Machine Learning Driven Contouring of High-Frequency Four-Dimensional Cardiac Ultrasound Data
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Ultrasound Data
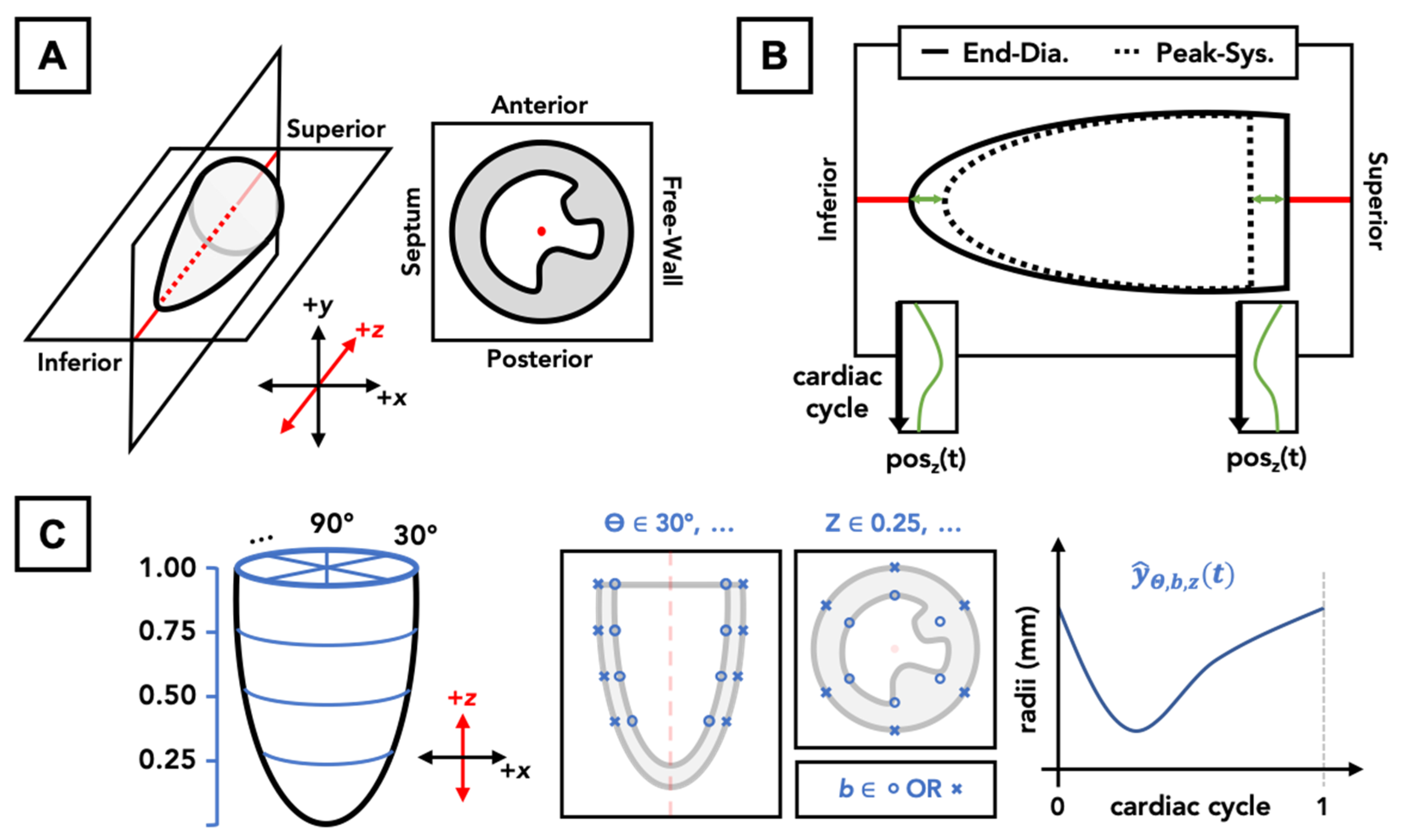
2.2. 4DUS Analysis and Contour Structure
2.3. Machine Learning Algorithms
2.3.1. Prediction Objective
2.3.2. Modeling Approach
2.3.3. Model Variants
2.3.4. Measuring Model Performance
2.4. Description of Metrics Derived from LV Mesh
3. Results and Discussion
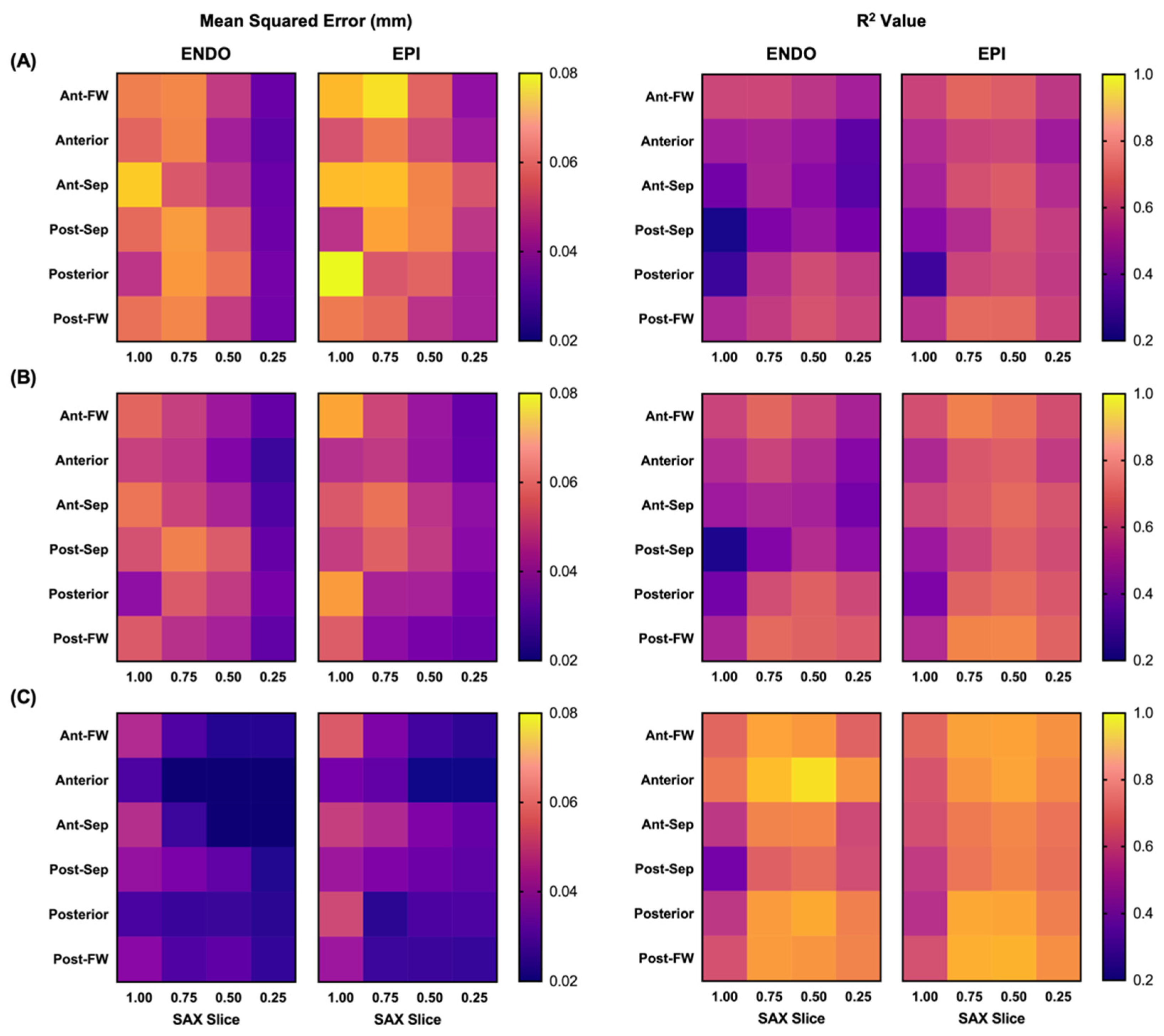
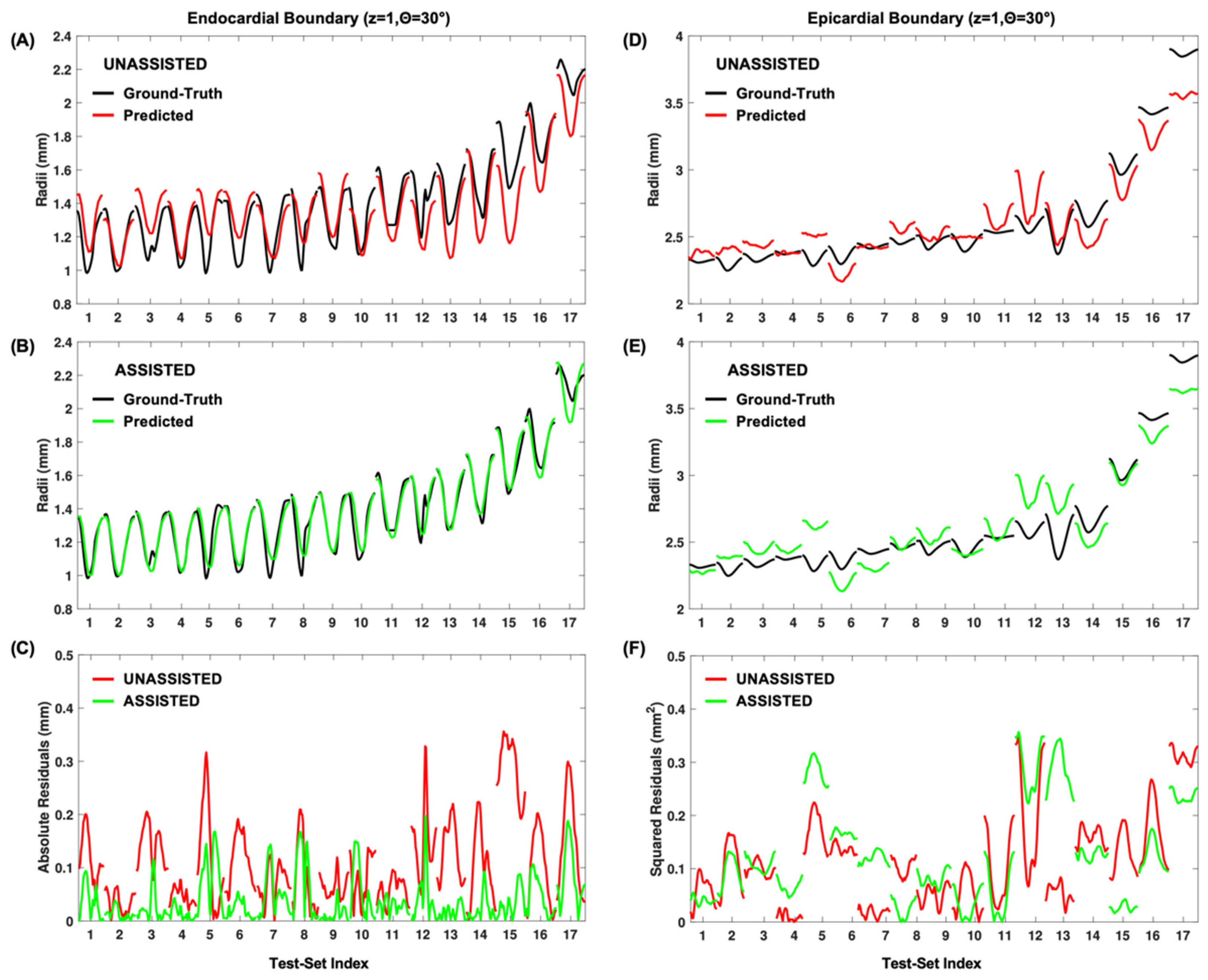
3.1. Model Fitting Results
3.2. Performance of Predication-Based Metrics
3.3. Limitations
3.4. Future Applications
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; Isasi, C.R.; et al. Heart disease and stroke statistics—2017 update: A report from the American heart association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Patten, R.D.; Hall-Porter, M.R. Small Animal Models of Heart Failure: Development of novel therapies, past and present. Circ. Heart Fail. 2009, 2, 138–144. [Google Scholar] [CrossRef] [PubMed]
- McConnell, B.K.; Fatkin, D.; Semsarian, C.; Jones, K.A.; Georgakopoulos, D.; Maguire, C.T.; Healey, M.J.; Mudd, J.O.; Moskowitz, I.P.G.; Conner, D.A.; et al. Comparison of Two Murine Models of Familial Hypertrophic Cardiomyopathy. Circ. Res. 2001, 88, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ceholski, D.K.; Liang, L.; Fish, K.; Hajjar, R.J. Variability in coronary artery anatomy affects consistency of cardiac damage after myocardial infarction in mice. Am. J. Physiol. Circ. Physiol. 2017, 313, H275–H282. [Google Scholar] [CrossRef]
- Muthuramu, I.; Lox, M.; Jacobs, F.; De Geest, B. Permanent Ligation of the Left Anterior Descending Coronary Artery in Mice: A Model of Post-myocardial Infarction Remodelling and Heart Failure. J. Vis. Exp. 2014, 94, 52206. [Google Scholar] [CrossRef] [PubMed]
