Abstract
Soil salinization and desertification due to climate change are the most relevant challenges for the agriculture of the 21st century. Soil compost amendment and plant growth promoting rhizobacteria (PGP-R) are valuable tools to mitigate salinization and desertification impacts on agricultural soils. Selection of novel halo/thermo-tolerant bacteria from the rhizosphere of glicophytes and halophytes, grown on soil compost amended and watered with 150/300 mM NaCl, was the main objective of our study. Beneficial effects on the biomass, well-being and resilience, exerted on the assayed crops (maize, tomato, sunflower and quinoa), were clearly observable when soils were amended with 20% compost despite the very high soil electric conductivity (EC). Soil compost amendment not only was able to increase crop growth and biomass, but also their resilience to the stress caused by very high soil EC (up to 20 dS m−1). Moreover, compost amendment has proved itself a valuable source of highly halo-(4.0 M NaCl)/thermo tolerant rhizobacteria (55 °C), showing typical PGP features. Among the 13 rhizobacterial isolates, molecularly and biochemically characterized, two bacterial strains showed several biochemical PGP features. The use of compost is growing all around the world reducing considerably for farmers soil fertilization costs. In fact, only in Italy its utilization has ensured, in the last years, a saving of 650 million euro for the farmers, without taking into account the environment and human health benefits. Furthermore, the isolation of halo/thermo-tolerant PGPR strains and their use will allow the recovery and cultivation of hundreds of thousands of hectares of saline and arid soils now unproductive, making agriculture more respectful of agro-ecosystems also in view of upcoming climate change.
1. Introduction
In the last decade, it is not uncommon to hear about climate change and global warming from both media and insiders (e.g., climatologists, ecologists, etc.). Climate change refers to variations in the Earth’s climate (i.e., variations at different spatial scales: regional, continental, hemispheric, and global) and historical-temporal (decennial, secular, millennial, and ultra-millennial) of one or more environmental and climatic parameters. Among the negatively effects related to climate change, the scientific community includes prolonged periods of drought and substantial depletion of surface aquifers (Technical Summary. Available online: https://www.ipcc.ch/site/assets/uploads/sites/4/2020/07/03_Technical-Summary-TS_V2.pdf (accessed on 01 February 2021)). The massive use of this resource not only leads to its depletion, but also to another relevant problem: the salinization of soils [1]. All soils contain salt and nutrients whose quantity depends on the pedo-climatic conditions of the territory [2], but when their amount exceeds the critical threshold crops are negatively affected and the soil is classified as saline. Salinization is a process typical of environments where rainfall is not sufficient to eliminate salts contained in the soil determining their increase [3]. It is estimated that worldwide about 20% of total cultivated lands and 33% of irrigated agricultural lands are afflicted by high salinity, and, moreover, 50% of the arable lands will be affected by high level of salts by 2050 [4].
Salinization inhibits plant growth since it limits their ability to supply water, causes nutritional imbalances and induces toxicity phenomena [5]. Saline soils can be classified into three classes based on salinity and sodium values, estimated by electrical conductivity (EC) and sodium adsorption ratio (SAR) or exchangeable sodium percentage (ESP): saline, saline- sodium and sodium. Soil is defined as saline when the EC is slightly greater than 4 deci-Siemens per meter (dS m−1), mild between 4 and 8 dS m−1, moderate between 8 and 16 dS m−1, and high above 16 dS m−1 [6]. Values above 4–8 dS m−1 reduce the growth of many crops except for halophytes (i.e., plants that tolerate or live on soils rich in salts) and only a few cultivated species have a high tolerance to salinity (e.g., beet). To solve the problem of soil salinization, several agronomic techniques can be adopted such as: reducing the excess of water that infiltrates the soil, the use of crop mutants, organic amendments, but also of microorganisms tolerating salinity [7].
Organic fertilizers intended also as soil amendments such as high-quality compost, obtained from the differentiated waste collection, or biochar, produced by means of pyrolysis processes, are able to promote soil health and stability, and reduce, in some cases, human health risks [8].
All the positive compost features above mentioned can be found only in certified compost that can guarantee its high-quality standards without any risk for crops and, consequently, human or animal health due to their use in agriculture. In the case of Italy is present on the national territory the Consorzio Italiano Compostatori (CIC. Available online: https://www.compost.it/en/circular-economy-for-biowaste-in-italy/who-we-are/ (accessed on 1 Februay 2021)) which is responsible for issuing a high-quality compost certificate to its members. CIC is also partner of European Compost Network (ECN. Available online: https://www.compostnetwork.info/ (accessed on 1 Februay 2021)) a membership organization with 64 members from 26 European Countries.
It is noteworthy that also biochar shows very interesting characteristics as a soil amendment, however, with respect to compost, it has high production costs which are extremely variable on the basis of place of production and seller (Boshir et al. [9] stated that: “…globally, the mean price for biochar in 2013 was 2650 USD per Mg−1; this ranged from as low as 90 USD per Mg−1 in the Philippines to as high as 8850 USD in the UK”; even if recently the latest development leads to a reduction in its production cost [10] ranging between 10 and 30 USD Mg−1) compared to 5–10 dollars for compost. Moreover, biochar in some cases can have possible risks of soil contamination, as illustrated by Johannes Lehmann (Professor of Soil Biogeochemistry and Soil Fertility Management at Cornell University), in a webinar dedicated to its use in agriculture (FAO. Available online: http://www.fao.org/energy/news/news-details/en/c/1295174/ (accessed on 1 February 2021)).
It is also noteworthy that high quality compost, in respect with biochar, is also a relevant source of beneficial plant microorganisms [11].
In the last decade, the study of soil microorganisms and, in particular those of the rhizosphere, that is that 1–2 mm layer of soil in close contact with the rhizoplane [6] have allowed to understand that many microorganisms (e.g., bacteria, fungi, etc.) play a fundamental role for the plant, also promoting the plant growth; these microorganisms are known as plant growth promoter microorganisms (PGPMs) [12]. Nowadays, different bacteria species and strains capable of improving the well-being and growth of plants in the most varied harsh and stressful conditions have been identified [13,14,15].
Plants support and select the microorganisms useful for its well-being, producing organic compounds derived from photosynthesis [16] and, on the other hand, the microorganisms improve the mobilization and uptake of macro and micronutrients from the soil matrices (e.g., nitrates, phosphates, etc.) [17,18], and also reduce plant stress breaking the positive feedback of the ethylene metabolic pathway [13,19,20].
Plant growth-promoting bacteria (PGPB) have been identified and characterized for the first time by Kloepper and Schroth [21], who showed the ability of PGPB to foster plant growth. In the last 40 years the number of species and strains of PGPB and PGPR (plant growth promoting rhizobacteria), isolated and characterized for their biochemical features useful to improve plant healthiness and resilience to abiotic and biotic stresses, has grown enormously. It has been shown that PGPR/PGPB, and even endophyte, bacteria which live inside the body of the plants, added singularly or as a bacterial consortium to pregerminated seeds or seedlings, were able to favor the plant growth undergoing biotic or abiotic stresses [22,23,24].
