Novel Structures and Applications of Graphene-Based Semiconductor Photocatalysts: Faceted Particles, Photonic Crystals, Antimicrobial and Magnetic Properties
Abstract
1. Introduction
2. Morphology Design of Graphene-Based Composites
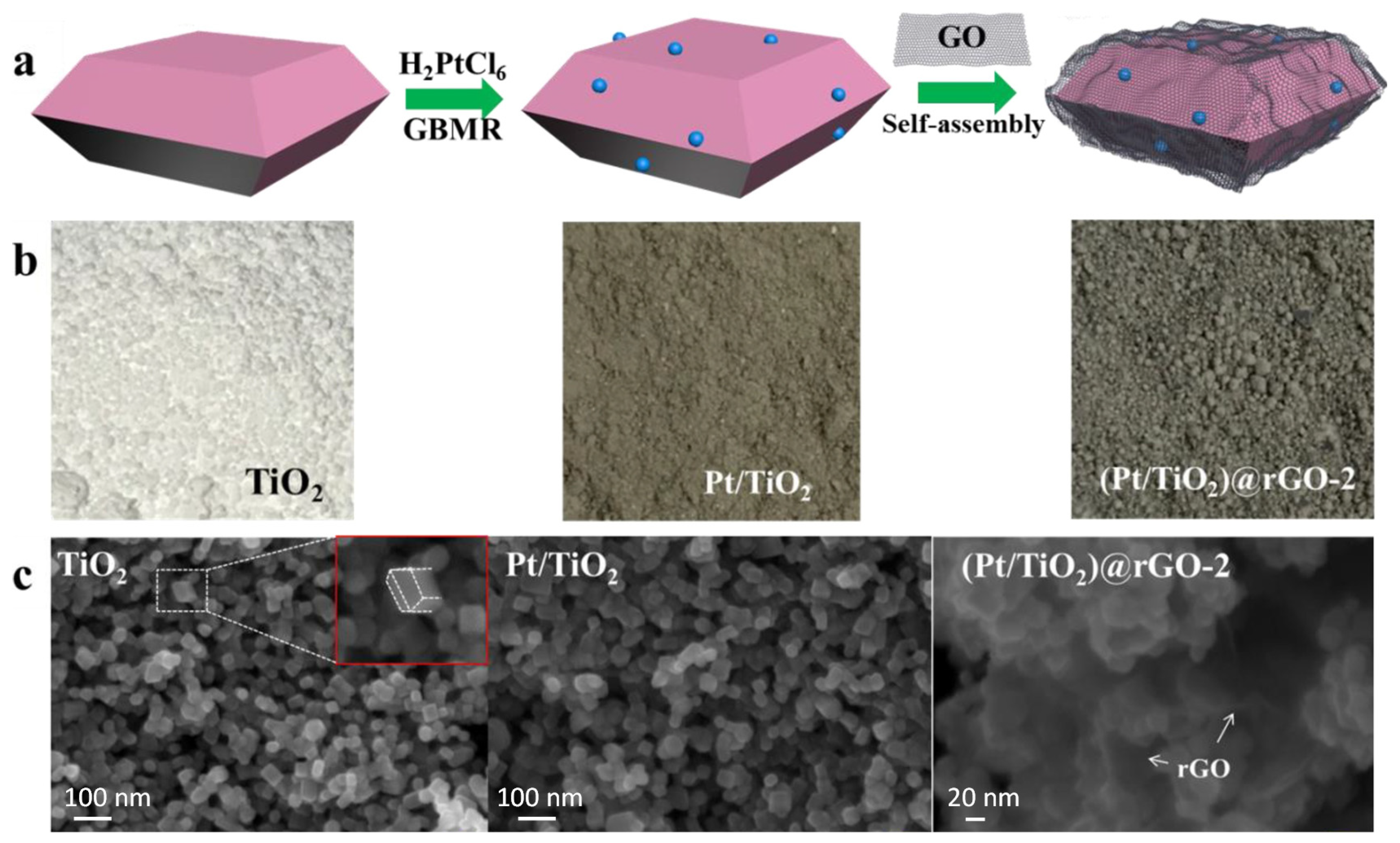
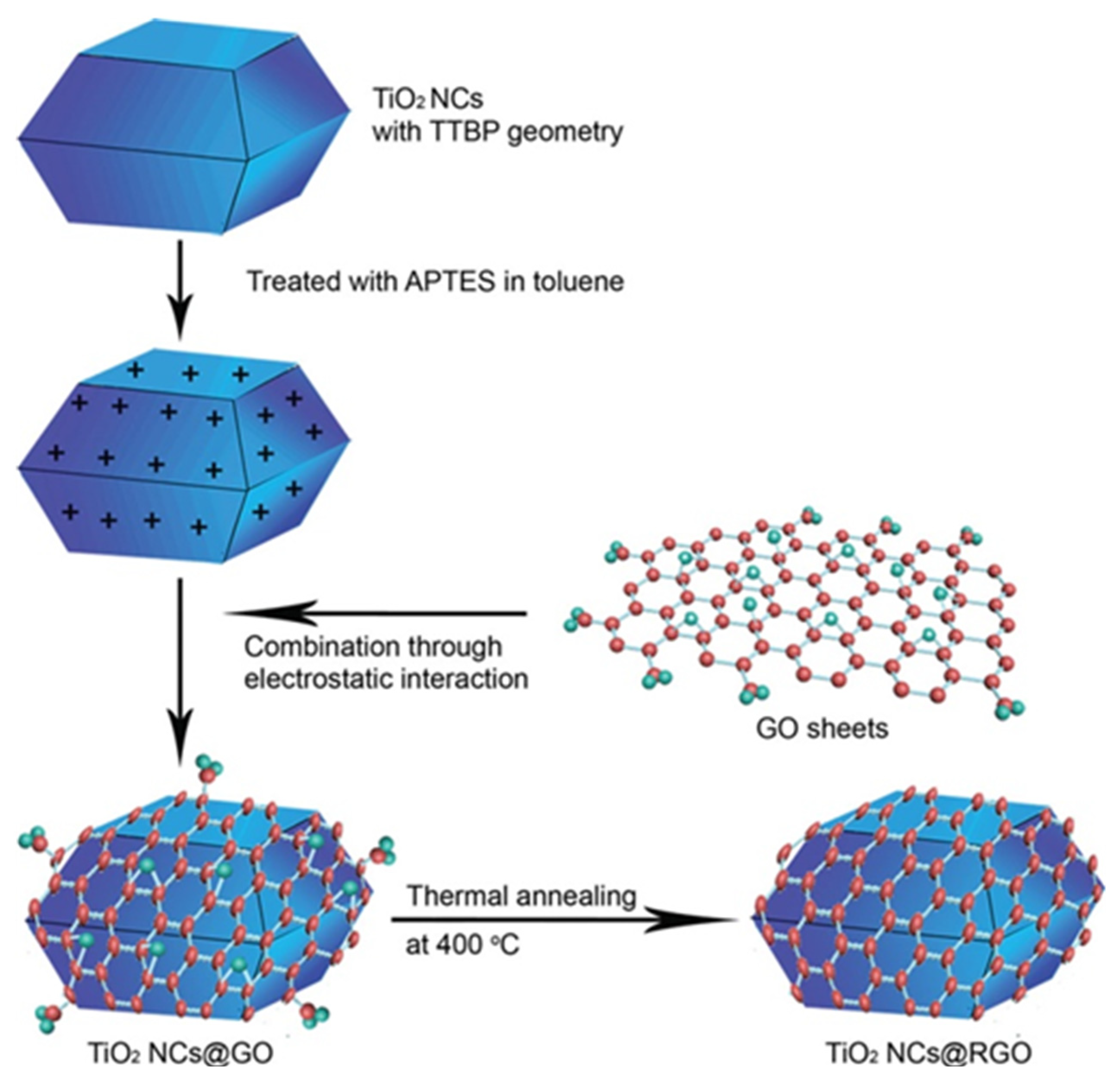
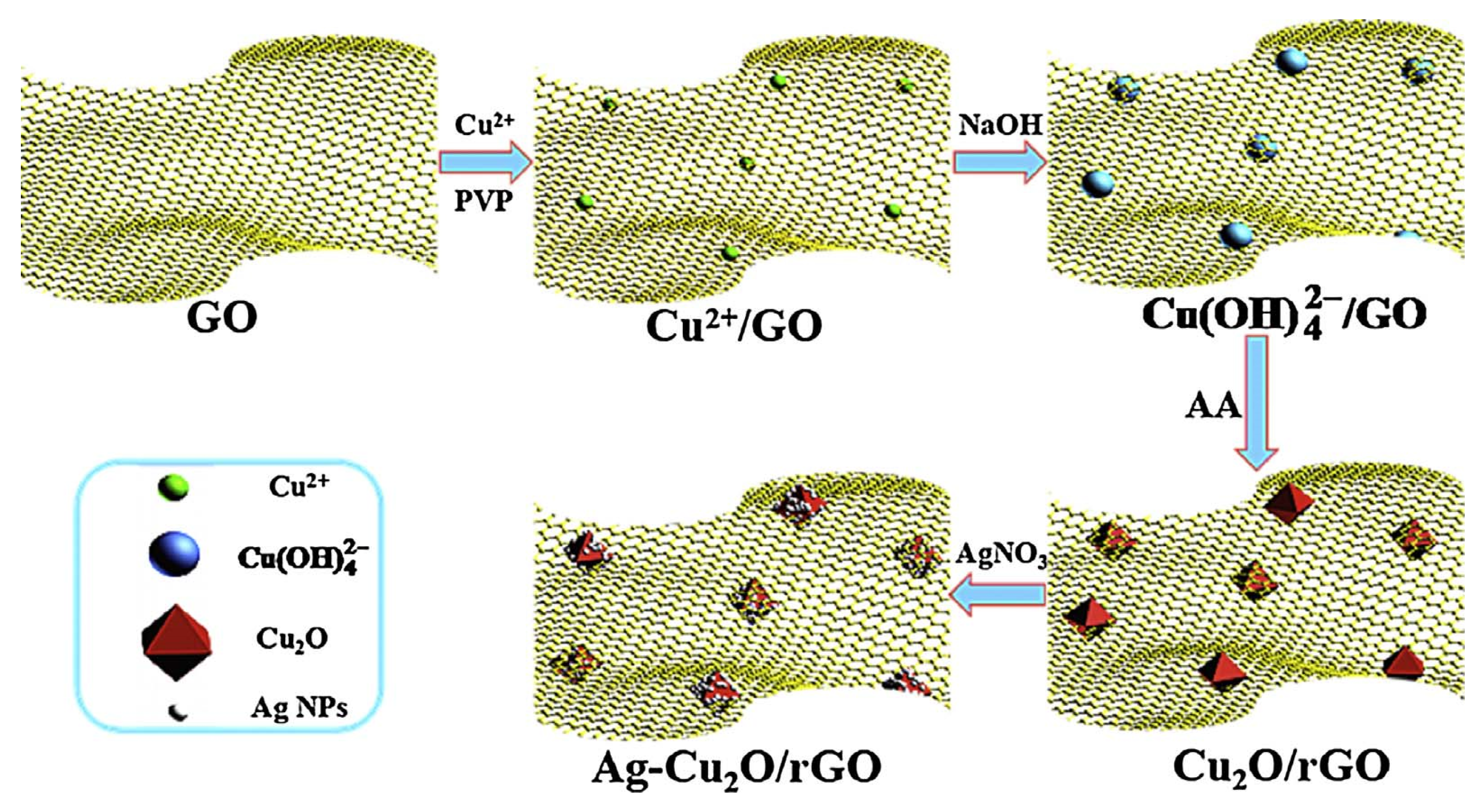
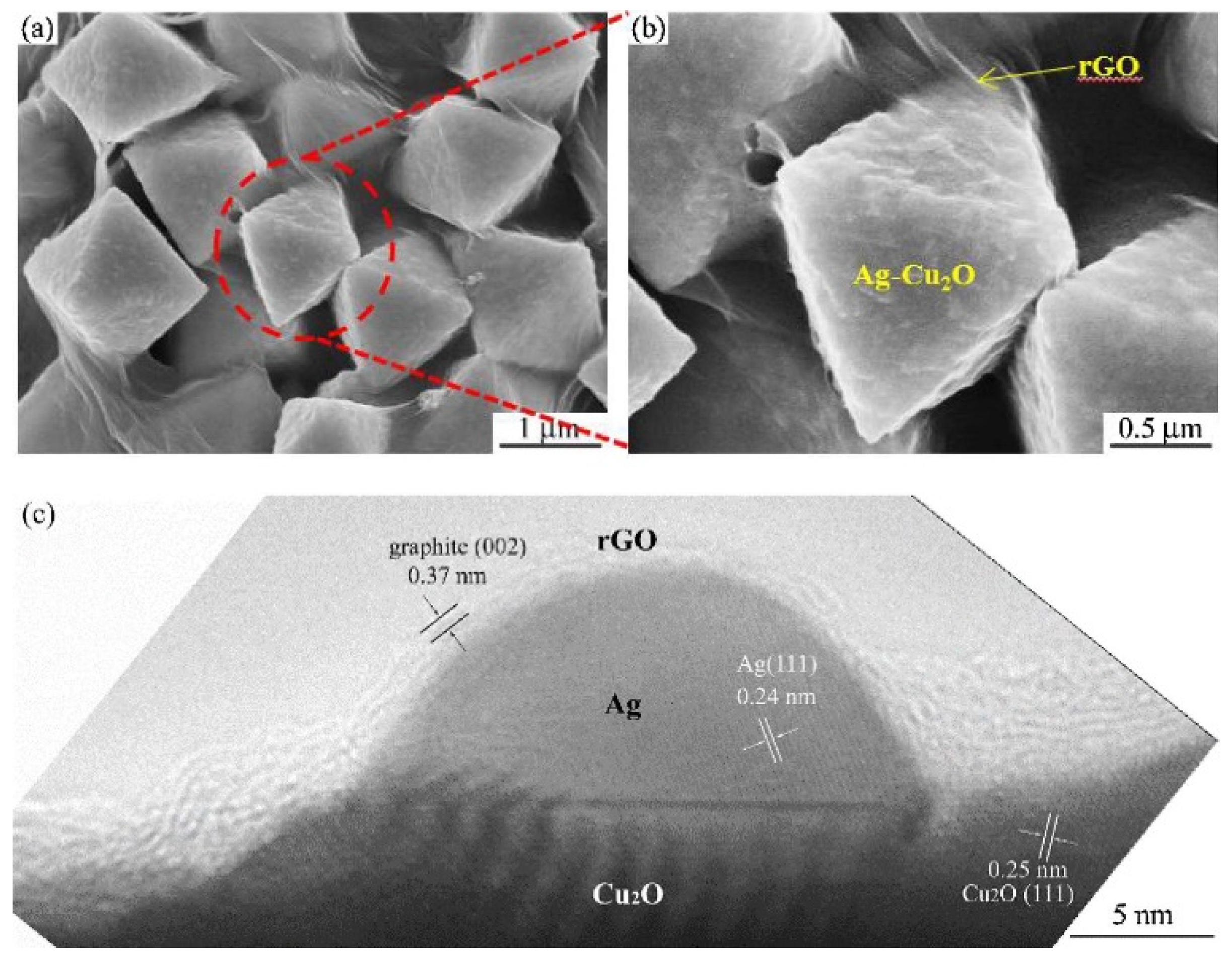
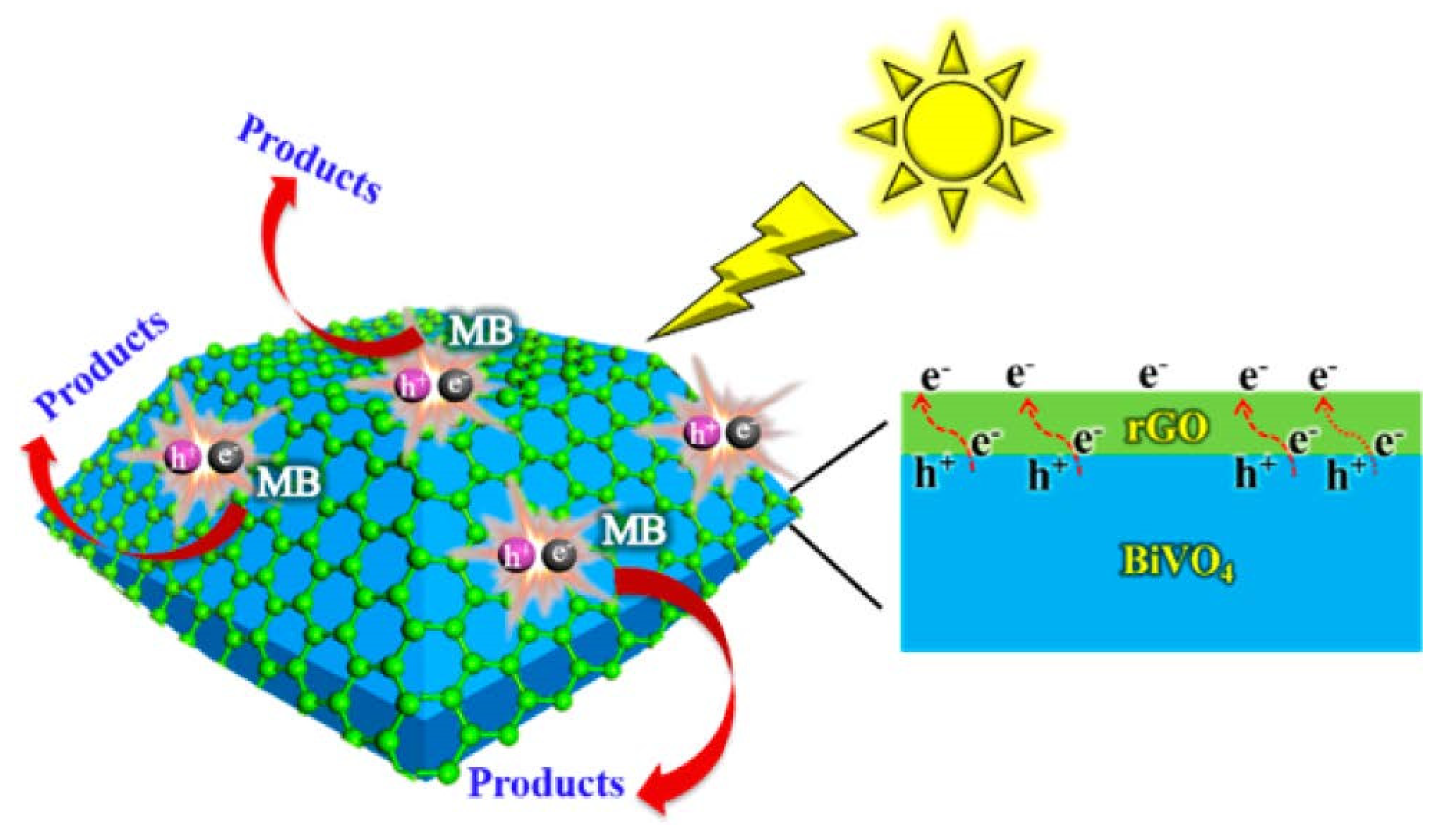
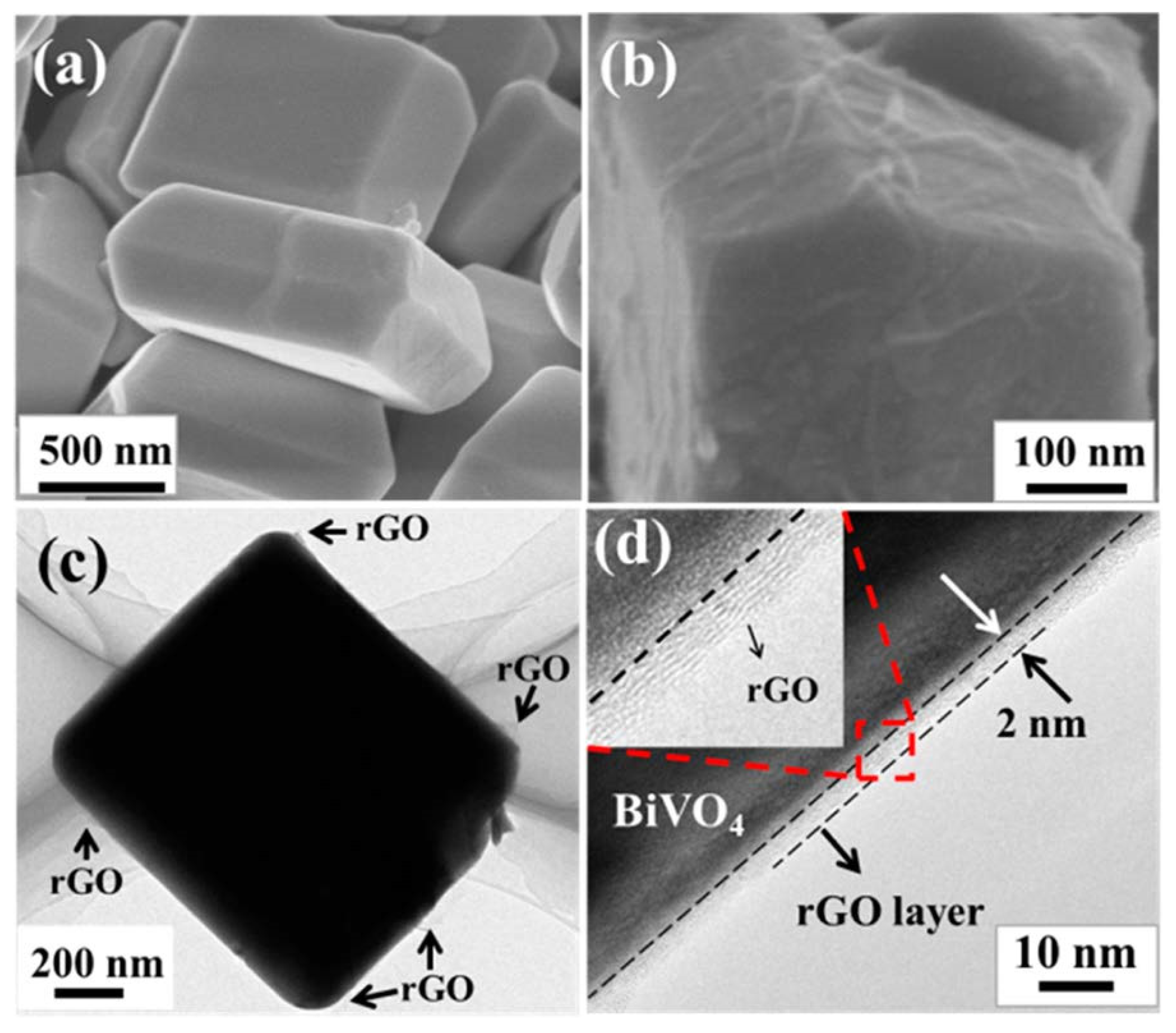
2.1. The Composites of Graphene with Faceted Particles
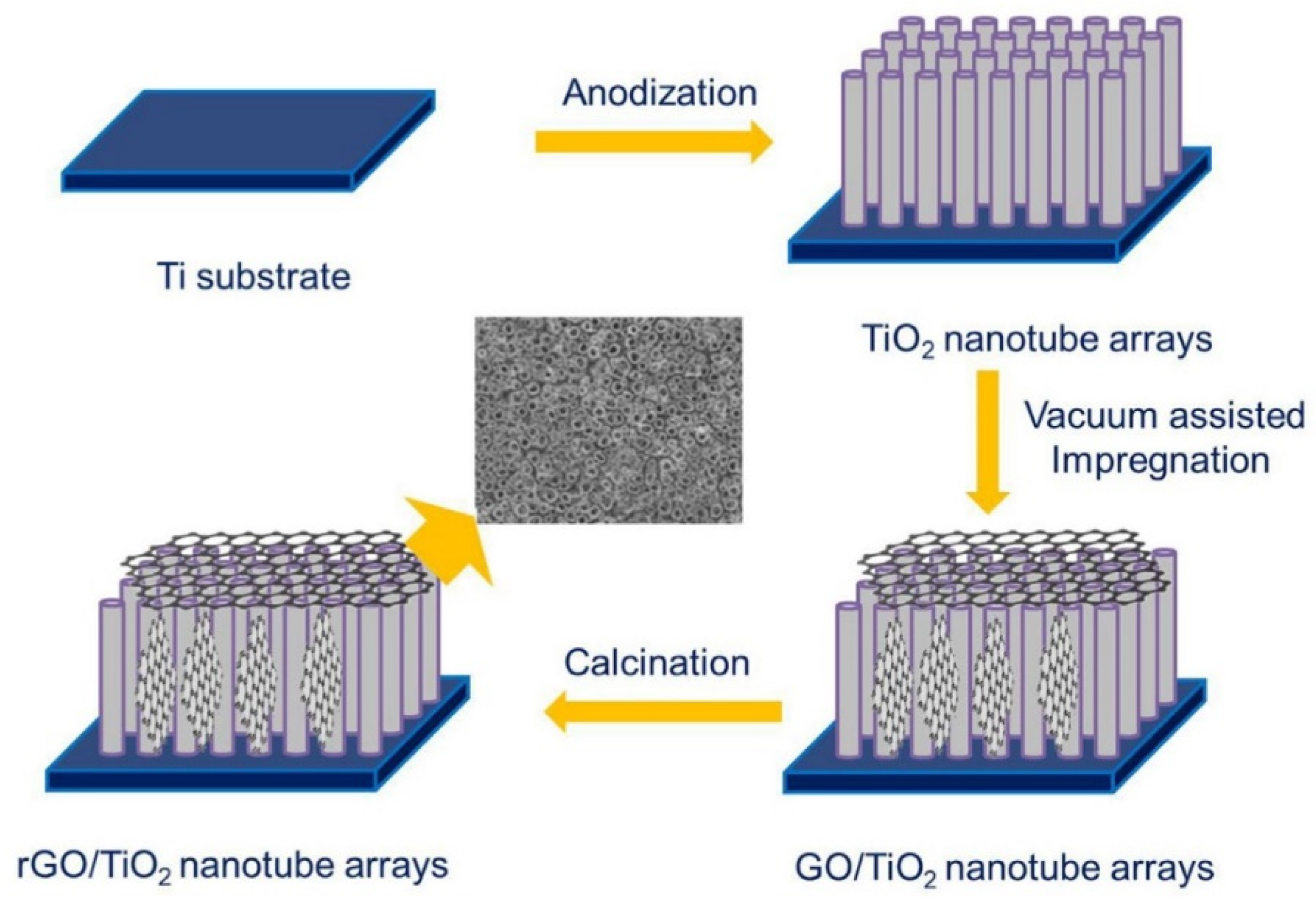
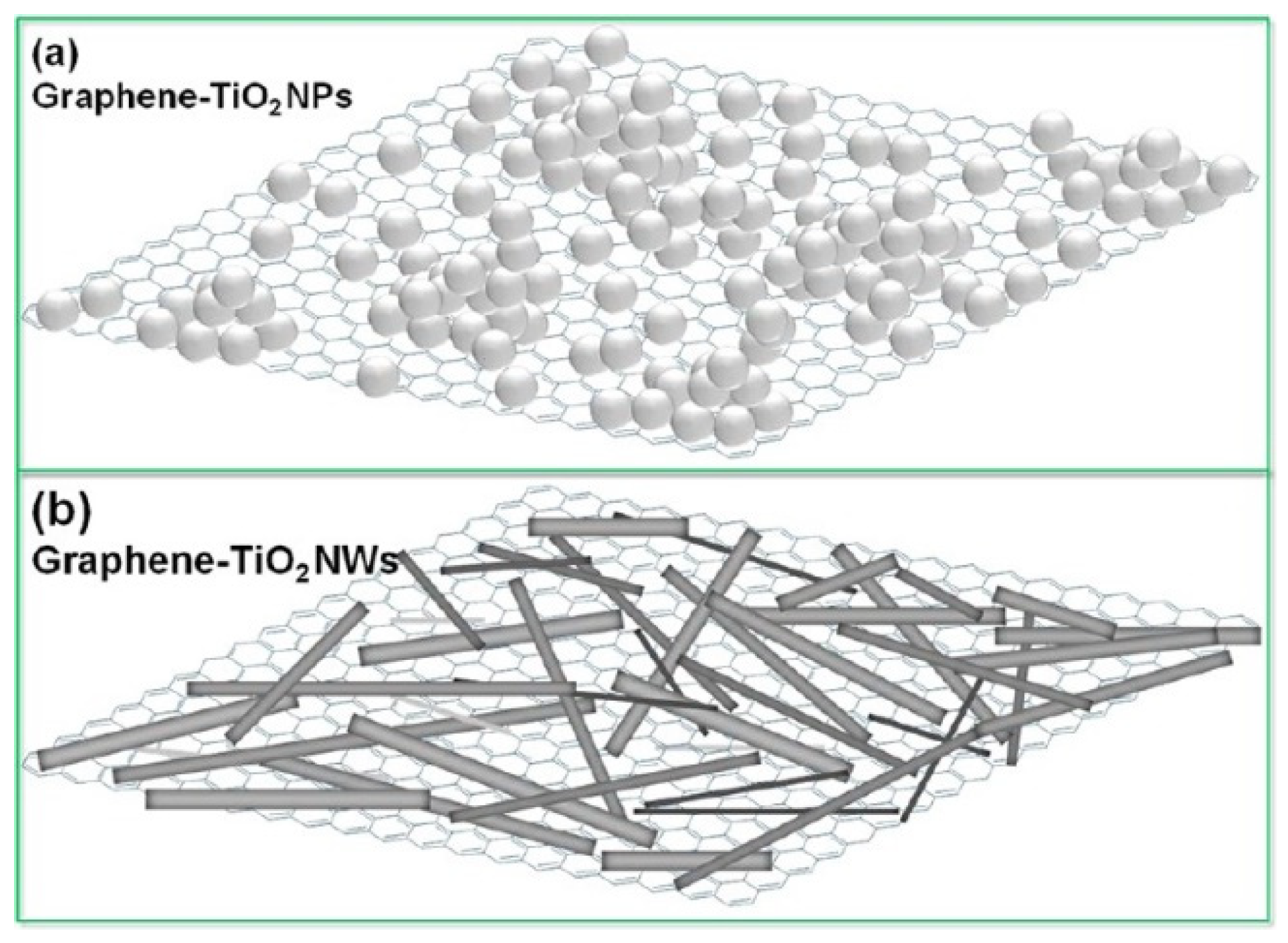
2.2. Titania-Graphene Composites (Not Faceted Titania)
2.3. Graphene-Based Photonic Crystals
3. Environmental Applications of Graphene-Based Photocatalysts
3.1. Antimicrobial Properties of Graphene-Based Composite Photocatalysts
3.2. Magnetically Separable Graphene-Based Composite Photocatalysts
4. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoffmann, M.R.; Martin, S.T.; Choi, W.Y.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Wang, K.L.; Janczarek, M.; Wei, Z.S.; Raja-Mogan, T.; Endo-Kimura, M.; Khedr, T.M.; Ohtani, B.; Kowalska, E. Morphology- and crystalline composition-governed activity of titania-based photocatalysts: Overview and perspective. Catalysts 2019, 9, 1054. [Google Scholar] [CrossRef]
- Zaleska, A. Doped-TiO2: A review. Rec. Pat. Eng. 2008, 2, 157–164. [Google Scholar] [CrossRef]
- Lambert, T.N.; Chavez, C.A.; Hernandez-Sachez, B.; Lu, P.; Bell, N.S.; Ambrosini, A.; Friedman, T.; Boyle, T.J.; Wheeler, D.R.; Hubert, D.L. Synthesis and Characterization of Titania-Graphene Nanocomposites. J. Phys. Chem. C 2009, 113, 19812–19823. [Google Scholar] [CrossRef]
- Kim, S.R.; Parvez, M.K.; Chhowalla, M. UV-reduced of graphene oxide and its application as an interfacial layer to reduce the back-transport reactions in dye-sensitized solar cells. Chem. Phys. Lett. 2009, 483, 124–127. [Google Scholar] [CrossRef]
- Williams, G.; Seger, B.; Kamat, P.V. TiO2-Graphene Nanocomposites. UV-Assisted Photocatalytic Reduction of Graphene Oxide. ACS Nano 2008, 2, 1487–1491. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, X.Y.; Hua, Z.L.; Ma, W.Q.; Dai, Z.Y.; Huang, X.; Gu, H.X. Uniformly distributed anatase TiO2 nanoparticles on graphene: Synthesis, characterization, and photocatalytic application. J. Alloys Compd. 2014, 599, 10–18. [Google Scholar] [CrossRef]
- Li, F.; Du, P.H.; Liu, W.; Li, X.S.; Ji, H.D.; Duan, J.; Zhao, D.Y. Hydrothermal synthesis of graphene grafted titania/titanate nanosheets for photocatalytic degradation of 4-chlorophenol: Solar-light-driven photocatalytic activity and computational chemistry analysis. Chem. Eng. J. 2018, 331, 685–694. [Google Scholar] [CrossRef]
- Stengl, V.; Bakardjieva, S.; Grygar, T.M.; Bludska, J.; Kormunda, M. TiO2-graphene oxide nanocomposite as advanced photocatalytic materials. Chem. Cent. J. 2013, 7, 41. [Google Scholar] [CrossRef]
- Nagaraju, G.; Ebeling, G.; Goncalves, R.V.; Teixeira, S.R.; Weibel, D.E.; Dupont, J. Controlled growth of TiO2 and TiO2-RGO composite nanoparticles in ionic liquids for enhanced photocatalytic H2 generation. J. Mol. Catal. A Chem. 2013, 378, 213–220. [Google Scholar] [CrossRef]
- Wang, K.L.; Endo-Kimura, M.; Belchi, R.; Zhang, D.; Habert, A.; Boucle, J.; Ohtani, B.; Kowalska, E.; Herlin-Boime, N. Carbon/graphene-modified titania with enhanced photocatalytic activity under UV and vis irradiation. Materials 2019, 12, 4158. [Google Scholar] [CrossRef]
- Neelgund, G.M.; Oki, A. Graphene-Coupled ZnO: A Robust NIR-Induced Catalyst for Rapid Photo-Oxidation of Cyanide. ACS Omega 2017, 2, 9095–9102. [Google Scholar] [CrossRef]
- Wang, X.W.; Li, Q.C.; Zhou, C.X.; Cao, Z.Q.; Zhang, R.B. ZnO rod/reduced graphene oxide sensitized by alpha-Fe2O3 nanoparticles for effective visible-light photoreduction of CO2. J. Colloid Interface Sci. 2019, 554, 335–343. [Google Scholar] [CrossRef]
- Giovannetti, R.; Rommozzi, E.; Zannotti, M.; D’Amato, C.A. Recent Advances in Graphene Based TiO2 Nanocomposites (GTiO2Ns) for Photocatalytic Degradation of Synthetic Dyes. Catalysts 2017, 7, 305. [Google Scholar] [CrossRef]
- Wei, Z.; Janczarek, M.; Wang, K.; Zheng, S.; Kowalska, E. Morphology-governed performance of plasmonic photocatalysts. Catalysts 2020, 10, 1070. [Google Scholar] [CrossRef]
- Selloni, A. Crystal growth—Anatase shows its reactive side. Nat. Mater. 2008, 7, 613–615. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef]
- Janczarek, M.; Kowalska, E.; Ohtani, B. Decahedral-shaped anatase titania photocatalyst particles: Synthesis in a newly developed coaxial-flow gas-phase reactor. Chem. Eng. J. 2016, 289, 502–512. [Google Scholar] [CrossRef]
- Wei, Z.; Janczarek, M.; Endo, M.; Wang, K.L.; Balcytis, A.; Nitta, A.; Mendez-Medrano, M.G.; Colbeau-Justin, C.; Juodkazis, S.; Ohtani, B.; et al. Noble metal-modified faceted anatase titania photocatalysts: Octahedron versus decahedron. Appl. Catal. B Environ. 2018, 237, 574–587. [Google Scholar] [CrossRef]
- Wei, Z.; Kowalska, E.; Verrett, J.; Colbeau-Justin, C.; Remita, H.; Ohtani, B. Morphology-dependent photocatalytic activity of octahedral anatase particles prepared by ultrasonication-hydrothermal reaction of titanates. Nanoscale 2015, 7, 12392–12404. [Google Scholar] [CrossRef]
- Amano, F.; Prieto-Mahaney, O.O.; Terada, Y.; Yasumoto, T.; Shibayama, T.; Ohtani, B. Decahedral single-crystalline particles of anatase titanium(IV) oxide with high photocatalytic activity. Chem. Mater. 2009, 21, 2601–2603. [Google Scholar] [CrossRef]
- Amano, F.; Yasumoto, T.; Prieto-Mahaney, O.O.; Uchida, S.; Shibayama, T.; Ohtani, B. Photocatalytic activity of octahedral single-crystalline mesoparticles of anatase titanium(IV) oxide. Chem. Commun. 2009, 17, 2311–2313. [Google Scholar] [CrossRef]
- Amano, F.; Yasumoto, T.; Mahaney, O.O.P.; Uchida, S.; Shibayama, T.; Terada, Y.; Ohtani, B. Highly Active Titania Photocatalyst Particles of Controlled Crystal Phase, Size, and Polyhedral Shapes. Top. Catal. 2010, 53, 455–461. [Google Scholar] [CrossRef]
- Sofianou, M.V.; Psycharis, V.; Boukos, N.; Vaimakis, T.; Yu, J.G.; Dillert, R.; Bahnemann, D.; Trapalis, C. Tuning the photocatalytic selectivity of TiO2 anatase nanoplates by altering the exposed crystal facets content. Appl. Catal. B Environ. 2013, 142, 761–768. [Google Scholar] [CrossRef]
- Dozzi, M.V.; Selli, E. Specific Facets-Dominated Anatase TiO2: Fluorine-Mediated Synthesis and Photoactivity. Catalysts 2013, 3, 455–485. [Google Scholar] [CrossRef]
