Comparisons of the Uncoupled Effects of CO2 on the CH4/O2 Counterflow Diffusion Flame under High Pressure
Abstract
1. Introduction
2. Numerical Simulation Model
3. Results
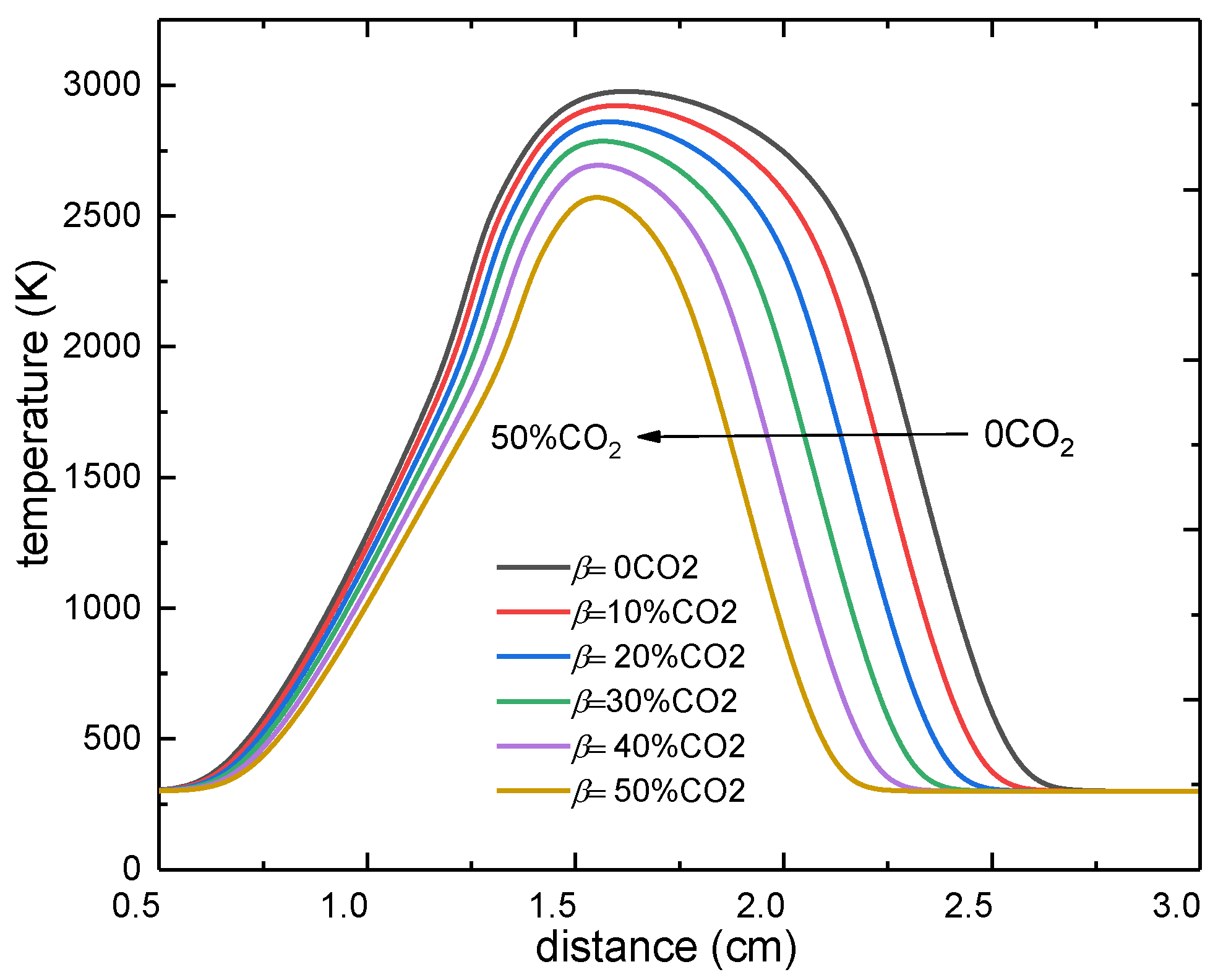
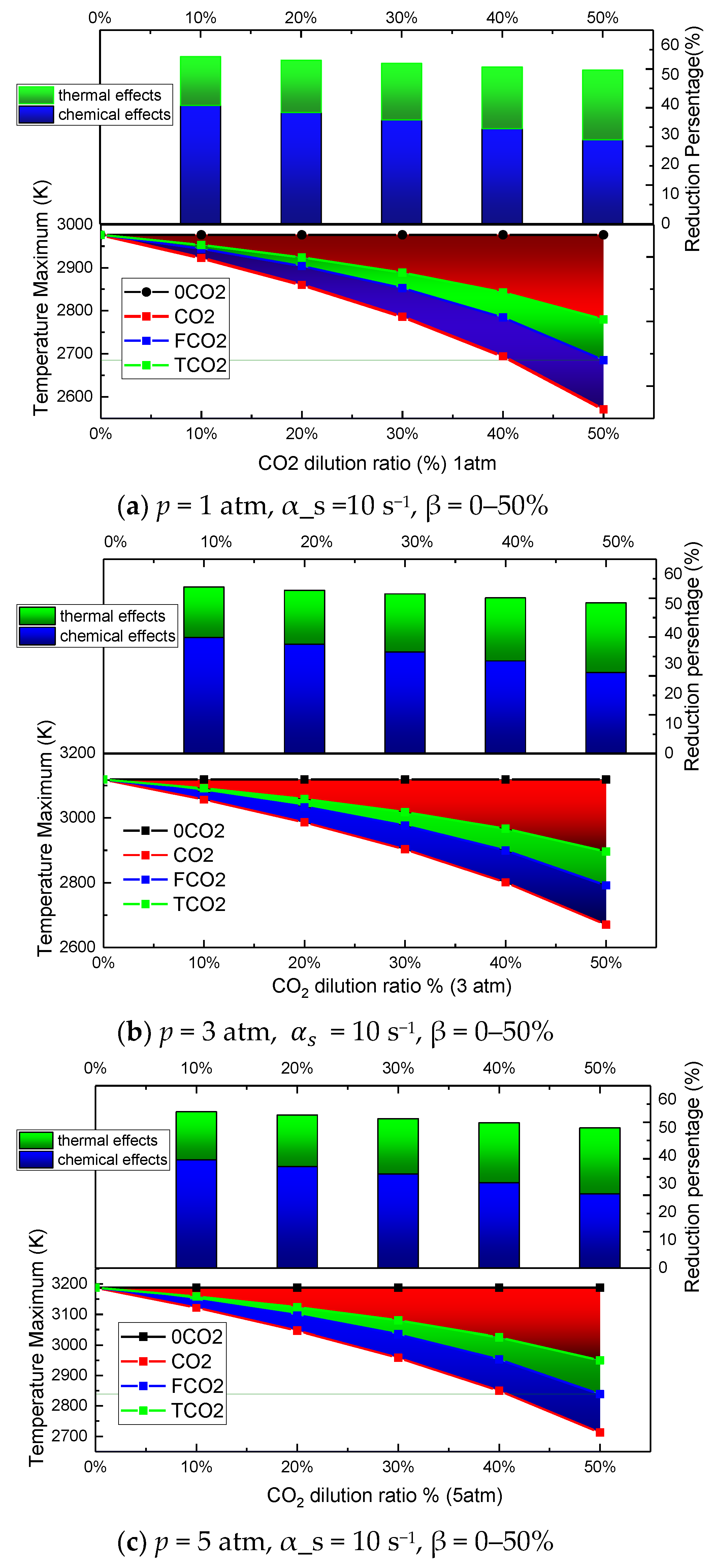
3.1. The Effect of CO2 Dilution on the Flame Temperature
