image analysis and an artificial neural network. Melanoma Res. 1998, 8, 261–266. [Google Scholar] [CrossRef]
- Burroni, M.; Corona, R.; Dell’Eva, G.; Sera, F.; Bono, R.; Puddu, P.; Perotti, R.; Nobile, F.; Andreassi, L.; Rubegni, P. Melanoma computer-aided diagnosis: Reliability and feasibility study. Clin. Cancer Res. 2004, 10, 1881–1886. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, I.A.; Koklu, M. Skin Lesion Classification using Machine Learning Algorithms. Int. J. Intell. Syst. Appl. Eng. 2017, 4, 285–289. [Google Scholar] [CrossRef]
- Bi, L.; Kim, J.; Ahn, E.; Feng, D.; Fulham, M. Automatic melanoma detection via multi-scale lesion-biased representation and joint reverse classification. In Proceedings of the 2016 IEEE 13th International Symposium on Biomedical Imaging (ISBI), Prague, Czech Republic, 13–16 April 2006; pp. 1055–1058. [Google Scholar]
- Hay, R.; Bendeck, S.E.; Chen, S.; Estrada, R.; Haddix, A.; McLeod, T.; Mahé, A. Skin diseases. In Disease Control Priorities in Developing Countries, 2nd ed.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2006. [Google Scholar]
- Author of Star Media Group. (2012, May 06, Oct 29). Itchy skin. Available online: https://www.thestar.com.my/lifestyle/health/2012/05/06/itchy-skin (accessed on 15 June 2020).
- Jinnai, S.; Yamazaki, N.; Hirano, Y.; Sugawara, Y.; Ohe, Y.; Hamamoto, R. The development of a skin cancer classification system for pigmented skin lesions using deep learning. Biomolecules 2020, 10, 1123. [Google Scholar] [CrossRef] [PubMed]
- Ercal, F.; Chawla, A.; Stoecker, W.V.; Lee, H.-C.; Moss, R.H. Neural Network Diagnosis of Malignant Melanoma From Color Images. IEEE Trans. Biomed. Eng. 1994, 41, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P. Segmentation of Digitized Dermatoscopic Images by Two-Dimensional Color Clustering. IEEE Trans. Med. Imaging 1999, 18, 164–171. [Google Scholar] [CrossRef]
- Hoshyar, A.N.; Al-Jumaily, A.; Sulaiman, R. Review on Automatic Early skin Cancer Detection. In Proceedings of the International Conference in Computer science and Service System (CSSS), Nanjing, China, 27–29 June 2011; pp. 4036–4039. [Google Scholar]
- Alam, N.; Munia, T.; Tavakolian, K.; Vasefi, V.; MacKinnon, N.; Fazel-Rezai, R. Automatic Detection and Severity Measurement of Eczema Using Image Processing. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, S.; Saboo, V. Dermatological Disease Detection Using Image Processing and Machine Learning. In Proceedings of the 2016 Third International Conference on Artificial Intelligence and Pattern Recognition (AIPR), Lodz, Poland, 19–21 September 2016. [Google Scholar] [CrossRef]
- Soliman, N.; ALEnezi, A. A Method of Skin Disease Detection Using Image Processing and Machine Learning. Procedia Comput. Sci. 2019, 163, 85–92. [Google Scholar]
- Bajwa, M.N.; Muta, K.; Malik, M.I.; Siddiqui, S.A.; Braun, S.A.; Homey, B.; Dengel, A.; Ahmed, S. Computer-Aided Diagnosis of Skin Diseases Using Deep Neural Networks. Appl. Sci. 2020, 10, 2488. [Google Scholar] [CrossRef]
- Codella, N.; Rotemberg, V.; Tschandl, P.; Celebi, M.E.; Dusza, S.; Gutman, D.; Helba, B.; Kalloo, A.; Liopyris, K.; Marchetti, M.; et al. Skin lesion analysis toward melanoma detection 2018: A challenge hosted by the international skin imaging collaboration (ISIC). arXiv Prepr. 2019; arXiv:1902.03368. [Google Scholar]
- Vestergaard, M.; Macaskill, P.; Holt, P.; Menzies, S. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: A meta-analysis of studies performed in a clinical setting. Br. J. Dermatol. 2008, 159, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Jameel, S.M.; Hashmani, M.A.; Rehman, M.; Budiman, A. Adaptive CNN Ensemble for Complex Multispectral Image Analysis. Complexity 2020, 2020, 8361989. [Google Scholar] [CrossRef]
