Research on Microstructure of Soft Clay under Various Artificial Ground Freezing Conditions Based on NMR
Abstract
1. Introduction
2. Influence of AGF on Soft Clay
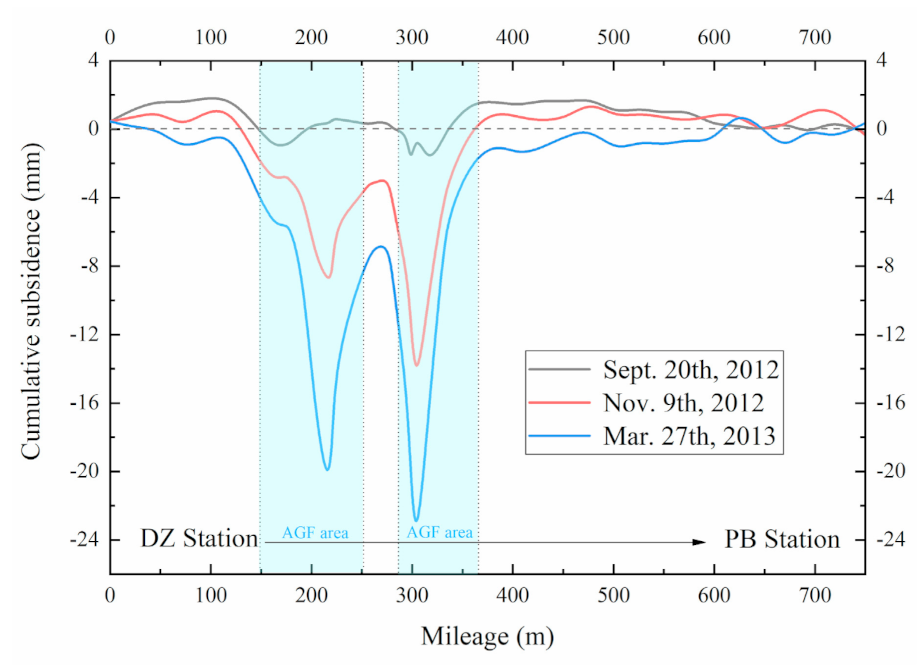
2.1. Measured Subsidence Data in AGF Area
2.2. Stiffness Variation after Freeze–Thaw Action
3. Materials and Methods
3.1. Soft Clay Samples
3.2. NMR Technique
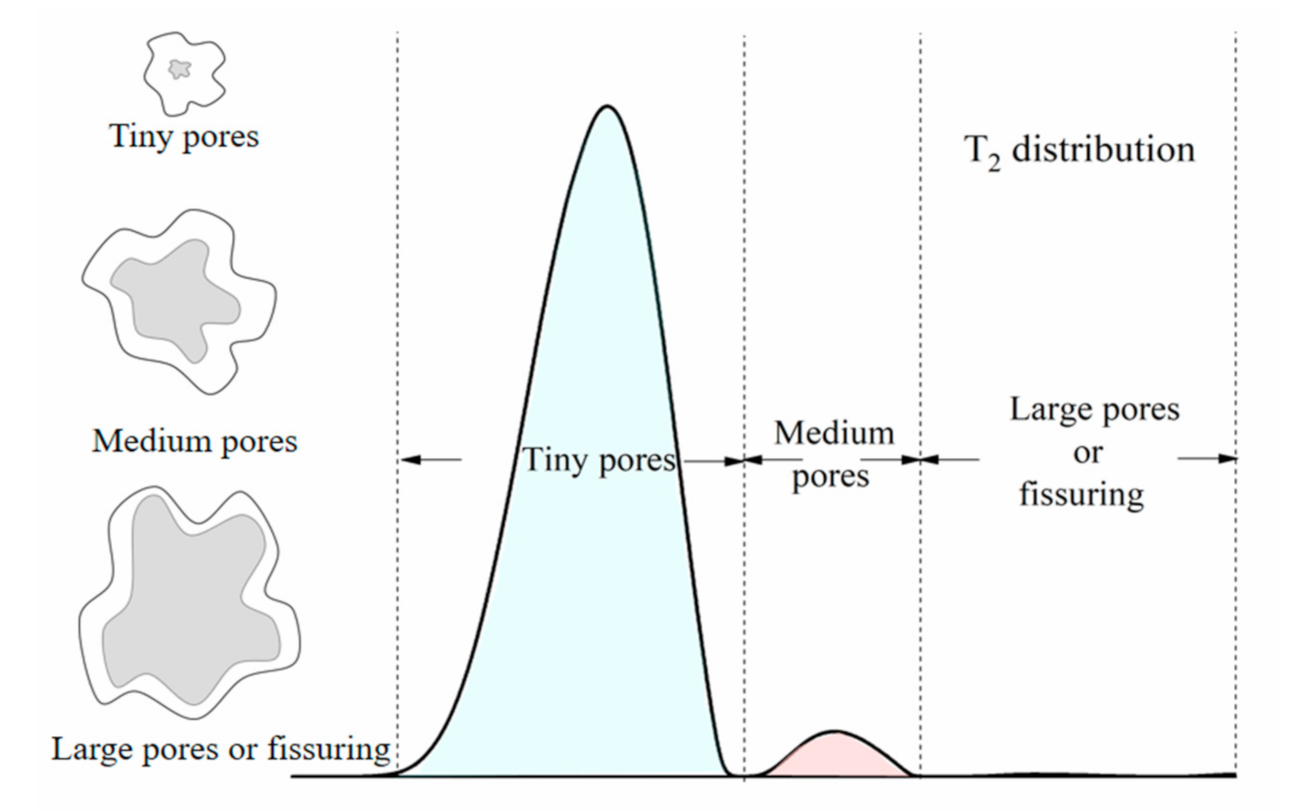
3.2.1. Principle of NMR Spectrometry
3.2.2. Surface Relaxation Ratio of Soft Clay
3.3. Freezing Plan
- Different freezing durations could show the progress of pore water freezing. Since there are three types of water to consider, including strong bound water, weak bound water, and free water, the freezing process is complicated compared with normal water.
