Modeling the Effects of Calcium Overload on Mitochondrial Ultrastructural Remodeling
Abstract
1. Introduction
2. Materials and Methods
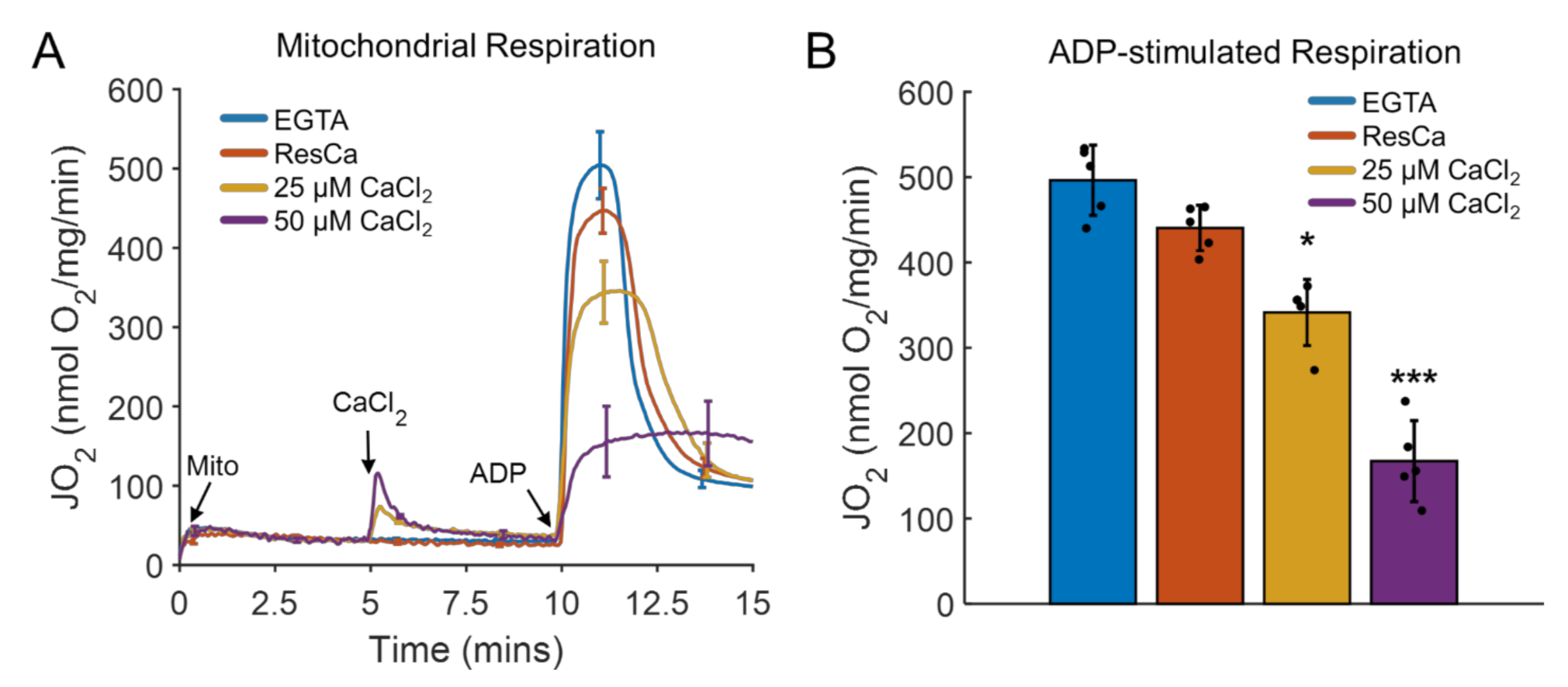
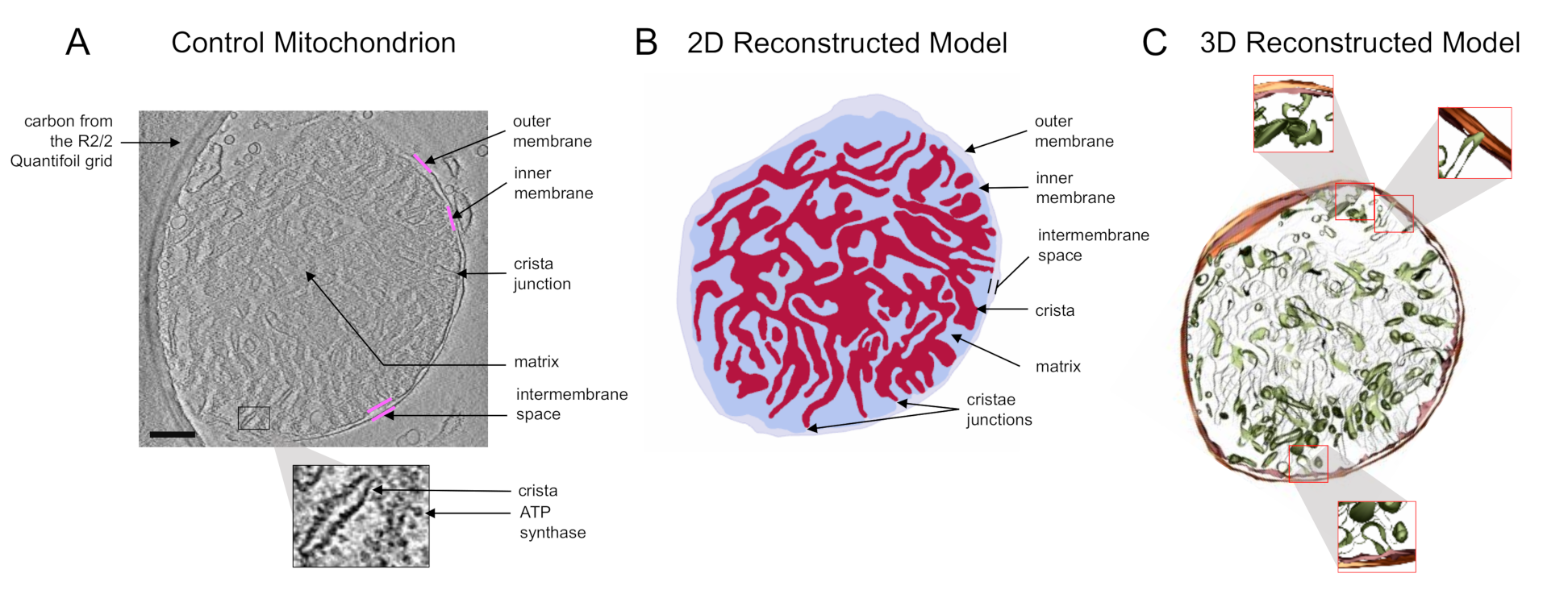
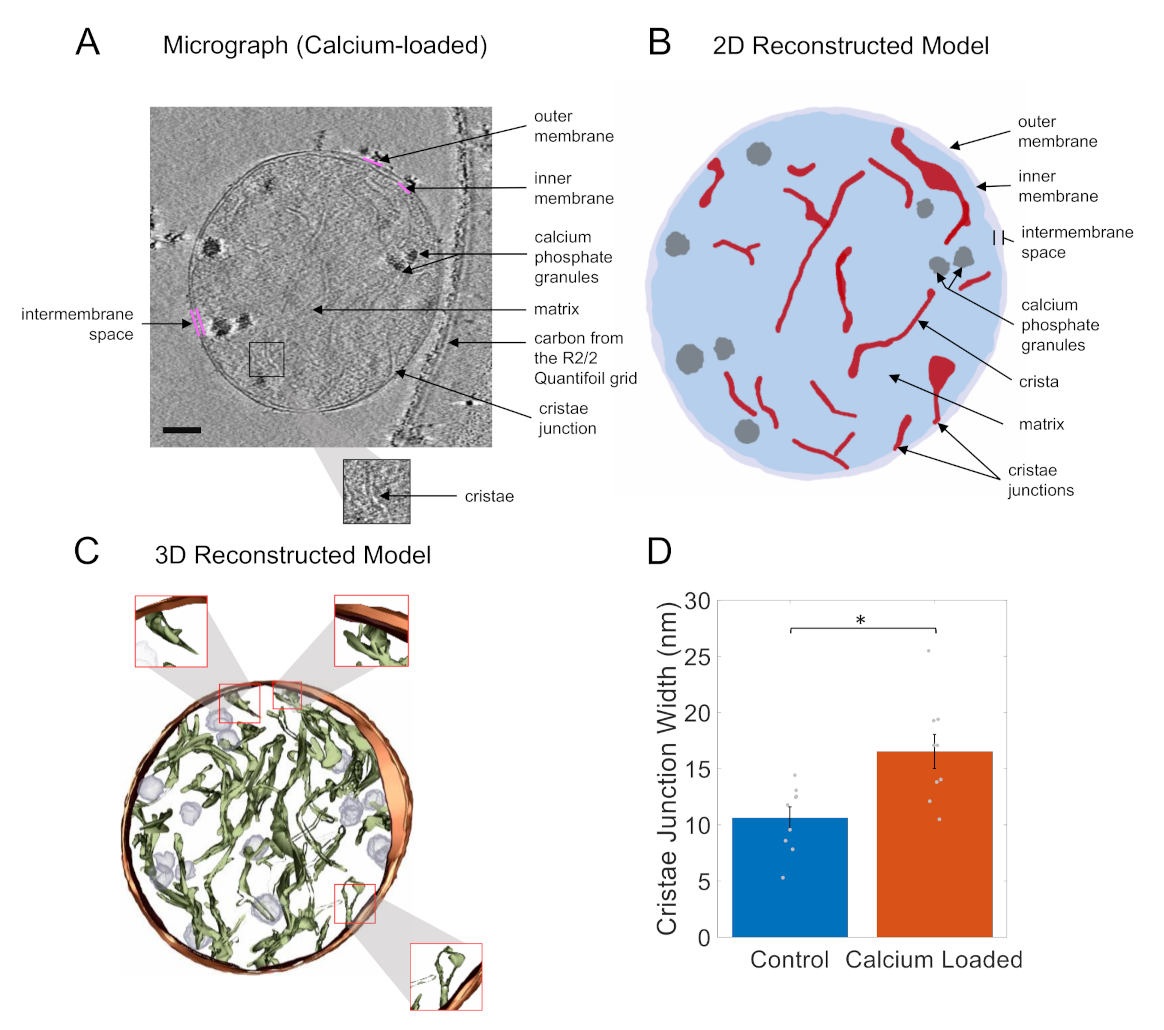
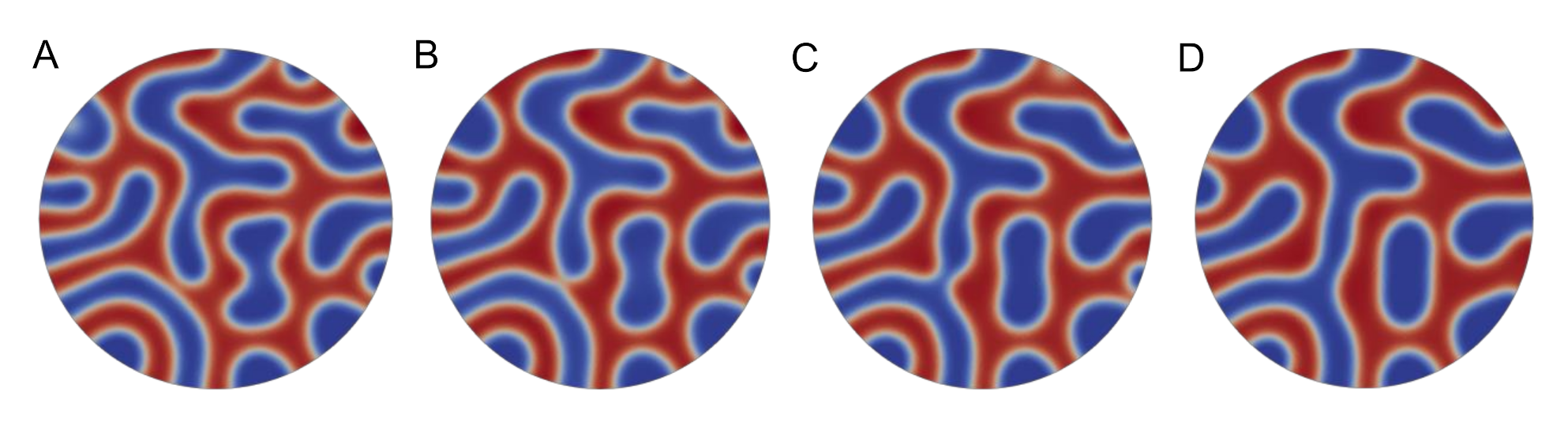
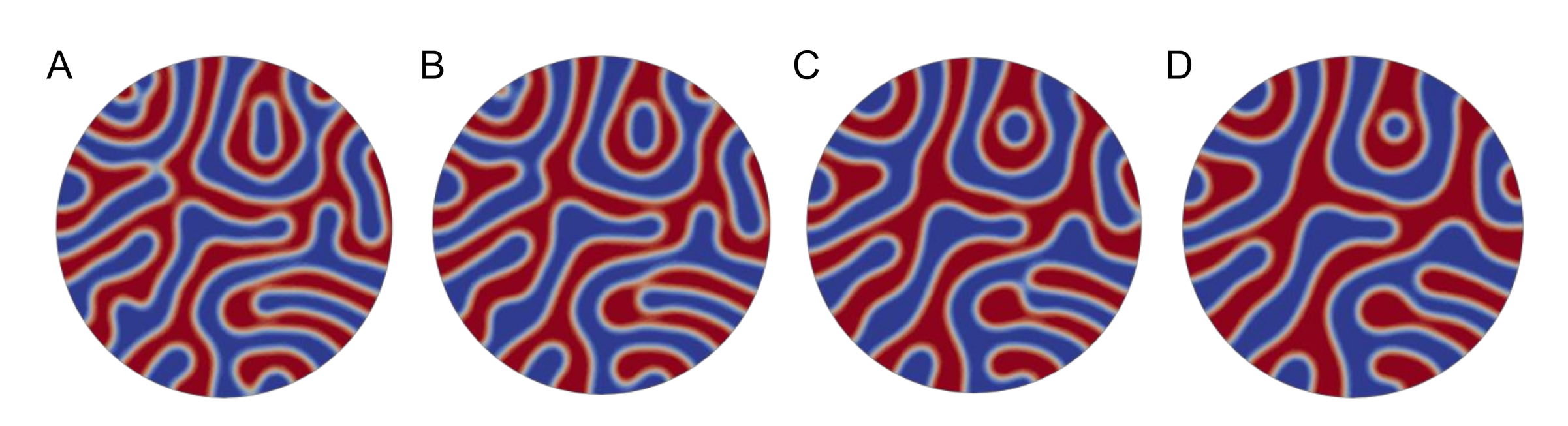
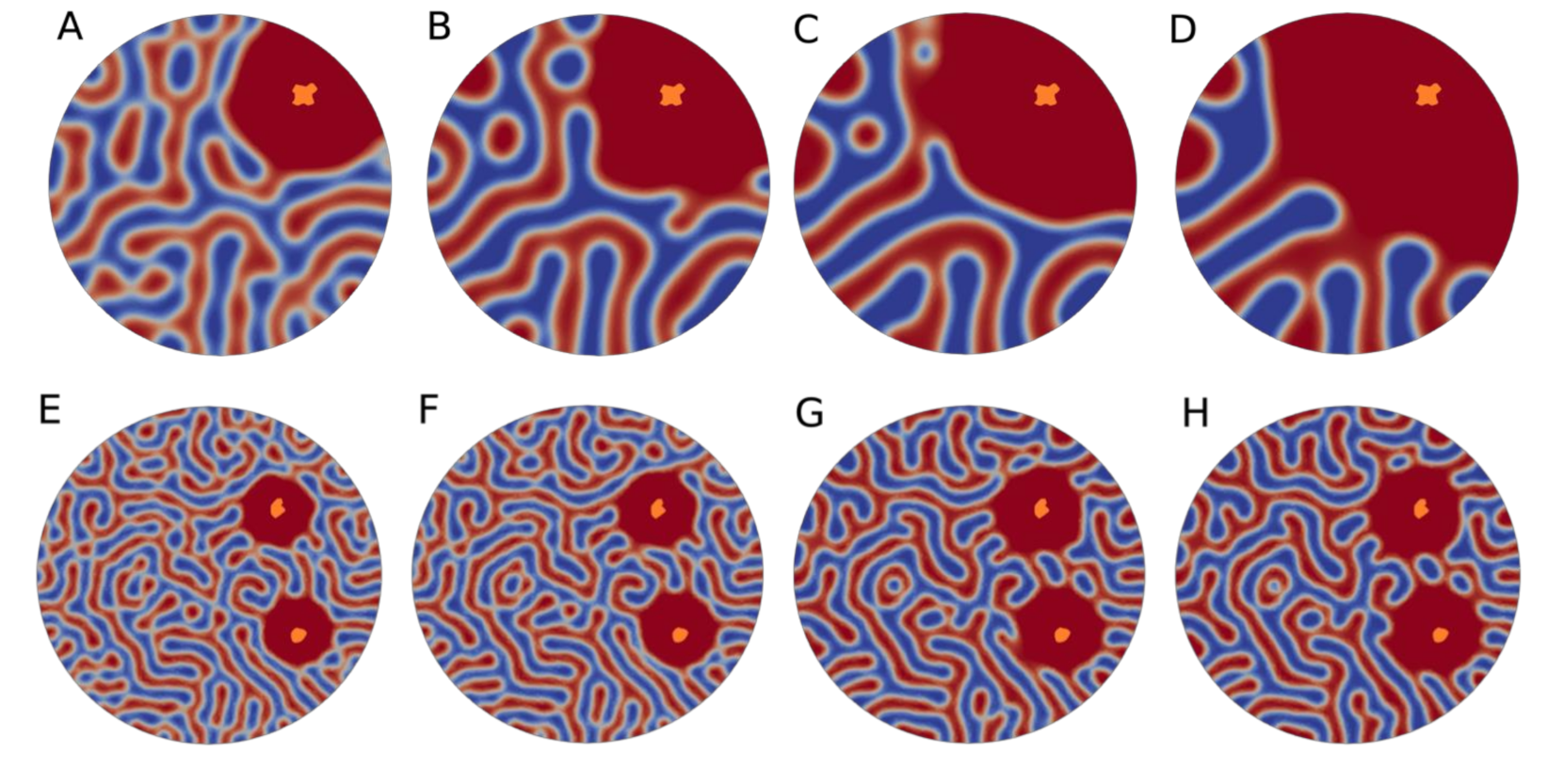
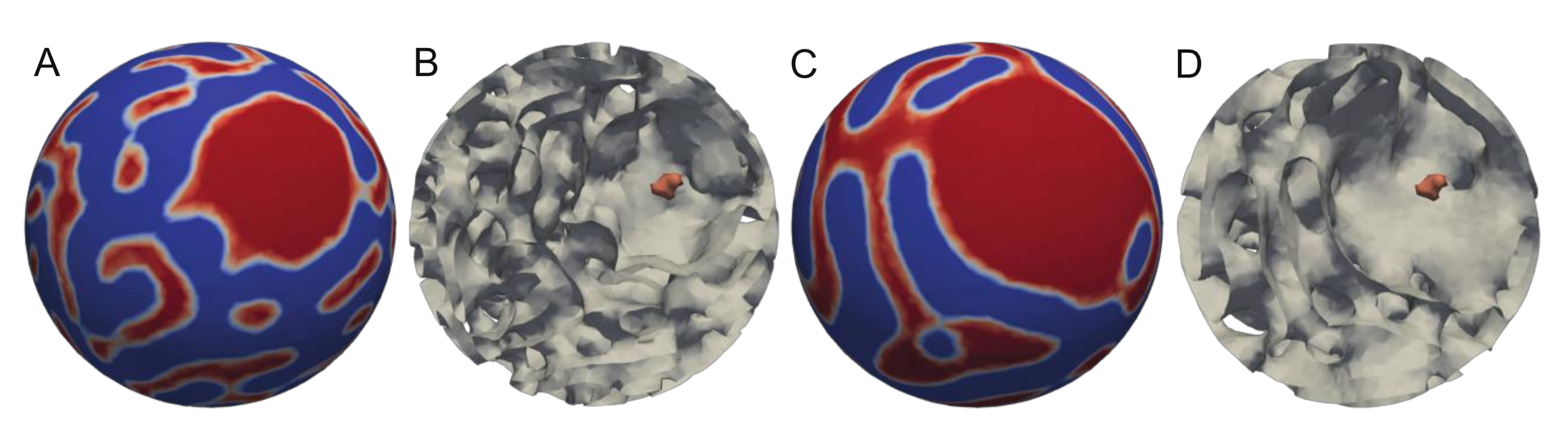
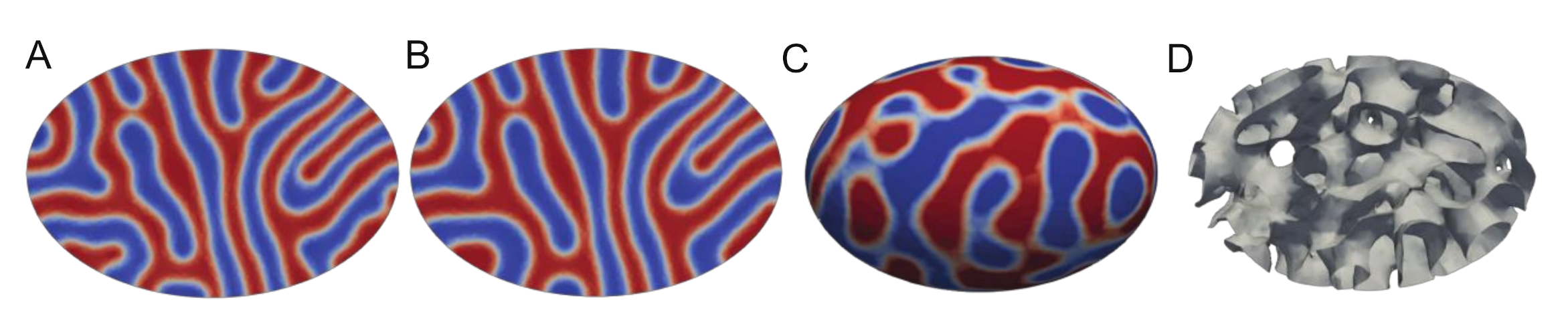
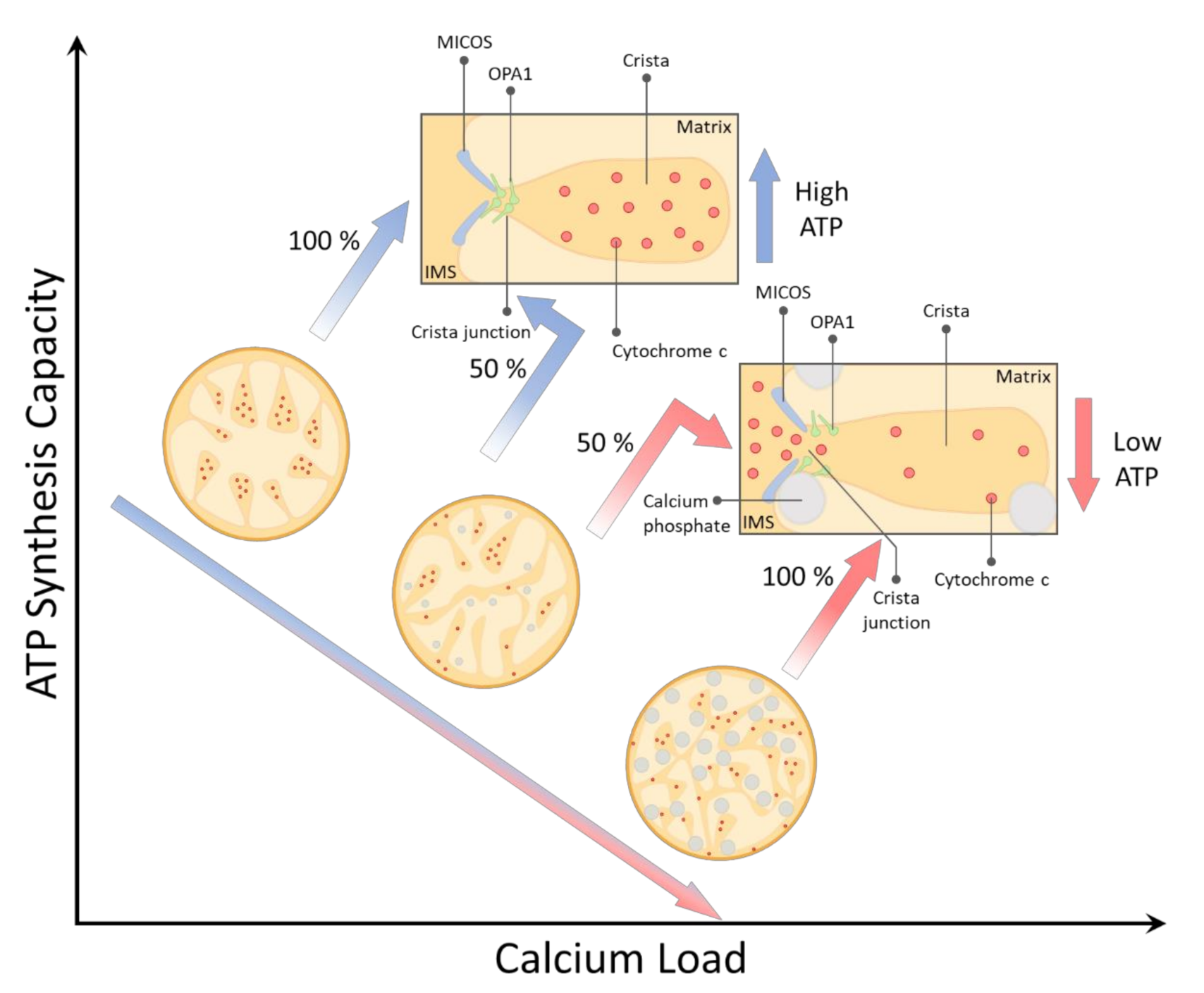
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stanley, W.C.; Recchia, F.A.; Lopaschuk, G.D. Myocardial substrate metabolism in the normal and failing heart. Physiol. Rev. 2005, 85, 1093–1129. [Google Scholar] [CrossRef]
- McCormack, J.G.; Denton, R.M. The role of intramitochondrial Ca2+ in the regulation of oxidative phosphorylation in mammalian tissues. Biochem. Soc. Trans. 1993, 21, 793–799. [Google Scholar] [CrossRef]
- Glancy, B.; Balaban, R.S. Role of mitochondrial Ca2+ in the regulation of cellular energetics. Biochemistry 2012, 51, 2959–2973. [Google Scholar] [CrossRef]
- Wescott, A.P.; Kao, J.P.Y.; Lederer, W.J.; Boyman, L. Voltage-energized calcium-sensitive ATP production by mitochondria. Nat. Metab. 2019, 1, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Abou-Khalil, S.; Abou-Khalil, W.H.; Yunis, A.A. Inhibition by Ca2+ of oxidative phosphorylation in myeloid tumor mitochondria. Arch. Biochem. Biophys. 1981, 209, 460–464. [Google Scholar] [CrossRef]
- Bernard, P.A.; Cockrell, R.S. Calcium transport by rat brain mitochondria and oxidation of 2-oxoglutarate. Biochim. Biophys. Acta 1984, 766, 549–553. [Google Scholar] [CrossRef]
- Brustovetsky, N.; Brustovetsky, T.; Jemmerson, R.; Dubinsky, J.M. Calcium-induced cytochrome c release from CNS mitochondria is associated with the permeability transition and rupture of the outer membrane. J. Neurochem. 2002, 80, 207–218. [Google Scholar] [CrossRef]
- Duong, Q.V.; Hoffman, A.; Zhong, K.; Dessinger, M.J.; Zhang, Y.; Bazil, J.N. Calcium overload decreases net free radical emission in cardiac mitochondria. Mitochondrion 2020, 51, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Fagian, M.M.; Da Silva, L.P.; Vercesi, A.E. Inhibition of oxidative phosphorylation by Ca2+ or Sr2+: A competition with Mg2+ for the formation of adenine nucleotide complexes. Biochim. Biophys. Acta 1986, 852, 262–268. [Google Scholar] [CrossRef]
- Fink, B.D.; Bai, F.; Yu, L.; Sivitz, W.I. Regulation of ATP production: Dependence on calcium concentration and respiratory state. Am. J. Physiol. Cell Physiol. 2017, 313, C146–C153. [Google Scholar] [CrossRef] [PubMed]