- Murakami, N.; Kurihara, Y.; Tsubota, T.; Ohno, T. Shape-Controlled Anatase Titanium(IV) Oxide Particles Prepared by Hydrothermal Treatment of Peroxo Titanic Acid in the Presence of Polyvinyl Alcohol. J. Phys. Chem. C 2009, 113, 3062–3069. [Google Scholar] [CrossRef]
- Su, Y.; Li, H.F.; Ma, H.B.; Robertson, J.; Nathan, A. Controlling surface termination and facet orientation in Cu2O nanoparticles for high photocatalytic activity: A combined experimental and density functional theory study. ACS Appl. Mater. Interfaces 2017, 9, 8100–8106. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.N.; Kao, J.C.; Zhong, X.Y.; Chan, S.J.; Patra, A.S.; Lo, Y.C.; Huang, M.H. Facet-specific photocatalytic activity enhancement of Cu2O polyhedra functionalized with 4-ethynylanaline resulting from band structure tuning. ACS Cent. Sci. 2020, 6, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.F.; Zhao, Q.Y.; Li, C.; Lyu, Y.; Dupuis, M. Photocatalytic facet selectivity in BiVO4 nanoparticles: Polaron electronic structure and thermodynamic stability considerations for photocatalysis. J. Phys. Chem. C 2019, 123, 20142–20151. [Google Scholar] [CrossRef]
- Lardhi, S.; Cavallo, L.; Harb, M. Significant impact of exposed facets on the BiVO4 material performance for photocatalytic water splitting reactions. J. Phys. Chem. Lett. 2020, 11, 5497–5503. [Google Scholar] [CrossRef]
- Wang, X.W.; Wang, W.Y.; Miao, Y.Q.; Feng, G.; Zhang, R.B. Facet-selective photodeposition of gold nanoparticles on faceted ZnO crystals for visible light photocatalysis. J. Colloid Interface Sci. 2016, 475, 112–118. [Google Scholar] [CrossRef]
- Yu, Z.Z.; Zhan, B.W.; Ge, B.H.; Zhu, Y.T.; Dai, Y.; Zhou, G.J.; Yu, F.P.; Wang, P.; Huang, B.B.; Zhan, J. Synthesis of high efficient and stable plasmonic photocatalyst Ag/AgNbO3 with specific exposed crystal-facets and intimate heterogeneous interface via combustion route. Appl. Surf. Sci. 2019, 488, 485–493. [Google Scholar] [CrossRef]
- Warmuth, L.; Ritschel, C.; Feldmann, C. Facet-, composition- and wavelength-dependent photocatalysis of Ag2MoO4. RSC Adv. 2020, 10, 18377–18383. [Google Scholar] [CrossRef]
- Dong, F.; Xiong, T.; Yan, S.; Wang, H.Q.; Sun, Y.J.; Zhang, Y.X.; Huang, H.W.; Wu, Z.B. Facets and defects cooperatively promote visible light plasmonic photocatalysis with Bi nanowires@BiOCl nanosheets. J. Catal. 2016, 344, 401–410. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.Z.; Sun, S.M.; Jiang, D.; Gao, E.P. Selective transport of electron and hole among {001} and {110} facets of BiOCl for pure water splitting. Appl. Catal. B Environ. 2015, 162, 470–474. [Google Scholar] [CrossRef]
- Liu, G.; Yu, J.C.; Lu, G.Q.; Cheng, H.M. Crystal facet engineering of semiconductor photocatalysts: Motivations, advances and unique properties. Chem. Commun. 2011, 47, 6763–6783. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.J.; Tian, C.G.; Pan, Q.J.; Jiang, Z.; Wang, J.Q.; Yan, W.S.; Fu, H.G. Enhanced photocatalytic activity and electron transfer mechanisms of graphene/TiO2 with exposed {001} facets. J. Phys. Chem. C 2011, 115, 23718–23725. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Wei, Y.C.; Wu, X.X.; Zheng, H.L.; Zhao, Z.; Liu, J.; Li, J.M. Graphene-wrapped Pt/TiO2 photocatalysts with enhanced photogenerated charges separation and reactant adsorption for high selective photoreduction of CO2 to CH4. Appl. Catal. B Environ. 2018, 226, 360–372. [Google Scholar] [CrossRef]
- Wang, C.; Meng, D.L.; Sun, J.H.; Memon, J.; Huang, Y.; Geng, J.X. Graphene wrapped TiO2 based catalysts with enhanced photocatalytic activity. Adv. Mater. Interfaces 2014, 1, 1. [Google Scholar] [CrossRef]
- Liu, H.; Liu, S.; Zhang, Z.L.; Dong, X.N.; Liu, T.T. Hydrothermal etching fabrication of TiO2@graphene hollow structures: Mutually independent exposed {001} and {101} facets nanocrystals and its synergistic photocaltalytic effects. Sci. Rep. 2016, 6, 33839. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, Y.; Qin, H.Y.; Wu, J.M.; Lu, Y.F.; Chi, H.Z.; Yang, F.; Zhou, B.; Yu, H.L.; Liu, J.B. Construction of rGO wrapping octahedral Ag-Cu2O heterostructure for enhanced visible light photocatalytic activity. Appl. Catal. B Environ. 2018, 227, 132–144. [Google Scholar] [CrossRef]
- Wang, T.; Li, C.J.; Ji, J.Y.; Wei, Y.J.; Zhang, P.; Wang, S.P.; Fan, X.B.; Gong, J.L. Reduced graphene oxide (rGO)/BiVO4 composites with maximized interfacial coupling for visible lght photocatalysis. ACS Sustain. Chem. Eng. 2014, 2, 2253–2258. [Google Scholar] [CrossRef]
- Wang, H.; Lu, J.R.; Liu, L.; Cui, W.Q.; Liang, Y.H. Ultra-thin rGO nanosheet modified TiO2 nanotube arrays for boosted photoelectrochemical performance. Appl. Surf. Sci. 2020, 506, 144966. [Google Scholar] [CrossRef]
- Pan, X.; Zhao, Y.; Liu, S.; Korzeniewski, C.L.; Wang, S.; Fan, Z.Y. Comparing graphene-TiO2 nanowire and graphene-TiO2 nanoparticle composite photocatalysts. ACS Appl. Mater. Interfaces 2012, 4, 3944–3950. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.G.; Han, J.; Xiong, H.X.; Guo, R.; Yin, Y.D. Nanostructured hybrid shells of r-GO/AuNP/m-TiO2 as highly active photocatalysts. ACS Appl. Mater. Interfaces 2015, 7, 6909–6918. [Google Scholar] [CrossRef]
- Yablonovitch, E. Photonic Band-Gap Structures. J. Opt. Soc. Am. B 1993, 10, 283–295. [Google Scholar] [CrossRef]
- Lima, A.W.; Sombra, A.S.B. Graphene-photonic crystal switch. Opt. Commun. 2014, 321, 150–156. [Google Scholar] [CrossRef]
- Raja-Mogan, T.; Ohtani, B.; Kowalska, E. Photonic crystals for plasmonic photocatalysis. Catalysts 2020, 10, 827. [Google Scholar] [CrossRef]
- Khaleque, A.; Hattori, H.T. Absorption enhancement in graphene photonic crystal structures. Appl. Opt. 2016, 55, 2936–2942. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, X.; Cheng, X.; Qiao, R.X.; Cheng, Y.; Liu, C.; Xie, Y.D.; Yu, W.T.; Yao, F.R.; Sun, Z.P.; et al. Graphene photonic crystal fibre with strong and tunable light-matter interaction. Nat. Photonics 2019, 13, 754–759. [Google Scholar] [CrossRef]
- Pourmahmoud, V.; Rezaei, B. Manipulation of Bragg and graphene photonic band gaps in one-dimensional photonic crystal containing graphene. Optik 2019, 185, 875–880. [Google Scholar] [CrossRef]
- Dash, J.N.; Jha, R. Graphene-based birefringent photonic crystal fiber sensor using surface plasmon resonance. IEEE Photonic Technol. Lett. 2014, 26, 1092–1095. [Google Scholar] [CrossRef]
- Sreekanth, K.V.; Zeng, S.W.; Yong, K.T.; Yu, T. Sensitivity enhanced biosensor using graphene-based one-dimensional photonic crystal. Sens. Actuators B Chem. 2013, 182, 424–428. [Google Scholar] [CrossRef]