- Jameel, S.M.; Hashmani, M.A.; Alhussain, H.; Rehman, M.; Budiman, A. An optimized deep convolutional neural network architecture for concept drifted image classification. In Intelligent Systems and Applications. IntelliSys 2019. Advances in Intelligent Systems and Computing; Bi, Y., Bhatia, R., Kapoor, S., Eds.; Springer: Cham, Switzerland, 2020; Volume 1037. [Google Scholar] [CrossRef]
- Jameel, S.M.; Hashmani, M.A.; Rehman, M.; Budiman, A. An Adaptive Deep Learning Framework for Dynamic Image Classification in the Internet of Things Environment. Sensors 2020, 20, 5811. [Google Scholar] [CrossRef]
- Gama, J.; Sebastião, R.; Rodrigues, P.P. On evaluating stream learning algorithms. Mach. Learn. 2013, 90, 317–346. [Google Scholar] [CrossRef]
- Sokolova, M.; Lapalme, G. A systematic analysis of performance measures for classification tasks. Inf. Process. Manag. 2009, 45, 427–437. [Google Scholar] [CrossRef]
- Iwashita, A.S.; Papa, J.P. An Overview on Concept Drift Learning. IEEE Access 2019, 7, 1532–1547. [Google Scholar] [CrossRef]
- Hashmani, M.A.; Muslim, S.; Alhussain, H.; Rehman, M.; Budiman, A. Accuracy Performance Degradation in Image Classification Models due to Concept Drift. Int. J. Adv. Comput. Sci. Appl. 2019, 10. [Google Scholar] [CrossRef]


| Training Hyper-Parameters | Tuning Values | Optimized Values |
|---|---|---|
| Mini-Batch Size | 16, 32, 64, 128, 256 | 120 |
| Learning Rate | 0.1, 0.01, 0.001 | 0.001 |
| L1 regularization (lambda parameter) | 0.001, 0.0003 | 0.0003 |
| Number of epochs | 10–100 | 100 |
| Optimization function | Adam | Adam |
| Cross-entropy | One-hot encoded | One-hot encoded |
| Model Configuration | Classification Accuracy (%) | Loss | ||
|---|---|---|---|---|
| Case 1 | Case 2 | Case 1 | Case 2 | |
| Model1_SD | 95.6 | 89.0 | 2.50 | 3.50 |
| Model Configuration | Precision | Recall | ||
|---|---|---|---|---|
| Case 1 | Case 2 | Case 1 | Case 2 | |
| Model1_SD | 0.95 | 0.90 | 0.95 | 0.91 |
| Classes | Mean | Variance | Standard Deviation |
|---|---|---|---|
| Class AK | 78.5 | 144.34 | 12.10 |
| Class BCC | 94.32 | 281.02 | 17.201 |
| Class NV | 99.85 | 160.55 | 12.6 |
| Class MEL | 87.12 | 134.6 | 11.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashmani, M.A.; Jameel, S.M.; Rizvi, S.S.H.; Shukla, S. An Adaptive Federated Machine Learning-Based Intelligent System for Skin Disease Detection: A Step toward an Intelligent Dermoscopy Device. Appl. Sci. 2021, 11, 2145. https://doi.org/10.3390/app11052145
Hashmani MA, Jameel SM, Rizvi SSH, Shukla S. An Adaptive Federated Machine Learning-Based Intelligent System for Skin Disease Detection: A Step toward an Intelligent Dermoscopy Device. Applied Sciences. 2021; 11(5):2145. https://doi.org/10.3390/app11052145
Chicago/Turabian StyleHashmani, Manzoor Ahmed, Syed Muslim Jameel, Syed Sajjad Hussain Rizvi, and Saurabh Shukla. 2021. "An Adaptive Federated Machine Learning-Based Intelligent System for Skin Disease Detection: A Step toward an Intelligent Dermoscopy Device" Applied Sciences 11, no. 5: 2145. https://doi.org/10.3390/app11052145
APA StyleHashmani, M. A., Jameel, S. M., Rizvi, S. S. H., & Shukla, S. (2021). An Adaptive Federated Machine Learning-Based Intelligent System for Skin Disease Detection: A Step toward an Intelligent Dermoscopy Device. Applied Sciences, 11(5), 2145. https://doi.org/10.3390/app11052145