The main aims of the research we conducted were to:
- Evaluate the effect of high-quality compost, obtained from the differentiated waste collection, on the growth of four crops cultivated worldwide such as maize, tomato, sunflower, and quinoa;
- Evaluate if soil compost amendment improved the resilience to salt stress in both glycophyte and halophyte crops here assayed;
- Isolate, select, and characterize (genetically and biochemically) novel highly salt resistant rhizobacteria from a glycophyte monocot (maize) and from a facultative halophyte dicot (quinoa) cultivated on a compost amended soil and added with increasing concentrations of sodium chloride (NaCl).
2. Materials and Methods
2.1. Soil Preparation and Crop Cultivation
The first step involved sowing maize, quinoa, tomato, or sunflower in a polystyrene seedbed filled with an agricultural soil (Table S1). A single seed was placed in each cavity of the seedbed. The growth of the seedlings was carried out in a climatic chamber set up with a photoperiod of 16 h of light and 8 of darkness and at constant temperature of 24 °C, and seedlings were irrigated periodically with tap water. After two weeks, the seedlings of all four crops had reached a suitable height and growth to be transferred into pots of 3.5 L of volume (θ 20 cm). The seedlings were transplanted on a garden soil previously characterized for its pedological and chemical features (see Table S1) and even amended with 20% of high-quality compost (Table S2). Four experimental treatments (five plants per each treatment), for each assayed crop, were prepared: non-composted soil considered as a control (C); amended soil (CC); amended soil irrigated with saline (NaCl) tap water solutions at 150 mM (CC150) or 300 mM (CC300). Each experimental group was irrigated seven times with tap water or 150 mM or 300 mM NaCl solutions by pouring 150 mL per pot, every 3–4 days, until reaching the 105 and 210 mmol in the case of CC150 and CC300, respectively.
2.2. Estimation of Soil Electrical Conductivity
At the end of the experiment (about 90 days after seed sowing), 10 g of soil from each pot were collected from the surface zone to measure its electrical conductivity in order to estimate the salt concentrations in the soils. Each soil sample was placed in a 50 mL tube and resuspended in distilled water (20 mL) in a 1:2 (w/v) ratio. All the prepared samples were placed on an orbital shaker at 180 RPM (rotations per minute) for about 24 h to favor the solubilization of the soil mineral salts. All the samples, at the end of the resuspension, were centrifuged at 280 RCF (relative centrifugal force) for 5 min to settle the soil particles, the supernatant solution recovered and transferred in new 50 mL falcon tubes. Subsequently, with the use of the YK30WA pure water tester conductivity meter (Lutron, Taipei, Taiwan), the EC values of each soil solutions were measured, and the salt concentration values estimated, comparing the obtained values with those of the calibration straight line.
2.3. Seedling Growth and Biomass Determination
About a week after the last addition of NaCl, the diameter and length of the stem of each seedling of the four tested experimental groups were measured. The measurement of the diameter was carried out with a vernier caliper, taking such measure at about 3/5 mm above the soil. At the later time, after three weeks, seedlings were removed from the pots, separated into the three organs, except maize for which leaves and stems were collected just as a single organ, and then dried to estimate the dry weight of stems, leaves and roots. Soil particles were carefully removed from the roots to decrease their damages and harvest, as much as possible, primary and fine roots, namely roots that have a diameter of less 2 mm. The dry biomass was obtained by drying the organs of each single seedling in an oven at a temperature of 70 °C, up to achieve a constant weight.
2.4. Statistical Analysis
Stem length, basal diameter and biomass of each seedling of the four experimental groups were statistically analyzed, the mean values and standard errors were calculated, and the data compared by analysis of variance (ANOVA) and the Tukey’s HSD post hoc test, with p < 0.05 as the significance cut-off. All data were analyzed with R software.
2.5. Isolation and Quantification of Rhizosphere Bacteria
The rhizosphere bacteria were isolated from the newly harvested fine roots. The procedure involved first preparing 5 g of fresh roots from the seedlings of maize (CC150) and quinoa (CC300) groups. One hundred mL of sterile saline solution (9.0 g·L−1 NaCl) were added to the 5 g of maize or quinoa fine roots in tubes. The tubes containing the fine roots and saline solution were then left to stir in an orbital shaker for a couple of hours at room temperature. The solutions, rich in bacteria, were recovered, transferred to a new sterile tube and then centrifuged at 30 RCF at 4 °C for two minutes to eliminate soil residues. The supernatants were recovered and centrifuged again at 550 RCF for 20 min always at 4 °C. Forty milliliters of saline solution were added to each bacteria pellet deposited on the bottom of the tube to remove residual debris and wash the bacteria; then the bacterial solutions were centrifuged at 550 RCF for other 20 min. Finally, after supernatant removal, the bacterial pellet was resuspended again in 4 mL of saline solution and then serially diluted up to 10−6. Five hundred µL of ND (not diluted) and of each dilution, from both maize and quinoa, were plated on 14 cm diameter Petri dishes containing 100 mL sterile PCA culture medium (plate count agar—5% peptone, 0.25% yeast extract, 0.1% glucose, 1.5% agar and deionized water q.b. to 1 L), or on PCA supplemented with 500 mM of NaCl (PCA + 500 mM of NaCl). Aliquot of undiluted bacterial pellet solutions (two mL) of both maize and quinoa rhizosphere were undergone to a temperature of 85 °C for 15 min and then 500 µL plated on 14 cm diameter Petri dishes containing 100 mL sterile YSA (yeast starch agar—1% starch, 0.2% yeast extract, 1.5% agar and deionized water q.b. to 1 L), or YSA medium added with 500 mM of NaCl (YSA + 500 mM NaCl) to isolate spore-forming bacterial colonies.
Petri dishes of each bacterial dilutions and ND, of both analyzed plant species, were incubated at 27 °C for one week and inspected daily. Bacteria population was quantified by determination of colony forming units (CFU) with agar plate method. During the incubation time morphologically different colonies (e.g., shape, size, and color) were collected with a sterile loop and streaked on a sterile 9 cm diameter Petri dish containing 20 mL of 1.0 M NaCl PCA solid medium.
2.6. Bacterial DNA Extraction
The bacterial colonies tolerant to NaCl concentration ranging between 2.0 and 4.0 M, distinguished on the basis of their morphology and growth rate at room temperature, were inoculated in sterile 15 mL tubes containing 5 mL of liquid PCA medium at the same selection concentrations of NaCl, and incubated overnight at 27 °C in a rotary incubator. The next morning, the liquid medium was centrifuged at 4 °C for 15 min at 1000 RCF to collect the bacteria; finally, the bacterial pellet was resuspended in 3 mL of liquid PCA medium. Aliquots of this were frozen at −80 °C in PCA 15% of glycerol, whilst the remaining were used for DNA extraction by means of REDExtract-N-Amp™ Tissue PCR kit (Merck Life Science Srl, Milan, Italy) following the supplier instructions.
