Spatially Related Sampling Uncertainty in the Assessment of Labile Soil Carbon and Nitrogen in an Irish Forest Plantation
Abstract
1. Introduction
2. Materials and Methods
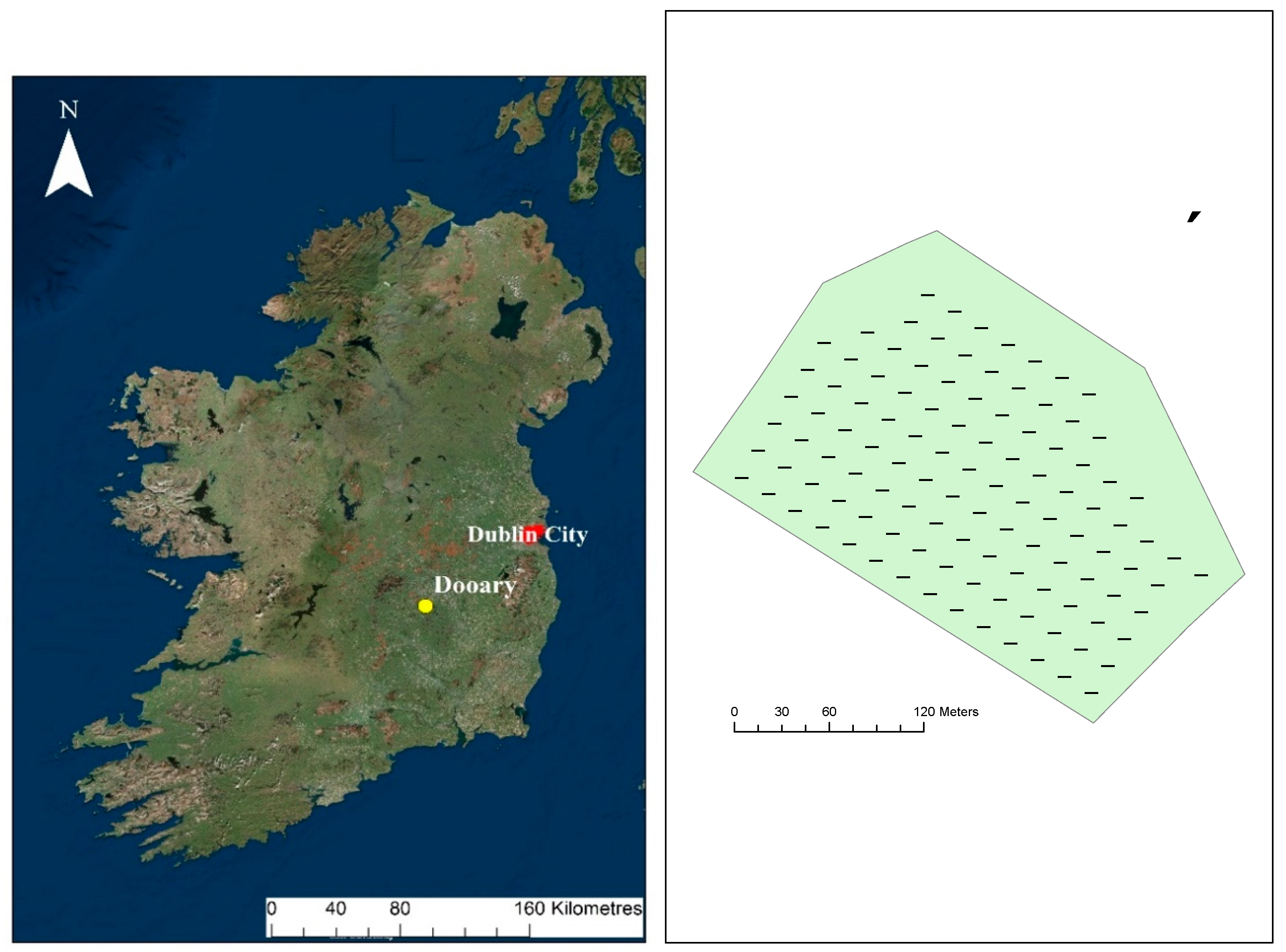
2.1. Site Description
2.2. Soil Sampling and Analysis
2.3. Data Analysis
3. Results
3.1. Descriptive Statistics for DOC and DTN
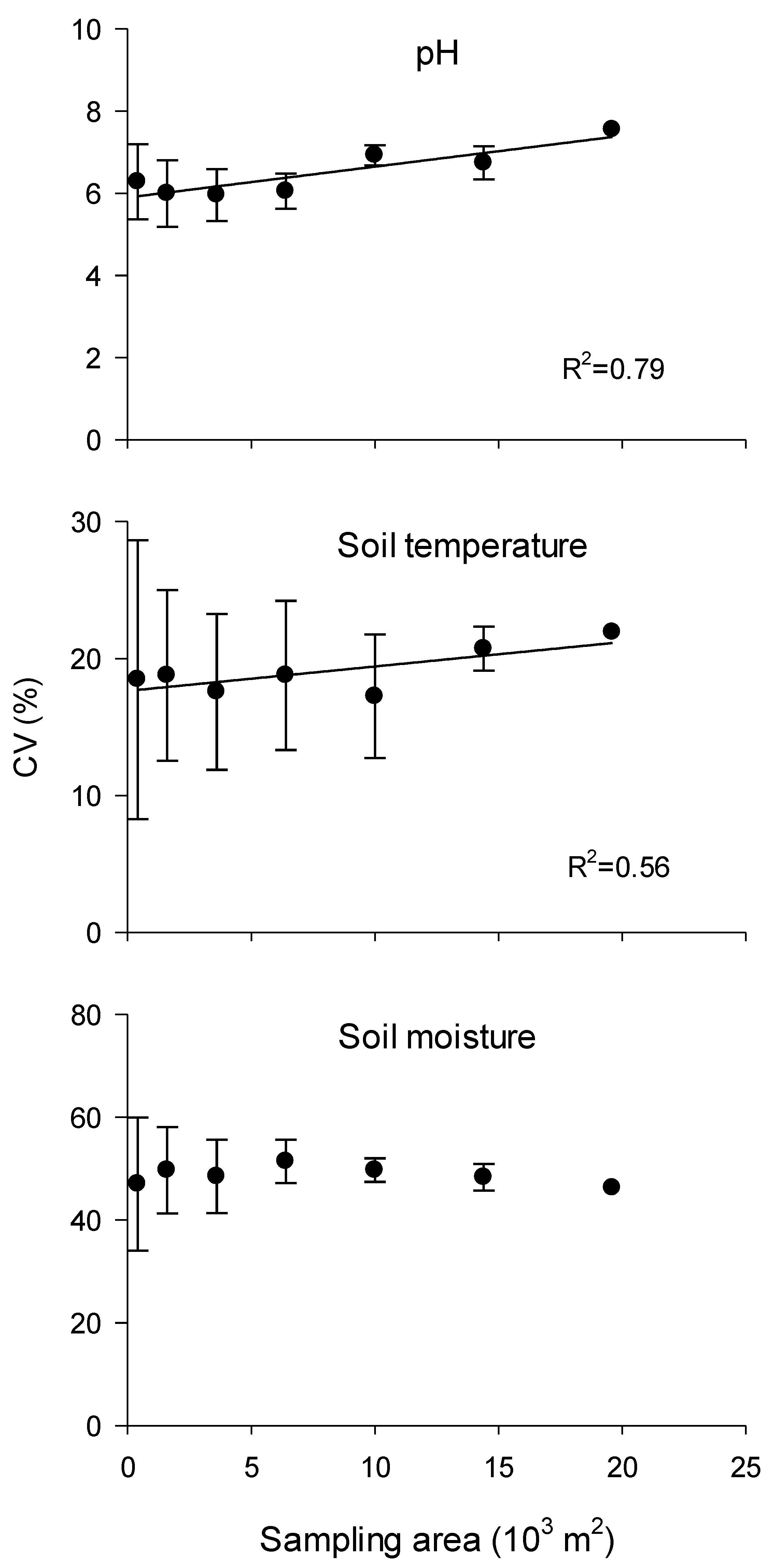
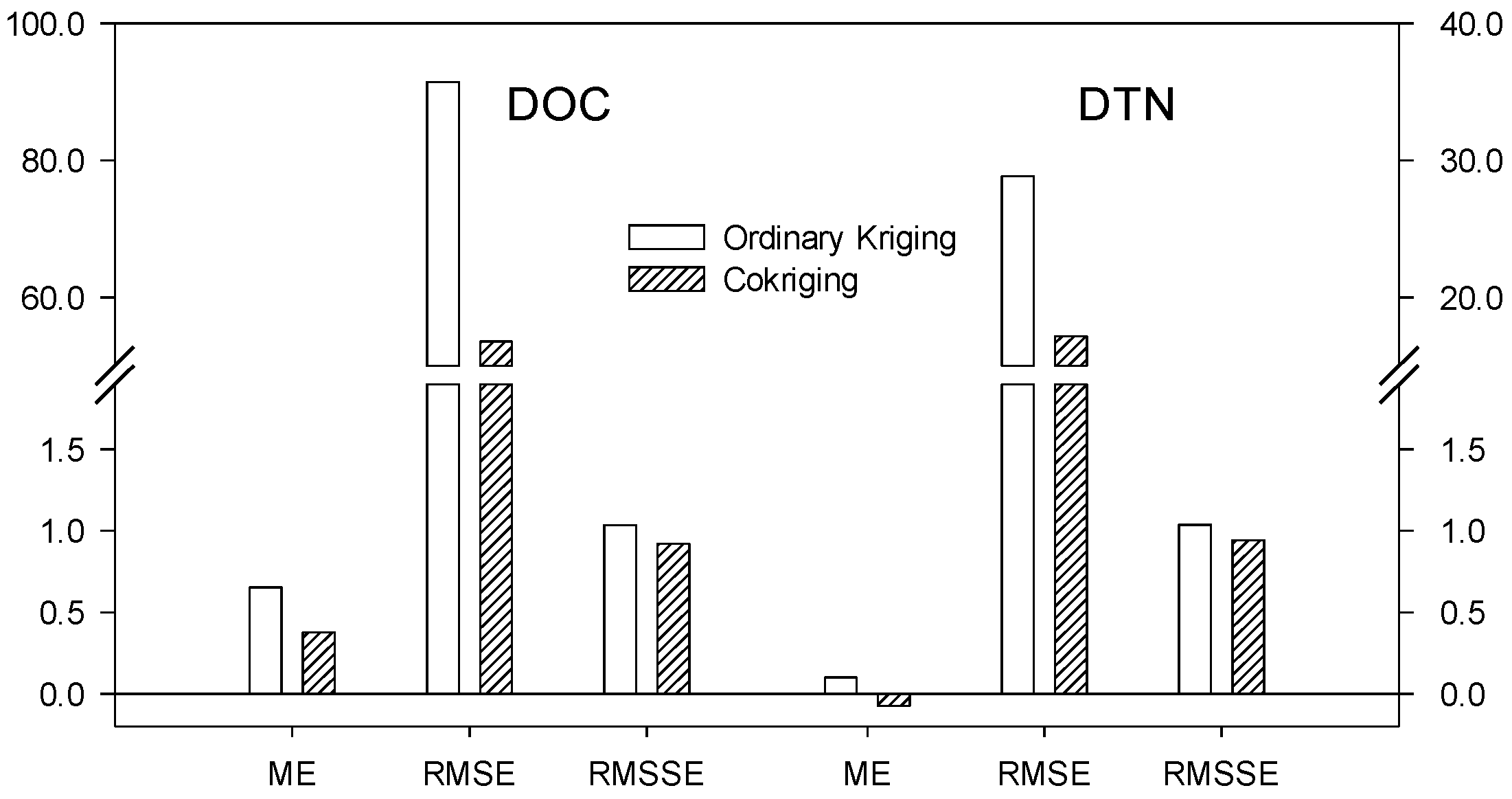
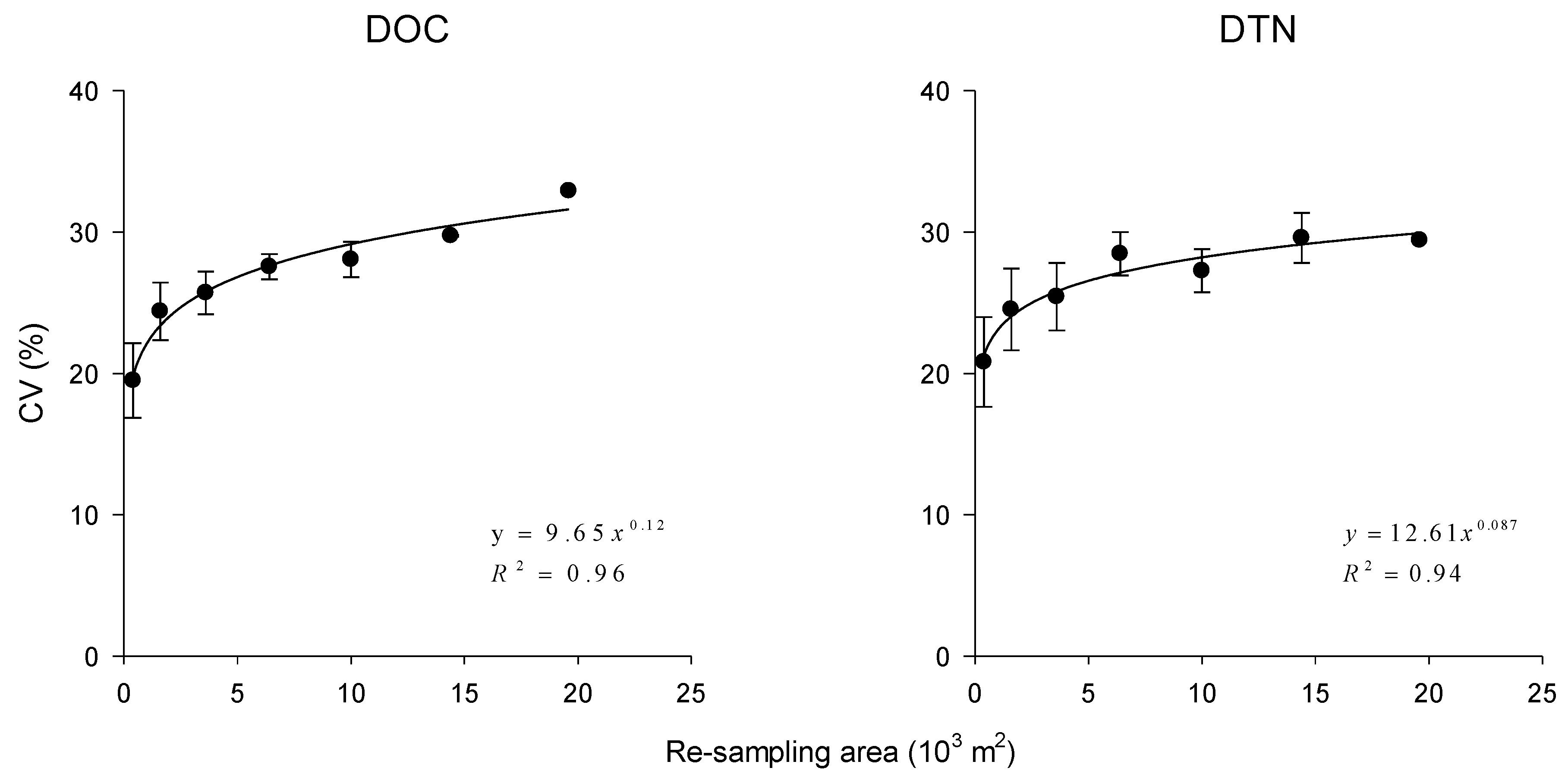
3.2. Spatial Variability of DOC and DTN
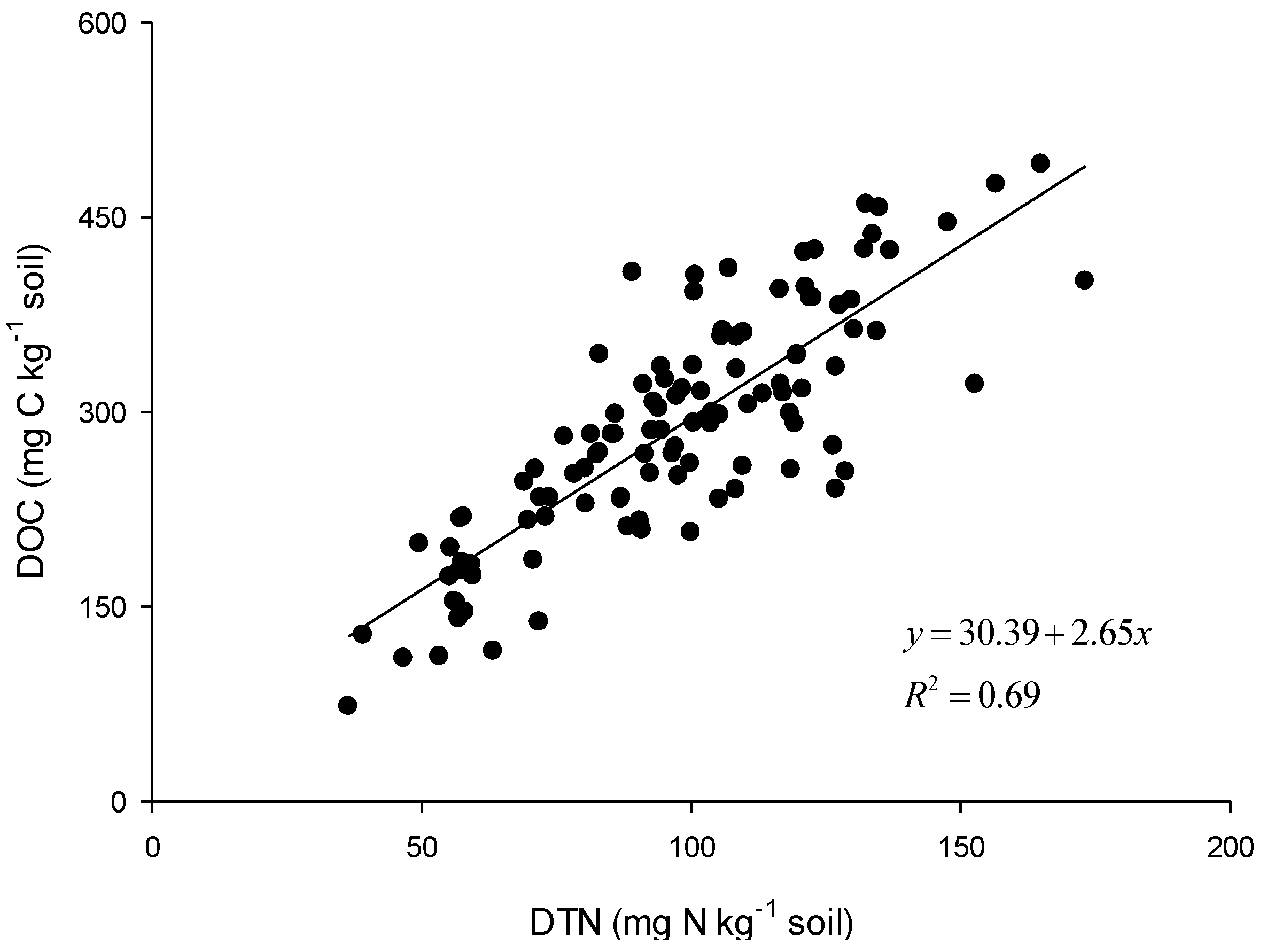
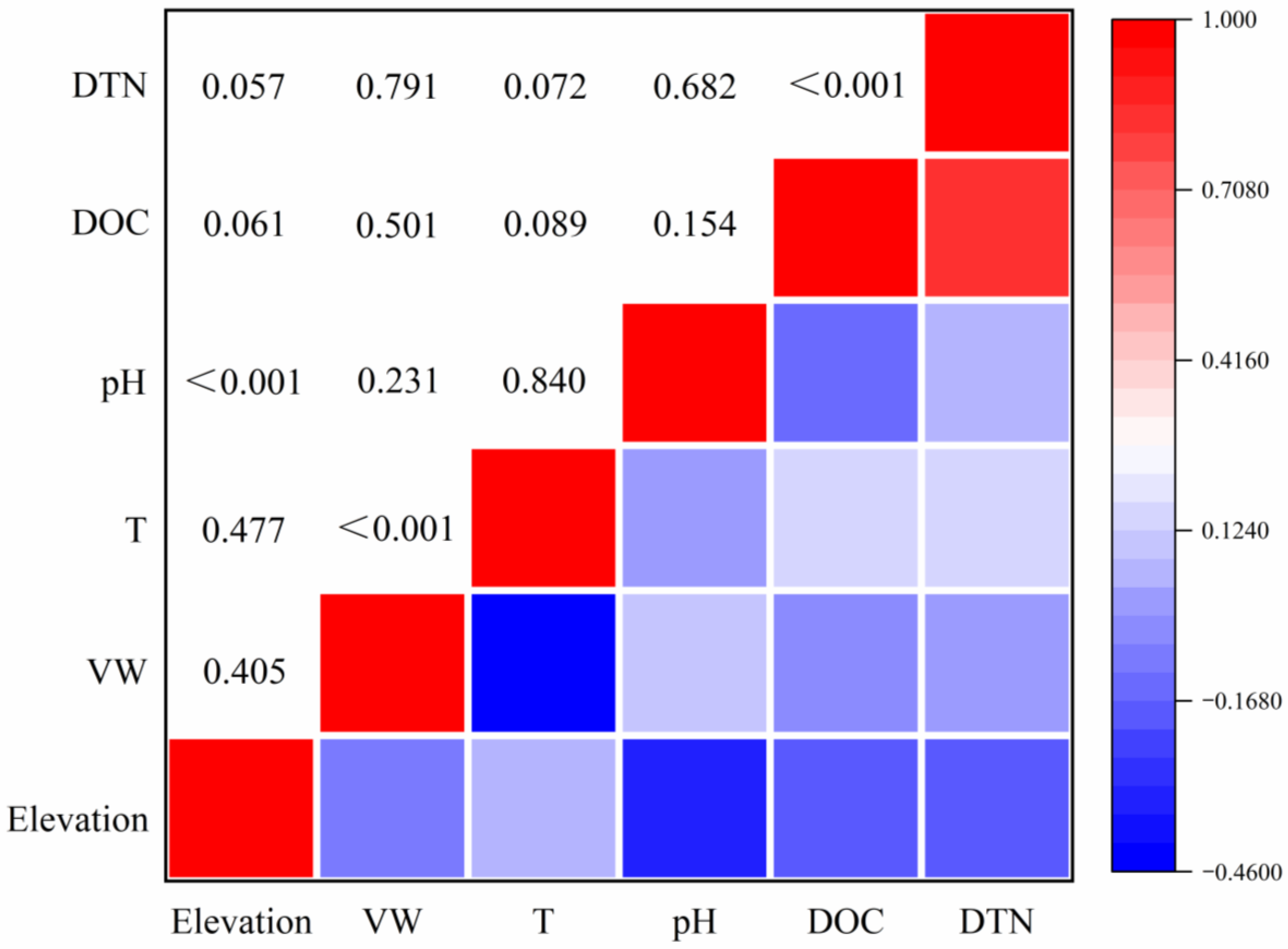
3.3. Spatial Distribution Characteristics of DOC and DTN and Their Correlation with Environmental Factors
4. Discussion
5. Conclusions
- (1)
- There was a moderate variation in DOC and DTN concentrations in the predominantly wet gley mineral soil of the Sitka spruce plantation, based on statistical and geostatistical analyses.
- (2)
- The variations in DOC and DTN concentration are scale-dependent and rise as the sampling area increases. Based on the analysis in this study, 40 sample locations would be required for adequately assessing labile C and N in this or other similar study areas.
- (3)
- Geostatistical analysis proved to be a powerful interpolation method for analysis of soil spatial variability and was useful for estimating DOC and DTN concentrations.
- (4)
- Cokriging techniques may have an advantage over ordinary kriging for predictions of highly corelated variables such as DOC and DTN in that they have higher prediction accuracy.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Size of Moving Windows (m2) | DOC_CV (%) | DTN_CV (%) | Precision | DOC_n | DTN_n | DOC_n | DTN_n |
|---|---|---|---|---|---|---|---|
| Confidence: 0.95 | Confidence: 0.99 | ||||||