- James, J.F.; Hewett, T.E.; Robbins, J. Cardiac physiology in transgenic mice. Circ. Res. 1998, 82, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Soepriatna, A.H.; Yeh, A.K.; Clifford, A.D.; Bezci, S.E.; O’Connell, G.D.; Goergen, C.J. Three-dimensional myocardial strain correlates with murine left ventricular remodelling severity post-infarction. J. R. Soc. Interface 2019, 16, 20190570. [Google Scholar] [CrossRef]
- Soepriatna, A.H.; Damen, F.W.; Vlachos, P.P.; Goergen, C.J. Cardiac and respiratory-gated volumetric murine ultrasound. Int. J. Cardiovasc. Imaging 2017, 34, 713–724. [Google Scholar] [CrossRef]
- Damen, F.W.; Berman, A.G.; Soepriatna, A.H.; Ellis, J.M.; Buttars, S.D.; Aasa, K.L.; Goergen, C.J. High Frequency Four-Dimensional Ultrasound (4DUS): A Reliable Method for Assessing Murine Cardiac Function. Tomography 2017, 3, 180–187. [Google Scholar] [PubMed]
- Chen, C.; Qin, C.; Qiu, H.; Tarroni, G.; Duan, J.; Bai, W.; Rueckert, D. Deep Learning for Cardiac Image Segmentation: A Review. Front. Cardiovasc. Med. 2020, 7, 25. [Google Scholar] [CrossRef]
- Leclerc, S.; Smistad, E.; Pedrosa, J.; Ostvik, A.; Cervenansky, F.; Espinosa, F.; Espeland, T.; Berg, E.A.R.; Jodoin, P.-M.; Grenier, T.; et al. Deep Learning for Segmentation Using an Open Large-Scale Dataset in 2D Echocardiography. IEEE Trans. Med. Imaging 2019, 38, 2198–2210. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, Y.; Yang, X.; Lei, B.; Liu, L.; Li, S.X.; Ni, D.; Wang, T. Deep Learning in Medical Ultrasound Analysis: A Review. Engineering 2019, 5, 261–275. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Z.; Zheng, J.; Huang, B.; Voiculescu, I.; Yang, G.-Z. Deep Learning in Medical Ultrasound Image Seg-mentation: A Review. arXiv 2020, arXiv:2002.07703. [Google Scholar]
- Brattain, L.J.; Telfer, B.A.; Dhyani, M.; Grajo, J.R.; Samir, A.E. Machine learning for medical ultrasound: Status, methods, and future opportunities. Abdom. Radiol. 2018, 43, 786–799. [Google Scholar] [CrossRef]