2.7. Amplification and Sequencing of 16S rDNA
The bacterial 16S rDNA gene was amplified by polymeras chain reaction (PCR) methods, using REDExtract-N-Amp TM tissue PCR kit (Merck Life Science Srl, Milan, Italy) and following the supplier instructions. Briefly, in a final volume of 20 mL were mixed 2.0 mL of DNA solution, 2.0 mL of 10X Red Extract-N- AmpPCR Ready Mix of the Sigma kit, 2.0 mL of 1.0 mM each universal primers 8-27F (5′ AGAGTTTGATCCTGGCTCAG 3′) and 1507-1492R (5′ TACCTTGTTACGACTT 3′). PCR conditions were: initial denaturation at 95 °C for 3 min, followed by 35 cycles of denaturation at 95 °C for 1 min, annealing at 55 °C for 1 min and elongation at 72 °C for 2 min, with additional final elongation step at 72 °C for 5 min.
The amplified PCR products were separated by electrophoresis on 1.0% agarose gel, stained with ethidium bromide and visualized under short-wavelength UV light. PCR products were purified with a GFX PCR DNA and Gel Band Purification Kit (GE Healthcare, Life Sciences) according to the manufacturer’s instructions, and finally sequenced. The 16S rDNA sequencing was performed by BMR Genomics Service (Padova, Italy). The sequences of the isolated strains (1300–1500 nucleotides) were identified by a similarity search using the BLAST function of GenBank at the National Center for Biotechnology Information (NCBI) electronic site (NCBI. Available online: http://www.ncbi.nlm.nih.gov/ (accessed on 26 February 21)).
2.8. Characterization of Selected Bacterial Strains
Salt and Temperature Tolerance
To evaluate the NaCl tolerance of each bacterial strain, suspensions of freshly grown bacteria were prepared in sterile saline solution (9.0 g·L−1 NaCl) at cellular density, measured by optical density at 600 nm (OD600) of 0.1. The same amounts of bacterial suspension (5.0 mL) were spotted, in triplicate, on PCA solid medium added with different NaCl concentrations (2.0 M, 2.5 M, 3.0 M, 3.5 M, 4.0 M) in parallel. Bacterial growth on PCA medium without salt was included as a positive control. Plates were incubated at 27 °C for 48–72 h.
To evaluate the best growth temperature range, the same amounts of bacterial suspension (5.0 mL) were spotted, in triplicate, on PCA solid medium and plates were incubated at 27, 37, 45, and 55 °C for 48–72 h.
Bacterial growth was evaluated qualitatively on bacterial spots (low, moderate, or high elevate growth).
2.9. PGP Features
2.9.1. Phosphate Solubilization Capacity
The Pikovskaya agar plate method was employed to estimate the bacterial strains capacity of solubilizing calcium tricalcium phosphate (Ca3(PO4)2). The Pikovskaya medium was prepared dissolving in one liter of ultra-pure water 10 g glucose, 5.0 g Ca3(PO4)2, 0.5 g (NH4)2SO4, 0.1 g MgSO4·7H2O, 0.2 g KCl, 0.2 g NaCl, 0.0001 g FeSO4·7H2O, 0.0001 g MnSO4, 0.5 g yeast extract, 15 g agar. Petri dishes of 90 mm diameter were prepared with 5.0 mL of Pikovskaya medium to allow the halo observation. Bacterial smear from culture plate was resuspended in saline solution and then 5.0 µL of it at 0.5 OD600 were spotted on the plates and then incubated for 7 days at 27 °C. Therefore, phosphate solubilizer strains were identified by observing the formation of a visible halo around the spotted bacterial strains [25].
2.9.2. Siderophore Production
Siderophores production in bacterial strains was screened using the chrome azurol S (CAS) agar plate method (A detailed description of the CAS agar preparation is given in Note SM1). Aliquots (5.0 μL) of the bacterial suspension (0.5 OD600) were inoculated on the CAS medium plates and incubated for seven days at 37 °C. The appearance and diameter of an orange halo in the CAS-agar around the bacterial colonies was indicative of entity and siderophore production [26].
2.9.3. Ammonia Production
Nessler’s method was used to estimate the ammonia production of bacterial strains. Aliquots (1.0 mL at 0.1 OD600) of the suspension of NaCl resistant strains were inoculated in 9.0 mL of peptone water (1.0 g·L−1 peptone, 5.0 g·L−1 NaCl). The inoculated peptone water solutions were incubated at 27 °C for 24 h under stirring at 135 RPM. At the end of the incubation time, the inoculated solutions were centrifuged at 5470 RCF for 20 min at room temperature, and 1.0 mL of the supernatant solution was mixed with 1.0 mL of Nessler’s reagent (VWR— Leuven, Belgium) and diluted up to 10.0 mL of ultra-pure distilled water. Finally, the amount of ammonia in the medium was quantified spectrophotometrically at 450 nm (Shimadzu, UV-1800) and comparing the absorbance values of each bacterial strain with an ammonium-sulfate ((NH4)2SO4) standard curve. The standard curve was prepared mixing eight different concentrations of ammonium-sulfate in peptone water, ranging between 1 and 200 µg·mL−1, following the procedure above (correlation factor R2 = 0.98). [27].
2.9.4. IAA Production
The Salkowski’s method was employed to estimate the indole acetic-3-acid (IAA). Aliquots (1 mL at 0.1 OD600) of the suspension of NaCl resistant strains were inoculated in 9.0 mL of PCA liquid medium (PCA, 5.0 g·L−1 tryptone, 1.0 g·L−1 glucose, 2.5 g·L−1 yeast extract) and incubated at 27 °C for 24 h under stirring at 135 RPM. At the end of the incubation time, the bacterial growth solutions were centrifuged at 5470 RCF for 20 min and 1.0 mL of supernatant was gently mixed with 2.0 mL of Salkowski’s reagent previously prepared mixing 1.0 mL of a 0.5 M FeCl3 solution with 49 mL HClO4 35%. Then, each prepared solution was incubated for 2 h in the dark at 27 °C, and then their absorbances were estimated spectrophotometrically at 530 nm (Shimadzu, UV-1800). The amount of IAA produced by each single bacterial strain was calculated comparing the absorbance data with an IAA standard curve. The standard curve was prepared mixing eight different concentrations of IAA in PCA liquid medium, ranging between 0.1 and 25 µg·mL−1, following the procedure above (correlation factor R2 = 0.99) [28].
3. Results
3.1. Soil Electrical Conductivity
By means of the knowledge of the electrical conductivity (EC) of the soil was then possible to even estimate the salt concentration with respect to a calibration curve, and then to classify each soil based on its own salt concentration. Since the saline solutions, obtained from the soils of each experimental group, showed a high EC, except those of the C group, they were diluted in order to obtain a more accurate measurement (see Section 2). The EC of each saline soil solutions is reported in Table 1.