| 400 | 19.50 | 20.81 | 0.05 | 14 | 16 | 15 | 17 |
| 1600 | 24.40 | 24.53 | 0.05 | 21 | 22 | 23 | 24 |
| 3600 | 25.70 | 25.43 | 0.05 | 24 | 23 | 26 | 25 |
| 6400 | 27.55 | 28.47 | 0.05 | 27 | 29 | 30 | 32 |
| 10,000 | 28.06 | 27.26 | 0.05 | 28 | 27 | 31 | 29 |
| 14,400 | 29.74 | 29.59 | 0.05 | 32 | 32 | 35 | 34 |
| 19,600 | 32.91 | 29.43 | 0.05 | 39 | 31 | 42 | 34 |
| 54,000 | 31.00 | 28.70 | 0.05 | 35 | 30 | 38 | 32 |
| 400 | 19.50 | 20.81 | 0.1 | 3 | 4 | 4 | 4 |
| 1600 | 24.40 | 24.53 | 0.1 | 5 | 5 | 6 | 6 |
| 3600 | 25.70 | 25.43 | 0.1 | 6 | 6 | 6 | 6 |
| 6400 | 27.55 | 28.47 | 0.1 | 7 | 7 | 7 | 8 |
| 10,000 | 28.06 | 27.26 | 0.1 | 7 | 7 | 8 | 7 |
| 14,400 | 29.74 | 29.59 | 0.1 | 8 | 8 | 9 | 9 |
| 19,600 | 32.91 | 29.43 | 0.1 | 10 | 8 | 11 | 8 |
| 54,000 | 31.00 | 28.70 | 0.1 | 9 | 7 | 9 | 8 |
| 400 | 19.50 | 20.81 | 0.15 | 2 | 2 | 2 | 2 |
| 1600 | 24.40 | 24.53 | 0.15 | 2 | 2 | 3 | 3 |
| 3600 | 25.70 | 25.43 | 0.15 | 3 | 3 | 3 | 3 |
| 6400 | 27.55 | 28.47 | 0.15 | 3 | 3 | 3 | 4 |
| 10,000 | 28.06 | 27.26 | 0.15 | 3 | 3 | 3 | 3 |
| 14,400 | 29.74 | 29.59 | 0.15 | 4 | 4 | 4 | 4 |
| 19,600 | 32.91 | 29.43 | 0.15 | 4 | 3 | 5 | 4 |
| 54,000 | 31.00 | 28.70 | 0.15 | 4 | 3 | 4 | 4 |

References
- Elliott, E.T. Aggregate structure and carbon, nitrogen, and phosphorus in native and cultivated soils. Soil Sci. Soc. Am. J. 1986, 50, 627–633. [Google Scholar] [CrossRef]
- Kucharik, C.J.; Brye, K.R.; Norman, J.M.; Foley, J.A.; Gower, S.T.; Bundy, L.G. Measurements and modeling of carbon and nitrogen cycling in agroecosystems of southern wisconsin: Potential for SOC sequestration during the next 50 years. Ecosystems 2001, 4, 237–258. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef]
