Abstract
The present study aimed to determine the kinetics of pollutant removal in biofilters with LECA filling (used as a buffer to prevent de-icing agents from being released into the environment with stormwater runoff). It demonstrated a significant effect of temperature and a C/N ratio on the rate of nitrification, denitrification, and organic compound removal. The nitrification rate was the highest (0.32 mg N/L·h) at 25 °C and C/N = 0.5, whereas the lowest (0.18 mg N/L·h) at 0 °C and C/N = 2.5 and 5.0. Though denitrification rate is mainly affected by the available quantity of organic substrate, it actually decreased as the C/N increased and was positively correlated with the temperature levels. Its value was found to be the highest (0.31 mg N/L·h) at 25 °C and C/N = 0.5, and the lowest (0.18 mg N/L·h) at 0 °C and C/N = 5.0. As the C/N increased, so did the content of organic compounds in the treated effluent. The lowest organic removal rates were noted for C/N = 0.5, ranging between 11.20 and 18.42 mg COD/L·h at 0 and 25 °C, respectively. The highest rates, ranging between 27.83 and 59.43 mg COD/L·h, were recorded for C/N = 0.5 at 0 and 25 °C, respectively.
1. Introduction
Air travel is considered to be one of the safest and fastest means of transport. The aviation industry stimulates the economic/cultural growth and creates many jobs. However, the day-to-day airport operations generate air, water, and soil pollution [1]. One of the main issues is the water runoff containing pollutants from runways, taxiways, washing and de-/anti-icing pads, trans-shipment points, fuel storage stations and hangars. These wastewater types contain petroleum-derived substances, surfactants, pavement de-icers, aircraft de-icing/anti-icing agents, and other organic and inorganic pollutants. In climate zones at risk of icing, the pollution caused by pavement de-icing poses a severe environmental problem. Urea, acetate, and sodium formate in the solid form, and acetate and potassium formate in the liquid form are the agents most commonly used for winter maintenance of airport pavements [2]. They release organic compounds and nitrogen into the environment while also increasing the salinity of water solutions and, thereby the salinity of areas adjacent to airports. Both the temperature [3] and the C/N ratio [4] are factors that significantly influence nitrogen conversion and contaminant removal in biofilters. Consequently, they have a major impact on the capacity to treat airport de-icing wastewater and protect the environment.
The issue of the treatment of wastewater containing de-icing agents remains unresolved. Most airports are not equipped with a wastewater treatment system. Only a few airports in the world have wetlands, which effectively remove pollutants. Unfortunately wetlands create favorable environmental conditions for birds, which may endanger airport operations. Other rarely used solutions include filters with zeolite and perlite, media made of crushed clay and granular activated carbon, a mixture of granular activated alumina and porous concrete, granular activated lignite, half-burnt dolomite, and granular ferric hydroxides. Considering sustainable development principles, the best solution would be to use the filling made of waste materials, e.g., light weight aggregates prepared from fly ash from sewage sludge thermal treatment. It is characterized by a large specific surface, resistance to physicochemical factors, low heat conductivity, good phosphorus-sorption properties, and facilitates the deammonification process. These attributes provide good conditions for biofilm growth even at low temperatures [5,6].
The breakdown of urea into ammonium (NH4+) or ammonia (NH3) is a well-known process that occurs in both aerobic and anaerobic conditions at a wide range of pH, temperature, and C/N values [7]. The resultant processes of nitrogen reduction and oxidation affect the natural environment.
The biological oxidation of NH4+ or ammonia NH3 to nitrate is known as nitrification. The nitrification of ammonium in a biofilter is a two-step process in which ammonium or ammonia is first converted to nitrite (NO2−) and then to nitrate (NO3−). Its conversion to nitrite is mainly conducted by a group of obligatory autotrophic bacteria. Also, a few heterotrophs have been reported to carry out nitrification in the environment, but usually at much lower rates than autotrophic bacteria [8]. Nitrification allows converting a relatively immobile ammonium-nitrogen to highly mobile nitrate, affecting environmental quality [9]. The most effective pathway for N removal from wastewater is nitrification followed by denitrification [4]. The biological denitrification is conducted by denitrifying microbes which use nitrate as a terminal electron acceptor, and organic and inorganic substances as electron donors and energy sources for sustaining the microbial growth [10]. In the course of autotrophic denitrification, microorganisms use sulfur compounds, hydrogen, and/or iron as energy sources, and carbon dioxide and hydrocarbons as carbon sources. Microorganisms that use organic carbon compounds are the most common denitrifiers in nature [11]. The heterotrophic biological denitrification is considered more economical, implementable on a large scale, and allowing for the ultimate reduction of nitrate to nitrogen gas with high selectivity [12]. The presence of organic compounds in wastewater significantly affects nitrification and denitrification, with a low organic content promoting effective nitrification [13] and a low C/N ratio significantly reducing denitrification [4]. The denitrification rate is also determined by the availability of nitrification products (nitrites and nitrates). Furthermore, the rate of biochemical conversion and the microbial activity are directly affected by the temperature of the wastewater treatment.