Table 1.
Electrical conductivity of soils (dS·m−1) reported as average ± standard deviation of the five replicates for each group.
The EC values were compared with the calibration line in order to estimate the salt concentrations of each soil solution. The results of this estimation are reported in Table 2.

Table 2.
Salt concentration in soil (M) estimated by the EC compared with the standard curve.
3.2. Seedling Growth and Dry Biomass
Crop stem length (Figure 1A) was positively influenced in some groups (tomato CC and CC150; quinoa CC, CC150 and CC300) by soil compost addition; however, it was negatively affected by salt spiked soil in almost all other groups. In particular, high salt concentration (300 mM) reduced the stem length of all crops with the exception of quinoa. On the other hand, stem diameter (Figure 1B) was always positively influenced by soil compost addition even in those groups irrigated with salty water, except maize and sunflower. In these last cases, at the highest salt dose (300 mM), the stem diameters were similar to that of the C group (no statistically significative difference). The response to salt addition of tomato group is noteworthy; in fact, even at the highest dose, no reduction of stem diameter was detected.
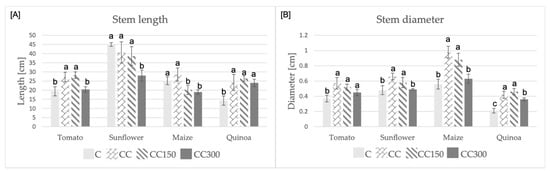
Figure 1.
Stem length (A) and stem diameter (B) of the four crops undergone the different experimental conditions. Statistical significance assayed for p = 0.05. The comparison was performed within the plant species. The letters: a, b, c, indicate the statistically significant differences.
The dry biomass of all organs in the four assayed crops was positively influenced by the addition of compost to the pot soils (Figure 2). In some case, the dry biomass increase was remarkable: up to five/six times, as for tomato roots and stems (Figure 2A, CC group), or for stems of quinoa grown on soils irrigated with salty waters (Figure 2D, CC150, and CC300 groups). A general reduction of the dry biomass was observed at the highest concentration of salt (300 mM) for all crops and organs, except for quinoa (Figure 2D). In fact, in the case of sunflower plants, they showed evident suffering symptoms (e.g., loss of cellular turgor, chlorosis, etc.), and, in the case of plants exposed to 300 mM NaCl, a biomass reduction for root, stem and leaf (Figure 2B). It is noteworthy that the dose of 300 mM of salt was so stressful for maize seedlings that all of them died at the end of the experiment (Figure 2C, CC300).
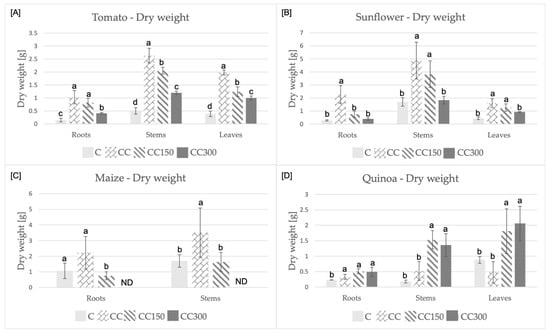
Figure 2.
Dry biomass produced by the four crops: (A) tomato, (B) sunflower, (C) maize, (D) quinoa in the different conditions. Statistical significance assayed for p = 0.05. The comparison was performed within each organ. The letters a, b, c, d indicate the statistically significant differences. ND indicates not detectable.
3.3. Isolation of Rhizosphere Bacteria
Differences between the two analyzed root microbiomes obtained by monocot glicophyte (maize) and a dicot halophyte (quinoa) plant exposed to salt were investigated. Bacterial pellets obtained by quinoa or maize plants irrigated with 300 or 150 mM NaCl solutions, respectively, were inoculated on PCA agar media (PCA), with or without the addition of 500 mM NaCl (PCA + 500 mM), to perform microbial counts of cultivable bacteria after one week of incubation at 27 °C. On PCA medium, bacterial pellets from maize and quinoa formed 22 × 104 and 93 × 104 CFU (colony forming units), respectively; while, on PCA plus NaCl, CFUs were 32 × 103 and 37 × 103 for maize and quinoa, respectively (Table 3).

Table 3.
Average CFU on control medium (PCA) and on PCA added with salt (PCA + 500 mM) related to the bacteria pellets obtained by quinoa or maize plants irrigated with 300 or 150 mM NaCl solutions, respectively.
Based on morphological differences a total of 50 bacterial colonies underwent NaCl selection as described in the Section 2. Among the 50 initially isolated colonies, resistant to 500 mM NaCl both from maize (25) and quinoa (25), only 13 colonies showed different degrees of resistance, as illustrated in Table 4.

Table 4.
Growth of NaCl resistant bacterial strains, identified by 16S rDNA sequencing, at difference sodium chloride concentration and different temperature.
3.4. Genetic Characterization of Bacteria
Ten different bacterial species (Table 4) were recognized out of the 13 NaCl resistant strains initially isolated. Four bacterial strains were selected from maize rhizosphere, while the remaining ones (9) were from that of quinoa. The most represented genus is the Halomonas one with five bacterial strains (Table 4) and three different species; the second most representative genus is Bacillus, with four different species (Table 4); finally, Brevibacterium and Staphylococcus genera were represented by two different bacterial strains each.
3.5. Salt and Temperature Tolerance, and PGP Features
The 13 NaCl resistant bacterial strains showed a clear different capability to tolerate this salt, ranging from 2.0 M up to 4.0 M (Table 4), as in the case of two strains of Staphylococcus succinus (MD6 and MB8) able to tolerate 4.0 M NaCl, or Brevibacterium (strain 128293) able to grow in the presence of 3.5 M NaCl. All the bacterial strains had the optimal growth temperature at 37 °C except Bacillus stratosphericus, which had its optimal growth temperature at 45 °C, but it was also able to grow at 55 °C.
The selected bacterial strains were also characterized by different and peculiar PGP features (ammonia, siderophore and IAA production or capacity to metabolize tricalcium phosphate). Among these strains, six showed PGP features (Table 5), whilst the others did not (data not shown). However, only two strains distinguish themselves for showing, contemporaneously, all four PGP features, namely: Staphylococcus succinus (strain JM40) and Bacillus stratosphericus (strain MPF-B2a).

Table 5.
PGP features
4. Discussion
Soil is considered saline if it exceeds 4 dS m−1 as EC (approximately 40 mM NaCl) at 25 °C, as already illustrated in the Section 1. In our experiment we reached mean values between 11 and 15, and 18 and 21 dS m−1 in the case of the experimental groups CC150 and CC300, respectively. These values are very far above the limit of 4 dS m−1 over which, plants and many crops feel the stress caused by salt excess. In particular, maize plants could not tolerate the saline concentrations reached by the soil irrigated with the solution 300 mM NaCl (around 21 dS m−1 mean value, Table 2), confirming that maize, being a glycophyte plant, was the most sensitive one among those tested. The effects of salt stress on crop growth, and especially in the case of maize, are mainly due to high osmotic imbalance caused by low external water potential, ion toxicity induced by Na+ and/or Cl−, photosynthesis inhibition, but also by an altered nutrition status which determines a reduction of essential element up-take [29]. Moreover, the presence of high level of these two ions in the plant tissues affects cellular and organelle membranes, primarily due to production of reactive oxygen species (ROS), limiting the plant growth and causing evident signs of phenotypic alterations before mortality, as we observed in the case of maize treated with 300 mM NaCl.