- Liang, L.L.; Eberwein, J.R.; Allsman, L.A.; Grantz, D.A.; Jenerette, G.D. Regulation of CO2 and N2O fluxes by coupled carbon and nitrogen availability. Environ. Res. Lett. 2015, 10, 034008. [Google Scholar] [CrossRef]
- Lizarraga, H.S.; Lai, C.G. Effects of spatial variability of soil properties on the seismic response of an embankment dam. Soil Dyn. Earthq. Eng. 2014, 64, 113–128. [Google Scholar] [CrossRef]
- Tao, S.; Lin, B. Water soluble organic carbon and its measurement in soil and sediment. Water Res. 2000, 34, 1751–1755. [Google Scholar] [CrossRef]
- Xi, M.; Kong, F.; Lyu, X.; Jiang, M.; Li, Y. Spatial variation of dissolved organic carbon in soils of riparian wetlands and responses to hydro-geomorphologic changes in Sanjiang Plain, China. Chin. Geogr. Sci. 2015, 25, 174–183. [Google Scholar] [CrossRef][Green Version]
- Xi, M.; Lu, X.; Li, Y.; Kong, F. Distribution characteristics of dissolved organic carbon in annular wetland soil-water solutions through soil profiles in the Sanjiang Plain, Northeast China. J. Environ. Sci. China 2007, 19, 1074–1078. [Google Scholar] [CrossRef]
- Zsolnay, Á. Dissolved organic matter: Artefacts, definitions, and functions. Geoderma 2003, 113, 187–209. [Google Scholar] [CrossRef]
- Grosso, F.; Temussi, F.; De Nicola, F. Water-extractable organic matter and enzyme activity in three forest soils of the Mediterranean area. Eur. J. Soil Biol. 2014, 64, 15–22. [Google Scholar] [CrossRef]
- Zsolnay, A. Dissolved Humus in Soil Waters. In Humic Substances in Terrestrial Ecosystems; Piccolo, A., Ed.; Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Batlle-Aguilar, J.; Brovelli, A.; Luster, J.; Shrestha, J.; Niklaus, P.A.; Barry, D.A. Analysis of carbon and nitrogen dynamics in riparian soils: Model validation and sensitivity to environmental controls. Sci. Total Environ. 2012, 429, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Glatzel, S.; Kalbitz, K.; Dalva, M.; Moore, T. Dissolved organic matter properties and their relationship to carbon dioxide efflux from restored peat bogs. Geoderma 2003, 113, 397–411. [Google Scholar] [CrossRef]