Some of these parameters may be regulated easily, but due to the high specific heat capacity of water, it is nearly impossible to influence temperature. Nitrification and denitrification are elements of the N-cycle critical to the removal of nitrogen from the treatment system. While the removal of organic compounds and nitrogen from municipal wastewater has been the subject of much research and is relatively well explored, there is limited knowledge on how to eliminate such pollution from airport wastewater. The previous authors’ research has shown that biofilters LECA filling could be an effective method for removing nitrogen and organic compounds from wastewater containing airfield deicing fluids. The nitrogen compounds were removed as a result of the simultaneous process of nitrification and denitrification, where the organic compounds present in the treated wastewater served as a carbon source [5,14]. To that end, the present paper provides the findings of a study aimed to determine the transformation kinetics of pollutants generated from airport maintenance, providing a means to estimate the retention time for de-icing wastewater in filters located near airports or in individual, local systems for removing such pollutants. The study was conducted under four different temperature profiles (0 °C, 4 °C, 8 °C, and 25 °C) and at 3 loading rates of de-icing agents used for winter maintenance of airport pavements, with C/N ratios of 0.5, 2.5, and 5.0.
2. Materials and Methods
2.1. Experimental Model
The experiment was performed in laboratory-scale models of biofilters operated at the Department of Environmental Engineering at the University of Warmia and Mazury in Olsztyn (Katedra Inżynierii Środowiska UWM w Olsztynie). The biofilters were loaded with a granulate having the structure of expanded-clay aggregate (with a diameter d60 = 8.2 mm, d60/d10 = 2.27, bulk density of 0.88 g/cm3, hydraulic conductivity of Kh 0.52 cm/s and resistance to crushing of 12.6 N/mm2) prepared from fly ash from sewage sludge thermal treatment in the “Dębogórze” Wastewater Treatment Plant in Gdynia (Poland). The granulate was prepared according to the method of mechanical plasticization and fragmentation of the raw material, followed by sintering of small balls in a rotary kiln at 1200 °C [15]. Biofilters consisted of a cylindrical polyethylene pipe and a cone-shaped bottom. In the bottom of the biofilter, which was outflow part, was a drain valve used to collect samples of wastewater. The technical parameters of the biofilter were: surface area 95 cm2, volume 2500 cm3, active volume 1552 cm3, total height 0.24 m and biofilter filling height 0.19 m [6]. Four temperature variants were adopted: 0 °C, 4 °C, 8 °C, and 25 °C (control biofilter). The experiment was conducted at C/N levels of 0.5, 2.5, and 5.0. C/N ratio affects the efficiency of the biofilters (in relation to C and N removal). The C/N ratio applied in the study, was aimed to determine the kinetics under conditions favoring nitrification (C/N = 0.5), denitrification (C/N = 5.0) and intermediate conditions (C/N = 2.5). Stable operating temperatures were maintained by a thermostatic chamber that regulated the temperature with an accuracy of ± 0.1 °C. Synthetic wastewater, prepared from a weighted sample including commonly used agents for de-icing airport pavements and tap water, was the substrate for the experiments. The composition and physicochemical parameters of the wastewater used in the study are given in Table 1.

Table 1.
Composition and parameters of the wastewater tested.
To promote nitrification, a low hydraulic load of 5 dm3/m2·d and hydraulic retention time (HRT) of 4 d were adopted, equivalent to the cycle of de-icing agent application in airports. The study on the kinetics of pollutant removal started at the end of technological research lasting four months. This four month research was preceded by three months adaptive period [6]. Samples were collected after 0.5, 6, and 12 h of operation; then, at 12 h intervals for 84 h, with the final sample taken after 96 h of operation (Figure 1). The size of the final sample was 5 cm3.
Figure 1.
Schedule of sampling for the kinetics assay during the experiment.
2.2. Analytical Procedures
Determinations of nitrate concentration, nitrite concentration, ammonium nitrogen concentration, Kjeldahl total nitrogen, and organic compound concentration (COD) were carried out according to the APHA [16]. The concentration of total nitrogen (TN) was analyzed using a TNM-L analyzer (Shimadzu Corporation, Kyoto, Japan) with the “oxidative combustion-chemiluminescence” method.
2.3. Kinetics
The results of the physicochemical testing were used to determine the order of reaction and rates of organic compound removal, ammonia nitrogen oxidation and oxidized nitrogen (nitrites and nitrates) reduction. The reaction rate constant was determined using the Statistica 13.1 PL package (TIBCO Software Inc., Palo Alto, CA, USA).
The ammonia nitrogen oxidation rate was described with the formula:
where Ct.Nox—concentration of oxidized nitrogen after time t [mgN/L]; Ci.Nox—initial concentration of oxidized nitrogen [mgN/L]; kNox—ammonia nitrogen oxidation rate constant [h−1].