However, plants/crops show a unique capacity to adapt to soil salinization, such as stomatal regulation, variation of hormone balance, activation of the antioxidant defense system, etc. [30]. Therefore, it can be hypothesized that the higher resilience observed for all assayed crops of our experiment, whenever grown on compost saline amended soils, is due to an improved adaptation of their physiology, metabolism, and morphology to the stress imposed by high soil EC, as mentioned above.
Compost is renewable resource which can also be obtained from separate collection of organic waste and it is employed to return organic matter to agricultural soils [31]. Its use as organic amendment dates back to the beginning of human civilization, in fact, animal manure was used when agriculture and cattle breeding were born, about 10–11,000 years ago. Thousands of scientific publications, in the last three decades, deal with compost, illustrating the beneficial effects on soils depleted of its organic matter due to the reckless use of inorganic fertilizers [32]. The benefits of compost on soil chemical-physical-biological properties, and on crop productivity have been widely demonstrated [33,34,35]. In our pot experiment the positive effect on the seedlings growth and biomass was confirmed once more, in almost all the treated crops and groups. Similar results were obtained by Cid et al. [36], showing that “compost improved soil fertility and microbial activity, including nodulation in soybean roots. Biomass production was strongly increased; content of chlorophyll, carotenes, sugars, and protein also increased”.
Soil salinity has always a detrimental effect on almost all vegetables and crops, as well illustrated by Machado and Serralheiro [7]. The authors, based on mathematical models related to ECe—electrical conductivity (EC) of saturated paste extract of soil and ECW—electrical conductivity (EC) of irrigation water, divided 19 vegetables and crops into four categories: Sensitive, moderately sensitive, moderately tolerant, or tolerant to soil salt content. Out of 19 vegetables and crops, considered by the two authors in their analysis, six were sensitive, ten moderately sensitive, two moderately tolerant, and only one tolerant. Based on this analysis, for instance, asparagus was tolerant, red beet was moderately tolerant, tomato was moderately sensitive, and beans were sensitive. In the last decades researchers focused their attention on the possibility that organic amendments, including compost, could cope with the damaging effects of soil salinity on crops and vegetables. Diacono and Montemurro [37] recently summarized the positive effect of compost on physical-chemical and biological soil characteristics and restoration of crop productivity. In our experiment we observed how compost soil amendment was able to cope with the detrimental effect, on the seedlings of all four tested crops (maize, tomato, sunflower, and quinoa), caused by the salty water irrigation, which increased the EC, and soil salt concentration up to 20 dS m−1 and 0.135 M, respectively. These values of EC and salt concentration are well known to usually cause reduction of growth, biomass, and yield to crops and vegetables [7], which are considered, except quinoa, glycophytes. The data we collected for tomato confirmed the analysis of Machado and Serralheiro, who considered this vegetable as moderately sensitive. However, compost amendment partly counteracted the negative effects of high salt soil content, in fact CC150 and CC300 showed a considerably greater organ biomass when compared to C group, and no substantial differences for stem length and diameter also when compared with CC group.
Seedlings of sunflower and maize C and CC groups did not show any significant difference in the case of the stem length, however their diameter increased significantly when compost was added to the soils, as well as all the other treated crops, suggesting that the seedlings of the C group of sunflower and maize were more streamlined respect to those amended with compost. Stem length and diameter were significantly reduced in almost all treatments CC300, except for stem length in the quinoa group.
The dry biomass of the three organs (roots, stems and leaves) increased in all CC groups when compared to the C group (control), while a slight or null decrease when the glycophyte crops were treated with NaCl 150 mM. It is probable that compost reduces the stress effect due to salt water irrigation. However, soil irrigation with NaCl 300 mM had, in general, a statistically significant effect on all the glycophytes, in particular on maize, which resulted to be the most sensitive one to NaCl treatments, and in particular to that of 300 mM; in fact, in this case (CC300) all seedlings were dead at the end of the experiment.
Hafez et al., has recently shown [38] that compost addition to a sodic soil improved quality, increasing nutrient uptake and stimulating its chemical properties. The resilience and growth promotion in wheat seedlings were attributed by the authors to a significant improvement in the relative water, chlorophyll, K+ leaf content, and, contemporary, to a reduction of the oxidative stress due to a more limited activities of the involved in the response to abiotic stresses enzymes (e.g., SOD, APX, etc.), and to the expression of the corresponding genes (CAT, APX, and MnSOD). In our experiment a quite similar situation was observed for the four assayed crops; their increased resilience to high soil EC was most likely due to the same kind of stress response that Hafez and co-workers showed in the case of wheat.
Soil irrigation with 150 or 300 mM NaCl water solutions, in the case of quinoa seedlings (CC150 and CC300), had an opposite effect respect to maize; in fact, they even showed a superior growth on saline soils, probably because, as stated by Guarino et al. [39], “the expression of salt stress-responsive genes, which are inducible in glycophytes, is, on the other hand, a salt tolerance trait in halophytes, as precisely quinoa is.”
Since the abiotic stress, induced by high salt irrigation to the assayed crops, was relevant, we supposed that a similar stress has been applied also to the rhizosphere microbial community as selection pressure and, consequently, allowed us to isolate the highly NaCl-tolerant rhizo-bacterial strains.
Many laboratories all around the world are isolating bacterial strains tolerant to NaCl, or salt in general. Halo-tolerant bacterial strains have been isolated from both balk and rhizosphere soils [40,41,42,43], but also from endophyte bacterial communities [24,44,45]. Several of these bacterial strains have proven some plant growth capabilities such as, indole acetic acid (IAA) and siderophore synthesis, solubilization of phosphate, 1-aminocyclopropane-1-carboxylate (ACC) deaminase [24,45,46]. The bacterial strains we isolated from maize and quinoa rhizosphere showed very high tolerance to NaCl between 2.0 and 4.0 M as in the case of the strains QB13 (3.5 M—Bacillus stratosphericus strain MPF-B2a) or MD6 (4.0 M—Staphylococcus succinus strain JM40), demonstrating that selection we applied to the rhizosphere community of the assayed crops and vegetables, amended with compost, was extended for a long time and strong enough to select highly NaCl tolerant bacterial strains.