- Hardy, L.; Clark, J.B.; Darley-Usmar, V.M.; Smith, D.R.; Stone, D. Reoxygenation-dependent decrease in mitochondrial NADH:CoQ reductase (Complex I) activity in the hypoxic/reoxygenated rat heart. Biochem. J. 1991, 274, 133–137. [Google Scholar] [CrossRef]
- Lai, J.C.K.; Cooper, A.J.L. Brain alpha ketoglutarate dehydrogenase complex: Kinetic properties, regional distribution, and effects of inhibitors. J. Neurochem. 1986, 47, 1376–1386. [Google Scholar] [CrossRef]
- Lai, J.C.K.; DiLorenzo, J.C.; Sheu, K.-F.R. Pyruvate dehydrogenase complex is inhibited in calcium-loaded cerebrocortical mitochondria. Neurochem. Res. 1988, 13, 1043–1048. [Google Scholar] [CrossRef]
- Malyala, S.; Zhang, Y.; Strubbe, J.O.; Bazil, J.N. Calcium phosphate precipitation inhibits mitochondrial energy metabolism. PLoS Comput. Biol. 2019, 15, e1006719. [Google Scholar] [CrossRef]
- Moreno-Sanchez, R. Inhibition of oxidative phosphorylation by a Ca2+-induced diminution of the adenine nucleotide translocator. Biochim. Biophys. Acta 1983, 724, 278–285. [Google Scholar] [CrossRef]
- Pandya, J.D.; Nukala, V.N.; Sullivan, P.G. Concentration dependent effect of calcium on brain mitochondrial bioenergetics and oxidative stress parameters. Front. Neuroenergetics 2013, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Rasbach, K.A.; Arrington, D.D.; Odejinmi, S.; Giguere, C.; Beeson, C.C.; Schnellmann, R.G. Identification and optimization of a novel inhibitor of mitochondrial calpain 10. J. Med. Chem. 2008, 52, 181–188. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roman, I.; Clark, A.; Swanson, P.D. The Interaction of calcium transport and ADP phosphorylation in brain mitochondria. Membr. Biochem. 1981, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.; Hu, Y.; Lesnefsky, E.J.; Chen, Q. Activation of mitochondrial calpain and increased cardiac injury: Beyond AIF release. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H376–H384. [Google Scholar] [CrossRef]
- Thorne, R.F.; Bygrave, F.L. Inhibition by calcium of adenine nucleotide translocation in mitochondria isolated from Ehrlich ascites tumour cells. FEBS Lett. 1974, 41, 118–121. [Google Scholar] [CrossRef]
- Vygodina, T.V.; Mukhaleva, E.; Azarkina, N.V.; Konstantinov, A.A. Cytochrome c oxidase inhibition by calcium at physiological ionic composition of the medium: Implications for physiological significance of the effect. Biochim. Biophys. Acta Gen. Subj. 2017, 1858, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Wollenman, L.C.; Ploeg, M.R.V.; Miller, M.L.; Zhang, Y.; Bazil, J.N. The effect of respiration buffer composition on mitochondrial metabolism and function. PLoS ONE 2017, 12, e0187523. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.M.; Murphy, E. Role of mitochondrial calcium and the permeability transition pore in regulating cell death. Circ. Res. 2020, 126, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Rasola, A.; Bernardi, P. Mitochondrial permeability transition in Ca2+-dependent apoptosis and necrosis. Cell Calcium 2011, 50, 222–233. [Google Scholar] [CrossRef]