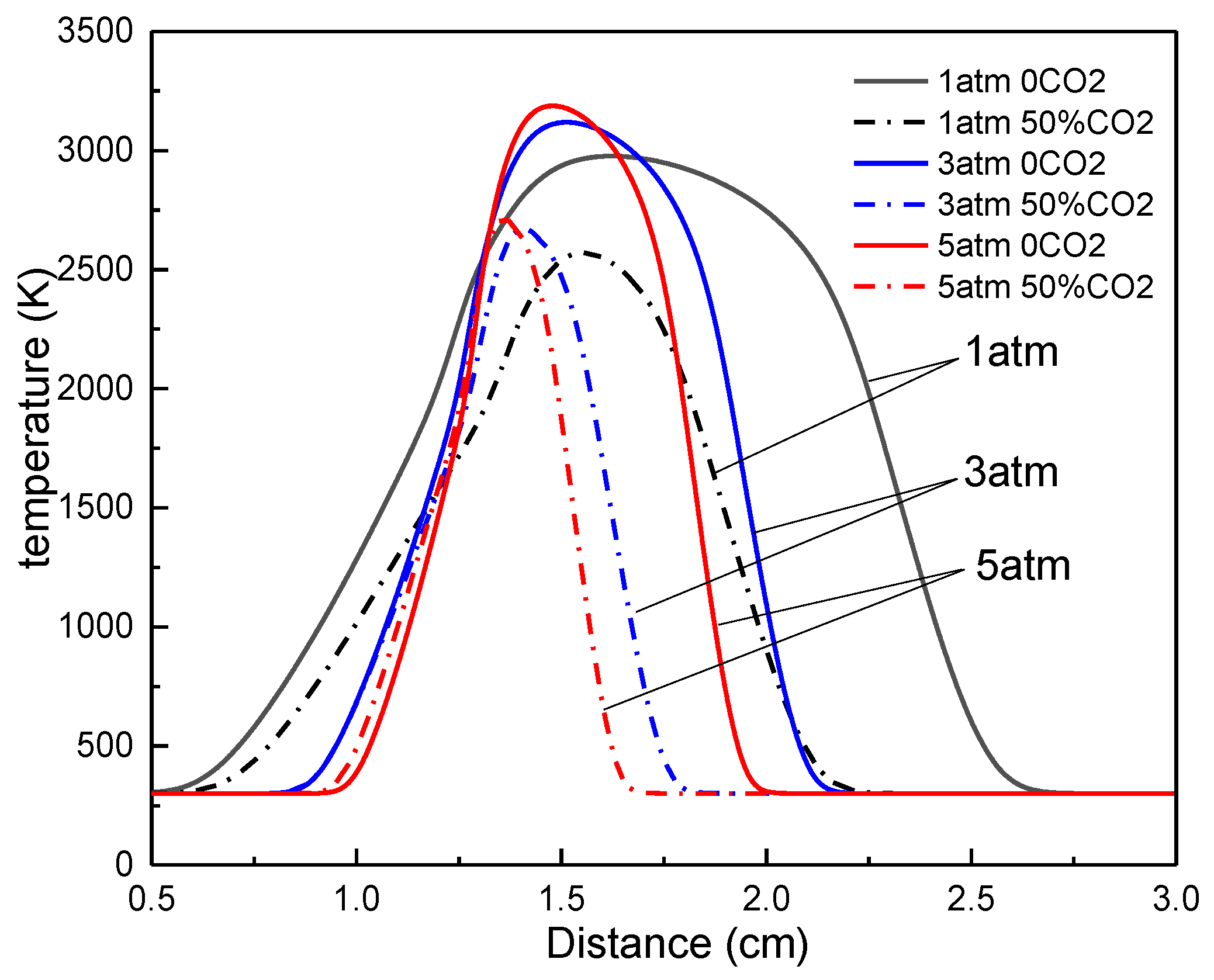
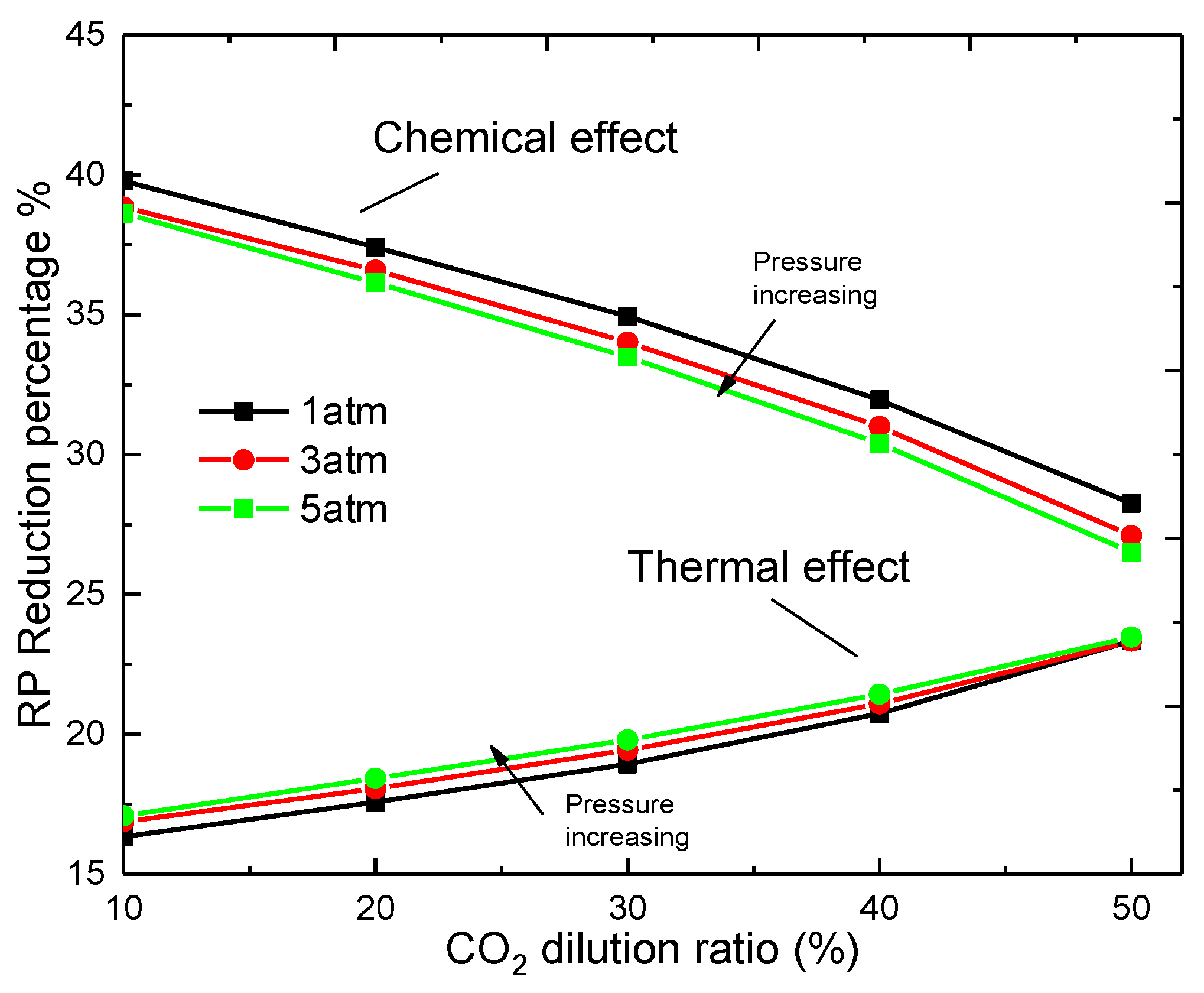
3.2. The Effect of Pressure on the Flame Temperature
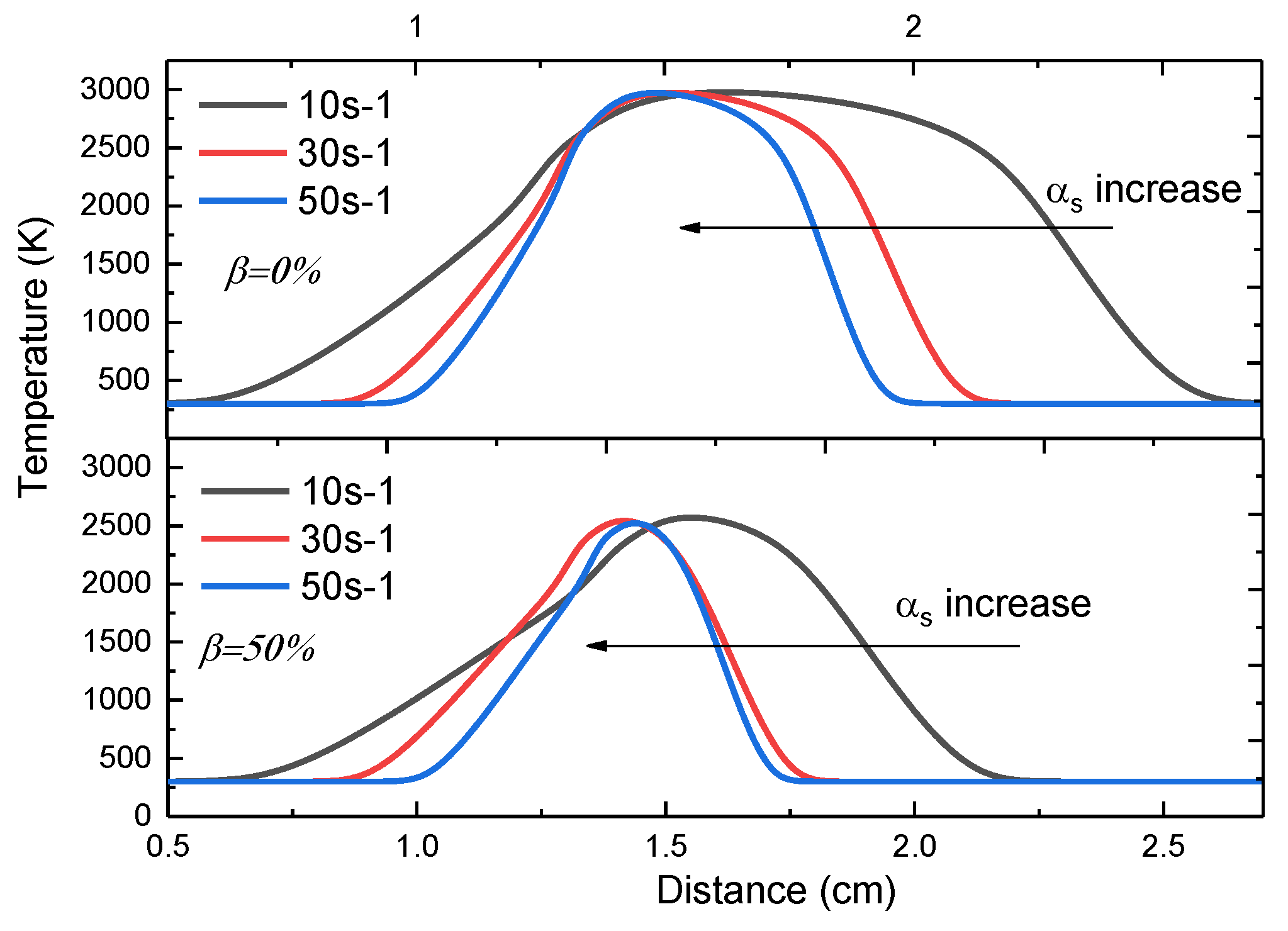
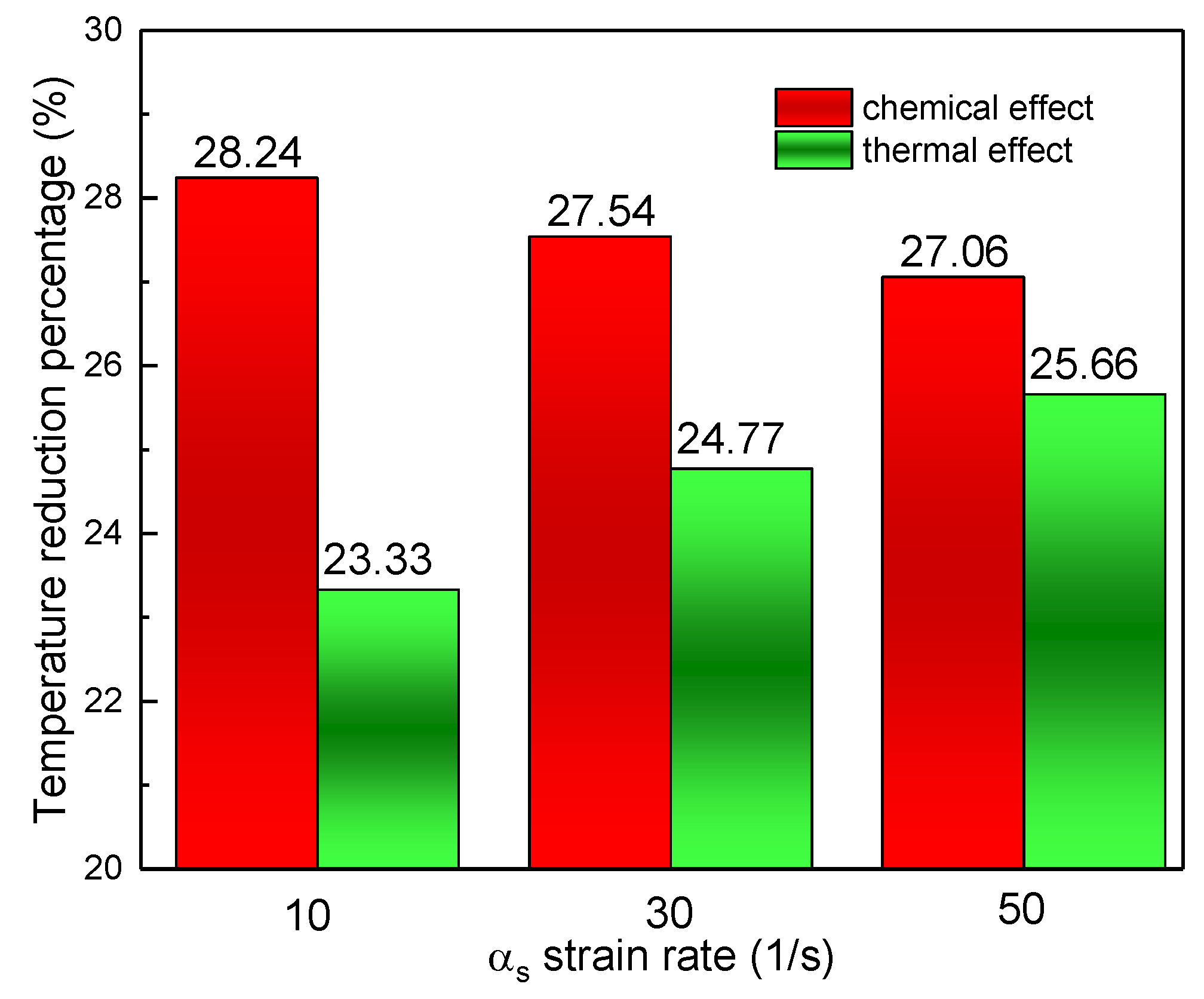
3.3. The Effect of the Strain Rates
4. Conclusions
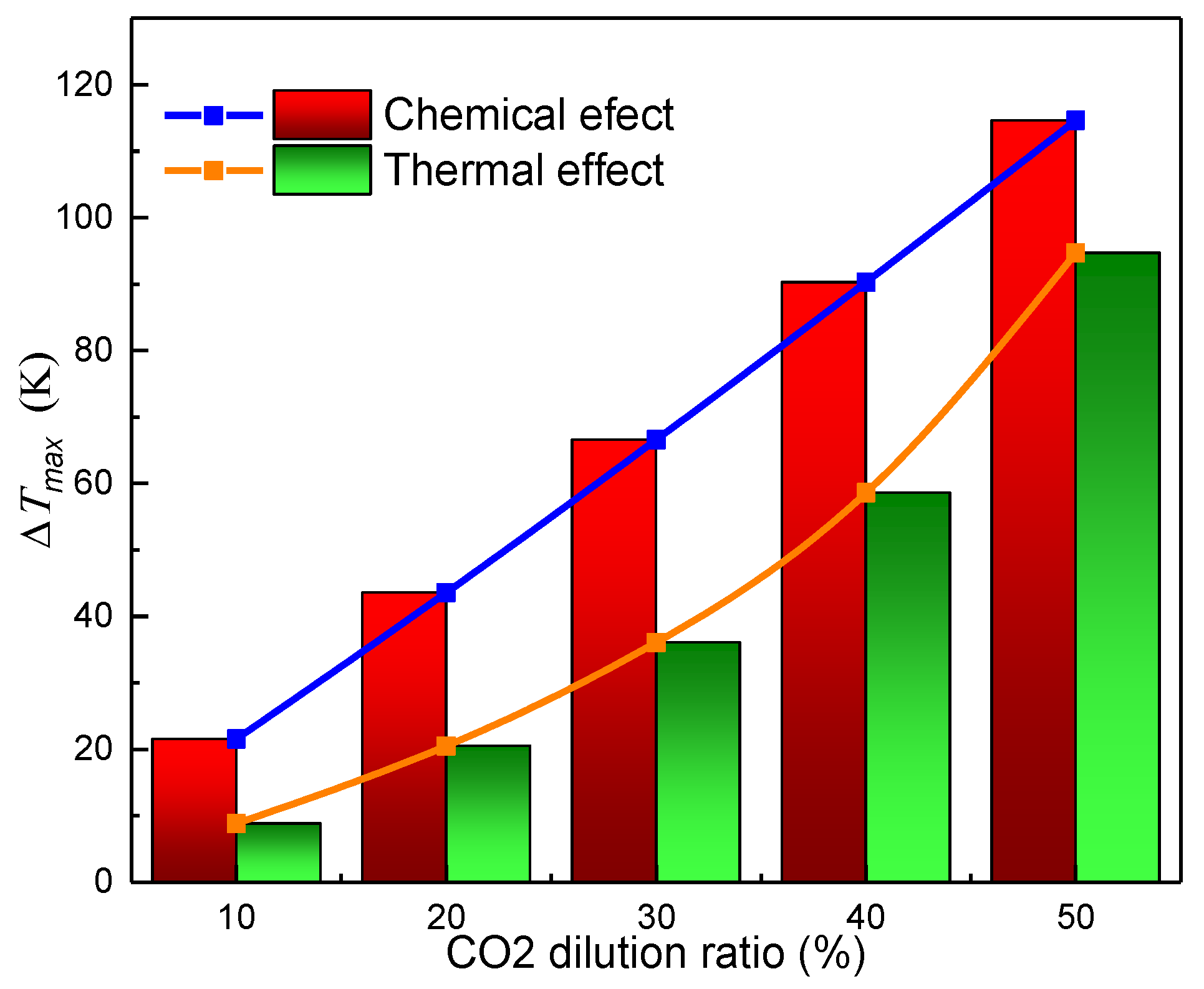
- Both the chemical effect and the thermal effect of CO2 dilution in the oxidizer side can decrease the flame temperature significantly, while the transport effect of CO2 on the flame temperature can be ignored.
- The decreases in value of the peak flame temperature caused by both the chemical effect and the thermal effect increased with the increasing CO2 dilution ratio, with linear increases for the former and exponential increases for the latter.
- The increasing pressure can narrow the reaction zone and elevate the peak value of the flame temperature. and behave in opposite ways along with the pressure. The former decreases, but the latter increases with the pressure.
- The increasing strain rate can narrow the reaction zone, but has no obvious influence on the peak value of the flame temperature. The change in and with the increasing strain rate shared similarities with the pressure influence.