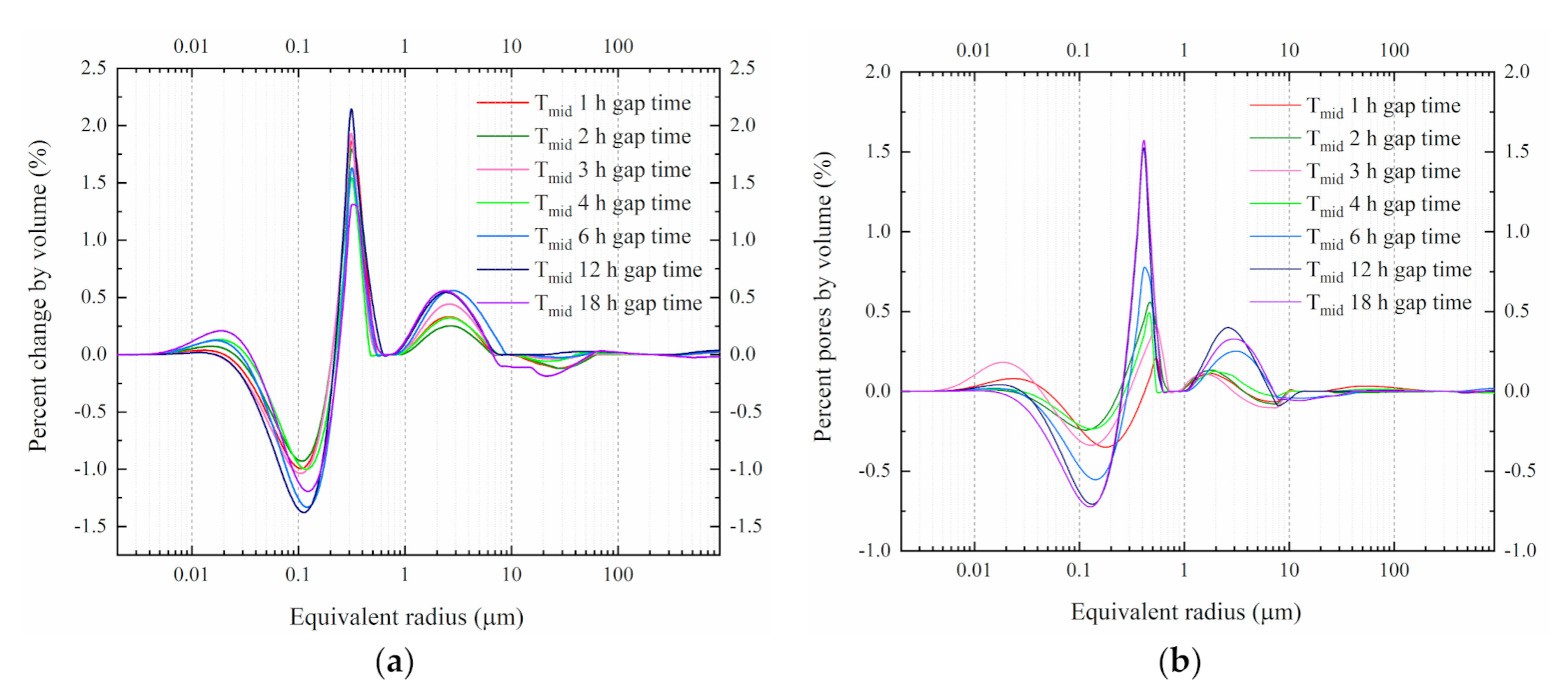
- Considering the risk of a water or electricity accident during construction, the freezing process could be interrupted for an uncertain time and understanding this impact is crucial. Therefore, in this section we characterized the influence of gap time on pore distribution. Time nodes to detect pore size are shown in Figure 10.
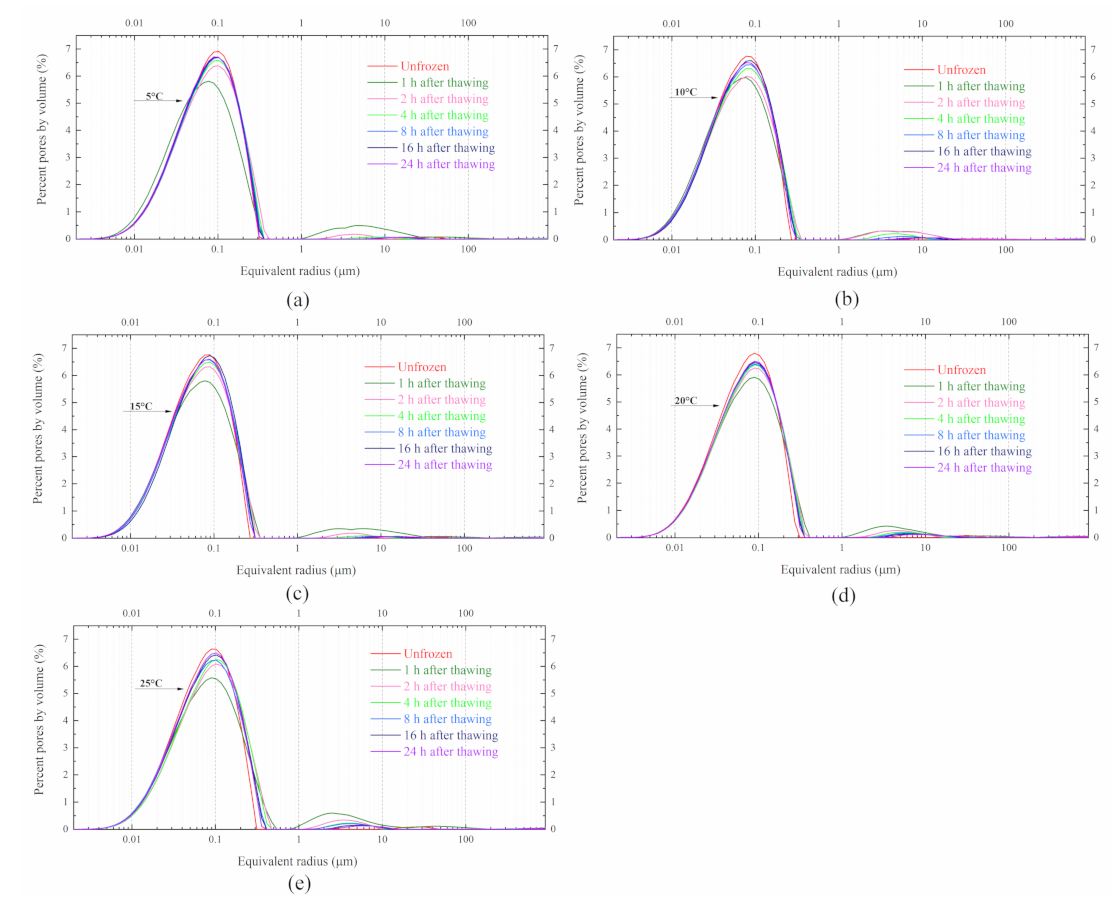
- The seasonal change in Hangzhou spans a temperature range from −10 to 40 °C, and the underground subway is buried at a depth of 7–10 m. Therefore, the thawing temperature ranging from 5 to 25 °C is reasonable.