- Papadakis, D.; Diamantopoulou, A.; Pantazopoulos, P.A.; Palles, D.; Sakellis, E.; Boukos, N.; Stefanou, N.; Likodimos, V. Nanographene oxide-TiO2 photonic films as plasmon-free substrates for surface-enhanced Raman scattering. Nanoscale 2019, 11, 21542–21553. [Google Scholar] [CrossRef]
- Apostolaki, M.A.; Toumazatou, A.; Antoniadou, M.; Sakellis, E.; Xenogiannopoulou, E.; Gardelis, S.; Boukos, N.; Falaras, P.; Dimoulas, A.; Likodimos, V. Graphene quantum dot-TiO2 photonic crystal films for photocatalytic applications. Nanomaterials 2020, 10, 2566. [Google Scholar] [CrossRef]
- Diamantopoulou, A.; Sakellis, E.; Gardelis, S.; Tsoutsou, D.; Glenis, S.; Boukos, N.; Dimoulas, A.; Likodimos, V. Advanced photocatalysts based on reduced nanographene oxide-TiO2 photonic crystal films. Materials 2019, 12, 2518. [Google Scholar] [CrossRef]
- Baghbadorani, H.K.; Barvestani, J.; Entezar, S.R. Biosensors based on Bloch surface waves in one-dimensional photonic crystal with graphene nanolayers. Appl. Opt. 2017, 56, 462–469. [Google Scholar] [CrossRef]
- Gan, X.T.; Mak, K.F.; Gao, Y.D.; You, Y.M.; Hatami, F.; Hone, J.; Heinz, T.F.; Englund, D. Strong enhancement of light-matter interaction in graphene coupled to a photonic crystal nanocavity. Nano Lett. 2012, 12, 5626–5631. [Google Scholar] [CrossRef]
- Ghasemi, F.; Entezar, S.R.; Razi, S. Graphene based photonic crystal optical filter: Design and exploration of the tunability. Phys. Lett. A 2019, 383, 2551–2560. [Google Scholar] [CrossRef]
- Zeng, S.W.; Yong, K.T.; Roy, I.; Dinh, X.Q.; Yu, X.; Luan, F. A Review on functionalized gold nanoparticles for biosensing applications. Plasmonics 2011, 6, 491–506. [Google Scholar] [CrossRef]
- Homola, J. Present and future of surface plasmon resonance biosensors. Anal. Bioanal. Chem. 2003, 377, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Khurgin, J.B.; Boltasseva, A. Reflecting upon the losses in plasmonics and metamaterials. MRS Bull. 2012, 37, 768–779. [Google Scholar] [CrossRef]
- Jovic, V.; Idriss, H.; Waterhouse, G.I.N. Slow photon amplification of gas-phase ethanol photo-oxidation in titania inverse opal photonic crystals. Chem. Phys. 2016, 479, 109–121. [Google Scholar] [CrossRef]
- Curti, M.; Mendive, C.B.; Grela, M.A.; Bahnemann, D.W. Stopband tuning of TiO2 inverse opals for slow photon absorption. Mater. Res. Bull. 2017, 91, 155–165. [Google Scholar] [CrossRef]
- Liu, J.T.; Liu, N.H.; Li, J.; Li, X.J.; Huang, J.H. Enhanced absorption of graphene with one-dimensional photonic crystal. Appl. Phys. Lett. 2012, 101, 052104. [Google Scholar] [CrossRef]
- Zhang, K.; Shi, X.; Kim, J.K.; Lee, J.S.; Park, J.H. Inverse opal structured alpha-Fe2O3 on graphene thin films: Enhanced photo-assisted water splitting. Nanoscale 2013, 5, 1939–1944. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.Y.; Lee, J.S.; Kim, K.; Bak, C.H.; Kim, S.I.; Kim, J.B.; Jang, J.H. Hematite-based photoelectrochemical water splitting supported by inverse opal structures of graphene. ACS Appl. Mater. Interfaces 2014, 6, 22634–22639. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.W.; Yuan, C.; Wang, Y. Nanocomposites of three-dimensionally ordered porous TiO2 decorated with Pt and reduced graphene oxide for the visible-light photocatalytic degradation of waterborne pollutants. ACS Appl. Nano Mater. 2019, 2, 2713–2724. [Google Scholar] [CrossRef]
- Boppella, R.; Kochuveedu, S.T.; Kim, H.; Jeong, M.J.; Mota, F.M.; Park, J.H.; Kim, D.H. Plasmon-sensitized graphene/TiO2 inverse opal nanostructures with enhanced charge collection efficiency for water splitting. ACS Appl. Mater. Interfaces 2017, 9, 7075–7083. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.C.; Xie, L.Z.; Wang, M.Z.; Ge, X.W. Preparation of three-dimensional inverse opal SnO2/graphene composite microspheres and their enhanced photocatalytic activities. J. Mater. Chem. A 2015, 3, 2991–2998. [Google Scholar] [CrossRef]
- Diamantopoulou, A.; Sakellis, E.; Romanos, G.E.; Gardelis, S.; Ioannidis, N.; Boukos, N.; Falaras, P.; Likodimos, V. Titania photonic crystal photocatalysts functionalized by graphene oxide nanocolloids. Appl. Catal. B Environ. 2019, 240, 277–290. [Google Scholar] [CrossRef]
- Wang, W.X.; Wang, X.X.; Xing, J.L.; Gong, Q.B.; Wang, H.H.; Wang, J.J.; Chen, Z.; Ai, Y.J.; Wang, X.K. Multi-heteroatom doped graphene-like carbon nanospheres with 3D inverse opal structure: A promising bisphenol-A remediation material. Environ. Sci. Nano 2019, 6, 809–819. [Google Scholar] [CrossRef]
- Raja-Mogan, T.; Lehoux, A.; Takashima, M.; Kowalska, E.; Ohtani, B. Slow photon-induced enhancement of photocatalytic activity of gold nanoparticle-incorporated titania in-verse opal. Chem. Lett. 2021, in press. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, Z.; Zhang, A.J.; Zhu, J.H.; Wei, X.R.; Guo, J.; Wu, W.D.; Chen, D.; Wu, Z.X. Surface-coating synthesis of nitrogen-doped inverse opal carbon materials with ultrathin micro/mesoporous graphene-like walls for oxygen reduction and supercapacitors. J. Mater. Chem. A 2017, 5, 25237–25248. [Google Scholar] [CrossRef]
- Chen, J.I.L.; Loso, E.; Ebrahim, N.; Ozin, G.A. Synergy of slow photon and chemically amplified photochemistry in platinum nanocluster-loaded inverse titania opals. J. Am. Chem. Soc. 2008, 130, 5420–5421. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.X.; Yang, B.F.; Xu, J.; Fu, Z.P.; Wu, M.; Li, F. Facile synthesis of Ag nanoparticles supported on TiO2 inverse opal with enhanced visible-light photocatalytic activity. Thin Solid Films 2012, 520, 3515–3522. [Google Scholar] [CrossRef]
- Chwalibog, A.; Sawosz, E.; Hotowy, A.; Szeliga, J.; Mitura, S.; Mitura, K.; Grodzik, M.; Orlowski, P.; Sokolowska, A. Visualization of interaction between inorganic nanoparticles and bacteria or fungi. Int. J. Nanomed. 2010, 5, 1085–1094. [Google Scholar] [CrossRef]
- Sambhy, V.; MacBride, M.M.; Peterson, B.R.; Sen, A. Silver bromide nanoparticle/polymer composites: Dual action tunable antimicrobial materials. J. Am. Chem. Soc. 2006, 128, 9798–9808. [Google Scholar] [CrossRef] [PubMed]
- Sunada, K.; Minoshima, M.; Hashimoto, K. Highly efficient antiviral and antibacterial activities of solid-state cuprous compounds. J. Hazard. Mater. 2012, 235, 265–270. [Google Scholar] [CrossRef]
- Matsunaga, T.; Tomoda, R.; Nakajima, T.; Wake, H. Photoelectrochemical sterilization of microbial-cells by semiconductor powders. FEMS Microbiol. Lett. 1985, 29, 211–214. [Google Scholar] [CrossRef]
