The Changes of Leaf Reflectance Spectrum and Leaf Functional Traits of Osmanthus fragrans Are Related to the Parasitism of Cuscuta japonica
Abstract
1. Introduction
2. Material and Methods
2.1. Research Area and Sample Collection
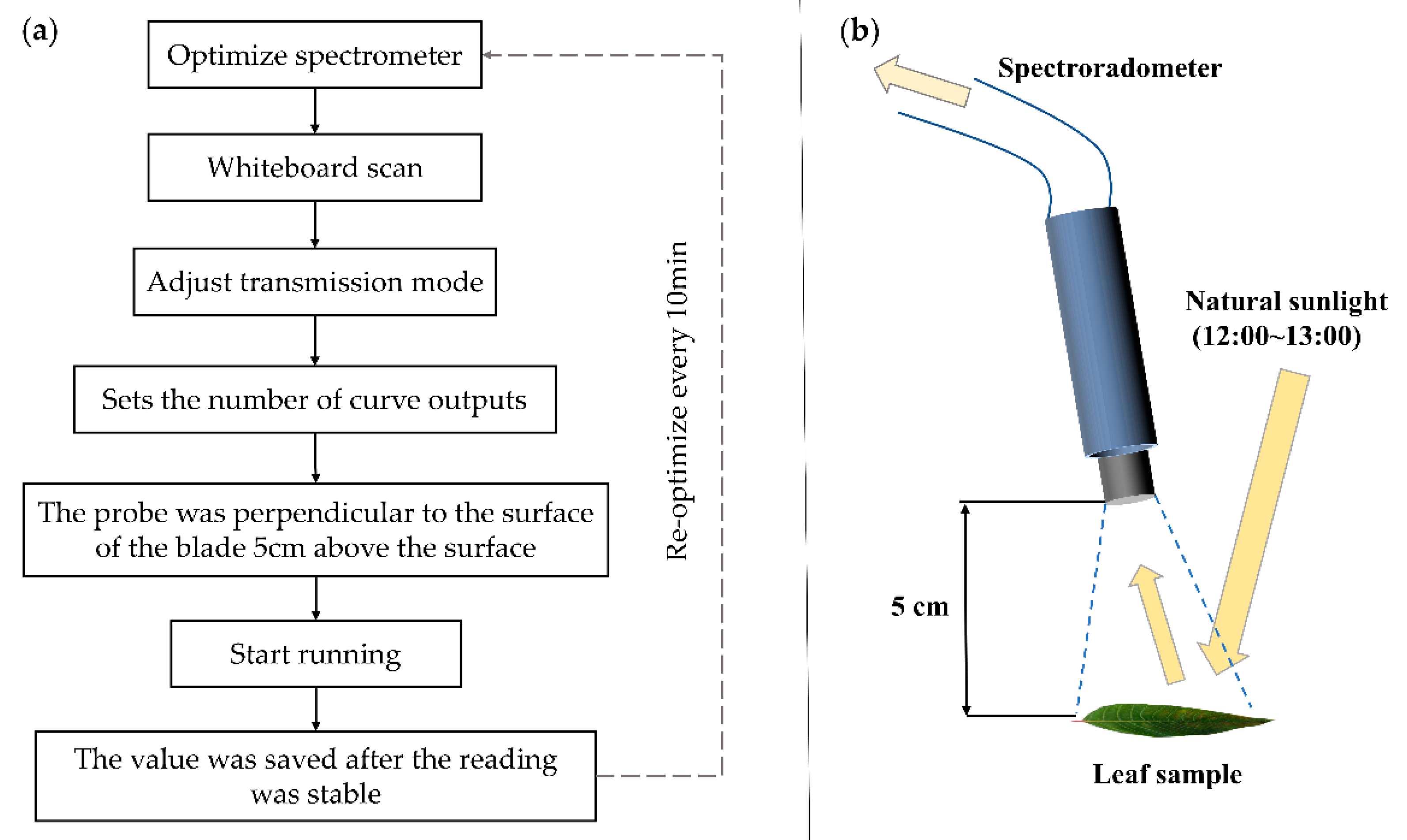
2.2. Leaf Reflectance Spectrum Collection and Calculation Method of Leaf Functional Traits
2.3. Data Analysis
3. Results and Discussion
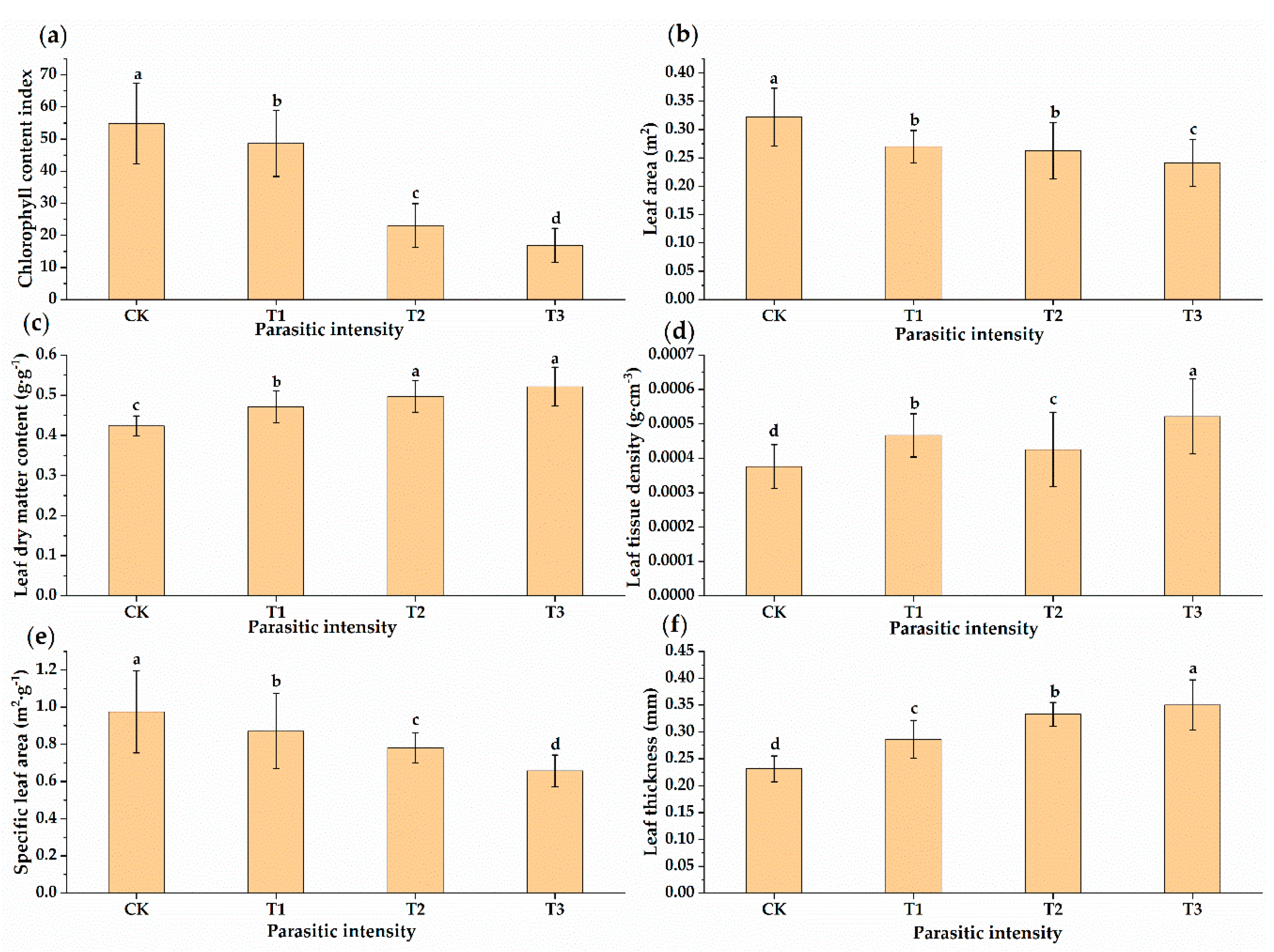