4. Results
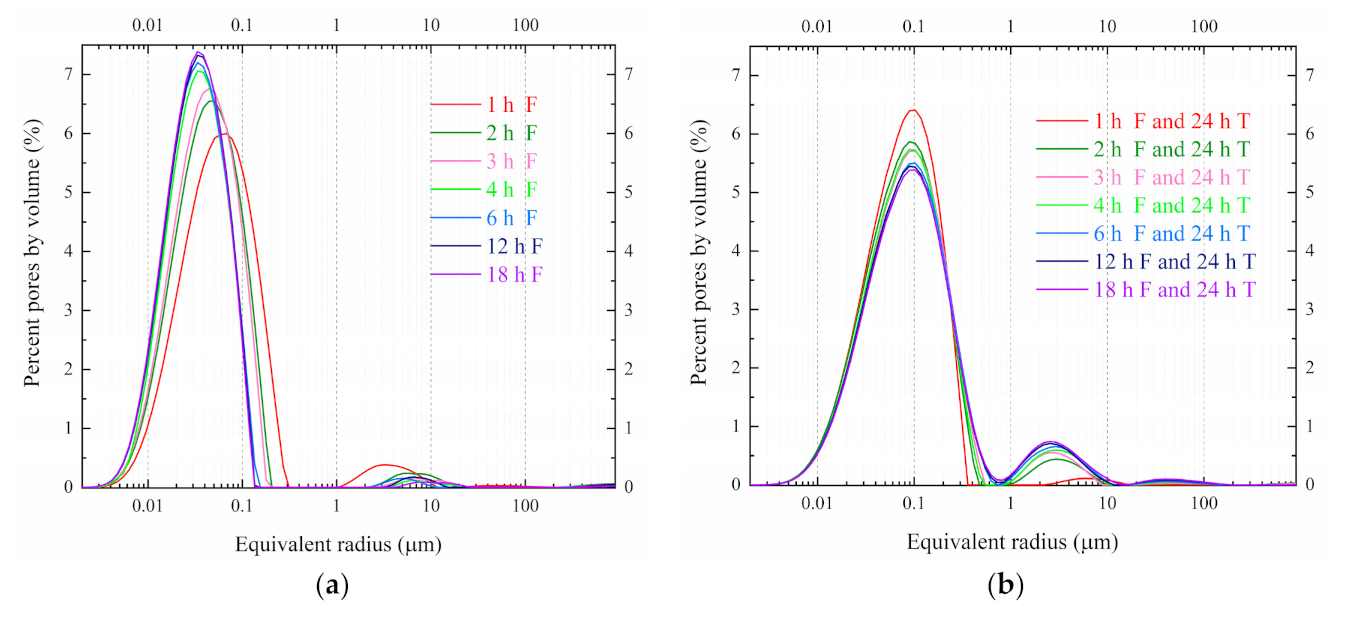
4.1. PSD under Different Freezing Durations at −20 °C
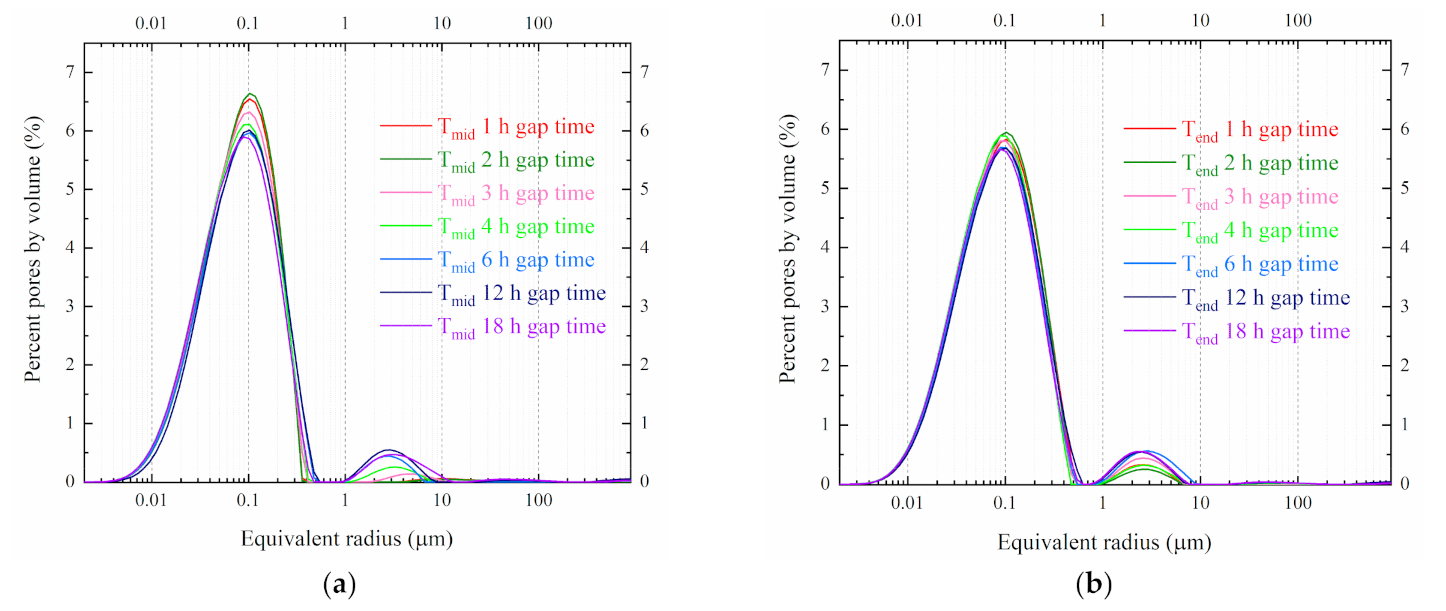
4.2. PSD under Different Freezing Gap Times at −20 °C
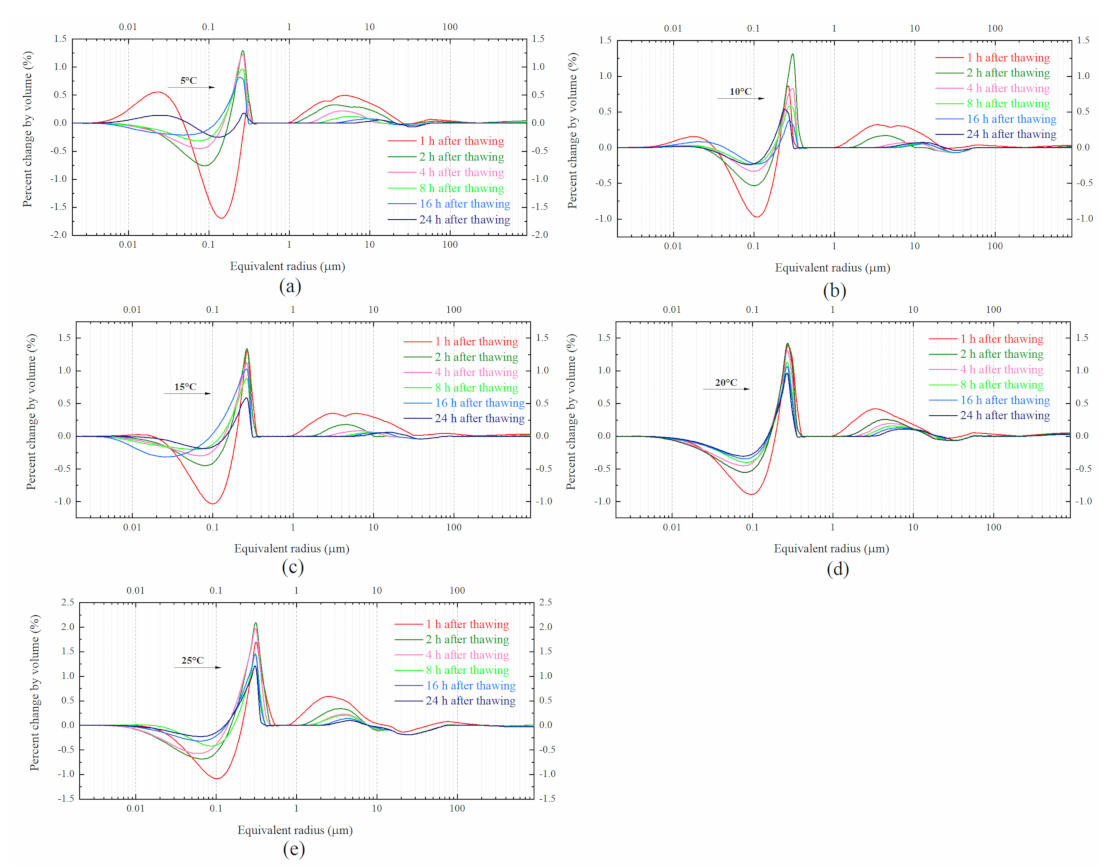
4.3. PSD under Different Thawing Temperature
5. Discussion
5.1. PSD Changes under Different Freezing Durations at −20 °C
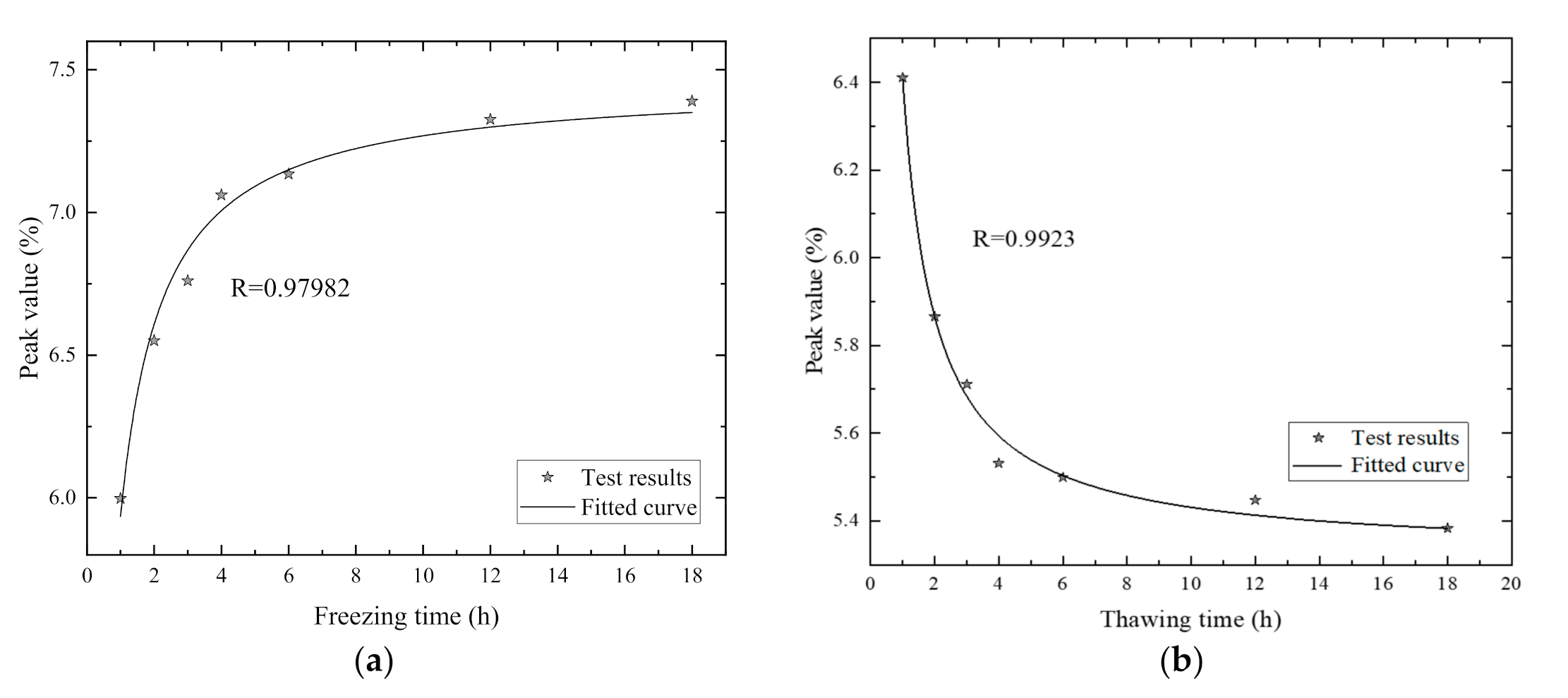
5.2. The Relationship of Freezing Duration and Pore Distribution Peak Value