- The flame temperature is predominantly influenced by the chemical effect of CO2, while the thermal effect increases with the increase of CO2 volume fraction, the increase of pressure, and the increase of stretch rate, and tends to exceed the chemical influence.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Strain Rate (s−1) | Fuel | Oxidant Composition | ρf (g/L) | ρO (g/L) | Vf (m/s) | VO (m/s) |
|---|---|---|---|---|---|---|
| 10 | Pure CH4 | 0%CO2 (FCO2, TCO2, XCO2) + 100%O2 | 0.7143 | 1.4286 | 10 | 14.14 |
| Pure CH4 | 10%CO2 (FCO2, TCO2, XCO2) + 90%O2 | 0.7143 | 1.4821 | 10 | 14.4 | |
| Pure CH4 | 20%CO2 (FCO2, TCO2, XCO2) + 80%O2 | 0.7143 | 1.5357 | 10 | 14.66 | |
| Pure CH4 | 30%CO2 (FCO2, TCO2, XCO2) + 70%O2 | 0.7143 | 1.5893 | 10 | 14.92 | |
| Pure CH4 | 40%CO2 (FCO2, TCO2, XCO2) + 60%O2 | 0.7143 | 1.6429 | 10 | 15.17 | |
| Pure CH4 | 50%CO2 (FCO2, TCO2, XCO2) + 50%O2 | 0.7143 | 1.6964 | 10 | 15.41 | |
| 30 | Pure CH4 | 0%CO2 (FCO2, TCO2, XCO2) + 100%O2 | 0.7143 | 1.4286 | 30 | 42.42 |
| Pure CH4 | 10%CO2 (FCO2, TCO2, XCO2) + 90%O2 | 0.7143 | 1.4821 | 30 | 43.2 | |
| Pure CH4 | 20%CO2 (FCO2, TCO2, XCO2) + 80%O2 | 0.7143 | 1.5357 | 30 | 43.98 | |
| Pure CH4 | 30%CO2 (FCO2, TCO2, XCO2) + 70%O2 | 0.7143 | 1.5893 | 30 | 44.76 | |
| Pure CH4 | 40%CO2 (FCO2, TCO2, XCO2) + 60%O2 | 0.7143 | 1.6429 | 30 | 45.51 | |
| Pure CH4 | 50%CO2 (FCO2, TCO2, XCO2) + 50%O2 | 0.7143 | 1.6964 | 30 | 46.23 | |
| 50 | Pure CH4 | 0%CO2 (FCO2, TCO2, XCO2) + 100%O2 | 0.7143 | 1.4286 | 50 | 70.7 |
| Pure CH4 | 10%CO2 (FCO2, TCO2, XCO2) + 90%O2 | 0.7143 | 1.4821 | 50 | 72 | |
| Pure CH4 | 20%CO2 (FCO2, TCO2, XCO2) + 80%O2 | 0.7143 | 1.5357 | 50 | 73.3 | |
| Pure CH4 | 30%CO2 (FCO2, TCO2, XCO2) + 70%O2 | 0.7143 | 1.5893 | 50 | 74.6 | |
| Pure CH4 | 40%CO2 (FCO2, TCO2, XCO2) + 60%O2 | 0.7143 | 1.6429 | 50 | 75.85 | |
| Pure CH4 | 50%CO2 (FCO2, TCO2, XCO2) + 50%O2 | 0.7143 | 1.6964 | 50 | 77.05 |
References
- Wall, T.F. Combustion processes for carbon capture. Proc. Combust. Inst. 2007, 31, 31–47. [Google Scholar] [CrossRef]
- Cormos, C. Integrated assessment of IGCC power generation technology with carbon capture and storage (CCS). Energy 2012, 42, 434–445. [Google Scholar] [CrossRef]
- Lasek, J.A.; Janusz, M.; Zuwała, J.; Głód, K.; Iluk, A. Oxy-fuel combustion of selected solid fuels under atmospheric and elevated pressures. Energy 2013, 62, 105–112. [Google Scholar] [CrossRef]
- Soloklou, M.N.; Golneshan, A.A. Effect of CO2 diluent on the formation of pollutant NOx in the laminar non-premixed methane-air flame. Int. J. Heat Mass Tran. 2020, 148, 119071. [Google Scholar] [CrossRef]
- Yang, Z.; Grace, J.R.; Lim, C.J.; Zhang, L. Combustion of Low-Concentration Coal Bed Methane in a Fluidized Bed. Energy Fuel 2011, 25, 975–980. [Google Scholar] [CrossRef]
- Gaber, C.; Schluckner, C.; Wachter, P.; Demuth, M.; Hochenauer, C. Experimental study on the influence of the nitrogen concentration in the oxidizer onNOx and CO emissions during the oxy-fuel combustion of natural gas. Energy 2021, 214, 118905. [Google Scholar] [CrossRef]
- Hu, X.; Yu, Q.; Liu, J.; Sun, N. Investigation of laminar flame speeds of CH4/O2/CO2 mixtures at ordinary pressure and kinetic simulation. Energy 2014, 70, 626–634. [Google Scholar] [CrossRef]
- Buhre, B.J.P.; Elliott, L.K.; Sheng, C.D.; Gupta, R.P.; Wall, T.F. Oxy-fuel combustion technology for coal-fired power generation. Prog. Energy Combust. 2005, 31, 283–307. [Google Scholar] [CrossRef]
- Li, X.; Jia, L.; Onishi, T.; Grajetzki, P.; Nakamura, H.; Tezuka, T.; Hasegawa, S.; Maruta, K. Study on stretch extinction limits of CH4/CO2 versus high temperature O2/CO2 counterflow non-premixed flames. Combust. Flame 2014, 161, 1526–1536. [Google Scholar] [CrossRef]
- Ren, F.; Xiang, L.; Chu, H.; Ya, Y.; Han, W.; Nie, X. Numerical investigation on the effect of CO2 and steam for the H2 intermediate formation and NOX emission in laminar premixed methane/air flames. Int. J. Hydrogen Energy 2020, 45, 3785–3794. [Google Scholar] [CrossRef]
- Saanum, I.; Ditaranto, M. Experimental Study of Oxy-fuel Combustion under Gas Turbine Conditions. Energy Fuels 2017, 31, 4445–4451. [Google Scholar] [CrossRef]
- Gascoin, N.; Yang, Q.; Chetehouna, K. Thermal effects of CO2 on the NOx formation behavior in the CH4 diffusion combustion system. Appl. Therm. Eng. 2017, 110, 144–149. [Google Scholar] [CrossRef]