Ct.Nox = Ci.Nox + kNox⋅t,
The rate of nitrogen removal through denitrification was described with the formula:
where Ct.Nre—concentration of nitrogen removed through denitrification after time t [mgN/L]; Ci.Nre—initial concentration of nitrogen removed through denitrification [mgN/L]; kNre—oxidized nitrogen removal rate constant [h−1].
Ct.Nre = Ci.Nre + kNre⋅t,
The organic compound removal rate (expressed as COD) was calculated with the formula:
where Ct.COD—concentration of organic compounds after time t [mg/L]; Ci.COD—initial concentration of organic compounds [mg/L]; kCOD—organic compound removal rate constant [h−1].
Ct.COD = Ci.COD ⋅e−kCOD⋅t,
3. Results and Discussion
The present study identified the kinetics of nitrification, denitrification, and organic pollutant removal from wastewater generated by wintertime airport maintenance. The experiment was conducted in four temperature variants and at different doses of the de-icing agents (sodium formate and potassium diacetate) characterized by C/N ratios, with the urea dose being constant. The use of biofilter filling promotes the formation of complex biofilms, providing favorable conditions for the development of both aerobic and anaerobic microorganisms [15]. A diverse range of parameters (which ensures the presence of various bacterial groups with different needs) creates favorable conditions for wastewater bio-treatment [17]. This, in the context of the present study, enabled the removal of organic pollutants and nitrogen species from airport de-icing wastewater. The reaction rates for the experimental variants and the goodness of fit for the model (R2) are given in Supplementary Table S1. Changes in nitrogen concentration due to nitrification and denitrification conformed to the zero-order reaction formula, whereas the organic removal rate followed the first-order reaction formula. A comparison between the reaction rates by temperature and the biofilter’s organic load was used to identify the impact of these parameters on the biofilter performance (Figure 2).
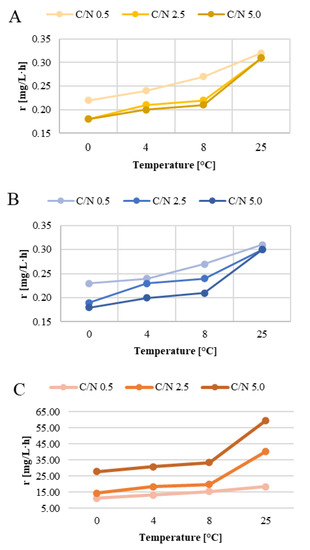
Figure 2.
Effect of temperature and C/N on the rates of nitrification (A), denitrification (B), and organic pollutant removal (C).
3.1. Nitrification
The nitrifier’s activity is very temperature-sensitive [3]. However, exposure to low temperatures did not impact it to the point of halting ammonia nitrogen oxidation, which followed the zero-order reaction, meaning that changes in activity levels over time were linear. Similar findings have been reported in the literature, indicating that longer HRTs may be used to improve removal performance at low temperatures [18]. The rate and efficiency of nitrification of airport de-icing wastewater increased at higher biofilter operating temperatures. In contrast, the higher organic matter content in series 2 and 3 caused a slight reduction in the ammonia nitrogen oxidation rate at lower biofilter operating temperatures (Figure 2 and Figure 3; Supplementary Figures S1–S3). At C/N = 0.5 group, the highest nitrification rate in the entire series, i.e., 0.32 mgN/L·h, was determined in the biofilter operating at 25 °C. Lowering biofilter operating temperatures, with the other technical parameters being equal, reduced the process rate by 31.3% (to 0.22 mgN/L·h) at 0 °C. In the two remaining reactors (T = 4 and 8 °C), the nitrification rates were 25.0 and 15.6% lower, respectively. It is noteworthy that the effect of temperature on reaction kinetics is much harder to determine in biofilm reactors than in suspended-growth biomass, and not as drastic as predicted by the van’t Hoff-Arrhenius equation [19]. Other factors, such as the reactor operating parameters, HRT or wastewater composition, may lessen the impact of temperature [20]. While lower temperatures do reduce nitrification rates, they also increase dissolved oxygen concentration in the water, meaning that the effect of temperature is mitigated by the higher oxygen availability. Thus, the resultant reduction in the biofilm nitrification rate was ultimately negligible (1.108% per 1 °C) [19]. The decreases in nitrification rates produced in the present experiment were similar. Autotrophic nitrification rates are, in large part, driven by high levels of readily digestible organic compounds, which promote heterotrophic bacterial growth [21]. According to De Pra et al. [22], fast-growing heterotrophic microorganisms compete for oxygen and ammonia nitrogen with nitrifying bacteria, and their metabolic intermediates may inhibit the activity of the latter. To achieve optimal nitrification conditions, the influent organic carbon must be kept under 2 kg COD/m3·d [22]. In an experiment by Zafarzadeh et al. [23], the highest nitrification rates were produced at C/N ratios under 6. Another study, by Mosquera-Corral et al. [24], showed that C/N values as low as 0.3 led to the competition between heterotrophs and autotrophs, impairing nitrification. In turn, Young et al. [25] reported that the inhibiting effect of high C/N ratios on ammoniacal nitrogen removal from treated wastewater was more pronounced at lower temperatures. This finding is corroborated by the present study, which showed that the reductions in nitrification rate and efficiency were higher at the lowest of the test temperatures (T = 0 °C), but minor at 25 °C and increased organic compound load. In series 2 (C/N = 2.5), the nitrification rate was similar to that of series 1 (0.31 mgN/L·h at 25 °C). As in series 1, the lowest nitrification rate, reaching 0.18 mgN/L·h (41.94% lower), was observed at the lowest treatment temperature (0 °C). The nitrification rates were similar in the 8 °C and 4 °C variants, reaching 0.22 and 0.21 mgN/L·h, respectively. With the higher organic carbon levels (C/N = 5) in series 3, the nitrification rate remained stable at 0.31 mgN/L·h in the control reactor (T = 25 °C). In the other reactors analyzed in this series, it was approx. one-third lower than in the control one and ranged from 0.18 mgN/L·h at 0 °C to 0.21 mg N/L·h at 8 °C.