5.3. PSD Changes under Different Freezing Gap Times at −20 °C
5.4. PSD Changes at Different Thawing Temperatures
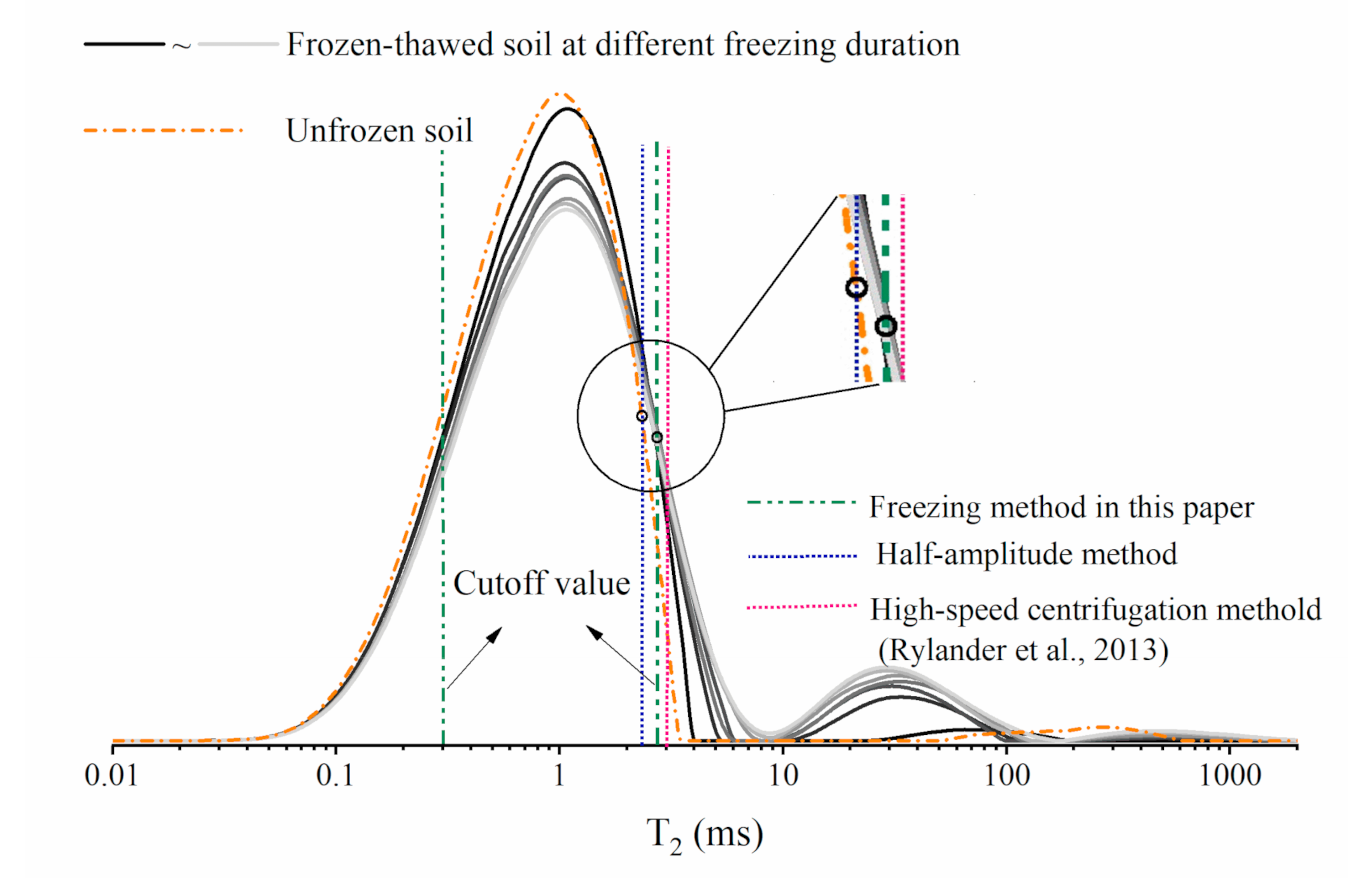
6. A New Method to Determine the T2 Cut-Off Value
7. Conclusions
- Under the freezing effect, the size of small pores in the soft clay was increased by frost heave. After thawing, the structure of soft clay was destroyed, and the structure went looser.