- Li, R.; Qu, M. Effects of dissolved organic matter on environment. Ecol. Environ. 2004, 13, 271–275. [Google Scholar]
- Kalbitz, K.; Solinger, S.; Park, J.-H.; Michalzik, B.; Matzner, E. Controls on the dynamics of dissolved organic matter in soils: A review. Soil Sci. 2000, 165, 277–304. [Google Scholar] [CrossRef]
- Camino-Serrano, M.; Graf Pannatier, E.; Vicca, S.; Luyssaert, S.; Jonard, M.; Ciais, P.; Guenet, B.; Gielen, B.; Peñuelas, J.; Sardans, J.; et al. Trends in soil solution dissolved organic carbon (DOC) concentrations across European forests. Biogeosciences 2016, 13, 5567–5585. [Google Scholar] [CrossRef]
- Hishi, T.; Hirobe, M.; Tateno, R.; Takeda, H. Spatial and temporal patterns of water-extractable organic carbon (WEOC) of surface mineral soil in a cool temperate forest ecosystem. Soil Biol. Biochem. 2004, 36, 1731–1737. [Google Scholar] [CrossRef]
- Grüneberg, E.; Schöning, I.; Kalko, E.K.V.; Weisser, W.W. Regional organic carbon stock variability: A comparison between depth increments and soil horizons. Geoderma 2010, 155, 426–433. [Google Scholar] [CrossRef]
- Zhang, P.; Shao, M. Spatial variability and stocks of soil organic carbon in the gobi desert of Northwestern China. PLoS ONE 2014, 9, e93584. [Google Scholar] [CrossRef]
- Kindler, R.; Siemens, J.; Kaiser, K.; Walmsley, D.C.; Bernhofer, C.; Buchmann, N.; Cellier, P.; Eugster, W.; Gleixner, G.; Grũnwald, T.; et al. Dissolved carbon leaching from soil is a crucial component of the net ecosystem carbon balance. Glob. Chang. Biol. 2011, 17, 1167–1185. [Google Scholar] [CrossRef]