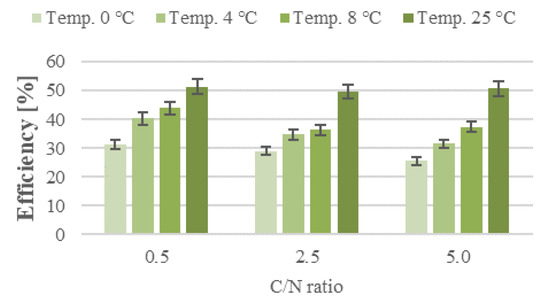
Figure 3.
Nitrification efficiency in the experiment depending on temperature and C/N ratio in storm-water containing airport de-icing agents.
The high nitrification efficiency obtained for wastewater samples with the higher organic compound levels may be explained by heterotrophic nitrification. Guo et al. [26] reported that numerous heterotrophic microorganisms (which are not limited to bacteria but extend to fungi and plants as well) were capable of nitrifying organic and inorganic nitrogen compounds. Such microorganisms grow faster, require lower oxygen concentrations, and tolerate higher C/N ratios than autotrophic nitrifying bacteria.
3.2. Nitrogen Removal
The activity of heterotrophic denitrifying bacteria is less dependent on the temperature than that of nitrifying bacteria [27]. Kadlec and Reddy [28] reported that 20–35 °C was the optimal temperature range for denitrification, which slows down considerably at temperatures lower than 10 °C and greater than 30 °C. Champagne et al. [29] noted that providing a source of readily biodegradable organic carbon helped maintain high nitrification efficiencies even at temperatures under 10 °C. In the present study, the denitrification rate increased at higher temperatures and decreased at higher C/N ratios (Figure 2; Supplementary Figures S4–S6), even though the presence of a readily available source of organic carbon is one of the main drivers of effective denitrification [30,31]. In series 1 (C/N = 0.5), oxidized nitrogen was reduced in the control reactor (T = 25 °C) at a rate of 0.31 mgN/L·h. Nitrogen removal was impeded by lowered biofilter operating temperatures, with the other technical parameters being equal. The lowest oxidized nitrogen reduction rates, at 0.22 mgN/L·h, were recorded for the biofilter operating at 0 °C. The denitrification rate was 0.30 mgN/L·h at 25 °C in series 2 and 3 (C/N = 2.5 and 5.0). Lowering the biofilter operating temperature to 0 °C reduced the performance by 36.7% and 40.0% against the control, respectively. These findings indicate that the nitrogen removal rates recorded in the study across the different experimental series did not correlate directly with the processing temperature or the C/N ratio—instead, the nitrification rate and efficiency served as the main factors. This pattern was the most strikingly evident in series 3, where the biofilters were fed with wastewater having the highest C/N ratio and were operating at optimal temperature conditions (T = 25 °C). A decreased ammonia nitrogen concentration in the effluent is indicative of intensive nitrification in the reactors. The difference between oxidized ammonia nitrogen and the nitrate/nitrite concentration in the treated wastewater shows how much nitrogen was removed. High rates of ammonia nitrogen oxidation and a low nitrite/nitrate content in the biofilter effluent, which did not exceed 12% TN in series 1 (no available organic substrate after approx. 24 h; Supplementary Figure S7) and 8% TN in the other series, suggest that nitrification and denitrification occurred simultaneously (Figure 4 and Figure 5). The main factor enabling simultaneous nitrification and denitrification is the gradient of dissolved oxygen levels, determined by inhibited diffusion in the film [32]. Puznava et al. [33] found that a dissolved oxygen concentration of 0.5–3.0 mgO2/dm3 reduced oxygen penetration and enabled denitrification in the internal layers. The authors obtained a denitrification efficiency of 71%, with the simultaneous nitrification reaching 96–98%. Another study, by Zinatizadeh and Ghaytoolin [34] obtained 46–50% nitrogen removal through simultaneous nitrification and denitrification at dissolved oxygen levels between 2.5 and 3.0 mgO2/dm3, depending on the filling used in the MBBR. The nitrogen removal rates recorded in the presented study ranged from 30.1 ± 1.5% (T = 0 °C) to 43.0 ± 2.1% (T = 25 °C) in series 1 (C/N = 0.5). In the series with the higher organic compound contents (higher C/N ratios), the biofilters operating between 0 and 8 °C exhibited lower performance. The nitrogen removal rate ranged from 26.5 ± 1.3% (T = 0 °C) to 35.9 ± 1.8% (T = 8 °C) for C/N = 2.5, and from 25.0 ± 1.3% (T = 0 °C) to 31.2 ± 1.6% (T = 8 °C) for C/N = 5.0. In the case of the biofilter operating at 25 °C, it was higher and reached 45.9 ± 2.3% and 46.7 ± 2.3% at C/N = 2.5 and 5.0, respectively.