- Strubbe-Rivera, J.O.; Schrad, J.R.; Pavlov, E.V.; Conway, J.F.; Parent, K.N.; Bazil, J.N. The mitochondrial permeability transition phenomenon elucidated by cryo-EM reveals the genuine impact of calcium overload on mitochondrial structure and function. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef]
- Liesa, M.; Shirihai, O.S. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 2013, 17, 491–506. [Google Scholar] [CrossRef]
- Montemurro, C.; Vadrevu, S.; Gurlo, T.; E Butler, A.; E Vongbunyong, K.; Petcherski, A.; Shirihai, O.S.; Satin, L.S.; Braas, D.; Butler, P.C.; et al. Cell cycle-related metabolism and mitochondrial dynamics in a replication-competent pancreatic beta-cell line. Cell Cycle 2017, 16, 2086–2099. [Google Scholar] [CrossRef]
- Moore, T.M.; Zhou, Z.; Cohn, W.; Norheim, F.; Lin, A.J.; Kalajian, N.; Strumwasser, A.R.; Cory, K.; Whitney, K.; Ho, T.; et al. The impact of exercise on mitochondrial dynamics and the role of DRP1 in exercise performance and training adaptations in skeletal muscle. Mol. Metab. 2019, 21, 51–67. [Google Scholar] [CrossRef]
- Pernas, L.; Scorrano, L. Mito-morphosis: Mitochondrial fusion, fission, and cristae remodeling as key mediators of cellular function. Annu. Rev. Physiol. 2016, 78, 505–531. [Google Scholar] [CrossRef]
- Kondadi, A.K.; Anand, R.; Hänsch, S.; Urbach, J.; Zobel, T.; Wolf, D.M.; Segawa, M.; Liesa, M.; Shirihai, O.S.; Weidtkamp-Peters, S.; et al. Cristae undergo continuous cycles of membrane remodelling in a MICOS-dependent manner. EMBO Rep. 2020, 21, e49776. [Google Scholar] [CrossRef]
- Cogliati, S.; Frezza, C.; Soriano, M.E.; Varanita, T.; Quintana-Cabrera, R.; Corrado, M.; Cipolat, S.; Costa, V.; Casarin, A.; Gomes, L.C.; et al. Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell 2013, 155, 160–171. [Google Scholar] [CrossRef]
- Eydt, K.; Davies, K.M.; Behrendt, C.; Wittig, I.; Reichert, A.S. Cristae architecture is determined by an interplay of the MICOS complex and the F1Fo ATP synthase via Mic27 and Mic10. Microb. Cell 2017, 4, 259–272. [Google Scholar] [CrossRef]
- Glytsou, C.; Calvo, E.; Cogliati, S.; Mehrotra, A.; Anastasia, I.; Rigoni, G.; Raimondi, A.; Shintani, N.; Loureiro, M.; Vazquez, J.; et al. Optic atrophy 1 is epistatic to the core MICOS component MIC60 in mitochondrial cristae shape control. Cell Rep. 2016, 17, 3024–3034. [Google Scholar] [CrossRef]