3.1. Changes in Leaf Functional Traits of Osmanthus Fragrans Leaves Parasitized by Cuscuta japonica Choisy
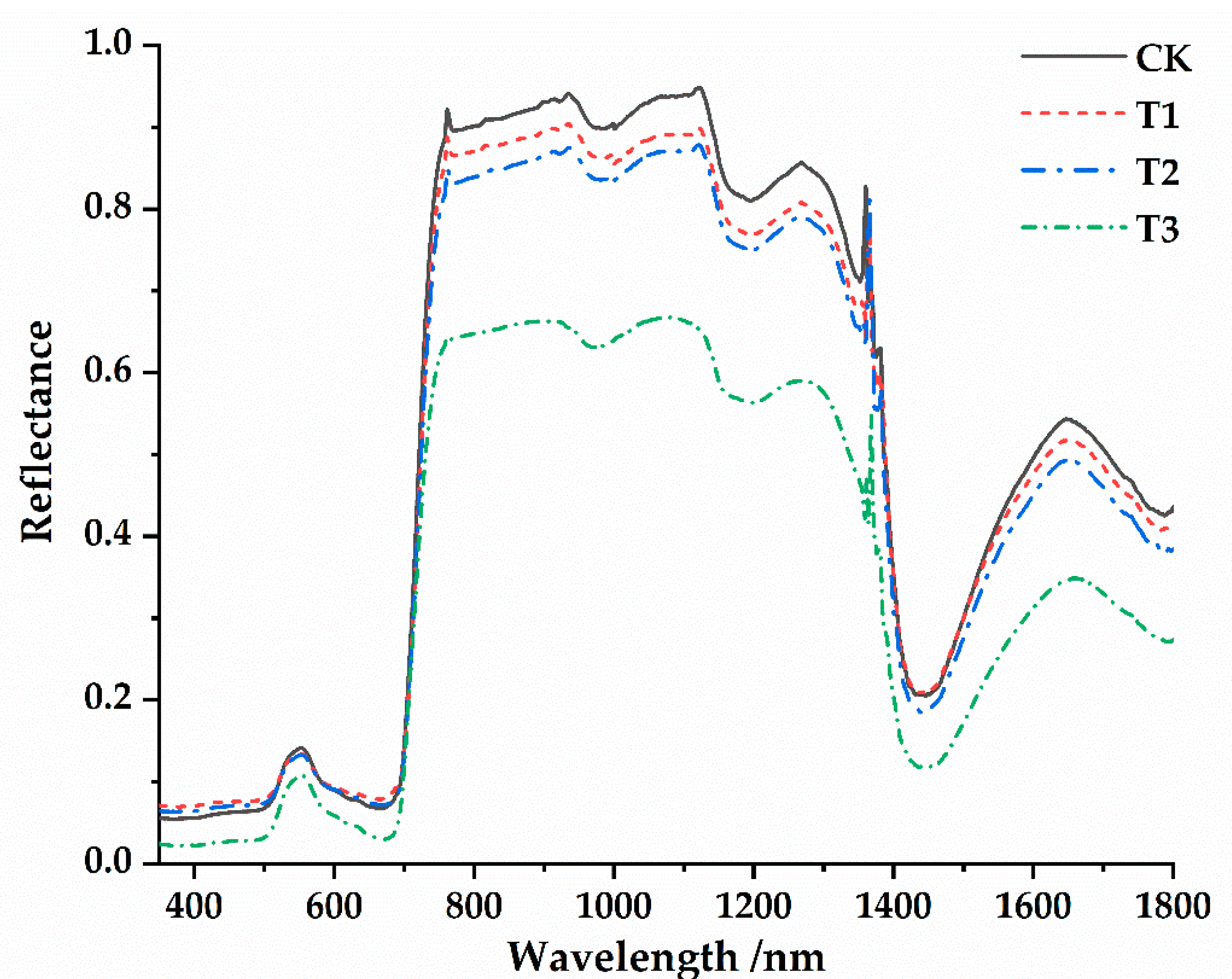
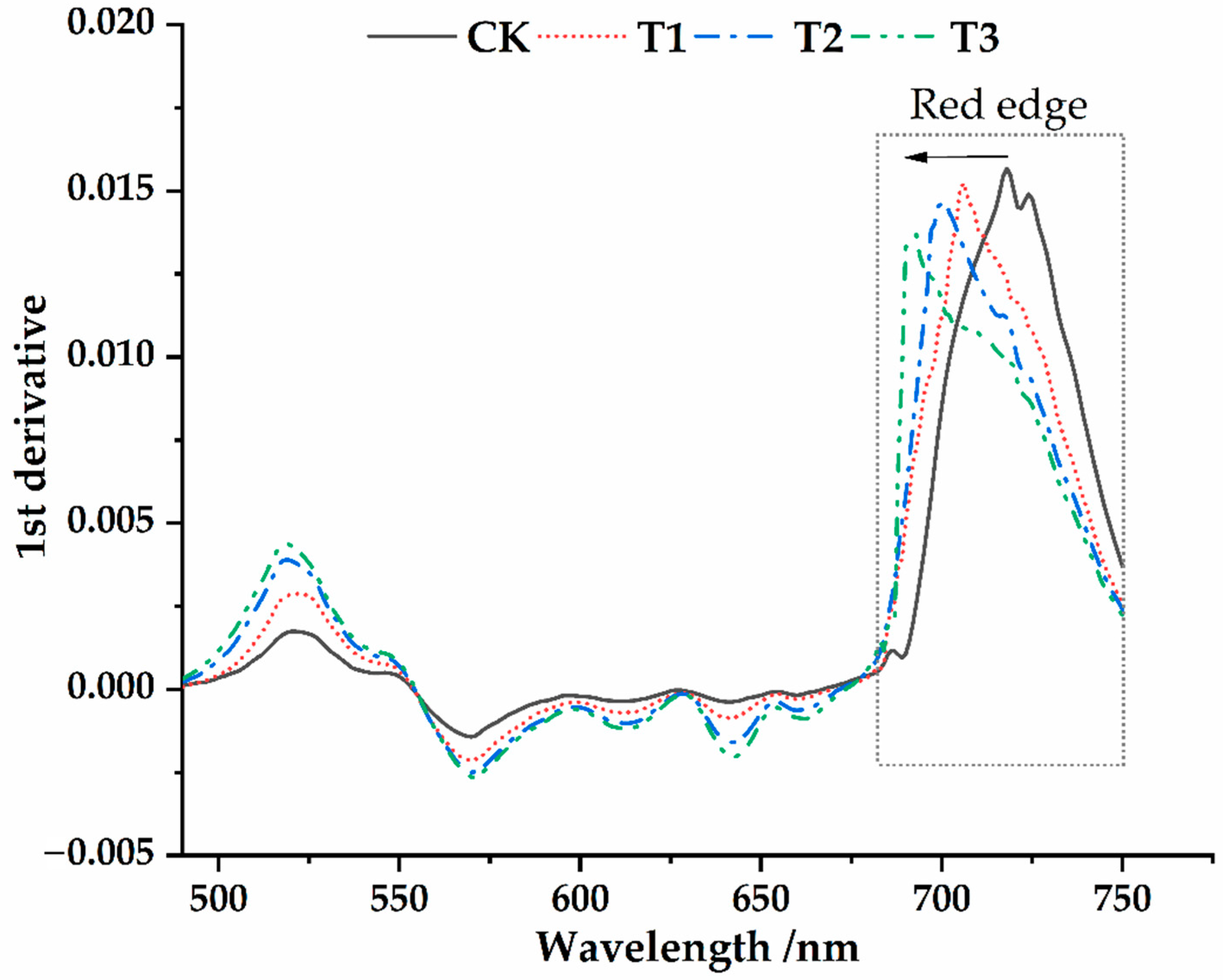
3.2. Spectral Characteristics of Osmanthus Fragrans Leaves under the Different Parasitic Intensity of Cuscuta japonica Choisy
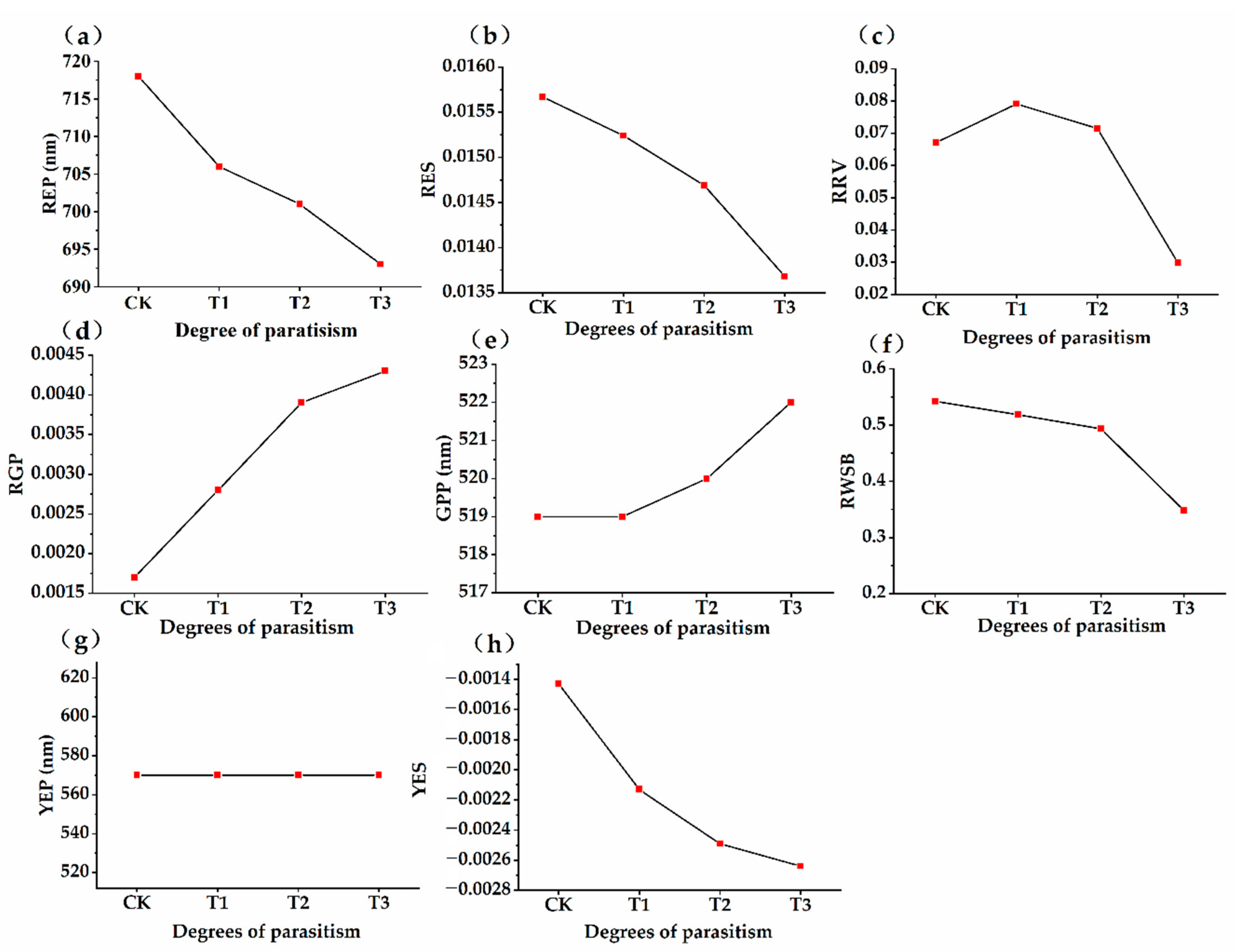
3.3. Dynamic Changes of Spectral Characteristic Parameters of Osmanthus fragrans in Different Parasitic Stages