- For the soft clay samples volume in this test, the freezing stage was mostly completed during the first four hours. During the second hour, the freezing rate reached the maximum, and slowed after four hours. So, there was also a turning point freezing duration in practical engineering.
- After the freezing progresses, some water in small pores and almost all water in medium pores was frozen; the frozen water in small pores interconnected and new medium pores were formed. The medium pores, especially from 0.03 to 0.2 µm, played a key role in the structural failure of soft clay.
- The second freezing action could disrupt the recover progress of the enlarged pores. The shorter the gap time is, the less damage cause by refreezing. Accidental breaks should be controlled in a specified range to avoid more microstructure damage.
- Considering the comprehensive effect of soft clay structure looseness and melting settlement, a thawing temperature above 15 °C was much better for the stable of soft clay structure. Therefore, arranging the cross-passage construction in spring and autumn was a good choice.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.M.B.; Cheng, Y. Design of the gongbei tunnel using a very large cross-section pipe-roof and soil freezing method. Tunn. Undergr. Sp. Technol. 2018, 72, 28–40. [Google Scholar] [CrossRef]
- Leung, C.K.W.; Leung, R.K.Y.; Cheung, A.K.K.; Chan, W.L.; Application of artificial ground freezing method for tunnel. HKIE Civ. Div. Int. Conf. 2012, 1–12. Available online: https://www.dsd.gov.hk/EN/Files/Technical_Manual/technical_papers/HATS1204.pdf (accessed on 18 January 2021).
- Chang, D.K.; Lacy, H.S. Artificial ground freezing in geotechnical engineering. In Proceedings of the International Conference on Case Histories in Geotechnical Engineering, Arlington, VA, USA, 14 August 2008; pp. 1–11. [Google Scholar]
- Cui, Z.D.; Tang, Y.Q.; Yan, X.X. Centrifuge modeling of land subsidence caused by the high-rise building group in the soft soil area. Environ. Earth Sci. 2009, 59, 1819–1826. [Google Scholar] [CrossRef]
- Casini, F.; Olivella, S.; Gens, A.; Viggiani, G.M.B. Artificial ground freezing of a volcanic ash: Laboratory tests and modelling. Environ. Geotech. 2016, 3, 141–154. [Google Scholar] [CrossRef]
- Anovitz, L.M.; Cole, D.R. Characterization and analysis of porosity and pore structures. Rev. Mineral. Geochem. 2015, 80, 61–164. [Google Scholar] [CrossRef]
- Wigger, C.; Gimmi, T.; Muller, A.; Loon, L.R. Van. The influence of small pores on the anion transport properties of natural argillaceous rocks—A pore size distribution investigation of Opalinus Clay and Helvetic Marl. Appl. Clay Sci. 2018, 156, 134–143. [Google Scholar] [CrossRef]
- Clarkson, C.R.; Freeman, M.; He, L.; Agamalian, M.; Melnichenko, Y.B.; Mastalerz, M.; Bustin, R.M.; Radliński, A.P.; Blach, T.P. Characterization of tight gas reservoir pore structure using USANS/SANS and gas adsorption analysis. Fuel 2012, 95, 371–385. [Google Scholar] [CrossRef]
- Diamond, S. Pore size distributions in clays. Clays Clay Miner. 2006, 18, 7–23. [Google Scholar] [CrossRef]
- Penumadu, D.; Dean, J. Compressibility effect in evaluating the pore-size distribution of kaolin clay using mercury intrusion porosimetry. Can. Geotech. J. 2000, 37, 393–405. [Google Scholar] [CrossRef]
- Spaans, E.J.A.; Baker, J.M. The soil freezing characteristic: Its measurement and similarity to the soil moisture charac-teristic. Soil Sci. Soc. Am. J. 1980, 60, 13–19. [Google Scholar] [CrossRef]