- Kaurov, I.; Vancová, M.; Schimanski, B.; Cadena, L.R.; Heller, J.; Bílý, T.; Potěšil, D.; Eichenberger, C.; Bruce, H.; Oeljeklaus, S.; et al. The diverged trypanosome MICOS complex as a hub for mitochondrial cristae shaping and protein import. Curr. Biol. 2018, 28, 3393–3407.e5. [Google Scholar] [CrossRef]
- Frezza, C.; Cipolat, S.; De Brito, O.M.; Micaroni, M.; Beznoussenko, G.V.; Rudka, T.; Bartoli, D.; Polishuck, R.S.; Danial, N.N.; De Strooper, B.; et al. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell 2006, 126, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Varanita, T.; Soriano, M.E.; Romanello, V.; Zaglia, T.; Quintana-Cabrera, R.; Semenzato, M.; Menabò, R.; Costa, V.; Civiletto, G.; Pesce, P.; et al. The OPA1-dependent mitochondrial cristae remodeling pathway controls atrophic, apoptotic, and ischemic tissue damage. Cell Metab. 2015, 21, 834–844. [Google Scholar] [CrossRef]
- Davies, K.M.; Anselmi, C.; Wittig, I.; Faraldo-Gómez, J.D.; Kühlbrandt, W. Structure of the yeast F1Fo-ATP synthase dimer and its role in shaping the mitochondrial cristae. Proc. Natl. Acad. Sci. USA 2012, 109, 13602–13607. [Google Scholar] [CrossRef] [PubMed]
- Quintana-Cabrera, R.; Quirin, C.; Glytsou, C.; Corrado, M.; Urbani, A.; Pellattiero, A.; Calvo, E.; Vázquez, J.; Enríquez, J.A.; Gerle, C.; et al. The cristae modulator Optic atrophy 1 requires mitochondrial ATP synthase oligomers to safeguard mitochondrial function. Nat. Commun. 2018, 9, 3399. [Google Scholar] [CrossRef] [PubMed]
- Salewskij, K.; Rieger, B.; Hager, F.; Arroum, T.; Duwe, P.; Villalta, J.; Colgiati, S.; Richter, C.P.; Psathaki, O.E.; Enriquez, J.A.; et al. The spatio-temporal organization of mitochondrial F1FO ATP synthase in cristae depends on its activity mode. Biochim. Biophys. Acta 2020, 1861, 148091. [Google Scholar] [CrossRef]
- Olichon, A.; ElAchouri, G.; Baricault, L.; Delettre, C.; Belenguer, P.; Lenaers, G. OPA1 alternate splicing uncouples an evolutionary conserved function in mitochondrial fusion from a vertebrate restricted function in apoptosis. Cell Death Differ. 2007, 14, 682–692. [Google Scholar] [CrossRef]
- Kojima, R.; Kakimoto, Y.; Furuta, S.; Itoh, K.; Sesaki, H.; Endo, T.; Tamura, Y. Maintenance of cardiolipin and crista structure requires cooperative functions of mitochondrial dynamics and phospholipid transport. Cell Rep. 2019, 26, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Rainbolt, T.K.; Lebeau, J.; Puchades, C.; Wiseman, R.L. Reciprocal degradation of YME1L and OMA1 adapts mitochondrial proteolytic activity during stress. Cell Rep. 2016, 14, 2041–2049. [Google Scholar] [CrossRef] [PubMed]