- McEvoy, J.G.; Zhang, Z.S. Antimicrobial and photocatalytic disinfection mechanisms in silver-modified photocatalysts under dark and light conditions. J. Photochem. Photobiol. C 2014, 19, 62–75. [Google Scholar] [CrossRef]
- Markowska-Szczupak, A.; Ulfig, K.; Morawski, W.A. The application of titanium dioxide for deactivation of bioparticulates: An overview. Catal. Today 2011, 161, 249–257. [Google Scholar] [CrossRef]
- Mitoraj, D.; Janczyk, A.; Strus, M.; Kisch, H.; Stochel, G.; Heczko, P.B.; Macyk, W. Visible light inactivation of bacteria and fungi by modified titanium dioxide. Photochem. Photobiol. Sci. 2007, 6, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Markowska-Szczupak, A.; Wang, K.L.; Rokicka, P.; Endo, M.; Wei, Z.S.; Ohtani, B.; Morawski, A.W.; Kowalska, E. The effect of anatase and rutile crystallites isolated from titania P25 photocatalyst on growth of selected mould fungi. J. Photochem. Photobiol. B 2015, 151, 54–62. [Google Scholar] [CrossRef]
- Markowska-Szczupak, A.; Endo-Kimura, M.; Paszkiewicz, O.; Kowalska, E. Are titania photocatalysts and titanium implants safe? Review on the toxicity of titanium compounds. Nanomaterials 2020, 10, 2065. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Photocatalytic reduction of graphene oxide nanosheets on TiO2 thin film for photoinactivation of bacteria in solar light irradiation. J. Phys. Chem. C 2009, 113, 20214–20220. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef]
- Khanam, P.N.; Hasan, A. Biosynthesis and characterization of graphene by using non-toxic reducing agent from Allium Cepa extract: Anti-bacterial properties. Int. J. Biol. Macromol. 2019, 126, 151–158. [Google Scholar] [CrossRef]
- Palmieri, V.; Bugli, F.; Lauriola, M.C.; Cacaci, M.; Torelli, R.; Ciasca, G.; Conti, C.; Sanguinetti, M.; Papi, M.; De Spirito, M. Bacteria meet graphene: Modulation of graphene oxide nanosheet interaction with human pathogens for effective antimicrobial therapy. ACS Biomater. Sci. Eng. 2017, 3, 619–627. [Google Scholar] [CrossRef]
- Jira, J.; Rezek, B.; Kriha, V.; Artemenko, A.; Matolinova, I.; Skakalova, V.; Stenclova, P.; Kromka, A. Inhibition of E. coli growth by nanodiamond and graphene oxide enhanced by Luria-Bertani medium. Nanomaterials 2018, 8, 140. [Google Scholar] [CrossRef]
- Gusev, A.; Zakharova, O.; Muratov, D.S.; Vorobeva, N.S.; Sarker, M.; Rybkin, I.; Bratashov, D.; Kolesnikov, E.; Lapanje, A.; Kuznetsov, D.V.; et al. Medium-dependent antibacterial properties and bacterial filtration ability of reduced graphene oxide. Nanomaterials 2019, 9, 1454. [Google Scholar] [CrossRef]
- Li, X.F.; Sun, J.Z.; Che, Y.L.; Lv, Y.; Liu, F. Antibacterial properties of chitosan chloride-graphene oxide composites modified quartz sand filter media in water treatment. Int. J. Biol. Macromol. 2019, 121, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Alayande, A.B.; Chae, S.; Kim, I.S. Surface morphology-dependent spontaneous bacterial behaviors on graphene oxide membranes. Sep. Purif. Technol. 2019, 226, 68–74. [Google Scholar] [CrossRef]
- Biswas, K.; De, D.; Bandyopadhyay, J.; Sen, P. Differential antibacterial response exhibited by graphene nanosheets toward gram-positive bacterium Staphylococcus aureus. IET Nanobiotechnol. 2018, 12, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.B.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Veerapandian, M.; Zhang, L.H.; Yun, K.; Kim, S.J. Antibacterial efficiency of graphene nanosheets against pathogenic bacteria via lipid peroxidation. J. Phys. Chem. C 2012, 116, 17280–17287. [Google Scholar] [CrossRef]
- Li, J.H.; Wang, G.; Zhu, H.Q.; Zhang, M.; Zheng, X.H.; Di, Z.F.; Liu, X.Y.; Wang, X. Antibacterial activity of large-area monolayer graphene film manipulated by charge transfer. Sci. Rep. 2014, 4, 4359. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Chaves-Lopez, C.; Oliveira, R.C.; Paparella, A.; Rodrigues, D.E. Cellular and metabolic approaches to investigate the effects of graphene and graphene oxide in the fungi Aspergillus flavus and Aspergillus niger. Carbon 2019, 143, 419–429. [Google Scholar] [CrossRef]
- Di Giulio, M.; Zappacosta, R.; Di Lodovico, S.; Di Campli, E.; Siani, G.; Fontana, A.; Cellini, L. Antimicrobial and antibiofilm efficacy of graphene oxide against chronic wound microorganisms. Antimicrob. Agents Chemother. 2018, 62, e00547-18. [Google Scholar] [CrossRef] [PubMed]
- Diez-Orejas, R.; Feito, M.J.; Cicuendez, M.; Casarrubios, L.; Rojo, J.M.; Portoles, M.T. Graphene oxide nanosheets increase Candida albicans killing by pro-inflammatory and reparative peritoneal macrophages. Colloid Surfaces B 2018, 171, 250–259. [Google Scholar] [CrossRef]
- Gandhi, P.R.; Chandramohan, G. Preparation of novel chitosan-graphene oxide/tin oxide nanocomposites for anti-bacterial activities. Dig. J. Nanomater. Biostruct. 2020, 15, 561–568. [Google Scholar]
- Yang, M.C.; Tseng, Y.Q.; Liu, K.H.; Cheng, Y.W.; Chen, W.T.; Chen, W.T.; Hsiao, C.W.; Yung, M.C.; Hsu, C.C.; Liu, T.Y. Preparation of amphiphilic chitosan-graphene oxide-cellulose nanocrystalline composite hydrogels and their biocompatibility and antibacterial properties. Appl. Sci. 2019, 9, 3051. [Google Scholar] [CrossRef]
- Bandara, P.C.; Nadres, E.T.; Pena-Bahamonde, J.; Rodrigues, D.F. Impact of water chemistry, shelf-life, and regeneration in the removal of different chemical and biological contaminants in water by a model polymeric graphene oxide nanocomposite membrane coating. J. Water Process. Eng. 2019, 32, 100967. [Google Scholar] [CrossRef]
- Bugli, F.; Cacaci, M.; Palmieri, V.; Di Santo, R.; Torelli, R.; Ciasca, G.; Di Vito, M.; Vitali, A.; Conti, C.; Sanguinetti, M.; et al. Curcumin-loaded graphene oxide flakes as an effective antibacterial system against methicillin-resistant Staphylococcus aureus. Interface Focus 2018, 8, 20170059. [Google Scholar] [CrossRef]
- Ghorbanzadeh, R.; Assadian, H.; Chiniforush, N.; Parker, S.; Pourakbari, B.; Ehsani, B.; Alikhani, M.Y.; Bahador, A. Modulation of virulence in Enterococcus faecalis cells surviving antimicrobial photodynamic inactivation with reduced graphene oxide-curcumin: An ex vivo biofilm model. Photodiagnosis Photodyn. Ther. 2020, 29, 101643. [Google Scholar] [CrossRef] [PubMed]