- Akkus, Z.; Cai, J.; Boonrod, A.; Zeinoddini, A.; Weston, A.D.; Philbrick, K.A.; Erickson, B.J. A Survey of Deep-Learning Applications in Ultrasound: Artificial Intelligence–Powered Ultrasound for Improving Clinical Workflow. J. Am. Coll. Radiol. 2019, 16, 1318–1328. [Google Scholar] [CrossRef]
- Dormer, J.D.; Guo, R.; Fei, B.; Shen, M.; Jiang, R.; Wagner, M.B. Ultrasound segmentation of rat hearts using a convolution neural network. In Proceedings of the Medical Imaging 2018: Ultrasonic Imaging and Tomography, Houston, TX, USA, 10–15 February 2018; p. 105801A. [Google Scholar]
- Dey, D.; Slomka, P.J.; Leeson, P.; Comaniciu, D.; Shrestha, S.; Sengupta, P.P.; Marwick, T.H. Artificial Intelligence in Cardiovascular Imaging. J. Am. Coll. Cardiol. 2019, 73, 1317–1335. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Xuan, W.; Kao, E.; Cao, P.; Tian, B.; Ordovas, K.; Saloner, D.; Liu, J. Fully automatic segmentation of 4D MRI for cardiac functional measurements. Med. Phys. 2019, 46, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Berhane, H.; Scott, M.; Elbaz, M.; Jarvis, K.; McCarthy, P.; Carr, J.; Malaisrie, C.; Avery, R.; Barker, A.J.; Robinson, J.D.; et al. Fully automated 3D aortic segmentation of 4D flow MRI for hemodynamic analysis using deep learning. Magn. Reson. Med. 2020, 84, 2204–2218. [Google Scholar] [CrossRef]
- Tran, P.V. A Fully Convolutional Neural Network for Cardiac Segmentation in Short-Axis MRI. arXiv 2016, arXiv:1604.00494. [Google Scholar]
- Commandeur, F.; Goeller, M.; Razipour, A.; Cadet, S.; Hell, M.M.; Kwiecinski, J.; Chen, X.; Chang, H.-J.; Marwan, M.; Achenbach, S.; et al. Fully Automated CT Quantification of Epicardial Adipose Tissue by Deep Learning: A Multicenter Study. Radiol. Artif. Intell. 2019, 1, e190045. [Google Scholar] [CrossRef]
- Militello, C.; Rundo, L.; Toia, P.; Conti, V.; Russo, G.; Filorizzo, C.; Maffei, E.; Cademartiri, F.; La Grutta, L.; Midiri, M.; et al. A semi-automatic approach for epicardial adipose tissue segmentation and quantification on cardiac CT scans. Comput. Biol. Med. 2019, 114, 103424. [Google Scholar] [CrossRef]