- Wang, F.; Li, S. Determination of the surface fractal dimension for porous media by capillary condensation. Ind. Eng. Chem. Res. 1997, 36, 1598–1602. [Google Scholar] [CrossRef]
- Moore, C.A.; Donaldson, C.F. Quantifying soil microstructure using fractals. Ghechnique 1995, 45, 105–116. [Google Scholar] [CrossRef]
- Yang, R.; He, S.; Yi, J.; Hu, Q. Nano-scale pore structure and fractal dimension of organic-rich Wufeng-Longmaxi shale from Jiaoshiba area, Sichuan Basin: Investigations using FE-SEM, gas adsorption and helium pycnometry. Mar. Pet. Geol. 2016, 70, 27–45. [Google Scholar] [CrossRef]
- Sun, W.; Zuo, Y.; Wu, Z.; Liu, H.; Xi, S.; Shui, Y. Fractal analysis of pores and the pore structure of the Lower Cambrian Niutitang shale in northern Guizhou province: Investigations using NMR, SEM and image analyses. Mar. Pet. Geol. 2019, 99, 416–428. [Google Scholar] [CrossRef]
- Shi, B.; Wu, Z.; Inyang, H.; Chen, J. Preparation of soil specimens for SEM analysis using freeze-cut-drying. Bull. Eng. Geol. Environ. 1999, 1, 1–7. [Google Scholar] [CrossRef]
- Hu, R.; Li, Z.; Du, T.; Hu, F.; Oyediran, I.A.; Huang, R.; Li, X. Study on pore structure characteristics of marine and continental shale in China. J. Nat. Gas Sci. Eng. 2016, 33, 143–152. [Google Scholar]
- Wang, S.; Yang (Joey), Z.; Yang, P. Structural change and volumetric shrinkage of clay due to freeze-thaw by 3D X-ray computed tomography. Cold Reg. Sci. Technol. 2017, 138, 108–116. [Google Scholar] [CrossRef]
- Zhou, J.; Tang, Y. Centrifuge experimental study of thaw settlement characteristics of mucky clay after artificial ground freezing. Eng. Geol. 2015, 190, 98–108. [Google Scholar] [CrossRef]
- Cui, Z.D.; Tang, Y. Microstructures of different soil layers caused by the high-rise building group in Shanghai. Environ. Earth Sci. 2011, 63, 109–119. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Zhou, W.H.; Liu, F.; Yi, S.; Geng, X. Microstructure and morphological characterization of lead-contaminated clay with nanoscale zero-valent iron (nZVI) treatment. Eng. Geol. 2019, 256, 84–92. [Google Scholar] [CrossRef]
- Morriss, C.; Rossini, D.; Straley, C.; Christian, P.; Vinegar, H. Core analysis by low field NMR. The Log Analyst. 1997, 38, 43–56. [Google Scholar]
- Jaeger, F.; Shchegolikhina, A.; Van As, H.; Schaumann, G.E. Proton NMR relaxometry as a useful too l to evaluate swelling processes in peat soils. Open Magn. Reson. J. 2010, 3, 27–45. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, D. Comparison of low-field NMR and mercury intrusion porosimetry in characterizing pore size distribu-tions of coals. Fuel 2012, 95, 152–158. [Google Scholar] [CrossRef]
- Gopinathan, N.; Yang, B.; Lowe, J.P.; Edler, K.J.; Rigby, S.P. NMR cryoporometry characterisation studies of the relation between drug release profile and pore structural evolution of polymeric nanoparticles. Int. J. Pharm. 2014, 469, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Schuleit, M.; Furó, I.; Daicic, J.; Petrov, O.; Domanig, R.; Plunkett, M. Pore size distributions of biodegradable polymer microparticles in aqueous environments measured by NMR cryoporometry. Int. J. Pharm. 2005, 309, 157–162. [Google Scholar]
- Masiello, C.A.; Valenza, J.J.; Ogbonnaya, U.; Teel, W.S.; Semple, K.T.; Corbett, P.; Song, Y.-Q.; Hu, Q.; Webber, J.B.W.; Fisher, Q.J. An NMR study of porous rock and biochar containing organic material. Microporous Mesoporous Mater. 2013, 178, 94–98. [Google Scholar]