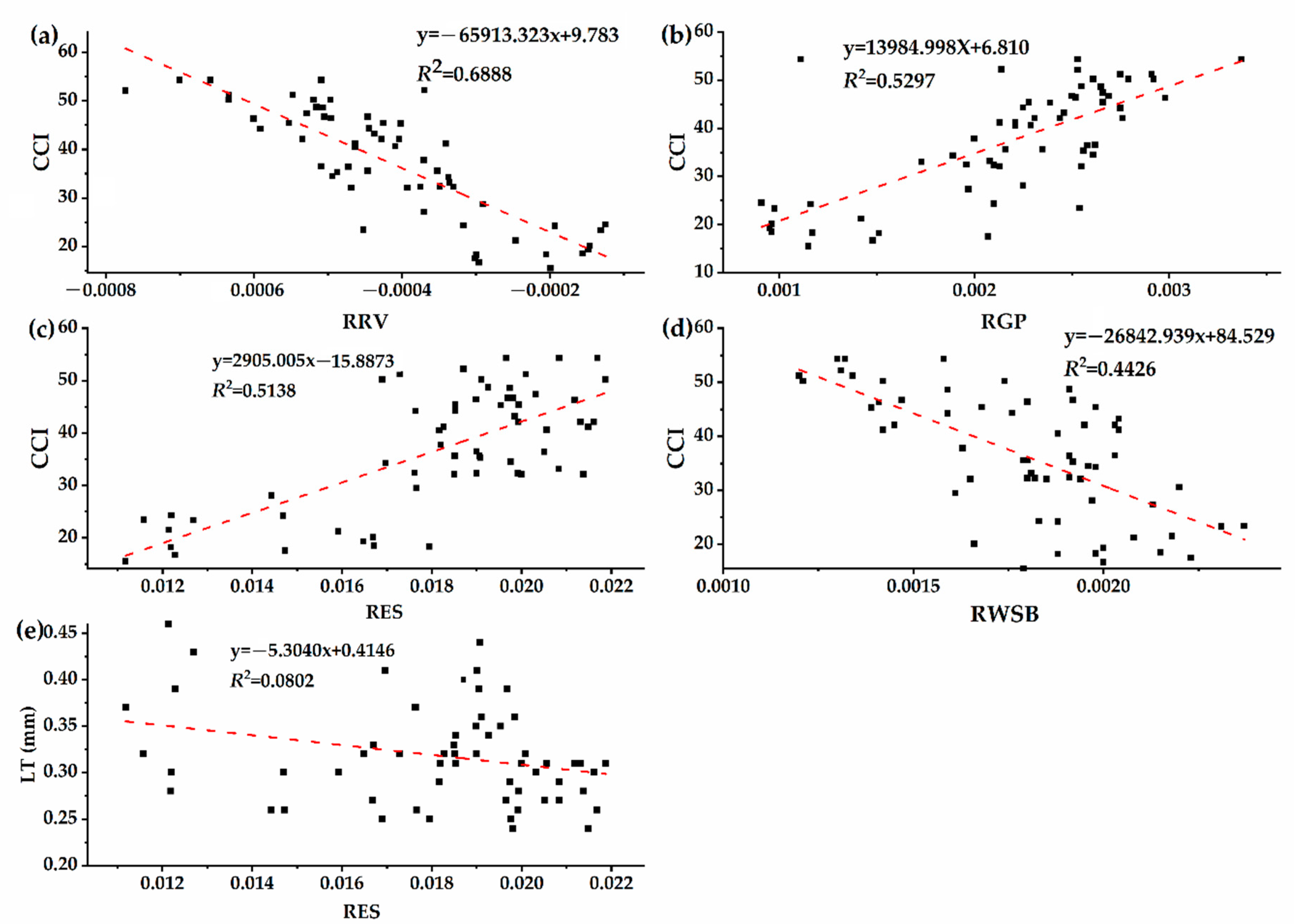
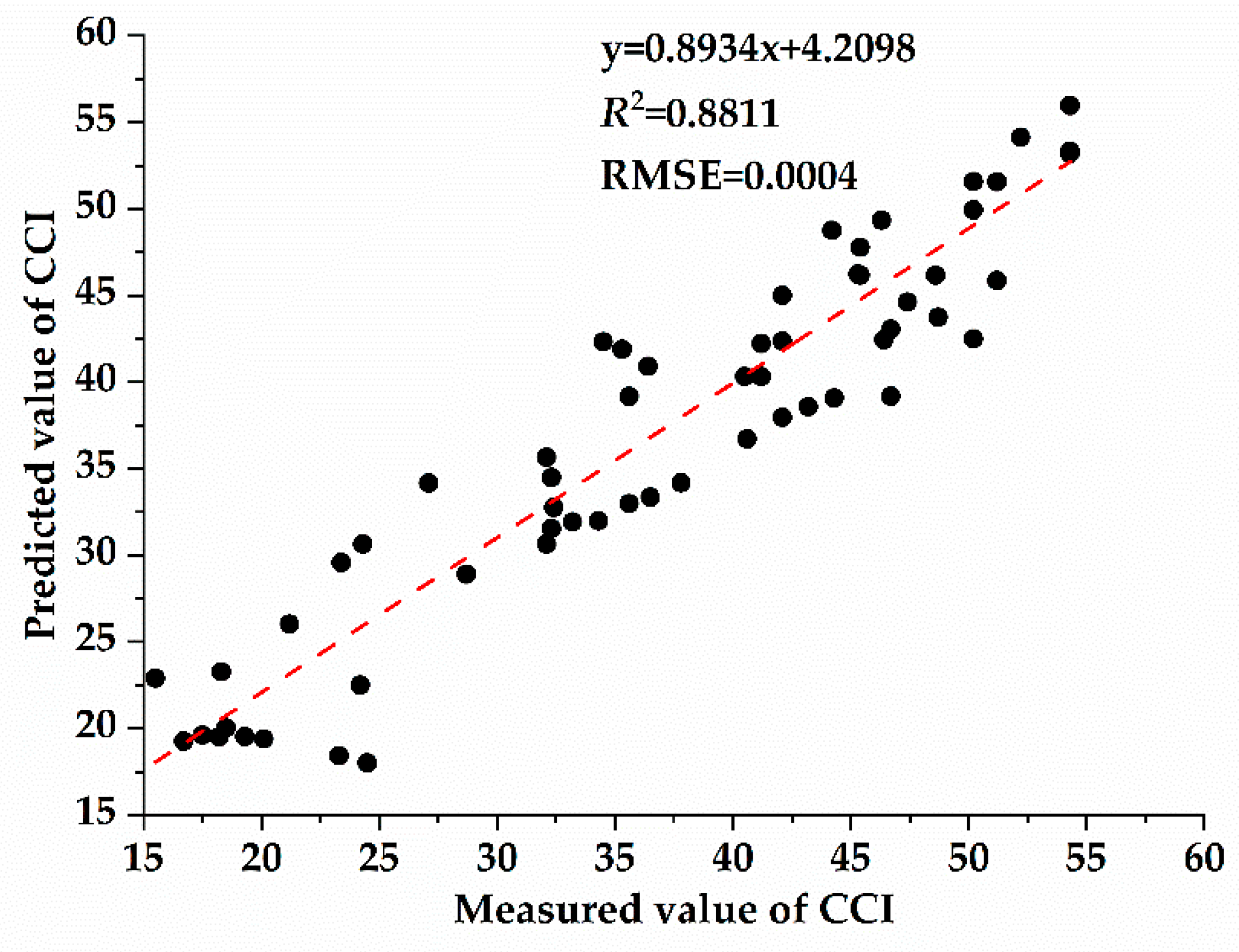
3.4. Correlation between Chlorophyll Content and Spectral Characteristic Parameters of Host Plants with Different Parasitic Degree of Cuscuta japonica Choisy
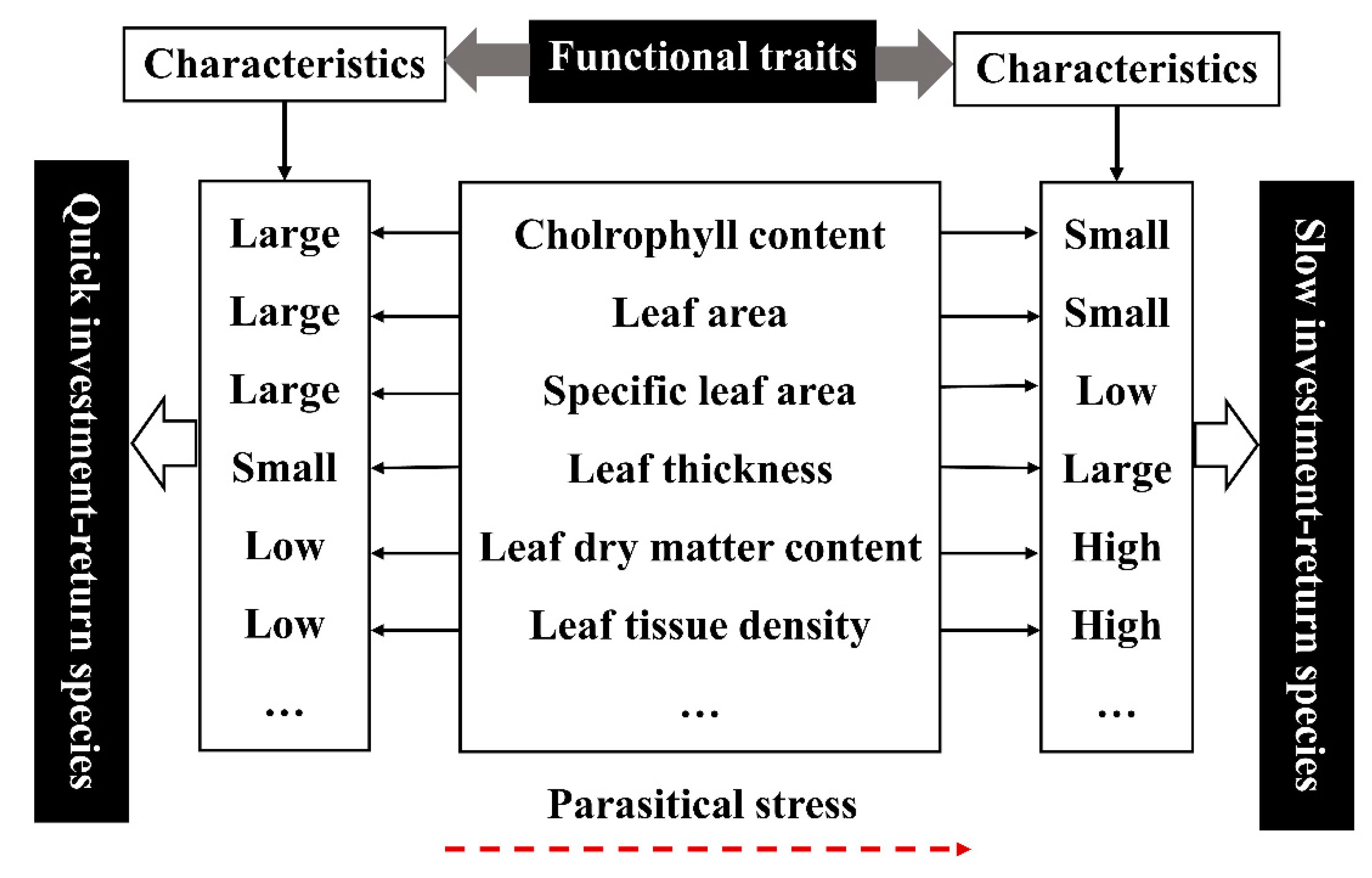
3.5. Effects of Parasitic Plants on the Correlation of Functional Traits of Osmanthus fragrans and Analysis of Leaf Economics Spectrum
4. Conclusions
- (1)
- The spectral reflectance was generally higher before parasitism than after parasitism. There were four main reflection peaks and five main absorption valleys in the spectral reflection curve (350~1800 nm). The near-infrared band (750~1400 nm) was the sensitive range of spectral response of host plants to parasitic infection. At the same time, such variation characteristics were universal under different parasitic degree conditions.
- (2)
- The position of red edge, slope of red edge, reflectance of a green peak, and reflectance of water stress band can well reflect the invasion status in different parasitic stages. After parasitism, the red edge position of the host plant spectrum shifted to shortwave direction. With the deepening of parasitic intensity, the moving distance of the red edge position to the short-wave direction increases.