- Furtado, M.B.; Wilmanns, J.C.; Chandran, A.; Perera, J.; Hon, O.; Biben, C.; Willow, T.J.; Nim, H.T.; Kaur, G.; Simonds, S.; et al. Point mutations in murine Nkx2-5 phenocopy human congenital heart disease and induce pathogenic Wnt signaling. JCI Insight 2017, 2, e88271. [Google Scholar] [CrossRef] [PubMed]
- Furtado, M.B.; Wilmanns, J.C.; Chandran, A.; Tonta, M.; Biben, C.; Eichenlaub, M.; Coleman, H.A.; Berger, S.; Bouveret, R.; Singh, R.; et al. A novel conditional mouse model for Nkx2-5 reveals transcriptional regulation of cardiac ion channels. Differentiation 2016, 91, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.W.; Guo, G.; Wolstein, O.; Vale, M.; Castro, M.L.; Wang, L.; Otway, R.; Riek, P.; Cochrane, N.; Furtado, M.; et al. Functional Characterization of a Novel Mutation in NKX2-5 Associated with Congenital Heart Disease and Adult-Onset Cardiomyopathy. Circ. Cardiovasc. Genet. 2013, 6, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Pereyra, A.S.; Hasek, L.Y.; Harris, K.L.; Berman, A.G.; Damen, F.W.; Goergen, C.J.; Ellis, J.M. Loss of cardiac carnitine palmitoyltransferase 2 results in rapamycin-resistant, acetylation-independent hypertrophy. J. Biol. Chem. 2017, 292, 18443–18456. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ellis, J.M.; Wolfgang, M.J. Adipose Fatty Acid Oxidation Is Required for Thermogenesis and Potentiates Oxidative Stress-Induced Inflammation. Cell Rep. 2015, 10, 266–279. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, L.; Ruan, L.; Quan, X.-Q.; Yang, J.; Lv, C.-X.; Zhang, C.-T. Speckle tracking echocardiography assessment of global and regional contraction dysfunction in the mice model of pressure overload. Acta Acad. Med. 2015, 35, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Cheng, S.; Jain, M.; Ngoy, S.; Theodoropoulos, C.; Trujillo, A.; Lin, F.-C.; Liao, R. Echocardiographic Speckle-Tracking Based Strain Imaging for Rapid Cardiovascular Phenotyping in Mice. Circ. Res. 2011, 108, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Pistner, A.; Belmonte, S.; Coulthard, T.; Blaxall, B.C. Murine echocardiography and ultrasound imaging. J. Vis. Exp. 2010, 42, e2100. [Google Scholar] [CrossRef]