- Heuvelink, G.B.M.; Webster, R. Modelling soil variation: Past, present, and future. Geoderma 2001, 100, 269–301. [Google Scholar] [CrossRef]
- Iqbal, J.; Thomasson, J.A.; Jenkins, J.N.; Owens, P.R.; Whisler, F.D. Spatial variability analysis of soil physical properties of alluvial soils. Soil Sci. Soc. Am. J. 2005, 69, 1338. [Google Scholar] [CrossRef]
- Zou, J.; Tobin, B.; Luo, Y.; Osborne, B. Differential responses of soil CO2 and N2O fluxes to experimental warming. Agric. For. Meteorol. 2018, 259, 11–22. [Google Scholar] [CrossRef]
- Huang, M.; Liang, T.; Ou-Yang, Z.; Wang, L.; Zhang, C.; Zhou, C. Leaching losses of nitrate nitrogen and dissolved organic nitrogen from a yearly two crops system, wheat-maize, under monsoon situations. Nutr. Cycl. Agroecosys. 2011, 91, 77. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Marschner, P. Retention and loss of water extractable carbon in soils: Effect of clay properties. Sci. Total Environ. 2014, 470, 400–406. [Google Scholar] [CrossRef]
- Walmsley, D.C.; Siemens, J.; Kindler, R.; Kaiser, K.; Saunders, M.; Fichtner, A.; Kaupenjohann, M.; Osborne, B.A. Reduced nitrate leaching from an Irish cropland soil under non-inversion tillage with cover cropping greatly outweighs increased dissolved organic nitrogen leaching. Agric. Ecosyst. Environ. 2018, 265, 340–349. [Google Scholar] [CrossRef]
- Zou, J.; Tobin, B.; Luo, Y.; Osborne, B.A. Response of soil respiration and its components to experimental warming and water addition in a temperate Sitka spruce forest ecosystem. Agric. Meteorol. 2018, 260, 204–215. [Google Scholar] [CrossRef]
- Olajuyigbe, S.; Tobin, B.; Saunders, M.; Nieuwenhuis, M. Forest thinning and soil respiration in a Sitka spruce forest in Ireland. Agric. Meteorol. 2012, 157, 86–95. [Google Scholar] [CrossRef]