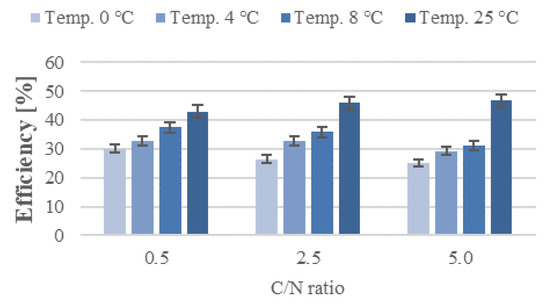
Figure 4.
Effectiveness of nitrogen removal in the experiment depending on temperature and C/N ratio in storm-water containing airport de-icing agents.
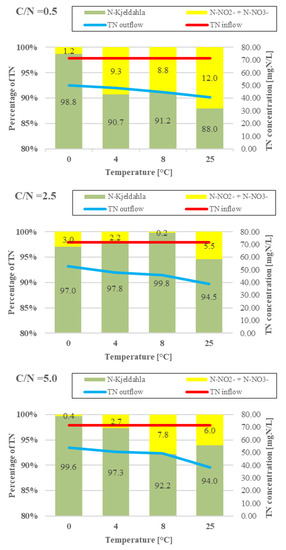
Figure 5.
TN and nitrate/nitrite in the biofilter effluent (column diagram—percentage of TN; line diagram—TN concentration).
3.3. Organic Removal Rate
The temperature not only affects the rate of substrate diffusion into the cell, but also the activity of enzymes, and thus the rate of biochemical processes, which determine how quickly pollutants are biodegraded. What is more, microorganisms are able to grow and metabolize organic compounds as long as the liquid pollutant is provided, meaning that organic compounds can be efficiently removed from wastewater even at low temperatures [35]. It is believed that aerobic digestion of organic compounds proceeds at exponentially increasing rates as the temperature rises from 0 °C to 32 °C. Its rate is stable between 32 and 40 °C, then declines sharply, reaching zero at 45 °C. This correlation holds true in the systems where the process rate is not limited by substrate concentration [36]. In addition, biofilms are less susceptible to adverse variations in temperature than activated sludge systems [37]. The rates of organic compound removal obtained in the present study increased with the C/N ratios and biofilter operating temperatures (Figure 2; Supplementary Figures S7–S9). The lowest rates of organic pollutant removal were recorded for series 1 reactors (C/N = 0.5), among which the control one (T = 25 °C) produced the highest reaction rate at 21.33 mgCOD/L·h. Lowering biofilter operating temperatures to 8, 4, and 0 °C, with the other technical parameters being equal, reduced its performance by 18.4, 32.7, and 48.0%, respectively. Increasing organic carbon (C/N = 2.5) in the T = 0 °C biofilter decreased organic compound removal by 65.5%, compared with the removal rates produced at 25 °C. The reaction rates were 51.8 and 57.3% lower in the other temperature variants (8 and 4 °C, respectively). The highest reaction rates were noted in the series with the greatest initial organic pollutant levels (C/N = 5.0). The peak value of organic compound removal, reaching 58.03 mgCOD/L·h, was reached in the control bioreactor (T = 25 °C). As in the C/N = 0.5 and 2.5 series, the organic removal performance in series 3 was the poorest at 0 °C (26.76 mgCOD/L·h)—being 53.9% lower than at T = 25 °C. The biofilters operating at 8 and 4 °C produced 41.5 and 45.7% lower reaction rates, respectively. Efficiencies and reaction rates were the highest in biofilters operating at 25 °C, and the lowest values in these operating at 0 °C (Figure 6).