- Merkwirth, C.; Dargazanli, S.; Tatsuta, T.; Geimer, S.; Löwer, B.; Wunderlich, F.T.; Von Kleist-Retzow, J.-C.; Waisman, A.; Westermann, B.; Langer, T. Prohibitins control cell proliferation and apoptosis by regulating OPA1-dependent cristae morphogenesis in mitochondria. Genes Dev. 2008, 22, 476–488. [Google Scholar] [CrossRef]
- Tatsuta, T.; Model, K.; Langer, T. Formation of membrane-bound ring complexes by prohibitins in mitochondria. Mol. Biol. Cell 2005, 16, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Demongeot, J.; Glade, N.; Hansen, O.; Moreira, A. An open issue: The inner mitochondrial membrane (IMM) as a free boundary problem. Biochimie 2007, 89, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Dieteren, C.E.J.; Gielen, S.C.A.M.; Nijtmans, L.G.J.; Smeitink, J.A.M.; Swarts, H.G.; Brock, R.; Willems, P.H.G.M.; Koopman, W.J.H. Solute diffusion is hindered in the mitochondrial matrix. Proc. Natl. Acad. Sci. USA 2011, 108, 8657–8662. [Google Scholar] [CrossRef]
- Elías-Wolff, F.; Lindén, M.; Lyubartsev, A.P.; Brandt, E.G. Curvature sensing by cardiolipin in simulated buckled membranes. Soft Matter 2019, 15, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Khalifat, N.; Fournier, J.-B.; Angelova, M.I.; Puff, N. Lipid packing variations induced by pH in cardiolipin-containing bilayers: The driving force for the cristae-like shape instability. Biochim. Biophys. Acta Biomembr. 2011, 1808, 2724–2733. [Google Scholar] [CrossRef]
- Khalifat, N.; Puff, N.; Bonneau, S.; Fournier, J.-B.; Angelova, M.I. Membrane deformation under local pH gradient: Mimicking mitochondrial cristae dynamics. Biophys. J. 2008, 95, 4924–4933. [Google Scholar] [CrossRef]
- Song, D.H.; Park, J.; Maurer, L.L.; Lu, W.; Philbert, M.A.; Sastry, A.M. Biophysical significance of the inner mitochondrial membrane structure on the electrochemical potential of mitochondria. Phys. Rev. E 2013, 88, 062723. [Google Scholar] [CrossRef]
- Stuchebrukhov, A.; Schäfer, J.; Berg, J.; Brzezinski, P. Kinetic advantage of forming respiratory supercomplexes. Biochim. Biophys. Acta 2020, 1861, 148193. [Google Scholar] [CrossRef] [PubMed]
- Sukhorukov, V.M.; Bereiter-Hahn, J. Anomalous diffusion induced by cristae geometry in the inner mitochondrial membrane. PLoS ONE 2009, 4, e4604. [Google Scholar] [CrossRef]
- Song, D.H.; Park, J.; Philbert, M.A.; Sastry, A.M.; Lu, W. Effects of local pH on the formation and regulation of cristae morphologies. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2014, 90, 022702. [Google Scholar] [CrossRef] [PubMed]