- Hartley, C.J.; Taffet, G.E.; Reddy, A.K.; Entman, M.L.; Michael, H. Noninvasive cardiovascular phenotyping in mice. ILAR J. 2002, 43, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.S.; McCabe, G.P.; Craig, B.A. Introduction to the Practice of Statistics, 7th ed.; W.H. Freeman & Co.: New York, NY, USA, 2012. [Google Scholar]
- Zhu, J.; Shi, F.; You, T.; Tang, C.; Chen, J. Global diastolic strain rate for the assessment of left ventricular diastolic dysfunction in young peritoneal dialysis patients: A case control study. BMC Nephrol. 2020, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Goodfellow, I.J.; Pouget-Abadie, J.; Mirza, M.; Xu, B.; Warde-Farley, D.; Ozair, S.; Courville, A.; Bengio, Y. Generative Adversarial Networks. arXiv 2014, arXiv:1406.2661. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, H.; Lu, H.; Won, D.; Yoon, S.W. Medical Image Synthesis with Generative Adversarial Networks for Tissue Recognition. In Proceedings of the 2018 IEEE International Conference on Healthcare Informatics (ICHI), New York, NY, USA, 4–7 June 2018; pp. 199–207. [Google Scholar]
- Singh, N.K.; Raza, K. Medical Image Generation Using Generative Adversarial Networks: A Review. Complex Netw. Appl. 2021, 932, 77–96. [Google Scholar]
- Han, C.; Rundo, L.; Araki, R.; Furukawa, Y.; Mauri, G.; Nakayama, H.; Hayashi, H. Infinite Brain MR Images: PGGAN-Based Data Augmentation for Tumor Detection. In Neural Approaches to Dynamics of Signal Exchanges; Esposito, A., Faundez-Zanuy, M., Morabito, F.C., Pasero, E., Eds.; Springer: Singapore, 2020; pp. 291–303. [Google Scholar]
- Yi, X.; Walia, E.; Babyn, P. Generative adversarial network in medical imaging: A review. Med. Image Anal. 2019, 58, 101552. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, M.; Zhu, Z. Automatic Data Augmentation for 3D Medical Image Segmentation. In Constructive Side-Channel Analysis and Secure Design; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 378–387. [Google Scholar]