- De Maio, F.; Palmieri, V.; Santarelli, G.; Perini, G.; Salustri, A.; Palucci, I.; Sali, M.; Gervasoni, J.; Primiano, A.; Ciasca, G.; et al. Graphene oxide-linezolid combination as potential new anti-tuberculosis treatment. Nanomaterials 2020, 10, 1431. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, J.C.; Ren, X.M.; Tan, X.L.; Hayat, T.; Alsaedi, A.; Cheng, C.; Chen, C.L. Impact of graphene oxide on the antibacterial activity of antibiotics against bacteria. Environ. Sci. Nano 2017, 4, 1016–1024. [Google Scholar] [CrossRef]
- Ramasamy, M.; Nanda, S.S.; Lee, J.H.; Lee, J. Construction of alizarin conjugated graphene oxide composites for inhibition of Candida albicans biofilms. Biomolecules 2020, 10, 565. [Google Scholar] [CrossRef] [PubMed]
- Goy, R.C.; de Britto, D.; Assis, O.B.G. A review of the antimicrobial activity of chitosan. Polimeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Helander, I.M.; Nurmiaho-Lassila, E.L.; Ahvenainen, R.; Rhoades, J.; Roller, S. Chitosan disrupts the barrier properties of the outer membrane of Gram-negative bacteria. Int. J. Food Microbiol. 2001, 71, 235–244. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.G.; Ryu, S.Y.; Lee, J. Calcium-chelating alizarin and other anthraquinones inhibit biofilm formation and the hemolytic activity of Staphylococcus aureus. Sci. Rep. 2016, 6, 19267. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, R.K.; Lee, J.H.; Kim, Y.G.; Lee, J. Alizarin and Chrysazin inhibit biofilm and hyphal formation by Candida albicans. Front. Cell. Infect. Microbiol. 2017, 7, 447. [Google Scholar] [CrossRef] [PubMed]
- Naeem, H.; Ajmal, M.; Qureshi, R.B.; Muntha, S.T.; Farooq, M.; Siddiq, M. Facile synthesis of graphene oxide-silver nanocomposite for decontamination of water from multiple pollutants by adsorption, catalysis and antibacterial activity. J. Environ. Manag. 2019, 230, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.M.; Lin, J.C.; Chen, Z.Y.; Wei, M.C.; Fu, Y.X.; Lu, S.S.; Yu, D.S.; Zhao, W. Enhanced antimicrobial activities of silver-nanoparticle-decorated reduced graphene nanocomposites against oral pathogens. Mater. Sci. Eng. C 2017, 71, 10–16. [Google Scholar] [CrossRef]
- Ko, K.; Kim, M.J.; Lee, J.Y.; Kim, W.; Chung, H. Effects of graphene oxides and silver-graphene oxides on aquatic microbial activity. Sci. Total Environ. 2019, 651, 1087–1095. [Google Scholar] [CrossRef]
- Cobos, M.; De-La-Pinta, I.; Quindos, G.; Fernandez, M.J.; Fernandez, M.D. Graphene oxide-silver nanoparticle nanohybrids: Synthesis, characterization, and antimicrobial properties. Nanomaterials 2020, 10, 376. [Google Scholar] [CrossRef] [PubMed]
- Cobos, M.; De-La-Pinta, I.; Quindos, G.; Fernandez, M.J.; Fernandez, M.D. Synthesis, physical, mechanical and antibacterial properties of nanocomposites based on poly(vinyl alcohol)/graphene oxide-silver nanoparticles. Polymers 2020, 12, 723. [Google Scholar] [CrossRef]
- Agarwalla, S.V.; Ellepola, K.; da Costa, M.C.F.; Fechine, G.J.M.; Morin, J.L.P.; Neto, A.H.C.; Seneviratne, C.J.; Rosa, V. Hydrophobicity of graphene as a driving force for inhibiting biofilm formation of pathogenic bacteria and fungi. Dent. Mater. 2019, 35, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Arshad, A.; Iqbal, J.; Mansoor, Q. NiO-nanoflakes grafted graphene: An excellent photocatalyst and a novel nanomaterial for achieving complete pathogen control. Nanoscale 2017, 9, 16321–16328. [Google Scholar] [CrossRef]
- Kozuszko, S.N.; Sanchez, M.A.; de Ferro, M.I.G.; Sfer, A.M.; Madrid, A.P.M.; Takabatake, K.; Nakano, K.; Nagatsuka, H.; Rodriguez, A.P. Antibacterial activity and biocompability of zinc oxide and graphite particles as endodontic materials. J. Hard Tissue Biol. 2017, 26, 311–318. [Google Scholar] [CrossRef]
- Ghanem, A.F.; Badawy, A.A.; Mohram, M.E.; Rehim, M.H.A. Synergistic effect of zinc oxide nanorods on the photocatalytic performance and the biological activity of graphene nano sheets. Heliyon 2020, 6, e03283. [Google Scholar] [CrossRef] [PubMed]
- Ficociello, G.; De Canis, M.G.; Trillo, G.; Cavallini, D.; Santo, M.S.; Uccelletti, D.; Mancini, P. Anti-candidal activity and in vitro cytotoxicity assessment of graphene nanoplatelets decorated with zinc oxide nanorods. Nanomaterials 2018, 8, 752. [Google Scholar] [CrossRef]
- Jiang, G.F.; Li, X.F.; Che, Y.L.; Lv, Y.; Liu, F.; Wang, Y.Q.; Zhao, C.C.; Wang, X.J. Antibacterial and anticorrosive properties of CuZnO@RGO waterborne polyurethane coating in circulating cooling water. Environ. Sci. Pollut. Res. 2019, 26, 9027–9040. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekar, M.; Jenefer, V.; Nambiar, R.B.; Babu, S.G.; Selvam, S.P.; Neppolian, B.; Bhat, S.V. Ambient light antimicrobial activity of reduced graphene oxide supported metal doped TiO2 nanoparticles and their PVA based polymer nanocomposite films. Mater. Res. Bull. 2018, 97, 238–243. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, X.K.; Hu, X.Y.; Hu, J.; Wang, Z.Y.; Yin, Y.C.; Sun, C.H.; Zhang, X.W. Two-dimensional g-C3N4/TiO2 nanocomposites as vertical Z-scheme heterojunction for improved photocatalytic water disinfection. Catal. Today 2019, 335, 243–251. [Google Scholar] [CrossRef]
- Cruz-Ortiz, B.R.; Hamilton, J.W.J.; Pablos, C.; Diaz-Jimenez, L.; Cortes-Hernandez, D.A.; Sharma, P.K.; Castro-Alferez, M.; Fernandez-Ibanez, P.; Dunlop, P.S.M.; Byrne, J.A. Mechanism of photocatalytic disinfection using titania-graphene composites under UV and visible irradiation. Chem. Eng. J. 2017, 316, 179–186. [Google Scholar] [CrossRef]
- Luo, R.; Xu, Z.Y.; Lin, X.M. Preparation and characterisation of silver nanoparticles in an aqueous suspension of TETA modified graphene oxide and their antibacterial activity. Micro Nano Lett. 2018, 13, 369–373. [Google Scholar] [CrossRef]
- Cobos, M.; De-La-Pinta, I.; Quindos, G.; Fernandez, M.J.; Fernandez, M.D. One-step eco-friendly synthesized silver-graphene oxide/poly(vinyl alcohol) antibacterial nanocomposites. Carbon 2019, 150, 101–116. [Google Scholar] [CrossRef]
- Xu, W.R.; Xie, W.J.; Huang, X.Q.; Chen, X.; Huang, N.; Wang, X.; Liu, J. The graphene oxide and chitosan biopolymer loads TiO2 for antibacterial and preservative research. Food Chem. 2017, 221, 267–277. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, X.J.; Li, Y.M.; Wang, Y.; Li, J.H. P25-graphene composite as a high performance photocatalyst. ACS Nano 2010, 4, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Paszkiwicz-Gawron, M.; Kowalska, E.; Endo-Kimura, M.; Zwara, J.; Pancielejko, A.; Wang, K.; Lisowski, W.; Luczak, J.; Zaleska-Medynska, A.; Gerabowska-Musial, E. Stannates, titanates and tantalates modified with carbon and graphene quantum dots for enhancement of visible-light photocatalytic activity. Appl. Surf. Sci. 2021, 541, 148425. [Google Scholar] [CrossRef]