- Saunders, M.; Tobin, B.; Black, K.; Gioria, M.; Nieuwenhuis, M.; Osborne, B.A. Thinning effects on the net ecosystem carbon exchange of a Sitka spruce forest are temperature-dependent. Agric. Meteorol. 2012, 157, 1–10. [Google Scholar] [CrossRef]
- Black, K.; Byrne, K.A.; Mencuccini, M.; Tobin, B.; Nieuwenhuis, M.; Reidy, B.; Bolger, T.; Saiz, G.; Green, C.; Farrell, E.T.; et al. Carbon stock and stock changes across a Sitka spruce chronosequence on surface-water gley soils. Forestry 2009, 82, 255–272. [Google Scholar] [CrossRef]
- Hu, W.; Shao, M.; Wang, Q. Scale-dependency of spatial variability of soil moisture on a degraded slope-land on the Loess Plateau. Nongye Gongcheng Xuebao 2005, 21, 11–16. [Google Scholar]
- Trangmar, B.B.; Yost, R.S.; Uehara, G. Application of geostatistics to spatial studies of soil properties. Adv. Agron. 1985, 38, 45–94. [Google Scholar]
- Eldeiry, A.A.; Garcia, L.A. Comparison of ordinary kriging, regression kriging, and cokriging techniques to estimate soil salinity using LANDSAT images. J. Irrig. Drain. Eng. 2010, 136, 355–364. [Google Scholar] [CrossRef]
- Marchetti, A.; Piccini, C.; Francaviglia, R.; Mabit, L. Spatial distribution of soil organic matter using geostatistics: A key indicator to assess soil degradation status in Central Italy. Pedosphere 2012, 22, 230–242. [Google Scholar] [CrossRef]
- Ge, P.; Da, L.J.; Wang, W.B.; Xu, X.N. Seasonal dynamics of dissolved organic carbon, nitrogen and other nutrients in soil of Pinus massoniana stands after pine wilt disease disturbance. J. Soil Sci. Plant. Nutr. 2014, 14, 75–87. [Google Scholar] [CrossRef]
- Song, L.-C.; Hao, J.-M.; Cui, X.-Y. Soluble organic nitrogen in forest soils of Northeast China. J. For. Res. 2008, 19, 53–57. [Google Scholar] [CrossRef]