- Ishizaki, T.; Maruyama, M.; Furukawa, Y.; Dash, J.G. Premelting of ice in porous silica glass. J. Cryst. Growth 1996, 163, 455–460. [Google Scholar] [CrossRef]
- Jeon, J.D.; Kim, S.J.; Kwak, S.Y. 1H nuclear magnetic resonance (NMR) cryoporometry as a tool to determine the pore size distribution of ultrafiltration membranes. J. Memb. Sci. 2008, 309, 233–238. [Google Scholar] [CrossRef]
- Li, X.; Kang, Y.; Haghighi, M. Investigation of pore size distributions of coals with di ff erent structures by nuclear magnetic resonance (NMR) and mercury intrusion porosimetry (MIP). Meas. 2018, 116, 122–128. [Google Scholar] [CrossRef]
- Tian, H.; Wei, C.; Tan, L. Effect of freezing-thawing cycles on the microstructure of soils: A two- dimensional NMR relaxation analysis. Cold Reg. Sci. Technol. 2019, 158, 106–116. [Google Scholar] [CrossRef]
- Lai, Y.; Wu, D.; Zhang, M. Crystallization deformation of a saline soil during freezing and thawing processes. Appl. Therm. Eng. 2017, 120, 463–473. [Google Scholar] [CrossRef]
- Watanabe, K.; Mizoguchi, M. Amount of unfrozen water in frozen porous media saturated with solution. Cold Reg. Sci. Technol. 2002, 34, 103–110. [Google Scholar] [CrossRef]
- Jackson, C.L.; McKenna, G.B. The melting behavior of organic materials confined in porous solids. J. Chem. Phys. 1990, 93, 9002–9011. [Google Scholar] [CrossRef]
- Petrov, O.V.; Furó, I. A joint use of melting and freezing data in NMR cryoporometry. Microporous Mesoporous Mater. 2010, 136, 83–91. [Google Scholar] [CrossRef]
- Liu, X.; Chen, H.; Kou, W.; Zhang, D. Robust anti-icing coatings via enhanced super hydrophobicity on fiberglass cloth. Cold Reg. Sci. Technol. 2017, 138, 18–23. [Google Scholar] [CrossRef]
- Nitao, J.J.; Bear, J. Potentials and their role in transport in porous media. Water Resour. Res. 1996, 32, 225–250. [Google Scholar] [CrossRef]
- Lowden, B.D.; Porter, M.J.; Powrie, L.S. T2 relaxation time versus mercury injection capillary pressure: Implications for NMR logging and reservoir characterization. Proc. Eur. Pet. Conf. 1998, 1, 323–327. [Google Scholar] [CrossRef]
- Liu, J.; Chang, D.; Yu, Q. Influence of freeze-thaw cycles on mechanical properties of a silty sand. Eng. Geol. 2016, 210, 23–32. [Google Scholar] [CrossRef]
- Mu, J.Q.; Pei, X.J.; Huang, R.Q.; Rengers, N.; Zou, X.Q. Degradation characteristics of shear strength of joints in three rock types due to cyclic freezing and thawing. Cold Reg. Sci. Technol. 2017, 138, 91–97. [Google Scholar] [CrossRef]
- Weihermu, L.; van Dusschoten, D. Characterization of unsaturated porous media by high-field and low-field NMR relaxometry. Ghechnique 2009, 45, 1–11. [Google Scholar]
- Rezaee, R.; Saeedi, A.; Clennell, B. Tight gas sands permeability estimation from mercury injection capillary pressure and nuclear magnetic resonance data. J. Pet. Sci. Eng. 2012, 88–89, 92–99. [Google Scholar] [CrossRef]
- Rylander, E.; Singer, P.M.; Jiang, T.; Lewis, R.; McLin, R.; Sinclair, S. NMR T2 distributions in the Eagle Ford shale: Reflections on pore size. Soc. Pet. Eng. SPE USA Unconv. Resour. Conf. 2013, 426–440. [Google Scholar]
- Zheng, S.; Yao, Y.; Liu, D.; Cai, Y. Nuclear magnetic resonance surface relaxivity of coals. Int. J. Coal Geol. 2019, 205, 1–13. [Google Scholar] [CrossRef]
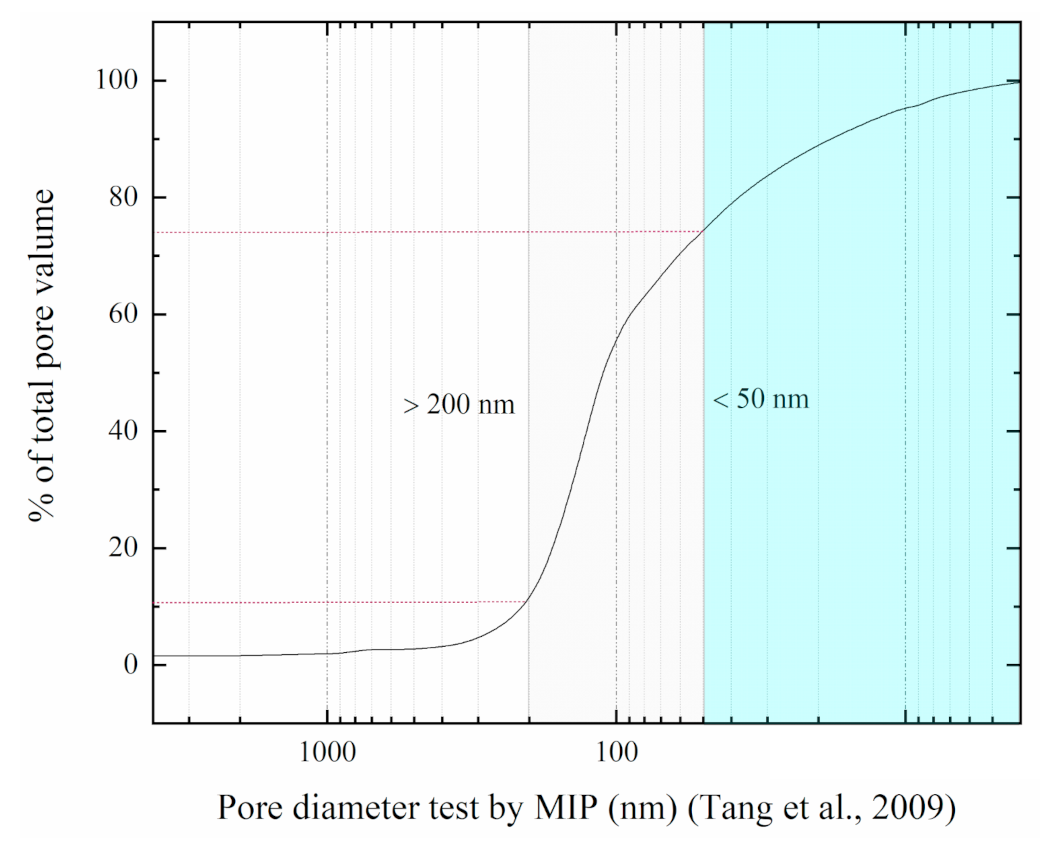
- Tang, Y.Q.; Zhao, S.K.; Yang, P.; Wang, J.X.; Zhang, X.Y. Quantitative analysis of the microscopic behavior of saturated soft clays under cyclic loading. Chin. Civ. Eng. J. 2009, 42, 98–103. (In Chinese) [Google Scholar]