- (3)
- With the increase of parasitic intensity, the relative content of chlorophyll in host plants gradually decreases, and the spectral characteristic parameters were significantly correlated with them. Chlorophyll inversion model based on red valley reflectance has the highest accuracy (y = −65913.323x + 9.783, R2 = 0.6888).
- (4)
- After parasitism, the leaf functional traits of host plant were characterized by large leaf thickness, small leaf area, small specific leaf area, low relative chlorophyll content, high leaf dry matter content, and high leaf tissue density. We suspect that there may be leaf economics spectrum (“slow investment-return”) in the parasitic environment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethics Approval and Consent to Participate
Abbreviations
References
- Mahlein, A.K.; Oerke, E.C.; Steiner, U.; Dehne, H.W. Recent advances in sensing plant diseases for precision crop protection. Eur. J. Plant. Pathol. 2012, 133, 197–209. [Google Scholar] [CrossRef]
- Press, M.C.; Phoenix, G.K. Impacts of parasitic plants on natural communities. New Phytolog. 2010, 166, 737–751. [Google Scholar] [CrossRef]
- Bouwmeester, H.J.; Roux, C.; Lopezraez, J.A.; Bécard, G. Rhizosphere communication of plants, parasitic plants and am fungi. Trends Plant Sci. 2007, 12, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Runyon, J.B.; Mescher, M.C.; Moraes, C.M.D. Volatile chemical cues guide host location and host selection by parasitic plants. Science 2006, 313, 1964–1967. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, K.; Xie, X.; Sekimoto, H.; Takeuchi, Y.; Ogasawara, S.; Akiyama, K.; Hayashi, H.; Yoneyama, K. Strigolactones, host recognition signals for root parasitic plants and arbuscular mycorrhizal fungi, from Fabaceae plants. New Phytol. 2008, 179, 484–494. [Google Scholar] [CrossRef]
- Yoneyama, K. Studies on the host recognition mechanism of root parasitic plants. J. Pestic. Sci. 2010, 35, 348–350. [Google Scholar] [CrossRef]
- Smith, J.L.; De Moraes, C.M.; Mescher, M.C. Jasmonate- and salicylate-mediated plant defense responses to insect herbivores, pathogens and parasitic plants. Pest. Manag. Sci. 2010, 65, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Bardgett, R.D.; Smith, R.S.; Shiel, R.S.; Peacock, S.; Simkin, J.M.; Quirk, H.; Hobbs, P.J. Parasitic plants indirectly regulate below–ground properties in grassland ecosystems. Nature 2009, 439, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Mower, J.P.; Stefanovi, S.; Young, G.J.; Palmer, J.D. Plant genetics, gene transfer from parasitic to host plants. Nature 2004, 432, 165–166. [Google Scholar] [CrossRef]
- Matvienko, M.; Wojtowicz, A.; Wrobel, R.; Jamison, D.; Goldwasser, Y.; Yoder, J.I. Quinone oxidoreductase message levels are differentially regulated in parasitic and non-parasitic plants exposed to allelopathic quinones. Plant J. 2010, 25, 375–387. [Google Scholar] [CrossRef]
- Pickett, J.A.; Hamilton, M.L.; Hooper, A.M.; Khan, Z.R.; Midega, C.A.O. Companion cropping to manage parasitic plants. Annu. Rev. Phytopathol. 2010, 48, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Rubiales, D. Parasitic plants, wild relatives and the nature of resistance. New Phytol. 2010, 160, 459–461. [Google Scholar] [CrossRef]
- Mahlein, A.-K.; Kuska, M.T.; Behmann, J.; Polder, G.; Walter, A. Hyperspectral Sensors and Imaging Technologies in Phytopathology: State of the Art. Annu. Rev. Phytopathol. 2018, 56, 535–558. [Google Scholar] [CrossRef]
- Yang, B.F.; Du, L.S.; Li, J.M. Effects of Cuscuta australis parasitism on the growth, reproduction and defense of Solidago canadensis. Chin. J. Appl. Ecol. 2015, 26, 3309–3314. [Google Scholar]
- Guo, S.M.; Li, J.M.; Li, Y.H.; Yan, M. The trade-off between growth and defense in Alternanthera philoxeroides parasitized by Cuscuta australis. Acta Ecol. Sin. 2014, 34, 4866–4873. [Google Scholar]
- Conn, C.E.; Bythell–Douglas, R.; Neumann, D.; Yoshida, S.; Whittington, B.; Westwood, J.H.; Shirasu, K.; Bond, C.S.; Dyer, K.A.; Nelson, D.C. Plant evolution convergent evolution of strigolactone perception enabled host detection in parasitic plants. Science 2015, 349, 540–543. [Google Scholar] [CrossRef]
- Press, M.C. Carbon acquisition and assimilation in parasitic plants. Funct. Ecol. 2015, 5, 278. [Google Scholar] [CrossRef]
- Roney, J.K.; Khatibi, P.A.; Westwood, J.H. Cross-species translocation of mRNA from host plants into the parasitic plant dodder. Plant Physiol. 2007, 143, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Hautier, Y.; Hector, A.; Vojtech, E.; Purves, D.; Turnbull, L.A. Modelling the growth of parasitic plants. J. Ecol. 2010, 98, 857–866. [Google Scholar] [CrossRef]
- Chin, J.A.; Wang, E.C.; Kibbe, M.R. Evaluation of hyperspectral technology for assessing the presence and severity of peripheral artery disease. J. Vasc. Surg. 2001, 54, 1679–1688. [Google Scholar] [CrossRef]
- Xie, X.; Ying, X.L.; Li, R.; Zhang, Y.; Huo, Y.; Bao, Y.; Shen, S. Hyperspectral characteristics and growth monitoring of rice Oryza sativa under asymmetric warming. Int. J. Remote Sens. 2013, 34, 8449–8462. [Google Scholar] [CrossRef]
- Cuevas, P. Effects of generalist and specialist parasitic plants (Loranthaceae) on the fluctuating asymmetry patterns of ruprestrian host plants. Basic Appl. Ecol. 2011, 12, 449–455. [Google Scholar] [CrossRef]