- Tam, Z.Y.; Cai, Y.H.; Gunawan, R. Elucidating cytochrome C release from mitochondria: Insights from an in silico three-dimensional model. Biophys. J. 2010, 99, 3155–3163. [Google Scholar] [CrossRef]
- Gurtin, M.E. Generalized Ginzburg-Landau and Cahn-Hilliard equations based on a microforce balance. Phys. D Nonlinear Phenom. 1996, 92, 178–192. [Google Scholar] [CrossRef]
- Kim, J.; Kang, K.; Lowengrub, J. Conservative multigrid methods for Cahn-Hilliard fluids. J. Comput. Phys. 2004, 193, 511–543. [Google Scholar] [CrossRef]
- Shen, J.; Yang, X. Numerical approximations of Allen-Cahn and Cahn-Hilliard equations. Discret. Contin. Dyn. Syst. A 2010, 28, 1669–1691. [Google Scholar] [CrossRef]
- Wei, G.-W.; Zhou, Y. Variational methods for biomolecular modeling. In Variational Methods in Molecular Modeling; Wu, J., Ed.; Springer: Singapore, 2017; pp. 181–221. [Google Scholar]
- Landolfi, G. New results on the Canham-Helfrich membrane model via the generalized Weierstrass representation. J. Phys. A Math. Gen. 2003, 36, 11937–11954. [Google Scholar] [CrossRef]
- Aranson, I.S.; Kramer, L. The world of the complex Ginzburg-Landau equation. Rev. Mod. Phys. 2002, 74, 99–143. [Google Scholar] [CrossRef]
- Taubes, C.H. The existence of a non-minimal solution to the SU (2) Yang-Mills-Higgs equations on R3. Part I. Commun. Math. Phys. 1982, 86, 257–298. [Google Scholar] [CrossRef]
- Dai, S.; Promislow, K. Geometric evolution of bilayers under the functionalized Cahn-Hilliard equation. Proc. R. Soc. A Math. Phys. Eng. Sci. 2013, 469, 20120505. [Google Scholar] [CrossRef]
- Novick-Cohen, A.; Segel, L.A. Nonlinear aspects of the Cahn-Hilliard equation. Phys. D Nonlinear Phenom. 1984, 10, 277–298. [Google Scholar] [CrossRef]
- Pego, R.L. Front migration in the nonlinear Cahn-Hilliard equation. Proc. R. Soc. Lond. A Math. Phys. Sci. 1997, 422, 261–278. [Google Scholar]
- Guan, S.; Lai, C.-H.; Wei, G. Fourier-Bessel analysis of patterns in a circular domain. Phys. D Nonlinear Phenom. 2001, 151, 83–98. [Google Scholar] [CrossRef]
- Guan, S.; Lai, C.-H.; Wei, G. Geometry and boundary control of pattern formation and competition. Phys. D Nonlinear Phenom. 2003, 176, 19–43. [Google Scholar] [CrossRef][Green Version]
- Bates, P.W.; Fife, P.C. The dynamics of nucleation for the Cahn-Hilliard equation. SIAM J. Appl. Math. 1993, 53, 990–1008. [Google Scholar] [CrossRef]
- Gránásy, L.; Oxtoby, D.W. Cahn-Hilliard theory with triple-parabolic free energy. II. Nucleation and growth in the presence of a metastable crystalline phase. J. Chem. Phys. 2000, 112, 2410–2419. [Google Scholar] [CrossRef]