Among 13 bacterial strains identified by 16S rDNA, we isolated 10 different species, suggesting the presence of a very heterogeneous bacterial community that populates the compost amended root soils of both maize and quinoa. Out of the 13 halo-tolerant strains, 9 were selected from quinoa rhizosphere, as well as the most represented bacterial genus, namely Halomonas. This observation would suggest that quinoa can establish and select a specific rhizospheric microbiome, enriched by compost, able to better tolerate high soil NaCl concentration when compared with that of maize. This consideration is supported also by the fact that all the assayed crop species had higher biomass when amended with compost and compared with the control (Group C), even when soils were irrigated with solutions containing high NaCl concentrations.
All the halotolerant NaCl bacterial species, we selected, belong to species and genera well known to be resistant to salts and have been isolated from different and peculiar environments as in the case of: Halomonas alkaliantarctica, originally isolated from the saline lake Cape Russell in Antarctica in 2001. It is an alkalophilic moderately halophilic, exopolysaccharide-producing bacterium [47]; Halomonas titanicae isolated in 2010 from a sample of rusticle obtained from the RMS Titanic, collected during an oceanic expedition in 1991, for its peculiar characteristics it could also have the potential to be used in bioremediation to accelerate the decomposition of shipwrecks littering the ocean and sea floor; [48] Bacillus mojavensis, whose name suggests the place where it was collected and isolated, such as the Mojave Desert in US [49]. It belongs to the Bacillus genus, in particular to the subgroup including Bacillus amyloliquefaciens, B. atrophaeus, B. licheniformis, Brevibacterium halotolerans, Paenibacillus lentimorbus, and P. popilliae, which have peculiar features such as: salt tolerance, resistance to metals and high temperatures, but also able to colonize the root vascular tissues and therefore considered as bacterial endophytes (B. mojavensis) [50]. It is relevant that we isolated also other two species belonging to this subgroup of the genus Bacillus as B. licheniformis and Brevibacterium spp (two strains). For all bacterial species we isolated, and listed in the Section 3, a brief description of their main microbiological features is given as note in the Supplementary Materials of the publication (Note S2).
The agricultural lands are going on reducing due mainly to anthropic activities, such as urbanization, climate change, soil salinization, etc. [51], so it is imperative to improve crop productivity and quality on the presently available agricultural surfaces avoiding the implementation of policies of deforestation and irresponsible use of natural resources. In the last decades, it has been possible to acquire knowledge on the relevance that rhizosphere microbiome and PGPRs, in particular, have in improving health, resilience, and productivity of the crops. Their identification, isolation, molecular classification and possessing relevant PGP features are essential for moving towards an agriculture more respectful of agroecosystems and consequently of human and animal health [52]. As stated above, salinization is one the main problem afflicting agricultural soils, however PGPRs can be of great help in reducing the impact of soil salinization and, at the same time, improving crop productivity and health [53,54]. The bacterial strains, we isolated and taxonomically classified in this research work, showed tolerance to very high NaCl concentrations, but also several PGP features, demonstrating, once more, that compost amendment of soil not only increases its fertility, but it is also a valuable source of rhizosphere bacteria able to promote health, resilience and plant productivity. However, we are still far away from being able to conclude the rhizobacteria we isolated can be considered real PGPR; in fact, they have to be tested in seed priming, and to even show effective plant growth-promoting features in pot experiments. At present, we are planning to test the most promising ones both singularly and in consortium on all four crops assayed in the experiment above illustrated. Moreover, once we will demonstrate their capacity to improve crop growth in pot experiment, also their effectiveness must, therefore, be assessed in field trials and in diversified soil and climatic conditions and, only then, will we be able to conclude that we have selected real PGPRs.
5. Conclusions
The data illustrated above confirm, once more, that compost soil amendment improves the capability of the assayed crop seedlings of both glycophytes (maize, sunflower, tomato) and halophytes (quinoa), to counteract abiotic stress due to, in our specific case, saline soil. The improved crop resilience to high soil EC common observed in saline or sodic soils is not only due to the nutrients that the compost adds to them, but also to the presence of high number of microorganisms with several biochemical features able to improve plant growth (siderophore and/or phosphatase production, etc.) and/or reduce abiotic stress, such as ACC deaminase. Therefore, compost, as we have demonstrated, is a great source of microorganisms which can counteract numerous biotic (fungal and bacterial diseases, e.g., Fusarium verticillioides causing Fusarium head blight in maize, Xylella fastidiosa causing the olive quick decline syndrome, etc.) or abiotic (e.g., drought, high temperature, heavy metals, etc.) plant/crop stresses, it is just a matter of searching for and selecting them carefully. For this reason, we suggest to all plant biologists to persevere with resolution to find novel rhizo-microorganisms with PGP features; if this happened in the near future their use will open the possibility to move towards a more sustainable and environment friendly agriculture in the perspective of the present and future climate challenges.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3417/11/5/2125/s1, Table S1. Agricultura soil characteristics; Table S2. Physical-chemical properties of compost; Note S1. CAS agar method preparation; Note S2. short description of bacterial species.
Author Contributions