- Barnes, M.L.; Breshears, D.D.; Law, D.J.; Van Leeuwen, W.J.D.; Monson, R.K.; Fojtik, A.C.; Barron Gafford, G.A.; Moore, D.J.P. Beyond greenness: Detecting temporal changes in photosynthetic capacity with hyperspectral reflectance data. PLoS ONE 2017, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Thenkabail, P.S.; Smith, R.B.; Pauw, E.D. Hyperspectral vegetation indices and their relationships with agricultural crop characteristics. Remote Sens. Environ. 2000, 71, 158–182. [Google Scholar] [CrossRef]
- Ge, S.; Everitt, J.; Carruthers, R.; Gong, P.; Anderson, G. Hyperspectral characteristics of canopy components and structure for phenological assessment of an invasive weed. Environ. Monit. Assess. 2006, 120, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Diaz, S.; Cabido, M.; Zak, M.; Martinez, C.E.; Aranibar, J. Plant functional traits, ecosystem structure and land–use history along a climatic gradient in central–western Argentina. J. Veg. Sci. 2010, 10, 651–660. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender–Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Vendramini, F.; Diaz, S.; Gurvich, D.E.; Wilson, P.J.; Thompson, K.; Hodgson, J.G. Leaf traits as indicators of resource–use strategy in floras with succulent species. New Phytol. 2002, 154, 147–157. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Cornelissen, J.H. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 2008, 11, 1065–1071. [Google Scholar] [CrossRef]
- Matusova, R. The strigolactone germination stimulants of the plant-parasitic striga and orobanche spp are derived from the carotenoid pathway. Plant Physiol. 2005, 139, 920–934. [Google Scholar] [CrossRef]
- Cheng, G.F.; Zhang, J.H.; Li, B.B.; Zhang, J.H.; Yang, S.B.; Xie, X.J. Hyper–Spectral and Red Edge Characteristics for Rice Under Different Temperature Stress Levels. Jiangsu J. Agric. Sci. 2008, 5, 573–580. (In Chinese) [Google Scholar]
- Huang, X.Y.; Xu, Z.H.; Lin, L.; Shi, W.C.; Yu, K.Y.; Liu, J.; Chen, C.C.; Zhou, H.K. Pantana Phyllostachysae Chao damage detection based on physical and chemical parameters of Moso Bamboo leaves. Spectrosc. Spect. Anal. 2019, 39, 857–864. [Google Scholar]
- Kim, S.R.; Lee, W.K.; Lim, C.H.; Kim, M.; Kafatos, M.C.; Lee, S.H.; Lee, S.S. Hyperspectral analysis of pine wilt disease to determine an optimal detection Index. Forests 2018, 9, 115. [Google Scholar] [CrossRef]
- Zhang, S.L.; Qin, J.; Tang, X.D.; Wang, Y.J.; Huang, J.L.; Song, Q.L.; Min, J.Y. Spectral characteristics and evaluation model of Pinus Massoniana suffering from Bursaphelenchus Xylophilus disease. Spectrosc. Spect. Anal. 2019, 39, 865–872. [Google Scholar]
- Everitt, J.H.; Escobar, D.E.; Appel, D.N.; Riggs, W.G.; Davis, M.R. Using airborne digital imagery for detecting Oak wilt disease. Plant Dis. 1999, 83, 502–505. [Google Scholar] [CrossRef]
- Wright, S.J. Leaf functional traits of tropical forest plants in telation to growth form. Funct. Ecol. 2010, 21, 19–27. [Google Scholar]
- Wickett, N.J.; Honaas, L.A.; Wafula, E.K.; Das, M.; Huang, K.; Wu, B.; Landherr, L.; Timko, M.P.; Yoder John Westwood, J.H.; de Pamphilis, C.W. Transcriptomes of the parasitic plant family Orobanchaceae reveal surprising conservation of chlorophyll synthesis. Curr. Biol. 2011, 21, 2098–2104. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N.; Lichtenthaler, H.K. Detection of red edge position and chlorophyll content by reflectance measurements near 700 nm. J. Plant. Physiol. 1996, 148, 501–508. [Google Scholar] [CrossRef]
- Cho, M.A.; Skidmore, A.K. A new technique for extracting the red edge position from hyperspectral data, the linear extrapolation method. Remote Sens. Environ. 2006, 101, 181–193. [Google Scholar] [CrossRef]
- Zhu, J.; Yu, Q.; Zhu, H.; He, W.; Xu, C.; Liao, J.; Qiu, Y.; Su, K. Response of dust particle pollution and construction of a leaf dust deposition prediction model based on leaf reflection spectrum characteristics. Environ. Sci. Pollut. Res. 2019, 26, 36764–36775. [Google Scholar] [CrossRef]
- Corney, H.J.; Sasse, J.M.; Ades, P.K. Assessment of salt tolerance in eucalypts using chlorophyll fluorescence attributes. New For. 2003, 26, 233–246. [Google Scholar] [CrossRef]
- Dodds, K.J.; Cooke, R.R.; Hanavan, R.P. The effects of silvicultural treatment on sirex noctilio attacks and tree health in northeastern United States. Forests 2014, 5, 2810–2824. [Google Scholar] [CrossRef]
- Lu, N.; Zhou, X.; Cui, M.; Yu, M.; Zhou, J.X.; Qin, Y.S.; Li, Y. Colonization with arbuscular mycorrhizal fungi promotes the growth of Morus alba L. seedlings under greenhouse conditions. Forests 2015, 6, 734–747. [Google Scholar] [CrossRef]
- Hegenauer, V.; Körner, M.; Albert, M. Plants under stress by parasitic plants. Curr. Opin. Plant. Biol. 2017, 38, 34. [Google Scholar] [CrossRef]
- Zhao, X.J.; Pan, Y.J.; Chen, H.F.; Tang, Z.H. Influence of holoparasitic plant Cuscuta japonica on growth and alkaloid content of its host shrub Catharanthus roseus, a field experiment. Arab. J. Sci. Eng. 2018, 43, 1–8. [Google Scholar] [CrossRef]
- Reynolds, G.J.; Gordon, T.R.; McRoberts, N. Quantifying the impacts of systemic acquired resistance to pitch canker on Monterey pine growth rate and hyperspectral reflectance. Forests 2016, 7, 20. [Google Scholar] [CrossRef]
- Westwood, J.H.; Bouwmeester, H. Parasitic plants tap into the main stream. New Phytol. 2010, 184, 284–287. [Google Scholar] [CrossRef]
- Johnson, B.I.; De Moraes, C.M.; Mescher, M.C. Manipulation of light spectral quality disrupts host location and attachment by parasitic plants in the genus Cuscuta. J. Appl. Ecol. 2016, 53, 794–803. [Google Scholar] [CrossRef]