- Boyanova, P.; Neytcheva, M. Efficient numerical solution of discrete multi-component Cahn-Hilliard systems. Comput. Math. Appl. 2014, 67, 106–121. [Google Scholar] [CrossRef]
- Li, Y.; Choi, J.-I.; Kim, J. Multi-component Cahn-Hilliard system with different boundary conditions in complex domains. J. Comput. Phys. 2016, 323, 1–16. [Google Scholar] [CrossRef]
- Kremer, J.R.; Mastronarde, D.N.; McIntosh, J.R. Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol. 1996, 116, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Azzolin, L.; Von Stockum, S.; Basso, E.; Petronilli, V.; Forte, M.A.; Bernardi, P. The mitochondrial permeability transition from yeast to mammals. FEBS Lett. 2010, 584, 2504–2509. [Google Scholar] [CrossRef]
- Bernardi, P.; Di Lisa, F. The mitochondrial permeability transition pore: Molecular nature and role as a target in cardioprotection. J. Mol. Cell. Cardiol. 2015, 78, 100–106. [Google Scholar] [CrossRef]
- Hurst, S.; Hoek, J.; Sheu, S.-S. Mitochondrial Ca2+ and regulation of the permeability transition pore. J. Bioenerg. Biomembr. 2017, 49, 27–47. [Google Scholar] [CrossRef]
- Parks, R.J.; Murphy, E.; Liu, J.C. Mitochondrial permeability transition pore and calcium handling. Methods Mol. Biol. 2018, 1782, 187–196. [Google Scholar]
- Mannella, C.A. Structure and dynamics of the mitochondrial inner membrane cristae. Biochim. Biophys. Acta 2006, 1763, 542–548. [Google Scholar] [CrossRef]
- Hackenbrock, C.R. Ultrastructural bases for metabolically linked mechanical activity in mitochondria. I. Reversible ultrastructural changes with change in metabolic steady state in isolated liver mitochondria. J. Cell Biol. 1966, 30, 269–297. [Google Scholar] [CrossRef]
- Hackenbrock, C.R. Energy-linked ultrastructural transformations in isolated liver mitochondria and mitoplasts. J. Cell Biol. 1972, 53, 450–465. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biochim. Biophys. Acta 2011, 1807, 1507–1538. [Google Scholar] [CrossRef] [PubMed]
- Scalettar, B.A.; Abney, J.R.; Hackenbrock, C.R. Dynamics, structure, and function are coupled in the mitochondrial matrix. Proc. Natl. Acad. Sci. USA 1991, 88, 8057–8061. [Google Scholar] [CrossRef] [PubMed]
- Rampelt, H.; Zerbes, R.M.; van der Laan, M.; Pfanner, N. Role of the mitochondrial contact site and cristae organizing system in membrane architecture and dynamics. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Prince, F.P. Lamellar and tubular associations of the mitochondrial cristae: Unique forms of the cristae present in steroid-producing cells. Mitochondrion 2002, 1, 381–389. [Google Scholar] [CrossRef]