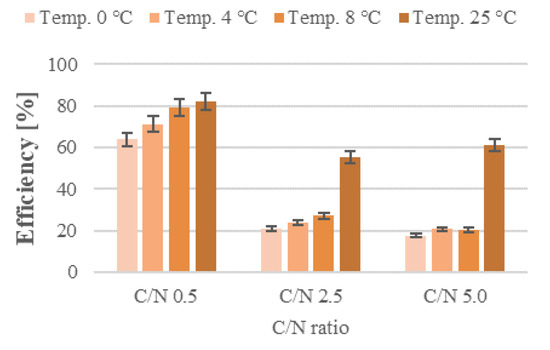
Figure 6.
Efficiency of organic compound removal from stormwater containing airport de-icing agents.
However, increased C/N ratios in the subsequent series also led to higher initial levels of organic compounds in the influent, necessitating the removal of higher pollutant loads. This caused the organic compound removal rates to decrease as the C/N increased, with the sole exception of the biofilter operating at T = 25 °C and C/N = 5.0, which ensured an increase in organic pollutant removal rate from 55.4 ± 2.8% (C/N = 2.5) to 61.2 ± 3.1% (C/N = 5.0). Nevertheless, the pollutant levels (as expressed by COD) observed were the highest in series 3 (289.3–614.0 mgCOD/L), and the lowest in series 1 (17.2–35.1 mgCOD/L). These findings are in line with literature data, which indicate that as the organic pollutant load increases, so does the COD of the treated effluent. Thus, one way to improve the treatment efficiency at low temperatures is to reduce the organic load rate [37].
4. Conclusions
The study showed that the rates of nitrification, denitrification, and organic compound removal were determined not only by the reactor operating temperature, but also by the organic carbon content (C/N ratios) in treated wastewater. Reaction rates were found to decrease at progressively lower temperatures in all of the experimental series. In terms of nitrification, the reaction rates fell as the organic compound levels (sodium formate and potassium acetate) rose. The nitrification rate was the highest (0.32 mg N/L·h) at 25 °C and C/N = 0.5, whereas the lowest rate (0.18 mg N/L·h) was produced at 0 °C for C/N = 2.5 and 5.0. The impact of the higher C/N ratios was more evident at lower biofilter operating temperatures. The study did not indicate that increased organic substrate levels led to improved denitrification rates, which were instead linked to the nitrification efficiency. The denitrification rate was found to be the highest (0.31 mg N/L·h) at 25 °C and C/N = 0.5, and the lowest (0.18 mg N/L·h) at 0 °C and C/N = 5.0. The lowest organic removal rates were noted for C/N = 0.5, ranging between 11.20 and 18.42 mg COD/L·h at 0 and 25 °C, respectively. The highest rates were recorded for C/N = 0.5, ranging between 27.83 and 59.43 mg COD/L·h at 0 and 25 °C, respectively. These results indicate that a treated wastewater with C/N = 0.5 does not inhibit the activity of nitrifying bacteria and actually reduces organic pollutant levels in the effluent, while also creating optimal conditions for simultaneous nitrification and denitrification, as long as suitable biofilter filling is provided.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3417/11/4/1724/s1, Figure S1: Effect of temperature on the nitrification rate—series 1 (C/N = 0.5), Figure S2: Effect of temperature on the nitrification rate—series 2 (C/N = 2.5), Figure S3: Effect of temperature on the nitrification rate—series 3 (C/N = 5.0), Figure S4: Effect of temperature on the denitrification rate—series 1 (C/N = 0.5), Figure S5: Effect of temperature on the denitrification rate—series 2 (C/N = 2.5), Figure S6: Effect of temperature on the denitrification rate—series 3 (C/N = 5.0), Figure S7: Effect of temperature on the organic removal rate—series 1 (C/N = 0.5), Figure S8: Effect of temperature on the organic removal rate—series 2 (C/N = 2.5), Figure S9: Effect of temperature on the organic removal rate—series 3 (C/N = 5.0), Table S1: Goodness of fit and the rates of nitrification, denitrification, and organic compound removal depending on the adopted operating parameters of the biofilters.