- Kuijt, J. Botanical enigmas. (book reviews, the biology of parasitic flowering plants). Science 1970, 168, 1081–1082. [Google Scholar]
- Filella, I.; Penuelas, J. The red edge position and shape as indicators of plant chlorophyll content, biomass and hydric status. Int. J. Remote Sens. 1994, 15, 1459–1470. [Google Scholar] [CrossRef]
- Zahn, L.M. Even Parasitic Plants Need Plastids. Science 2011, 331, 1243. [Google Scholar] [CrossRef]
- Yoneyama, K.; Awad, A.A.; Xie, X.; Takeuchi, Y. Strigolactones as germination stimulants for root parasitic plants. Plant Cell Physiol. 2010, 51, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Han, L. Estimating chlorophyll-a concentration using first-derivative spectra in coastal water. Int. J. Remote Sens. 2005, 26, 5235–5244. [Google Scholar] [CrossRef]
- Jiang, J.; Steven, M.D.; He, R.; Chen, Y.; Du, P.; Guo, H. Identifying the spectral responses of several plant species under Co2 leakage and waterlogging stresses. Int J. Greenh. Gas. Con. 2015, 37, 1–11. [Google Scholar] [CrossRef]
- Xu, H.C.; Luo, Y.Q.; Zhang, T.T.; Shi, Y.J. Changes of Reflectance Spectra of Pine Needles in Different Stage after Being Infected by Pine Wood Nematode. Spectrosc. Spect. Anal. 2011, 31, 1352–1356. [Google Scholar]
- Liu, W.; Chang, Q.R.; Guo, M.; Xing, D.X.; Yuan, Y.S. Extraction of first derivative spectrum features of soil organic matter via wavelet denoising. Spectrosc. Spect. Anal. 2011, 31, 100–104. [Google Scholar]
- Liu, L.; Wang, J.; Huang, W.; Zhao, C.; Zhang, B.; Tong, Q. Estimating winter wheat plant water content using red edge parameters. Int. J. Remote Sens. 2003, 25, 1688–1691. [Google Scholar] [CrossRef]
- Wang, Z.H.; Ding, L.X. Tree species discrimination based on leaf-level hyperspectral characteristic analysis. Spectrosc. Spect. Anal. 2010, 30, 1825–1829. [Google Scholar]
- Atkinson, L.J.; Campbell, C.D.; Zaragoza-Castells, J.; Hurry, V.; Atkin, O.K. Impact of growth temperature on scaling relationships linking photosynthetic metabolism to leaf functional traits. Funct. Ecol. 2010, 24, 1181–1191. [Google Scholar] [CrossRef]
- Ülo, N. Leaf age dependent changes in within–canopy variation in leaf functional traits, a meta-analysis. J. Plant Res. 2016, 129, 313–338. [Google Scholar]
- Lemoine, N.P.; Shue, J.; Verrico, B.; Erickson, D.; Kress, W.J.; Parker, J.D. Phylogenetic relatedness and leaf functional traits, not introduced status, influence community assembly. Ecology 2016, 96, 2605–2612. [Google Scholar] [CrossRef] [PubMed]
- Lópezsampson, A.; Cernusak, L.A.; Page, T. Relationship between leaf functional traits and productivity in Aquilaria crassna (Thymelaeaceae) plantations, a tool to aid in the early selection of high–yielding trees. Tree Physiol. 2017, 37, 1–9. [Google Scholar]

| Spectral Parameter | Description |
|---|---|
| RES | The maximum 1st derivative of reflectance in the red band (680~750 nm). |
| REP | The wavelength position corresponding to the maximum reflectance in the wavelength band 680~750 nm. |
| RRV | The minimum band reflectance in the range of 640~700 nm. |
| RGP | The maximum band reflectance in the range of 510~580 nm. |
| GPP | The wavelength position corresponding to the green peak reflectance in the wavelength band 510~580 nm. |
| RWSB | The maximum band reflectance in the range of wavelengths from 1550~1750 nm. |
| YES | The maximum 1st derivative of reflectance in the yellow band (550–582 nm). |
| YEP | The wavelength position corresponding to the maximum reflectance in the wavelength band 550~582 nm. |
| RRV | RGP | RES | RWSB | |
|---|---|---|---|---|
| LT | 0.25218 | −0.1787 | −0.28318 * | 0.09577 |
| LA | −0.01651 | 0.18462 | −0.14292 | 0.0353 |
| LDMC | 0.01136 | −0.00523 | 0.03048 | 0.09512 |
| SLA | 0.20281 | −0.24112 | −0.06407 | 0.0641 |
| LTD | −0.19553 | 0.17124 | 0.13662 | −0.01367 |
| CCI | −0.82993 ** | 0.72953 ** | 0.65295 ** | −0.56967 ** |
| LT | LA | SLA | LDMC | LTD | CCI | |
|---|---|---|---|---|---|---|
| LT | 1 | |||||
| LA | −0.1696 | 1 | ||||
| SLA | −0.1502 | 0.3581 * | 1 | |||
| LDMC | −0.1293 | −0.4246 * | −0.6991 ** | 1 | ||
| LTD | −0.5436 ** | −0.4218 * | −0.5950 ** | 0.7517 ** | 1 | |
| CCI | 0.2566 | 0.2623 | 0.4993 * | −0.2201 | −0.4456 * | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Xu, Q.; Yao, J.; Zhang, X.; Xu, C. The Changes of Leaf Reflectance Spectrum and Leaf Functional Traits of Osmanthus fragrans Are Related to the Parasitism of Cuscuta japonica. Appl. Sci. 2021, 11, 1937. https://doi.org/10.3390/app11041937
Zhu J, Xu Q, Yao J, Zhang X, Xu C. The Changes of Leaf Reflectance Spectrum and Leaf Functional Traits of Osmanthus fragrans Are Related to the Parasitism of Cuscuta japonica. Applied Sciences. 2021; 11(4):1937. https://doi.org/10.3390/app11041937
Chicago/Turabian StyleZhu, Jiyou, Qing Xu, Jiangming Yao, Xinna Zhang, and Chengyang Xu. 2021. "The Changes of Leaf Reflectance Spectrum and Leaf Functional Traits of Osmanthus fragrans Are Related to the Parasitism of Cuscuta japonica" Applied Sciences 11, no. 4: 1937. https://doi.org/10.3390/app11041937
APA StyleZhu, J., Xu, Q., Yao, J., Zhang, X., & Xu, C. (2021). The Changes of Leaf Reflectance Spectrum and Leaf Functional Traits of Osmanthus fragrans Are Related to the Parasitism of Cuscuta japonica. Applied Sciences, 11(4), 1937. https://doi.org/10.3390/app11041937