- Chen, C.R.; Xu, Z.H.; Zhang, S.L.; Keay, P. Soluble organic nitrogen pools in forest soils of subtropical Australia. Plant. Soil 2005, 277, 285–297. [Google Scholar] [CrossRef]
- Wang, J.; Fu, B.; Qiu, Y.; Chen, L. Soil nutrients in relation to land use and landscape position in the semi-arid small catchment on the loess plateau in China. J. Arid Environ. 2001, 48, 537–550. [Google Scholar] [CrossRef]
- Western, A.W.; Blöschl, G. On the spatial scaling of soil moisture. J. Hydrol. 1999, 217, 203–224. [Google Scholar] [CrossRef]
- Cambardella, C.A.; Moorman, T.B.; Novak, J.M.; Parkin, T.B.; Karlen, D.L.; Turco, R.F.; Konopka, A.E. Field-scale variability of soil properties in central Iowa soils. Soil Sci. Soc. Am. J. 1994, 58, 1501–1511. [Google Scholar] [CrossRef]
- Wang, J.; Fu, B.; Qiu, Y.; Chen, L. Analysis on soil nutrient characteristics for sustainable land use in Danangou catchment of the Loess Plateau, China. Catena 2003, 54, 17–29. [Google Scholar] [CrossRef]
- Ceddia, M.B.; Villela, A.L.O.; Pinheiro, É.F.M.; Wendroth, O. Spatial variability of soil carbon stock in the Urucu river basin, Central Amazon-Brazil. Sci. Total Environ. 2015, 526, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Xiong, Y.; Zhu, J.; Ye, Y.; Ye, M. Soil organic carbon content and distribution in a small landscape of Dongguan, South China. Pedosphere 2006, 16, 10–17. [Google Scholar] [CrossRef]
- Tack, F.M.G.; Verloo, M.G.; Vanmechelen, L.; Van Ranst, E. Baseline concentration levels of trace elements as a function of clay and organic carbon contents in soils in Flanders (Belgium). Sci. Total Environ. 1997, 201, 113–123. [Google Scholar] [CrossRef]

| Soil Properties | n | Mean | Standard Deviation |
|---|---|---|---|
| Bulk density (g cm−3) | 6 | 0.83 | 0.10 |
| pH | 6 | 4.28 | 0.29 |