- Xu, H.; Liu, F.; Sun, S.; Zhao, Y.; Meng, S.; Tang, W. Effects of H2O and CO2 diluted oxidizer on the structure and shape of laminar coflow syngas diffusion flames. Combust. Flame 2017, 177, 67–78. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; He, Y.; Han, X.; Sun, Z.; Zhu, Y.; Costa, M. Laminar burning velocities of CH4/O2/N2 and oxygen-enriched CH4/O2/CO2 flames at elevated pressures measured using the heat flux method. Fuel 2020, 259, 116152. [Google Scholar] [CrossRef]
- De Persis, S.; Foucher, F.; Pillier, L.; Osorio, V.; Gökalp, I. Effects of O2 enrichment and CO2 dilution on laminar methane flames. Energy 2013, 55, 1055–1066. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, J.; Zhang, M.; Gong, J.; Jin, W.; Huang, Z. Experimental and Numerical Study on Laminar Flame Characteristics of Methane Oxy-fuel Mixtures Highly Diluted with CO2. Energ Fuel 2013, 27, 6231–6237. [Google Scholar] [CrossRef]
- Hassan, M.I.; Aung, K.T.; Faeth, G.M. Measured and predicted properties of laminar premixed methane/air flames at various pressures. Combust. Flame 1998, 115, 539–550. [Google Scholar] [CrossRef]
- Zhou, Q.; Cheung, C.S.; Leung, C.W.; Li, X.; Huang, Z. Effects of diluents on laminar burning characteristics of bio-syngas at elevated pressure. Fuel 2019, 248, 8–15. [Google Scholar] [CrossRef]
- Lu, X.; Hu, E.; Huang, S.; Xu, Z.; Li, X.; Huang, Z. Experimental and kinetic study on laminar burning velocities of 1,3-butadiene at pressures up to 1.5 MPa. Fuel 2019, 246, 222–231. [Google Scholar] [CrossRef]
- Liu, F.; Guo, H.; Smallwood, G.J.; Gülder, Ö.L. The chemical effects of carbon dioxide as an additive in an ethylene diffusion flame: Implications for soot and NOx formation. Combust. Flame 2001, 125, 778–787. [Google Scholar] [CrossRef]
- Liu, F.; Guo, H.; Smallwood, G.J. The chemical effect of CO2 replacement of N2 in air on the burning velocity of CH4 and H2 premixed flames. Combust. Flame 2003, 133, 495–497. [Google Scholar] [CrossRef]
- Liu, F.; Consalvi, J.; Fuentes, A. Effects of water vapor addition to the air stream on soot formation and flame properties in a laminar coflow ethylene/air diffusion flame. Combust. Flame 2014, 161, 1724–1734. [Google Scholar] [CrossRef]
- Wang, P.; Guo, T.; Xu, H.; Zhao, Y.; Meng, S.; Feng, D.; Sun, S. Study on the effect of H2O on the formation of CO in the counterflow diffusion flame of H2/CO syngas in O2/H2O. Fuel 2018, 234, 516–525. [Google Scholar] [CrossRef]
- Glarborg, P.; Bentzen, L.L.B. Chemical Effects of a High CO2 Concentration in Oxy-Fuel Combustion of Methane. Energy Fuel 2008, 22, 291–296. [Google Scholar] [CrossRef]
- Galmiche, B.; Halter, F.; Foucher, F.; Dagaut, P. Effects of Dilution on Laminar Burning Velocity of Premixed Methane/Air Flames. Energy Fuel 2011, 25, 948–954. [Google Scholar] [CrossRef]
- Hu, E.; Jiang, X.; Huang, Z.; Iida, N. Numerical Study on the Effects of Diluents on the Laminar Burning Velocity of Methane–Air Mixtures. Energy Fuel 2012, 26, 4242–4252. [Google Scholar] [CrossRef]
- Hu, X.; Yu, Q.; Liu, J. Chemical effect of CO2 on the laminar flame speeds of oxy-methane mixtures in the condition of various equivalence ratios and oxygen concentrations. Int. J. Hydrogen Energy 2016, 41, 15068–15077. [Google Scholar] [CrossRef]
- Shih, H.; Hsu, J. Dilution effects analysis of opposed-jet H2/CO syngas diffusion flames. Proc. Combust. Inst. 2005, 30, 1–19. [Google Scholar] [CrossRef]
- Shih, H.; Hsu, J. A computational study of combustion and extinction of opposed-jet syngas diffusion flames. Int. J. Hydrogen Energy 2011, 36, 15868–15879. [Google Scholar] [CrossRef]
- Xiang, L.; Jiang, H.; Ren, F.; Chu, H.; Wang, P. Numerical study of the physical and chemical effects of hydrogen addition on laminar premixed combustion characteristics of methane and ethane. Int. J. Hydrogen Energy 2020, 45, 20501–20514. [Google Scholar] [CrossRef]
- Xiang, L.; Chu, H.; Ren, F.; Gu, M. Numerical analysis of the effect of CO2 on combustion characteristics of laminar premixed methane/air flames. J. Energy Inst. 2019, 92, 1487–1501. [Google Scholar] [CrossRef]
- Shih, H. Computed extinction limits and flame structures of H2/O2 counterflow diffusion flames with CO2 dilution. Int. J. Hydrogen Energy 2009, 34, 4005–4013. [Google Scholar] [CrossRef]
- Lutz, A.E.; Kee, R.J.; Grcar, J.F.; Rupley, F.M. OPPDIF: A Fortran Program for Computing Opposed-Flow Diffusion Flames; Sandia National Labs.: Livermore, CA, USA, 1997. [CrossRef]
- Smith, G.P.; Golden, D.M.; Frenklach, M.; Moriarty, N.W.; Eiteneer, B.; Goldenberg, M.; Bowman, C.T.; Hanson, R.K.; Song, S.; Gardiner, W.C., Jr. GRI-Mech 3.0 [J]. 1999. Available online: http://www.me.berkeley.edu/gri_mech/ (accessed on 9 February 2021).