- Cetin, H.; Fener, M.; Söylemez, M.; Günaydin, O. Soil structure changes during compaction of a cohesive soil. Eng. Geol. 2007, 92, 38–48. [Google Scholar] [CrossRef]
- Papakonstantinou, S.; Anagnostou, G.; Pimentel, E. Evaluation of ground freezing data from the Naples subway. Proc. Inst. Civ. Eng. Geotech. Eng. 2013, 166, 280–298. [Google Scholar] [CrossRef]
- Cicchetti, L. Thermo-Hydro-Mechanical Simulations of Artificial Ground Freezing. Master’s Thesis, Norwegian University of Science and Technology, Trondheim, Norway, June 2018. Available online: http://hdl.handle.net/11250/2507933 (accessed on 18 January 2021).
- Tian, H.; Wei, C.; Wei, H.; Zhou, J. Freezing and thawing characteristics of frozen soils: Bound water content and hysteresis phenomenon. Cold Reg. Sci. Technol. 2014, 103, 74–81. [Google Scholar] [CrossRef]
- Kruse, A.M.; Darrow, M.M. Adsorbed cation effects on unfrozen water in fine-grained frozen soil measured using pulsed nuclear magnetic resonance. Cold Reg. Sci. Technol. 2017, 142, 42–54. [Google Scholar] [CrossRef]
- Chai, M.; Zhang, J.; Zhang, H.; Mu, Y.; Sun, G.; Yin, Z. A method for calculating unfrozen water content of silty clay with consideration of freezing point. Appl. Clay Sci. 2018, 161, 474–481. [Google Scholar] [CrossRef]
- Borre, M.K.; Coffey, B.P. Multi-stage cleaning for routine core analysis in heavy oil-bearing carbonates, Campos Basin, Brazil. Soc. Pet. Eng. SPE Heavy Oil Conf. Can. 2014, 3, 1801–1809. [Google Scholar]
- Kuptsov, K.; Griffiths, R.; Maggs, D. Technology update: Magnetic resonance-while-drilling system improves under-standing of complex reservoirs. J. Pet. Technol. 2015, 67, 26–29. [Google Scholar] [CrossRef]

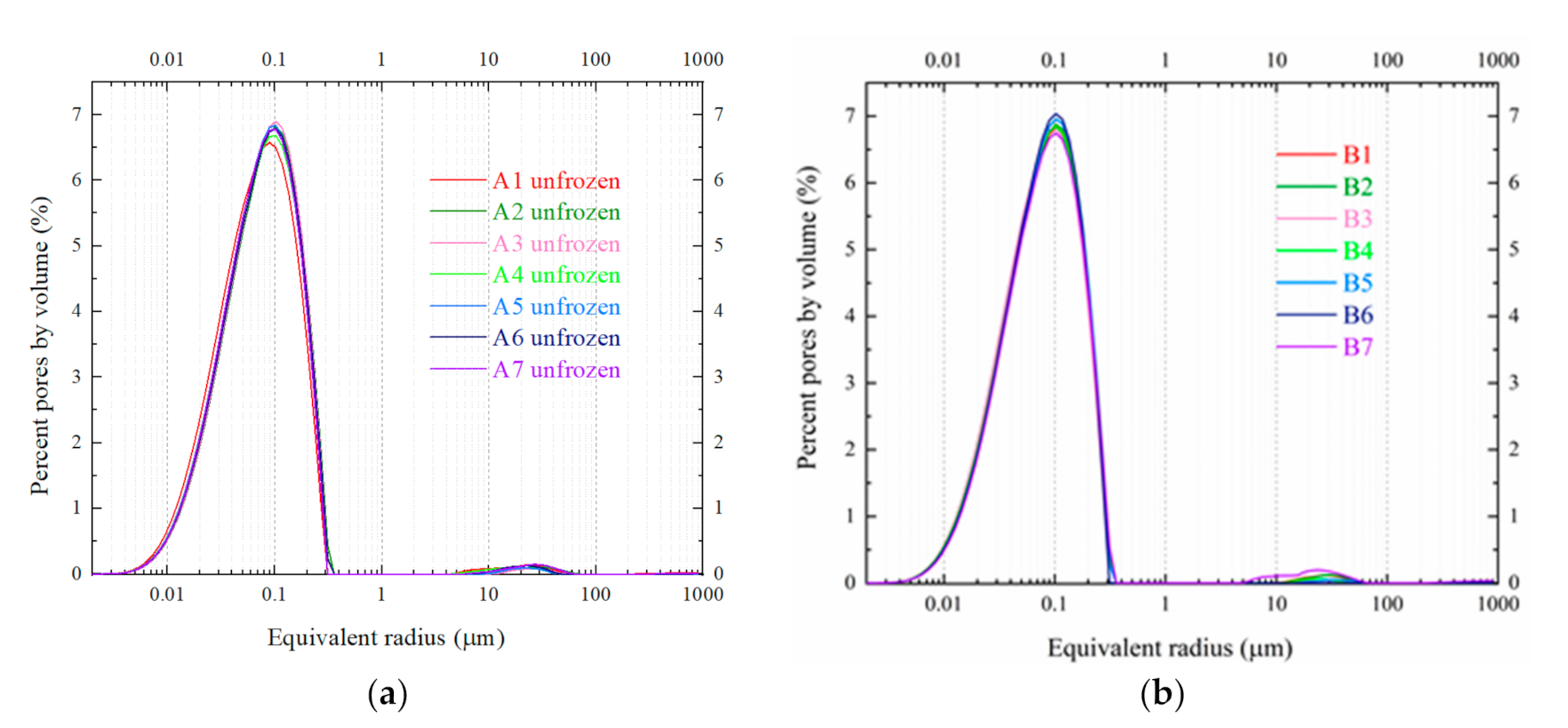

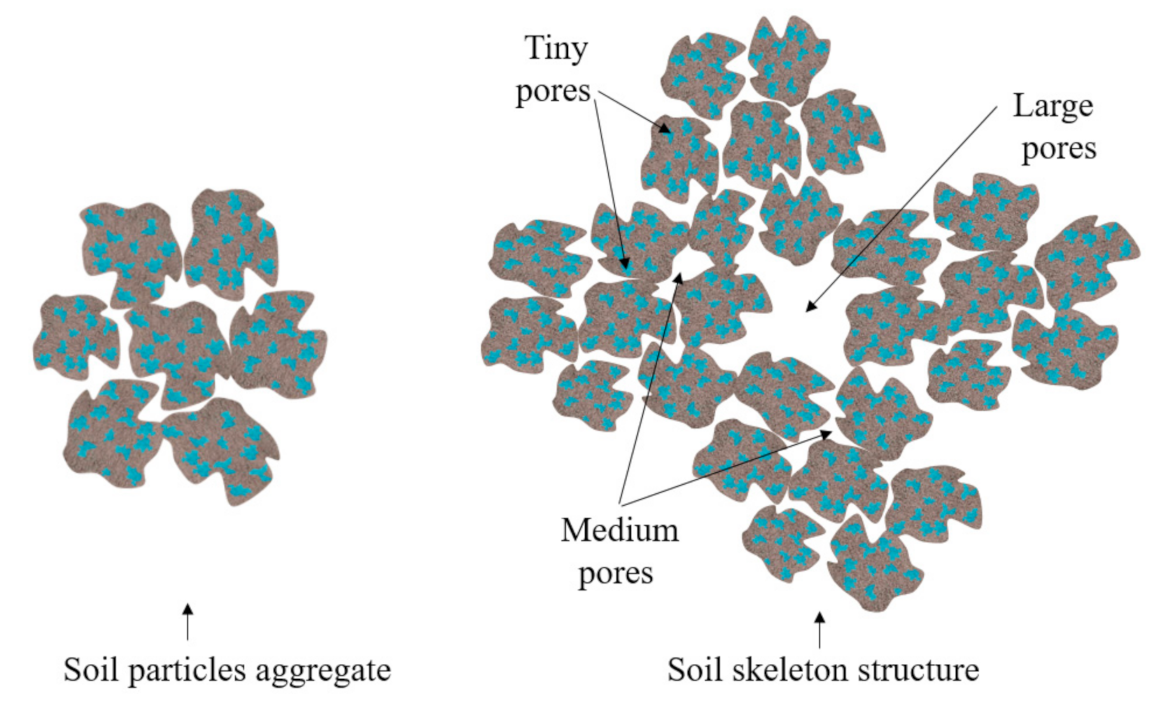



| Sample No. | Sample Size /mm | Freezing Duration /h | Gap Time /h | Thawing Temperature /°C |
|---|---|---|---|---|
| A1 | 38 × 38 | 1 | - | 25 |
| A2 | 38 × 38 | 2 | - | 25 |
| A3 | 38 × 38 | 3 | - | 25 |
| A4 | 38 × 38 | 4 | - | 25 |
| A5 | 38 × 38 | 6 | - | 25 |
| A6 | 38 × 38 | 12 | - | 25 |
| A7 | 38 × 38 | 18 | - | 25 |
| B1 | 38 × 38 | 24 | 1 | 25 |
| B2 | 38 × 38 | 24 | 2 | 25 |
| B3 | 38 × 38 | 24 | 3 | 25 |
| B4 | 38 × 38 | 24 | 4 | 25 |
| B5 | 38 × 38 | 24 | 6 | 25 |
| B6 | 38 × 38 | 24 | 12 | 25 |
| B7 | 38 × 38 | 24 | 18 | 25 |
| B8 | 38 × 38 | 24 | 24 | 25 |
| C1 | 38 × 38 | 24 | - | 5 |
| C2 | 38 × 38 | 24 | - | 10 |
| C3 | 38 × 38 | 24 | - | 15 |
| C4 | 38 × 38 | 24 | - | 20 |
| C5 | 38 × 38 | 24 | - | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, B.; He, S.; Xia, T.; Ding, Z. Research on Microstructure of Soft Clay under Various Artificial Ground Freezing Conditions Based on NMR. Appl. Sci. 2021, 11, 1810. https://doi.org/10.3390/app11041810
Kong B, He S, Xia T, Ding Z. Research on Microstructure of Soft Clay under Various Artificial Ground Freezing Conditions Based on NMR. Applied Sciences. 2021; 11(4):1810. https://doi.org/10.3390/app11041810
Chicago/Turabian StyleKong, Bowen, Shaoheng He, Tangdai Xia, and Zhi Ding. 2021. "Research on Microstructure of Soft Clay under Various Artificial Ground Freezing Conditions Based on NMR" Applied Sciences 11, no. 4: 1810. https://doi.org/10.3390/app11041810
APA StyleKong, B., He, S., Xia, T., & Ding, Z. (2021). Research on Microstructure of Soft Clay under Various Artificial Ground Freezing Conditions Based on NMR. Applied Sciences, 11(4), 1810. https://doi.org/10.3390/app11041810