- Chen, S.; Ma, K.; Zheng, Y. Med3D: Transfer Learning for 3D Medical Image Analysis. arXiv 2019, arXiv:1904.00625. [Google Scholar]
- Muraru, D.; Niero, A.; Rodriguez-Zanella, H.; Cherata, D.; Badano, L. Three-dimensional speckle-tracking echocardiography: Benefits and limitations of integrating myocardial mechanics with three-dimensional imaging. Cardiovasc. Diagn. Ther. 2018, 8, 101–117. [Google Scholar] [CrossRef] [PubMed]

| Endocardial | Epicardial | |||||||
|---|---|---|---|---|---|---|---|---|
| MSE (mm2) | R2 | vs. M1 (%) | vs. M2 (%) | MSE (mm2) | R2 | vs. M1 (%) | vs. M2 (%) | |
| Model 1 | 0.069 ± 0.054 | 0.41 | —- | —- | 0.068 ± 0.044 | 0.51 | —- | —- |
| Model 2 | 0.060 ± 0.049 | 0.49 | 48.7 | —- | 0.058 ± 0.039 | 0.59 | 54.4 | —- |
| Model 3 | 0.030 ± 0.021 | 0.71 | 88.0 | 83.5 | 0.037 ± 0.020 | 0.71 | 81.9 | 71.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damen, F.W.; Newton, D.T.; Lin, G.; Goergen, C.J. Machine Learning Driven Contouring of High-Frequency Four-Dimensional Cardiac Ultrasound Data. Appl. Sci. 2021, 11, 1690. https://doi.org/10.3390/app11041690
Damen FW, Newton DT, Lin G, Goergen CJ. Machine Learning Driven Contouring of High-Frequency Four-Dimensional Cardiac Ultrasound Data. Applied Sciences. 2021; 11(4):1690. https://doi.org/10.3390/app11041690
Chicago/Turabian StyleDamen, Frederick W., David T. Newton, Guang Lin, and Craig J. Goergen. 2021. "Machine Learning Driven Contouring of High-Frequency Four-Dimensional Cardiac Ultrasound Data" Applied Sciences 11, no. 4: 1690. https://doi.org/10.3390/app11041690
APA StyleDamen, F. W., Newton, D. T., Lin, G., & Goergen, C. J. (2021). Machine Learning Driven Contouring of High-Frequency Four-Dimensional Cardiac Ultrasound Data. Applied Sciences, 11(4), 1690. https://doi.org/10.3390/app11041690