Author Contributions
Conceptualization, W.J. and J.R.; Methodology, J.R.; Software, A.M.; Validation, K.O., J.R. and A.M.; Formal Analysis, J.R.; Investigation, K.O.; Resources, K.O. and W.J.; Data Curation, K.O. and A.M.; Writing—Original Draft Preparation, A.M. and W.J.; Writing—Review & Editing, J.R.; Visualization, A.M.; Supervision, W.J.; Project Administration, K.O.; Funding Acquisition, K.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Project No. 29.610.023 of the University of Warmia and Mazury in Olsztyn, Poland.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Sulej, A.; Polkowska, Z.; Namieśnik, J. Contaminants in airport runoff water in the vicinities of two international airports in Poland. Polish J. Environ. Stud. 2012, 21, 725–739. [Google Scholar]
- Switzenbaum, M.S.; Veltman, S.; Mericas, D.; Wagoner, B.; Schoenberg, T. Best management practices for airport deicing stormwater. Chemosphere 2001, 43, 1051–1062. [Google Scholar] [CrossRef]
- Kinyage, J.P.H.; Pedersen, L.F. Impact of temperature on ammonium and nitrite removal rates in RAS moving bed biofilters. Aquac. Eng. 2016, 75, 51–55. [Google Scholar] [CrossRef]
- Yu, G.; Peng, H.; Fu, Y.; Yan, X.; Du, C.; Chen, H. Enhanced nitrogen removal of low C/N wastewater in constructed wetlands with co-immobilizing solid carbon source and denitrifying bacteria. Bioresour. Technol. 2019, 280, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Rodziewicz, J.; Mielcarek, A.; Janczukowicz, W.; Bryszewski, K.; Ostrowska, K. Treatment of wastewater containing runway de-icing agents in biofilters as a part of airport environment management system. Sustainability 2020, 12, 3608. [Google Scholar] [CrossRef]
- Rodziewicz, J.; Mielcarek, A.; Janczukowicz, W.; Ostrowska, K.; Jóźwiakowski, K.; Bugajski, P.; Jucherski, A. Biofilter with innovative filling for low-temperature treatment of sewage from de-icing airport runways. Sep. Purif. Technol. 2020, 242, 116761. [Google Scholar] [CrossRef]
- Katipoglu-Yazan, T.; Ubay Cokgor, E.; Insel, G.; Orhon, D. Is ammonification the rate limiting step for nitrification kinetics? Bioresour. Technol. 2012, 114, 117–125. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Sahrawat, K.L.; Nakahara, K.; Ishikawa, T.; Kishii, M.; Rao, I.M.; Hash, C.T.; George, T.S.; Srinivasa Rao, P.; Nardi, P.; et al. Biological nitrification inhibition-a novel strategy to regulate nitrification in agricultural systems. In Advances in Agronomy; Elsevier Inc.: Amsterdam, The Netherlands, 2012; Volume 114, pp. 249–302. [Google Scholar]
- Sahrawat, K.L. Factors affecting nitrification in soils. Commun. Soil Sci. Plant Anal. 2008, 39, 1436–1446. [Google Scholar] [CrossRef]
- Ghafari, S.; Hasan, M.; Aroua, M.K. Bio-electrochemical removal of nitrate from water and wastewater-A review. Bioresour. Technol. 2008, 99, 3965–3974. [Google Scholar] [CrossRef] [PubMed]
- Van Rijn, J.; Tal, Y.; Schreier, H.J. Denitrification in recirculating systems: Theory and applications. Aquac. Eng. 2006, 34, 364–376. [Google Scholar] [CrossRef]
- Wang, J.; Chu, L. Biological nitrate removal from water and wastewater by solid-phase denitrification process. Biotechnol. Adv. 2016, 34, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Chen, S. Effects of organic carbon on nitrification rate in fixed film biofilters. Aquac. Eng. 2001, 25, 1–11. [Google Scholar] [CrossRef]
- Rodziewicz, J.; Ostrowska, K.; Janczukowicz, W.; Mielcarek, A. Effectiveness of nitrification and denitrification processes in biofilters treating waste water from de-icing airport runways. Water 2019, 11, 630. [Google Scholar] [CrossRef]
- Rodziewicz, J.; Mielcarek, A.; Janczukowicz, W.; Białowiec, A.; Gotkowska-Płachta, A.; Proniewicz, M. Ammonia Nitrogen Transformations in a Reactor with Aggregate made of Sewage Sludge Combustion Fly Ash. Water Environ. Res. 2016, 88, 715–723. [Google Scholar] [CrossRef]
- Federation, W.E. ; APHA Association. Standard Methods for Examination of Water and Wastewater (Standard Methods for the Examination of Water and Wastewater); American Public Health Association (APHA): Washington, DC, USA, 2012; ISBN 9780875532356. [Google Scholar]
- Mielcarek, A.; Rodziewicz, J.; Janczukowicz, W.; Struk-Sokołowska, J. The impact of biodegradable carbon sources on nutrients removal in post-denitrification biofilm reactors. Sci. Total Environ. 2020, 720, 137377. [Google Scholar] [CrossRef]