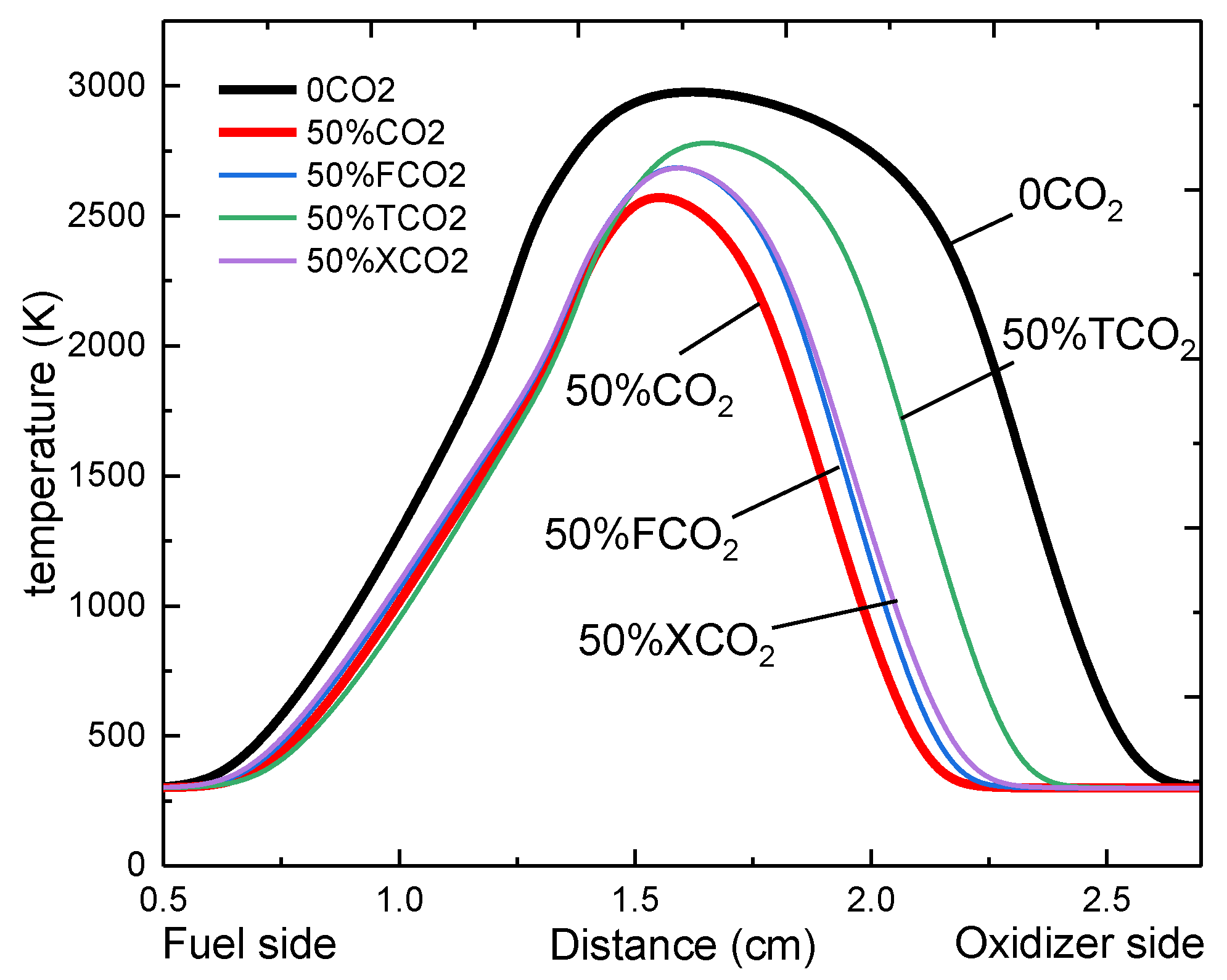
| Chemical | Thermal | Transport | |
|---|---|---|---|
| CO2 | √ | √ | √ |
| FCO2 | - | √ | √ |
| TCO2 | - | N2 | √ |
| XCO2 | - | √ | N2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Wang, J.; Zhang, X.; Li, C. Comparisons of the Uncoupled Effects of CO2 on the CH4/O2 Counterflow Diffusion Flame under High Pressure. Appl. Sci. 2021, 11, 1768. https://doi.org/10.3390/app11041768
Chen Y, Wang J, Zhang X, Li C. Comparisons of the Uncoupled Effects of CO2 on the CH4/O2 Counterflow Diffusion Flame under High Pressure. Applied Sciences. 2021; 11(4):1768. https://doi.org/10.3390/app11041768
Chicago/Turabian StyleChen, Ying, Jingfu Wang, Xiaolei Zhang, and Conghao Li. 2021. "Comparisons of the Uncoupled Effects of CO2 on the CH4/O2 Counterflow Diffusion Flame under High Pressure" Applied Sciences 11, no. 4: 1768. https://doi.org/10.3390/app11041768
APA StyleChen, Y., Wang, J., Zhang, X., & Li, C. (2021). Comparisons of the Uncoupled Effects of CO2 on the CH4/O2 Counterflow Diffusion Flame under High Pressure. Applied Sciences, 11(4), 1768. https://doi.org/10.3390/app11041768