- Whitehead, K.A.; Vaidya, M.; Liauw, C.M.; Brownson, D.A.C.; Ramalingam, P.; Kamieniak, J.; Rowley-Neale, S.J.; Tetlow, L.A.; Wilson-Nieuwenhuis, J.S.T.; Brown, D.; et al. Antimicrobial activity of graphene oxide-metal hybrids. Int. Biodeterior. Biodegrad. 2017, 123, 182–190. [Google Scholar] [CrossRef]
- Pulingam, T.; Thong, K.L.; Ali, M.E.; Appaturi, J.N.; Dinshaw, I.J.; Ong, Z.Y.; Leo, B.F. Graphene oxide exhibits differential mechanistic action towards Gram-positive and Gram-negative bacteria. Colloids Surf. B 2019, 181, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Stanford, M.G.; Li, J.T.; Chen, Y.D.; McHugh, E.A.; Liopo, A.; Xiao, H.; Tour, J.M. Self-sterilizing laser-induced graphene bacterial air filter. ACS Nano 2019, 13, 11912–11920. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Dwivedi, N.; Dhand, C.; Khan, R.; Sathish, N.; Gupta, M.K.; Kumar, R.; Kumar, S. Potential of graphene-based materials to combat COVID-19: Properties, perspectives, and prospects. Mater. Today Chem. 2020, 18, 100385. [Google Scholar] [CrossRef]
- Alegret, N.; Criado, A.; Prato, M. Recent advances of graphene-based hybrids with magnetic nanoparticles for biomedical applications. Curr. Med. Chem. 2017, 24, 529–536. [Google Scholar] [CrossRef]
- Orfanakis, G.; Patila, M.; Catzikonstantinou, A.V.; Lyra, K.M.; Kouloumpis, A.; Spyrou, K.; Katapodis, P.; Paipetis, A.; Rudolf, P.; Gournis, D.; et al. Hybrid nanomaterials of magnetic iron nanoparticles and graphene oxide as matrices for the immobilization of beta-glucosidase: Synthesis, characterization, and biocatalytic properties. Front. Mater. 2018, 5, 25. [Google Scholar] [CrossRef]
- Xu, K.J.; Wang, Y.Z.; Ding, X.Q.; Huang, Y.H.; Li, N.; Wen, Q. Magnetic solid-phase extraction of protein with deep eutectic solvent immobilized magnetic graphene oxide nanoparticles. Talanta 2016, 148, 153–162. [Google Scholar] [CrossRef]
- Kim, S.E.; Tieu, M.V.; Hwang, S.Y.; Lee, M.H. Magnetic particles: Their applications from sample preparations to biosensing platforms. Micromachines 2020, 11, 302. [Google Scholar] [CrossRef]
- Mishra, A.K.; Ramaprabhu, S. Ultrahigh arsenic sorption using iron oxide-graphene nanocomposite supercapacitor assembly. J. Appl. Phys. 2012, 112, 104315. [Google Scholar] [CrossRef]
- Kim, S.; Park, C.M.; Jang, M.; Son, A.; Her, N.; Yu, M.; Snyder, S.; Kim, D.H.; Yoon, Y. Aqueous removal of inorganic and organic contaminants by graphene-based nanoadsorbents: A review. Chemosphere 2018, 212, 1104–1124. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, B.A.; Zhang, L.M.; Huang, J.; Chen, F.H.; Yang, Z.P.; Yao, J.L.; Zhang, Z.J. Controlled assembly of Fe3O4 magnetic nanoparticles on graphene oxide. Nanoscale 2011, 3, 1446–1450. [Google Scholar] [CrossRef]
- Xiang, Q.J.; Yu, J.G.; Jaroniec, M. Graphene-based semiconductor photocatalysts. Chem. Soc. Rev. 2012, 41, 782–796. [Google Scholar] [CrossRef]
- Fu, Y.S.; Chen, Q.; He, M.Y.; Wan, Y.H.; Sun, X.Q.; Xia, H.; Wang, X. Copper ferrite-graphene hybrid: A multifunctional heteroarchitecture for photocatalysis and energy storage. Ind. Eng. Chem. Res. 2012, 51, 11700–11709. [Google Scholar] [CrossRef]
- Cheng, R.L.; Fan, X.Q.; Wang, M.; Li, M.L.; Tian, J.J.; Zhang, L.X. Facile construction of CuFe2O4/g-C3N4 photocatalyst for enhanced visible-light hydrogen evolution. RSC Adv. 2016, 6, 18990–18995. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Hosseini-Monfared, H.; Abbasi, V. Silver ferrite-graphene nanocomposite and its good photocatalytic performance in air and visible light for organic dye removal. Appl. Organomet. Chem. 2017, 31, e3589. [Google Scholar] [CrossRef]
- Mrotek, E.; Dudziak, S.; Malinowska, I.; Pelczarski, S.; Ryzynska, Z.; Zielinska-Jurek, A. Improved degradation of etodolac in the presence of core-shell ZnFe2O4/SiO2/TiO2 magnetic photocatalyst. Sci. Total. Environ. 2020, 724, 138167. [Google Scholar] [CrossRef]
- Zielinska-Jurek, A.; Bielan, Z.; Wysocka, I.; Strychalska, J.; Janczarek, M.; Klimczuk, T. Magnetic semiconductor photocatalysts for the degradation of recalcitrant chemicals from flow back water. J. Environ. Manag. 2017, 195, 157–165. [Google Scholar] [CrossRef]
- Dai, J.F.; Xian, T.; Di, L.J.; Yang, H. Preparation of BiFeO3-graphene nanocomposites and their enhanced photocatalytic activities. J. Nanomater. 2013, 2013, 642897. [Google Scholar] [CrossRef]
- Beydoun, D.; Amal, R.; Low, G.K.C.; McEvoy, S. Novel photocatalyst: Titania-coated magnetite. Activity and photodissolution. J. Phys. Chem. B 2000, 104, 4387–4396. [Google Scholar] [CrossRef]
- Bavarsiha, F.; Rajabi, M.; Montazeri-Pour, M. Synthesis of SrFe12O19/SiO2/TiO2 composites with core/shell/shell nano-structure and evaluation of their photo-catalytic efficiency for degradation of methylene blue. J. Mater. Sci. Mater. Electron. 2018, 29, 1877–1887. [Google Scholar] [CrossRef]
- Park, C.M.; Kim, Y.M.; Kim, K.H.; Wang, D.J.; Su, C.M.; Yoon, Y. Potential utility of graphene-based nano spinel ferrites as adsorbent and photocatalyst for removing organic/inorganic contaminants from aqueous solutions: A mini review. Chemosphere 2019, 221, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Bao, N.Z.; Shen, L.M.; Wang, Y.H.; Padhan, P.; Gupta, A. A facile thermolysis route to monodisperse ferrite nanocrystals. J. Am. Chem. Soc. 2007, 129, 12374–12375. [Google Scholar] [CrossRef] [PubMed]
- Naskar, A.; Khan, H.; Jana, S. One pot low temperature synthesis of graphene coupled Gd-doped ZnFe2O4 nanocomposite for effective removal of antibiotic levofloxacin drug. J. Sol-Gel Sci. Technol. 2018, 86, 599–609. [Google Scholar] [CrossRef]
- Yamaguchi, N.U.; Bergamasco, R.; Hamoudi, S. Magnetic MnFe2O4-graphene hybrid composite for efficient removal of glyphosate from water. Chem. Eng. J. 2016, 295, 391–402. [Google Scholar] [CrossRef]
- Luciano, A.J.R.; Soletti, L.D.; Ferreira, M.E.C.; Cusioli, L.F.; de Andrade, M.B.; Bergamasco, R.; Yamaguchi, N.U. Manganese ferrite dispersed over graphene sand composite for methylene blue photocatalytic degradation. J. Environ. Chem. Eng. 2020, 8, 104191. [Google Scholar] [CrossRef]
- Jelokhani, F.; Sheibani, S.; Ataie, A. Adsorption and photocatalytic characteristics of cobalt ferrite-reduced graphene oxide and cobalt ferrite-carbon nanotube nanocomposites. J. Photochem. Photobiol. A 2020, 403, 112867. [Google Scholar] [CrossRef]