- Daija, L.; Selberg, A.; Rikmann, E.; Zekker, I.; Tenno, T.; Tenno, T. The influence of lower temperature, influent fluctuations and long retention time on the performance of an upflow mode laboratory-scale septic tank. Desalin. Water Treat. 2016, 57, 18679–18687. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, S. The impact of temperature on nitrification rate in fixed film biofilters. Aquac. Eng. 2002, 26, 221–237. [Google Scholar] [CrossRef]
- Zhou, H.; Li, X.; Xu, G.; Yu, H. Overview of strategies for enhanced treatment of municipal/domestic wastewater at low temperature. Sci. Total Environ. 2018, 643, 225–237. [Google Scholar] [CrossRef]
- Ge, S.; Wang, S.; Yang, X.; Qiu, S.; Li, B.; Peng, Y. Detection of nitrifiers and evaluation of partial nitrification for wastewater treatment: A review. Chemosphere 2015, 140, 85–98. [Google Scholar] [CrossRef] [PubMed]
- De Prá, M.C.; Kunz, A.; Bortoli, M.; Perondi, T.; Chini, A. Simultaneous removal of TOC and TSS in swine wastewater using the partial nitritation process. J. Chem. Technol. Biotechnol. 2012, 87, 1641–1647. [Google Scholar] [CrossRef]
- Zafarzadeh, A.; Bina, B.; Nikaeen, M.; Attar, H.M.; Khiadani, M.H. Effect of dissolved oxygen and chemical oxygen demand to nitrogen ratios on the partial nitrification/denitrification process in moving bed biofilm reactors. Iran. J. Biotechnol. 2011, 9, 197–205. [Google Scholar]
- Mosquera-Corral, A.; González, F.; Campos, J.L.; Méndez, R. Partial nitrification in a SHARON reactor in the presence of salts and organic carbon compounds. Process Biochem. 2005, 40, 3109–3118. [Google Scholar] [CrossRef]
- Young, B.; Delatolla, R.; Kennedy, K.; Laflamme, E.; Stintzi, A. Low temperature MBBR nitrification: Microbiome analysis. Water Res. 2017, 111, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Peng, Y.; Huang, H.; Wang, S.; Ge, S.; Zhang, J.; Wang, Z. Short- and long-term effects of temperature on partial nitrification in a sequencing batch reactor treating domestic wastewater. J. Hazard. Mater. 2010, 179, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Elgood, Z.; Robertson, W.D.; Schiff, S.L.; Elgood, R. Nitrate removal and greenhouse gas production in a stream-bed denitrifying bioreactor. Ecol. Eng. 2010, 36, 1575–1580. [Google Scholar] [CrossRef]
- Kadlec, R.H.; Reddy, K.R. Temperature Effects in Treatment Wetlands. Water Environ. Res. 2001, 73, 543–557. [Google Scholar] [CrossRef]
- Champagne, P.; Liu, L.; Howell, M. Aerobic Treatment in Cold-Climate Countries. In Current Developments in Biotechnology and Bioengineering: Biological Treatment of Industrial Effluents; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 161–201. ISBN 9780444636768. [Google Scholar]
- Mielcarek, A.; Rodziewicz, J.; Janczukowicz, W.; Dabrowska, D.; Ciesielski, S.; Thornton, A.; Struk-Sokołowska, J. Citric acid application for denitrification process support in biofilm reactor. Chemosphere 2017, 171, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Rodziewicz, J.; Janczukowicz, W.; Mielcarek, A.; Filipkowska, U.; Kłodowska, I.; Ostrowska, K.; Duchniewicz, S. Anaerobic rotating disc batch reactor nutrient removal process enhanced by volatile fatty acid addition. Environ. Technol. 2015, 36, 953–958. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, C.; Rong, H.; Zheng, G.; Zhao, L. The effect of dissolved oxygen concentration (DO) on oxygen diffusion and bacterial community structure in moving bed sequencing batch reactor (MBSBR). Water Res. 2017, 108, 86–94. [Google Scholar] [CrossRef]
- Puznava, N.; Payraudeau, M.; Thornberg, D. Simultaneous nitrification and denitrification in biofilters with real time aeration control. Water Sci. Technol. 2001, 43, 269–276. [Google Scholar] [CrossRef]
- Zinatizadeh, A.A.L.; Ghaytooli, E. Simultaneous nitrogen and carbon removal from wastewater at different operating conditions in a moving bed biofilm reactor (MBBR): Process modeling and optimization. J. Taiwan Inst. Chem. Eng. 2015, 53, 98–111. [Google Scholar] [CrossRef]
- Di Trapani, D.; Christensson, M.; Torregrossa, M.; Viviani, G.; Ødegaard, H. Performance of a hybrid activated sludge/biofilm process for wastewater treatment in a cold climate region: Influence of operating conditions. Biochem. Eng. J. 2013, 77, 214–219. [Google Scholar] [CrossRef]
- Lewandowski, Z.; Boltz, J.P. Biofilms in Water and Wastewater Treatment. In Treatise on Water Science; Elsevier: Amsterdam, The Netherlands, 2011; Volume 4, pp. 529–570. ISBN 9780444531933. [Google Scholar]
- Yao, S.; Ni, J.; Ma, T.; Li, C. Heterotrophic nitrification and aerobic denitrification at low temperature by a newly isolated bacterium, Acinetobacter sp. HA2. Bioresour. Technol. 2013, 139, 80–86. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).