- Harner, M.E.; Unger, A.-K.; Geerts, W.J.; Mari, M.; Izawa, T.; Stenger, M.; Geimer, S.; Reggiori, F.; Westermann, B.; Neupert, W. An evidence based hypothesis on the existence of two pathways of mitochondrial crista formation. eLife 2016, 5, e18853. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, B.; Klec, C.; Leitinger, G.; Bernhart, E.; Rost, R.; Bischof, H.; Madreiter-Sokolowski, C.T.; Radulović, S.; Eroglu, E.; Sattler, W.; et al. MICU1 controls cristae junction and spatially anchors mitochondrial Ca2+ uniporter complex. Nat. Commun. 2019, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; López-Doménech, G.; Halff, E.F.; Covill-Cooke, C.; Ivankovic, D.; Melandri, D.; Arancibia-Cárcamo, I.L.; Burden, J.J.; Lowe, A.R.; Kittler, J.T. Miro clusters regulate ER-mitochondria contact sites and link cristae organization to the mitochondrial transport machinery. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Wang, L.-J.; Hsu, T.; Lin, H.-L.; Fu, C.-Y. Drosophila MICOS knockdown impairs mitochondrial structure and function and promotes mitophagy in muscle tissue. Biol. Open 2020, 9, 054262. [Google Scholar] [CrossRef]
- Kushnareva, Y.E.; Gerencser, A.A.; Bossy, B.; Ju, W.-K.; White, A.D.; Waggoner, J.; Ellisman, M.H.; Perkins, G.; Bossy-Wetzel, E. Loss of OPA1 disturbs cellular calcium homeostasis and sensitizes for excitotoxicity. Cell Death Differ. 2013, 20, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Waldeck-Weiermair, M.; Malli, R.; Parichatikanond, W.; Gottschalk, B.; Madreiter-Sokolowski, C.T.; Klec, C.; Rost, R.; Graier, W.F. Rearrangement of MICU1 multimers for activation of MCU is solely controlled by cytosolic Ca2+. Sci. Rep. 2015, 5, 15602. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strubbe-Rivera, J.O.; Chen, J.; West, B.A.; Parent, K.N.; Wei, G.-W.; Bazil, J.N. Modeling the Effects of Calcium Overload on Mitochondrial Ultrastructural Remodeling. Appl. Sci. 2021, 11, 2071. https://doi.org/10.3390/app11052071
Strubbe-Rivera JO, Chen J, West BA, Parent KN, Wei G-W, Bazil JN. Modeling the Effects of Calcium Overload on Mitochondrial Ultrastructural Remodeling. Applied Sciences. 2021; 11(5):2071. https://doi.org/10.3390/app11052071
Chicago/Turabian StyleStrubbe-Rivera, Jasiel O., Jiahui Chen, Benjamin A. West, Kristin N. Parent, Guo-Wei Wei, and Jason N. Bazil. 2021. "Modeling the Effects of Calcium Overload on Mitochondrial Ultrastructural Remodeling" Applied Sciences 11, no. 5: 2071. https://doi.org/10.3390/app11052071
APA StyleStrubbe-Rivera, J. O., Chen, J., West, B. A., Parent, K. N., Wei, G.-W., & Bazil, J. N. (2021). Modeling the Effects of Calcium Overload on Mitochondrial Ultrastructural Remodeling. Applied Sciences, 11(5), 2071. https://doi.org/10.3390/app11052071