- Sharma, R.; Bansal, S.; Singhal, S. Tailoring the photo-Fenton activity of spinel ferrites (MFe2O4) by incorporating different cations (M = Cu, Zn, Ni and Co) in the structure. RSC Adv. 2015, 5, 6006–6018. [Google Scholar] [CrossRef]
- Gan, L.; Shang, S.M.; Yuen, C.W.M.; Jiang, S.X.; Hu, E.L. Hydrothermal synthesis of magnetic CoFe2O4/graphene nanocomposites with improved photocatalytic activity. Appl. Surf. Sci. 2015, 351, 140–147. [Google Scholar] [CrossRef]
- Chen, P. Synthesis and photocatalysis of novel magnetic reduced graphene oxide-ZnFe2O4 nanocomposites with highly efficient interface-induced effect. J. Sol-Gel Sci. Technol. 2017, 82, 397–406. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.H.; Meng, Z.L.; Tong, W.S.; Yu, X.L.; An, Q. Constructing the magnetic bifunctional graphene/titania nanosheet-based composite photocatalysts for enhanced visible-light photodegradation of MB and electrochemical ORR from polluted water. Sci. Rep. 2017, 7, 12296. [Google Scholar] [CrossRef]
- Houshiar, M.; Zebhi, F.; Razi, Z.J.; Alidoust, A.; Askari, Z. Synthesis of cobalt ferrite (CoFe2O4) nanoparticles using combustion, coprecipitation, and precipitation methods: A comparison study of size, structural, and magnetic properties. J. Magn. Magn. Mater. 2014, 371, 43–48. [Google Scholar] [CrossRef]
- Lee, J.S.; Cha, J.M.; Yoon, H.Y.; Lee, J.K.; Kim, Y.K. Magnetic multi-granule nanoclusters: A model system that exhibits universal size effect of magnetic coercivity. Sci. Rep. 2015, 5, 12135. [Google Scholar] [CrossRef]
- Wu, W.; Jiang, C.Z.; Roy, V.A.L. Recent progress in magnetic iron oxide-semiconductor composite nanomaterials as promising photocatalysts. Nanoscale 2015, 7, 38–58. [Google Scholar] [CrossRef]
- Andersen, H.L.; Saura-Muzquiz, M.; Granados-Miralles, C.; Canevet, E.; Lock, N.; Christensen, M. Crystalline and magnetic structure-property relationship in spinel ferrite nanoparticles. Nanoscale 2018, 10, 14902–14914. [Google Scholar] [CrossRef]
- Aslibeiki, B.; Kameli, P.; Ehsani, M.H.; Salamati, H.; Muscas, G.; Agostinelli, E.; Foglietti, V.; Casciardi, S.; Peddis, D. Solvothermal synthesis of MnFe2O4 nanoparticles: The role of polymer coating on morphology and magnetic properties. J. Magn. Magn. Mater. 2016, 399, 236–244. [Google Scholar] [CrossRef]
- Dey, S.; Dey, S.K.; Majumder, S.; Poddar, A.; Dasgupta, P.; Banerjee, S.; Kumar, S. Superparamagnetic behavior of nanosized Co0.2Zn0.8Fe2O4 synthesized by a flow rate controlled chemical coprecipitation method. Physica B 2014, 448, 247–252. [Google Scholar] [CrossRef]
- Rani, G.J.; Rajan, M.A.J.; Kumar, G.G. Reduced graphene oxide/ZnFe2O4 nanocomposite as an efficient catalyst for the photocatalytic degradation of methylene blue dye. Res. Chem. Intermed. 2017, 43, 2669–2690. [Google Scholar] [CrossRef]
- Wu, L.K.; Wu, H.; Zhang, H.B.; Cao, H.Z.; Hou, G.Y.; Tang, Y.P.; Zheng, G.Q. Graphene oxide/CuFe2O4 foam as an efficient absorbent for arsenic removal from water. Chem. Eng. J. 2018, 334, 1808–1819. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wang, A.Q.; Ma, J. Visible-light-driven photocatalytic removal of antibiotics by newly designed C3N4@MnFe2O4-graphene nanocomposites. J. Hazard. Mater. 2017, 336, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Santhosh, C.; Kollu, P.; Felix, S.; Velmurugan, V.; Jeong, S.K.; Grace, A.N. CoFe2O4 and NiFe2O4@graphene adsorbents for heavy metal ions—Kinetic and thermodynamic analysis. RSC Adv. 2015, 5, 28965–28972. [Google Scholar] [CrossRef]
- Yin, Y.C.; Zeng, M.; Liu, J.; Tang, W.K.; Dong, H.R.; Xia, R.Z.; Yu, R.H. Enhanced high-frequency absorption of anisotropic Fe3O4/graphene nanocomposites. Sci. Rep. 2016, 6, 25075. [Google Scholar] [CrossRef]
- Mahmood, M.; Yousuf, M.A.; Baig, M.M.; Imran, M.; Suleman, M.; Shahid, M.; Khan, M.A.; Warsi, M.F. Spinel ferrite magnetic nanostructures at the surface of graphene sheets for visible light photocatalysis applications. Physica B 2018, 550, 317–323. [Google Scholar] [CrossRef]
- Fakhri, H.; Farzadkia, M.; Boukherroub, R.; Srivastava, V.; Sillanpaa, M. Design and preparation of core-shell structured magnetic graphene oxide@MIL-101(Fe): Photocatalysis under shell to remove diazinon and atrazine pesticides. Sol. Energy 2020, 208, 990–1000. [Google Scholar] [CrossRef]
- Chandel, N.; Sharma, K.; Sudhaik, A.; Raizada, P.; Hosseini-Bandegharaei, A.; Thakur, V.K.; Singh, P. Magnetically separable ZnO/ZnFe2O4 and ZnO/CoFe2O4 photocatalysts supported onto nitrogen doped graphene for photocatalytic degradation of toxic dyes. Arab. J. Chem. 2020, 13, 4324–4340. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Liu, G.J.; Cao, Y. Synthesis, characterization and photocatalytic activity of inexpensive and non-toxic Fe2O3-Fe3O4 nano-composites supported by montmorillonite and modified by graphene. Compos. Part B Eng. 2017, 114, 211–222. [Google Scholar] [CrossRef]
- Bajpai, O.P.; Mandal, S.; Ananthakrishnan, R.; Mandal, P.; Khastgir, D.; Chattopadhyay, S. Structural features, magnetic properties and photocatalytic activity of bismuth ferrite nanoparticles grafted on graphene nanosheets. New J. Chem. 2018, 42, 10712–10723. [Google Scholar] [CrossRef]
- Fu, Y.S.; Chen, H.Q.; Sun, X.Q.; Wang, X. Combination of cobalt ferrite and graphene: High-performance and recyclable visible-light photocatalysis. Appl. Catal. B Environ. 2012, 111, 280–287. [Google Scholar] [CrossRef]
- Shakir, I.; Sarfraz, M.; Ali, Z.; Aboud, M.F.A.; Agboola, P.O. Magnetically separable and recyclable graphene-MgFe2O4 nanocomposites for enhanced photocatalytic applications. J. Alloys Compd. 2016, 660, 450–455. [Google Scholar] [CrossRef]
- Cao, M.H.; Wang, P.F.; Ao, Y.H.; Wang, C.; Hou, J.; Qian, J. Visible light activated photocatalytic degradation of tetracycline by a magnetically separable composite photocatalyst: Graphene oxide/magnetite/cerium-doped titania. J. Colloid Interface Sci. 2016, 467, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Guediri, M.K.; Chebli, D.; Bouguettoucha, A.; Bourzami, R.; Amrane, A. Novel Fe2TiO5/reduced graphene oxide heterojunction photocatalyst with improved adsorption capacity and visible light photoactivity: Experimental and DFT approach. Environ. Sci. Pollut. Res. 2021, 28, 8507–8519. [Google Scholar] [CrossRef] [PubMed]

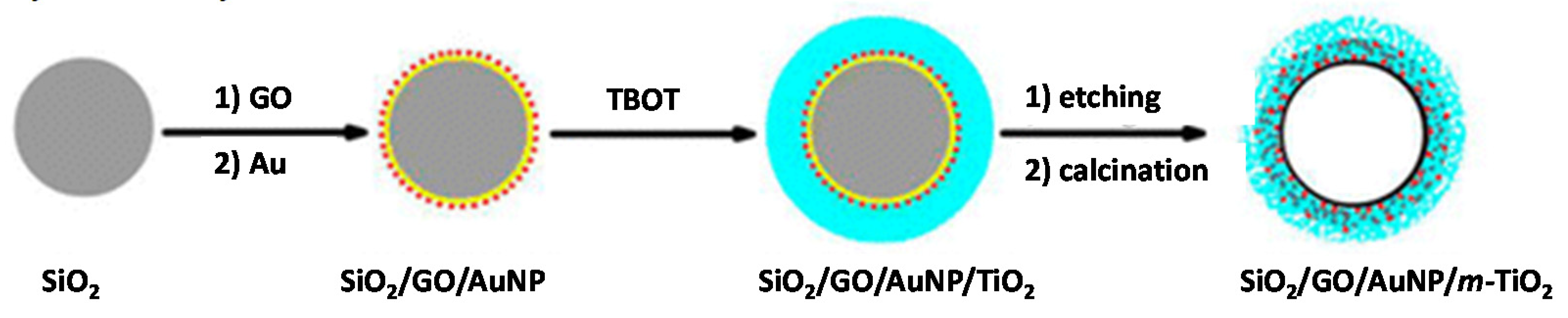

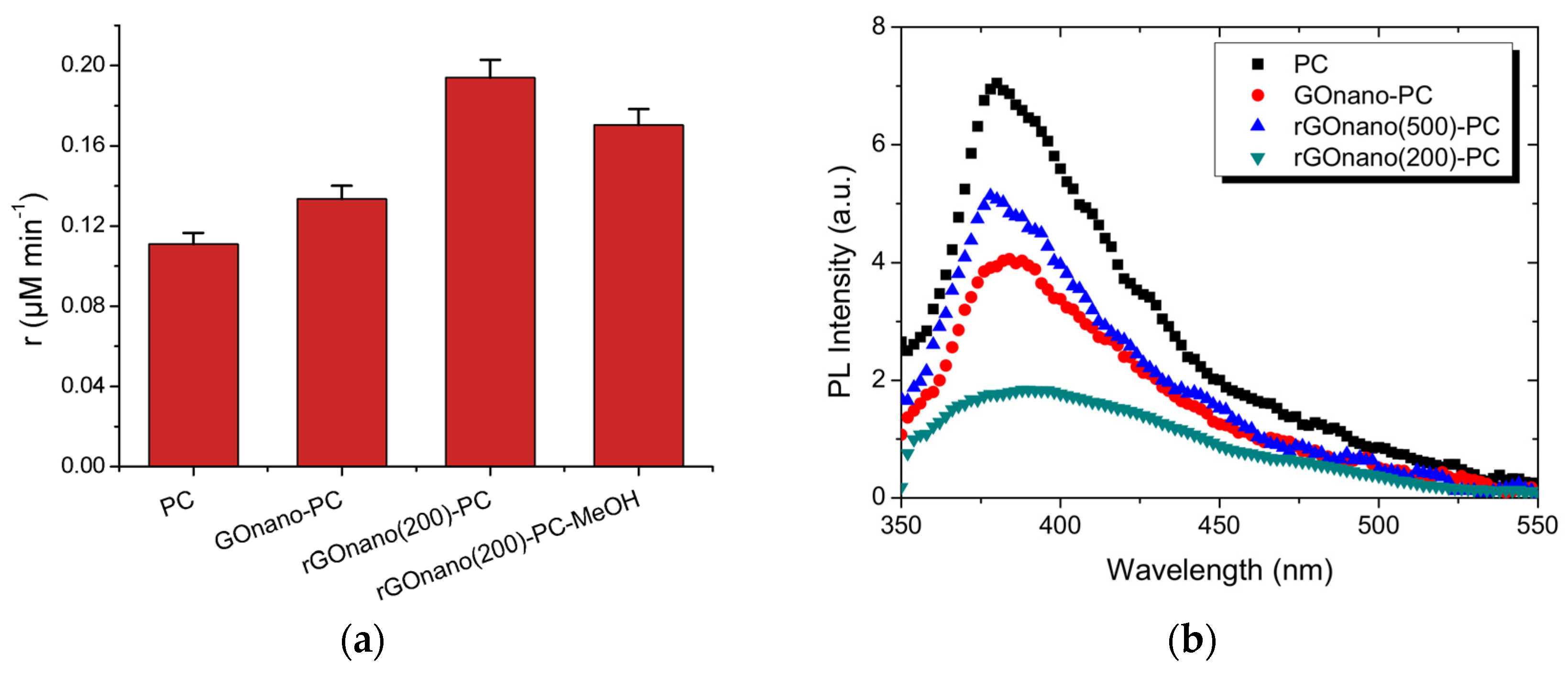
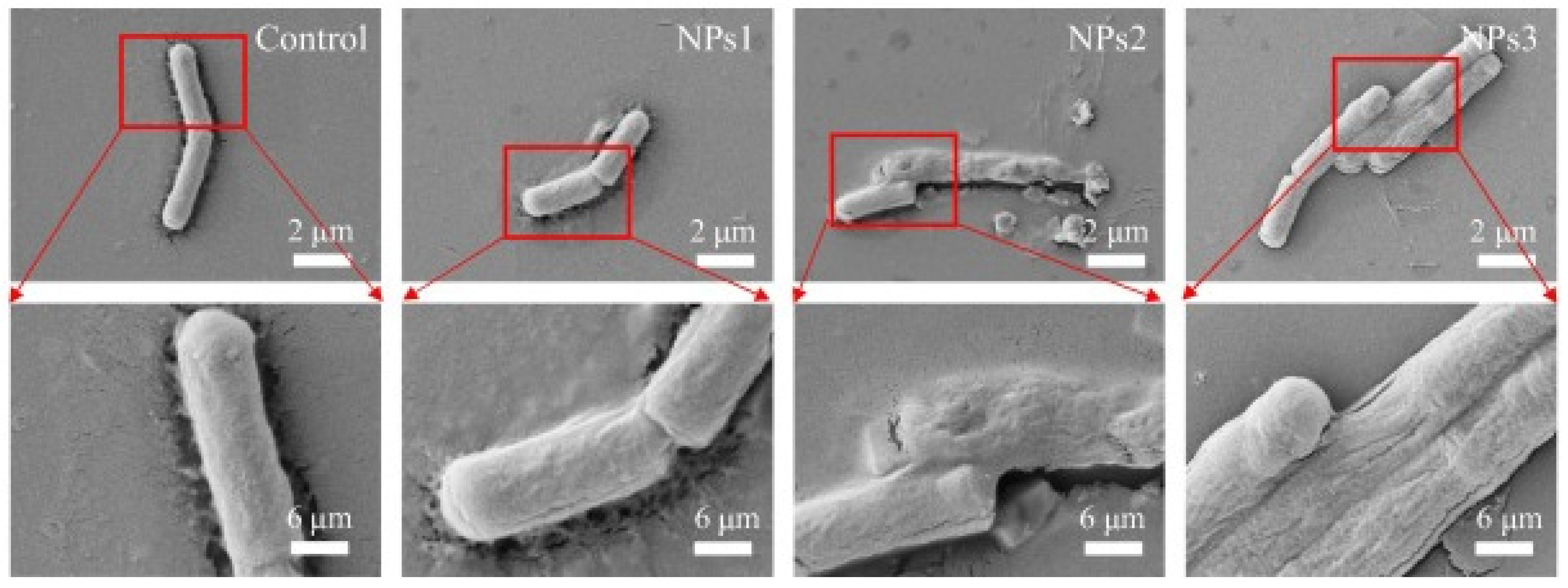
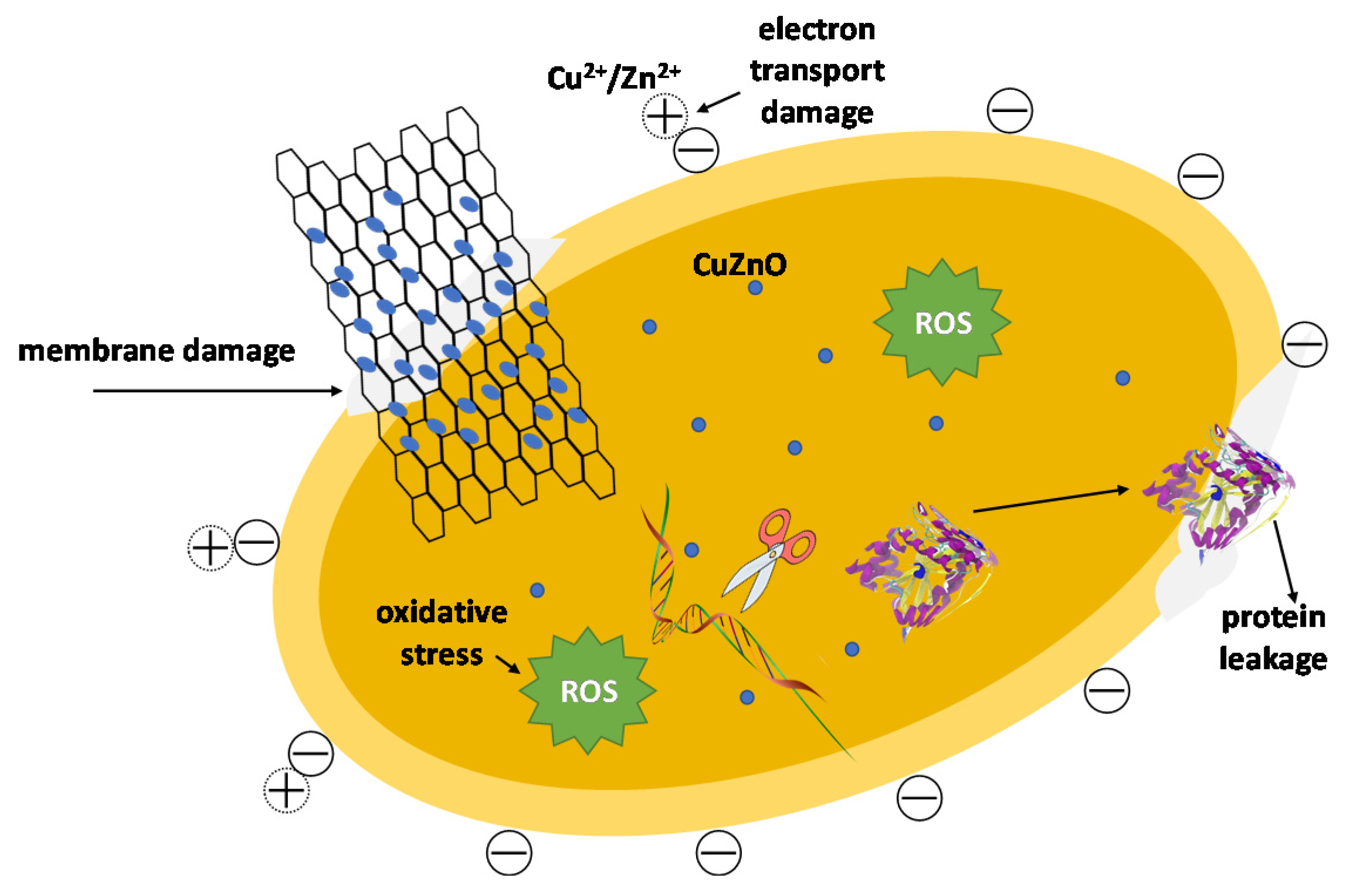


| PCs | Loading Method | Applications | Findings | Ref. |
|---|---|---|---|---|
| (GQD)-TiO2 film | TiO2 immersion in GQD suspension | SA degradation; λ > 400 nm | enhanced activity | [55] |