Conceptualization: S.C., A.C., F.G., G.L.; methodology: S.C., G.V., G.L., E.G.; software: G.O., F.G.; validation: S.C., A.C., G.V., F.G.; formal analysis: G.O., G.N.; investigation: G.O., G.N.; resources: S.C.; data curation: G.O., G.V., S.C.; writing—original draft preparation: S.C., G.O., A.C., F.G.; writing—review and editing: E.G., G.L.; supervision: S.C., A.C., F.G.; funding acquisition: A.C. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by FARB 2017 (Fondi di Ateneo per la Ricerca di Base) to AC.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Libutti, A.; Cammerino, A.R.B.; Monteleone, M. Risk Assessment of Soil Salinization Due to Tomato Cultivation in Mediterranean Climate Conditions. Water 2018, 10, 1503. [Google Scholar] [CrossRef]
- Brombin, V.; Mistri, E.; De Feudis, M.; Forti, C.; Salani, G.M.; Natali, C.; Falsone, G.; Antisari, L.V.; Bianchini, G. Soil carbon investigation in three pedoclimatic and agronomic settings of Northern Italy. Sustainability 2020, 12, 10539. [Google Scholar] [CrossRef]
- Welle, P.D.; Mauter, M.S. High-resolution model for estimating the economic and policy implications of agricultural soil salinization in California. Environ. Res. Lett. 2017, 12, 094010. [Google Scholar] [CrossRef]
- Jamil, A.; Riaz, S.; Ashraf, M.; Foolad, M.R. gene expression profiling of plants under salt stress. Crit. Rev. Plant Sci. 2011, 30, 435–458. [Google Scholar] [CrossRef]
- Jesus, J.M.; Danko, A.S.; Fiúza, A.; Borges, M.-T. Phytoremediation of salt-affected soils: A review of processes, applicability, and the impact of climate change. Environ. Sci. Pollut. Res. 2015, 22, 6511–6525. [Google Scholar] [CrossRef]
- Qadir, M.; Ghafoor, A.; Murtaza, G. Amelioration strategies for saline soils: A review. Land Degrad. Dev. 2000, 11, 501–521. [Google Scholar] [CrossRef]
- Machado, R.M.; Serralheiro, R.P. Soil salinity: Effect on vegetable crop growth. management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Abbas, A.; Naveed, M.; Azeem, M.; Yaseen, M.; Ullah, R.; Alamri, S.; Farooq, Q.U.; Siddiqui, M.H. Efficiency of wheat straw biochar in combination with compost and biogas slurry for enhancing nutritional status and productivity of soil and plant. Plants 2020, 9, 1516. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Insight into biochar properties and its cost analysis. Biomass- Bioenergy 2016, 84, 76–86. [Google Scholar] [CrossRef]
- Maroušek, J.; Strunecký, O.; Stehel, V. Biochar farming: Defining economically perspective applications. Clean Technol. Environ. Policy 2019, 21, 1389–1395. [Google Scholar] [CrossRef]
- Guarino, F.; Improta, G.; Triassi, M.; Cicatelli, A.; Castiglione, S. Effects of zinc pollution and compost amendment on the root microbiome of a metal tolerant poplar clone. Front. Microbiol. 2020, 11, 1677. [Google Scholar] [CrossRef] [PubMed]
- Cicatelli, A.; Ferrol, N.; Rozpadek, P.; Castiglione, S. Editorial: Effects of plant-microbiome interactions on phyto- and bio-remediation capacity. Front. Plant Sci. 2019, 10, 533. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Ahmad, M.; Zahir, Z.A.; Javaid, A.; Ashraf, M. The role of mycorrhizae and plant growth promoting rhizobacteria (pgpr) in improving crop productivity under stressful environments. Biotechnol. Adv. 2014, 32, 429–448. [Google Scholar] [CrossRef]
- Forni, C.; Duca, D.; Glick, B.R. Mechanisms of plant response to salt and drought stress and their alteration by rhizobacteria. Plant Soil 2017, 410, 335–356. [Google Scholar] [CrossRef]
- Chandra, P.; Enespa, F. Singh R soil salinity and its alleviation using Plant Growth–Promoting Fungi. In Agriculturally Important Fungi for Sustainable Agriculture; Yadav, A., Mishra, S., Kour, D., Yadav, N., Kumar, A., Eds.; Springer: Cham, Switzerland, 2020; Volume 2. [Google Scholar]
- Vives-Peris, V.; de Ollas, C.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Root exudates: From plant to rhizosphere and beyond. Plant Cell Rep. 2020, 39, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Khanghahi, M.Y.; Ricciuti, P.; Allegretta, I.; Terzano, R.; Crecchio, C. Solubilization of insoluble zinc compounds by zinc solubilizing bacteria (ZSB) and optimization of their growth conditions. Environ. Sci. Pollut. Res. 2018, 25, 25862–25868. [Google Scholar] [CrossRef]
- Kadmiri, I.M.; Chaouqui, L.; Azaroual, S.E.; Sijilmassi, B.; Yaakoubi, K.; Wahby, I. Phosphate-solubilizing and auxin-producing rhizobacteria promote plant growth under saline conditions. Arab. J. Sci. Eng. 2018, 43, 3403–3415. [Google Scholar] [CrossRef]
- Wu, Z.S.; Yue, H.T.; Lu, J.J.; Li, C. Characterization of rhizobacterial strain rs-2 with ACC Deaminase activity and its performance in promoting cotton growth under salinity stress. World J. Microbiol. Biotechnol. 2012, 28, 2383–2393. [Google Scholar] [CrossRef]
- Gamalero, E.; Berta, G.; Massa, N.; Glick, B.R.; Lingua, G. Interactions between Pseudomonas putida UW4 and Gigaspora rosea BEG9 and their consequences for the growth of cucumber under salt-stress conditions. J. Appl. Microbiol. 2010, 108, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Kloepper, J.; Schroth, M.N. Plant Growth-Promoting Rhizobacteria on radishes. In Proceedings of the IV International Conference on Plant Pathogenic Bacteria, INRA, Angers, France, 2 September 1978; pp. 879–882. [Google Scholar]
- Moreira, H.; Pereira, S.I.; Vega, A.; Castro, P.M.; Marques, A.P. Synergistic effects of arbuscular mycorrhizal fungi and plant growth-promoting bacteria benefit maize growth under increasing soil salinity. J. Environ. Manag. 2020, 257, 109982. [Google Scholar] [CrossRef] [PubMed]
- Silambarasan, S.; Logeswari, P.; Cornejo, P.; Kannan, V.R. Role of Plant Growth–Promoting Rhizobacterial consortium in improving the Vigna radiata growth and alleviation of aluminum and drought stresses. Environ. Sci. Pollut. Res. 2019, 26, 27647–27659. [Google Scholar] [CrossRef]
- Gamalero, E.; Favale, N.; Bona, E.; Novello, G.; Cesaro, P.; Massa, N.; Glick, B.R.; Orozco-Mosqueda, M.D.C.; Berta, G.; Lingua, G. Screening of bacterial endophytes able to promote plant growth and increase salinity tolerance. Appl. Sci. 2020, 10, 5767. [Google Scholar] [CrossRef]
- Nautiyal, C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Letters 1999, 170, 265–270. [Google Scholar] [CrossRef]
- Alexander, D.B.; Zuberer, D.A. Use of Chrome Azurol-S reagents to evaluate siderophore production by rhizosphere bacteria. Biol.Fertil. Soils 1991, 12, 39–45. [Google Scholar] [CrossRef]
- Goswami, D.; Vaghela, H.; Parmar, S.; Dhandhukia, P.; Thakker, J.N. Plant growth promoting potentials of Pseudomonas Spp. strain og isolated from marine water. J. Plant Interact. 2013, 8, 281–290. [Google Scholar]
- Mohite, B. Isolation and characterization of Indole Acetic Acid (IAA) producing bacteria F\from rhizospheric soil and its effect on plant growth. J. Soil Sci. Plant Nutr. 2013, 13, 638–649. [Google Scholar] [CrossRef]