| Total C (%) | 6 | 4.84 | 1.42 |
| Total N (%) | 6 | 0.27 | 0.02 |
| Sand (%) | 6 | 27 | 5.63 |
| Silt (%) | 6 | 25 | 4.65 |
| Clay (%) | 6 | 48 | 6.86 |
| Ammonium (mg N kg−1 soil) | 6 | 6.52 | 2.03 |
| Nitrate (mg N kg−1 soil) | 6 | 1.67 | 2.60 |
| Variables | n | Mean | SD | CV | Skewness | Kurtosis | Minimum | Maximum | p of Shapiro–Wilk Test |
|---|---|---|---|---|---|---|---|---|---|
| DOC (mg C kg−1 soil) | 111 | 288.14 | 89.31 | 31.00 | 0.00 | −0.39 | 73.15 | 490.74 | 0.8395 |
| DTN (mg N kg−1 soil) | 111 | 97.26 | 27.97 | 28.76 | 0.09 | −0.24 | 36.43 | 173.14 | 0.4147 |
| Variable | Model | Nugget C0 | Sill (C0 + C) | C0/(C0 + C) | Range (m) | R2 | RSS |
|---|---|---|---|---|---|---|---|
| DOC | Exponential | 740.00 | 8140.00 | 0.09 | 34.20 | 0.53 | 488333 |
| DTN | Exponential | 84.00 | 812.10 | 0.10 | 35.10 | 0.56 | 5008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, J.; Osborne, B. Spatially Related Sampling Uncertainty in the Assessment of Labile Soil Carbon and Nitrogen in an Irish Forest Plantation. Appl. Sci. 2021, 11, 2139. https://doi.org/10.3390/app11052139
Zou J, Osborne B. Spatially Related Sampling Uncertainty in the Assessment of Labile Soil Carbon and Nitrogen in an Irish Forest Plantation. Applied Sciences. 2021; 11(5):2139. https://doi.org/10.3390/app11052139
Chicago/Turabian StyleZou, Junliang, and Bruce Osborne. 2021. "Spatially Related Sampling Uncertainty in the Assessment of Labile Soil Carbon and Nitrogen in an Irish Forest Plantation" Applied Sciences 11, no. 5: 2139. https://doi.org/10.3390/app11052139
APA StyleZou, J., & Osborne, B. (2021). Spatially Related Sampling Uncertainty in the Assessment of Labile Soil Carbon and Nitrogen in an Irish Forest Plantation. Applied Sciences, 11(5), 2139. https://doi.org/10.3390/app11052139