| α-Fe2O3/graphene | spin coating and thermal treatment | ICPE measurement; Xe lamp | 2.6-fold higher than reference | [66] |
| graphene | - | optical absorption | enhanced abs. | [49] |
| graphene PC fiber | - | optoelectronics | stronger broadband response | [50] |
| α-Fe2O3/GIO | carburization; nickel oxide IO and CVD | PEC water splitting | 1.4-fold higher photocurrent den. | [67] |
| rGO/Pt/3DOM TiO2 | immersion and calcination to form r-GO | MO degradation; λ > 420 nm; | 4-fold increase | [68] |
| TiO2@ rGO@Au | immersion and calcination | PEC water splitting; 300-W Xe lamp | 2-fold enhancement | [69] |
| IO-SnO2/G microspheres | immersion and calcination | MO degradation 300-W Hg lamp; λ = 365 nm | enhanced activity | [70] |
| graphene | - | biosensor | 14.8 times higher sensitivity * | [53] |
| GO/TiO2 IO | immersion in GO nanocolloid | MB degradation 150-W Xe lamp with 305 nm and 400 nm cut off filter | enhanced activity | [71] |
| GO/TiO2 IO film | immersion in GO nanocolloid | SERS detection | lower LOD | [54] |
| rGO/TiO2 | immersion and calcination | MB and SA degradation 150-W Xe lamp with 305 nm and 400 nm cut off filter | improved photodegradation | [56] |
| NPS-IOC | - | organic pollutants adsorbents | excellent adsorption | [72] |
| Sample | Preparation Method | Experimental Conditions | Findings | Ref. |
|---|---|---|---|---|
| GO G | HM (GO)/ GO red. by Allium cepa extract (G) | PA; Streptococcus sp., Staphylococcus sp., E. coli or P. aeruginosa. | activity: GO > G | [88] |
| GO | sonication of commercial GO to reduce sample polydispersity | CC; E. coli or S. aureus | low conc.: enh. growth; high conc.: inh. growth. | [89] |
| GO rGO | graphite oxidation by BaM (GO) and BrM (rGO) | CC; E. coli cultured in LB or NB media | in LB 35% and 45% CFU red.; in NB 80% and 85% CFU red. for GO and rGO, respectively (24 h) | [90] |
| rGO | graphite oxidation by KMnO4 (GO); GO red. by L-ascorbic acid (rGO) | Fluorescence analysis using GFP-labelled E. coli | in NaCl no effect; in NB inh. growth (nutrient ads.) | [91] |
| CS@GO | HM (GO); GO and CSCl mixing, evaporating, freeze-drying (CS@GO). | CC; E. coli or S. aureus | 96% inhibition for 0.6% GO; stable after 3 times (>90%) | [92] |
| Sample | Preparation Method | Experimental Conditions | Findings | Ref. |
|---|---|---|---|---|
| GO-Ag | AgNO3 aq. added to GO under constant stirring | DD; E. coli or S. aureus | conc. dependent inhibition zone; E. coli > S. aureus | [113] |
| GO Ag-GO | HM (GO); GO, PVP, α-D-glucose, Ag/NH3 at 45 °C. | EEAA, NR, LDH, ROS; AMNRLW | Ag-GO > GO, dec. EEAA/NR, LDH release and ROS generation | [115] |
| AgNPs/TETA-GO | HM (GO)/ self-assembly using TETA as a bridging agent | CC; E. coli or S. aureus | MIC: 0.125 mg/mL (E. coli), 0.25 mg/mL (S. aureus) | [127] |
| PVA/AgNPs-GO | HM (GO)/ Ag+ red. on PVA-GO by L-ascorbic acid | EUCAST; E. coli or S. aureus | 2 wt% Ag—4 log red. E. coli/S. aureus (24 h) | [128] |
| GO/CS/TiO2 | HM (GO)/ GO dropwise into CS, TiO2 addition | DD, L/D; B. subtilis and A. niger | inh. colony formation; most active GO:CS:TiO2 = 1:20:4 | [129] |
| Cu2O-TiO2/rGO | USR followed by WI | DD and MIC; S. aureus, E. coli, S. oralis, Pseudomonas aeruginosa under vis | bactericidal activity: S. aureus > P. aeruginosa ≈ E. coli > S. oralis. | [124] |
| Photocatalyst | Pollutants | Experimental Conditions | Findings | Ref. |
|---|---|---|---|---|
| Co0.7Zn0.3ErxFe2−xO4-rGO (x = 0.01–0.05) | MB | 1-h vis (Xe lamp) | 100% dec.; 55% deg. | [173] |
| AFG@MIL-101(Fe) (amine-func. Fe3O4 wrapped with rGO) | DIZ, ATZ pesticides | HPHg (400 W, λ = 546.8); 30 ppm; 105 min | 100% rem. (DIZ, alk. pH), 81% rem. (ATZ, aci. pH); TOC rem.; 4 cycles—almost same act. | [174] |
| ZnO/ZnFe2O4/N-doped G ZnO/CoFe2O4/N-doped G | MO MG | 10-W LED (400–700 nm); 10 ppm; 70 min (MG), 140 min (MO) | 99% rem. ZnO/CoFe2O4/N-doped G (pH 4–6). 10 cycles—almost same act. | [175] |
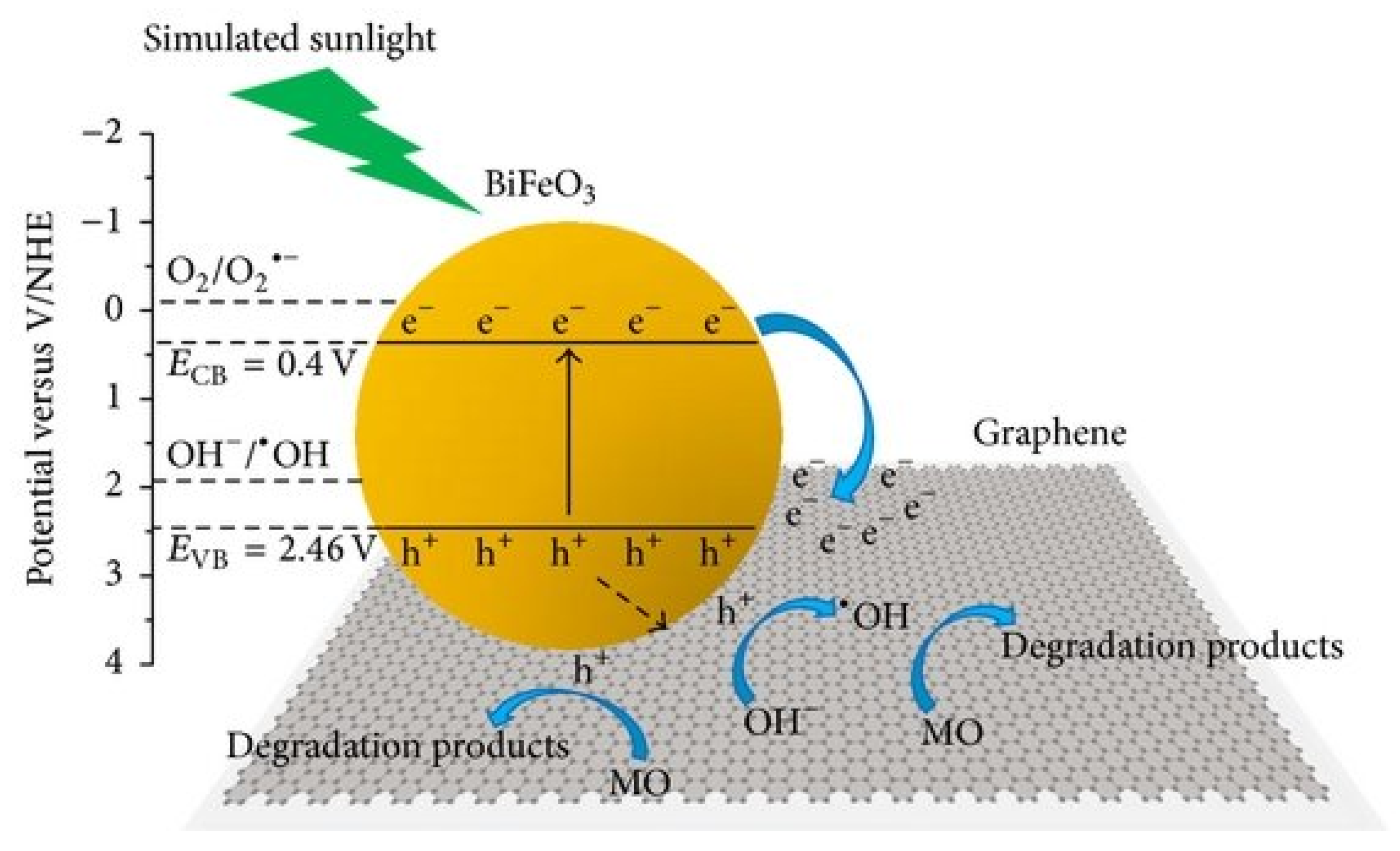
| BiFeO3-G (G: 1–9 wt%) | MO | SSol (200-W Xe lamp); 10 ppm; 6 h | 50% MO deg. on BiFeO3-5%G; e− captured by G, h+ oxidative species | [149] |
| Fe2O3-Fe3O4-M-G (M—montmorillonite) | MO | vis (300-W Xe lamp); 20 ppm | 100% MO deg. (175 min) on Fe2O3-Fe3O4-M-G; 98.8% recycling with magnetic field | [176] |
| BiFeO3-g-GNS | MOMB | vis (λ ≥ 420 nm, 300-W halogen lamps); 20 ppm; 60 min | 87% deg. MB, 35.9% deg. MO; activity increase with increase of BiFeO3 content on GNS | [177] |
| CoFe2O4-G (G = 15–50%) | MB, RhB, MO, BL-G, RGB | vis (500-W Xe lamp, JB450 cut-off filter; 20 ppm; 240 min | 95% deg. MB on CoFe2O4-G(40%) | [178] |
| MgFe2O4-G | MB | UV/vis; 40 min | ~100% deg. MB | [179] |
| GO/magnetite/Ce-doped TiO2 | TC | vis (300-W Xe lamp, 400-nm cut-off filter); 25 ppm; 60 min. | 89.92% deg. on MGO-Ce-TiO2; 4 cycles-decrease activity to 60% | [180] |
| Fe2TiO5/rGOx (x = 0–15%) | MB | vis (50-W LED); 10 ppm; 150 min | 40% deg. on Fe2TiO5/rGOx (x = 10%) | [181] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janczarek, M.; Endo-Kimura, M.; Wei, Z.; Bielan, Z.; Mogan, T.R.; Khedr, T.M.; Wang, K.; Markowska-Szczupak, A.; Kowalska, E. Novel Structures and Applications of Graphene-Based Semiconductor Photocatalysts: Faceted Particles, Photonic Crystals, Antimicrobial and Magnetic Properties. Appl. Sci. 2021, 11, 1982. https://doi.org/10.3390/app11051982
Janczarek M, Endo-Kimura M, Wei Z, Bielan Z, Mogan TR, Khedr TM, Wang K, Markowska-Szczupak A, Kowalska E. Novel Structures and Applications of Graphene-Based Semiconductor Photocatalysts: Faceted Particles, Photonic Crystals, Antimicrobial and Magnetic Properties. Applied Sciences. 2021; 11(5):1982. https://doi.org/10.3390/app11051982
Chicago/Turabian StyleJanczarek, Marcin, Maya Endo-Kimura, Zhishun Wei, Zuzanna Bielan, Tharishinny R. Mogan, Tamer M. Khedr, Kunlei Wang, Agata Markowska-Szczupak, and Ewa Kowalska. 2021. "Novel Structures and Applications of Graphene-Based Semiconductor Photocatalysts: Faceted Particles, Photonic Crystals, Antimicrobial and Magnetic Properties" Applied Sciences 11, no. 5: 1982. https://doi.org/10.3390/app11051982
APA StyleJanczarek, M., Endo-Kimura, M., Wei, Z., Bielan, Z., Mogan, T. R., Khedr, T. M., Wang, K., Markowska-Szczupak, A., & Kowalska, E. (2021). Novel Structures and Applications of Graphene-Based Semiconductor Photocatalysts: Faceted Particles, Photonic Crystals, Antimicrobial and Magnetic Properties. Applied Sciences, 11(5), 1982. https://doi.org/10.3390/app11051982