- Iqbal, S.; Hussain, S.; Qayyaum, M.A.; Ashraf, M.; Saifullah, M. The response of maize physiology under salinity stress and its coping strategies. In Plant Stress Physiology; Hossain, A., Ed.; IntechOpen Limited: London, UK, 2021. [Google Scholar]
- Acosta-Motos, J.R.; Ortuno, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Vallini, G.; Caimi, V.; Centemero, M.; Favoino, E.; Lanz, A.M.; Cortellini, L.; Ghini, C. Caratteristiche Tecniche e Settori Di Impiego Del Compost: Importanza Per L’Agricoltura Italiana. In Il Recupero Di Sostanza Organica Dai Rifiuti Per La Produzione Di Ammendanti Di Qualita’; Vallini, G., Caimi, V., Centemero, M., Favoino, E., Lanz, A.M., Cortellini, L., Ghini, C., Eds.; Agenzia Nazionale Protezione Ambiente: Rome, Italy, 2002. [Google Scholar]
- Diacono, M.; Montemurro, F. Effectiveness of organic wastes as fertilizers and amendments in salt-affected soils. Agriculture 2015, 5, 221–230. [Google Scholar] [CrossRef]
- Baldantoni, D.; Morra, L.; Saviello, G.; Alfani, A. Nutrient and toxic element soil concentrations during repeated mineral and compost fertilization treatments in a Mediterranean agricultural soil. Environ. Sci. Pollut. Res. 2016, 23, 25169–25179. [Google Scholar] [CrossRef] [PubMed]
- Baldantoni, D.; Saviello, G.; Alfani, A. Nutrients and non-essential elements in edible crops following long-term mineral and compost fertilization of a Mediterranean agricultural soil. Environ. Sci. Pollut. Res. 2018, 26, 35353–35364. [Google Scholar] [CrossRef]
- Siedt, M.; Schäffer, A.; Smith, K.E.; Nabel, M.; Roß-Nickoll, M.; Van Dongen, J.T. Comparing straw, compost, and biochar regarding their suitability as agricultural soil amendments to affect soil structure, nutrient leaching, microbial communities, and the fate of pesticides. Sci. Total. Environ. 2021, 751, 141607. [Google Scholar] [CrossRef] [PubMed]
- Cid, C.V.; Ferreyroa, G.V.; Pignata, M.L.; Rodriguez, J.H. Biosolid compost amendment increases soil fertility and soybean growth. J. Plant Nutr. 2020, 1–10. [Google Scholar]
- Diacono, M.; Montemurro, F. Long-term effects of organic amendments on soil fertility. A review. Agron. Sustain. Dev. 2010, 30, 401–422. [Google Scholar] [CrossRef]
- Hafez, E.M.; Omara, A.E.D.; Alhumaydhi, F.A.; El-Esawi, M.A. Minimizing hazard impacts of soil salinity and water stress on wheat plants by soil application of vermicompost and biochar. Physiol. Plant. 2020, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Guarino, F.; Ruiz, K.B.; Castiglione, S.; Cicatelli, A.; Biondi, S. The combined effect of Cr(III) and NaCl determines changes in metal uptake, nutrient content, and gene expression in quinoa (Chenopodium quinoa Willd.). Ecotoxicol. Environ. Saf. 2020, 193, 110345. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Zahir, Z.A.; Asghar, H.N.; Asghar, M. Inducing salt tolerance in mung bean through coinoculation with rhizobia and plant-growth-promoting rhizobacteria containing 1-aminocyclopropane-1-carboxylate deaminase. Can. J. Microbiol. 2011, 57, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Gururani, M.A.; Upadhyaya, C.P.; Baskar, V.; Venkatesh, J.; Nookaraju, A.; Park, S.W. Plant Growth-Promoting Rhizobacteria enhance abiotic stress tolerance in Solanum tuberosum through inducing changes in the expression of ROS-scavenging enzymes and improved photosynthetic performance. J. Plant Growth Regul. 2013, 32, 245–258. [Google Scholar] [CrossRef]
- Bharti, N.; Pandey, S.S.; Barnawal, D.; Patel, V.K.; Kalra, A. Plant Growth Promoting Rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci. Rep. 2016, 6, 34768. [Google Scholar] [CrossRef]
- Yasin, N.A.; Akram, W.; Khan, W.U.; Ahmad, S.R.; Ahmad, A.; Ali, A. Halotolerant Plant-Growth Promoting Rhizobacteria modulate gene expression and osmolyte production to improve salinity tolerance and growth in Capsicum annum L. Environ. Sci. Pollut. Res. 2018, 25, 23236–23250. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.L.; Waqas, M.; Asaf, S.; Kamran, M.; Shahzad, R.; Bilal, S.; Khan, M.A.; Kang, S.-M.; Kim, Y.-H.; Yun, B.-W.; et al. Plant growth-promoting endophyte Sphingomonas sp. LK11 alleviates salinity stress in Solanum pimpinellifolium. Environ. Exp. Bot. 2017, 133, 58–69. [Google Scholar] [CrossRef]
- Afridi, M.S.; Amna; Sumaira; Mahmood, T.; Salam, A.; Mukhtar, T.; Mehmood, S.; Ali, J.; Khatoon, Z.; Bibi, M.; et al. Induction of tolerance to salinity in wheat genotypes by plant growth promoting endophytes: Involvement of ACC deaminase and antioxidant enzymes. Plant Physiol. Biochem. 2019, 139, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Park, H.G.; Jeong, M.H.; Ahn, Y.S. Inoculation with Bacillus licheniformis MH48 to improve Camellia japonica seedling development in coastal lands. Turkish J.Agric. For. 2017, 41, 381–388. [Google Scholar] [CrossRef]
- Poli, A.; Esposito, E.; Orlando, P.; Lama, L.; Giordano, A.; De Appolonia, F.; Nicolaus, B.; Gambacorta, A. Halomonas alkaliantarctica sp. nov., isolated from saline lake Cape Russell in Antarctica, an alkalophilic moderately halophilic, exopolysaccharide-producing bacterium. Syst. Appl. Microbiol. 2007, 30, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Porro, C.; Kaur, B.; Mann, H.; Ventosa, A. Halomonas titanicae sp. nov., a halophilic bacterium isolated from the RMS Titanic. Int. J. Syst. Evol. Microbiol. 2010, 60, 2768–2774. [Google Scholar] [CrossRef]
- Roberts, M.S.; Nakamura, L.K.; Cohan, F.M. Bacillus mojavensis sp. nov., Distinguishable from Bacillus subtilis by sexual isolation, divergence in dna sequence, and differences in fatty acid composition. Int. J. Syst. Bacteriol. 1994, 44, 256–264. [Google Scholar] [CrossRef]
- Bacon, C.W.; Hinton, D.M. Endophytic and biological control potential of Bacillus mojavensis and related species. Biol. Control. 2002, 23, 274–284. [Google Scholar] [CrossRef]
- Arora, N.K. Impact of climate change on agriculture production and its sustainable solutions. Environ. Sustain. 2019, 2, 95–96. [Google Scholar] [CrossRef]
- Tkacz, A.; Poole, P. Role of root microbiota in plant productivity. J. Exp. Bot. 2015, 66, 2167–2175. [Google Scholar] [CrossRef]
- Qin, Y.; Druzhinina, I.S.; Pan, X.; Yuan, Z. Microbially mediated plant salt tolerance and microbiome-based solutions for saline agriculture. Biotechnol. Adv. 2016, 34, 1245–1259. [Google Scholar] [CrossRef]
- Kerbab, S.; Silini, A.; Bouket, A.C.; Cherif-Silini, H.; Eshelli, M.; Rabhi, N.E.H.; Belbahri, L. Mitigation of NaCl stress in wheat by rhizosphere engineering using salt habitat adapted PGPR halotolerant bacteria. Appl. Sci. 2021, 11, 1034. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).