Semi-Continuous Adsorption Processes with Multi-Walled Carbon Nanotubes for the Treatment of Water Contaminated by an Organic Textile Dye
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Adsorbent Columns
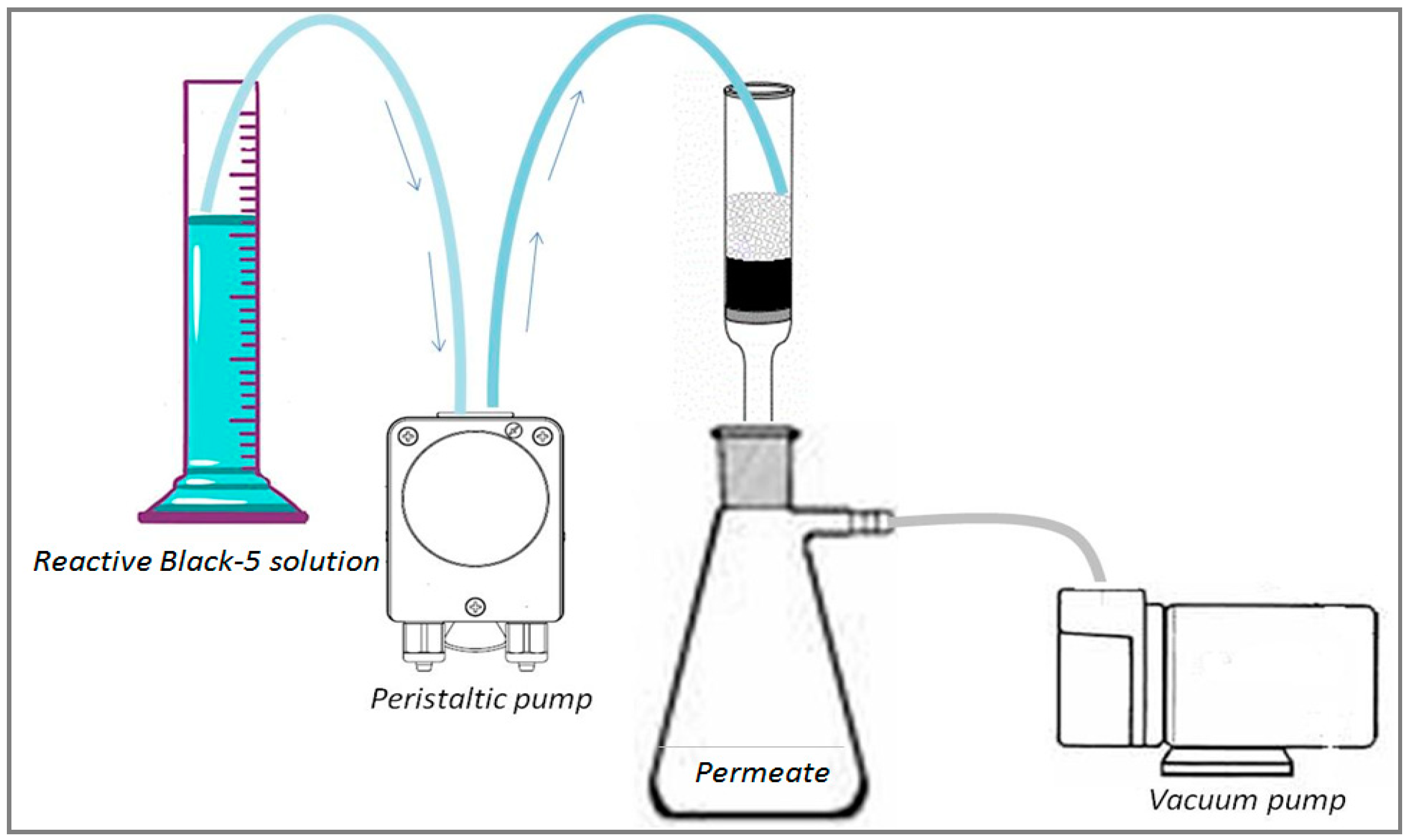
2.3. Adsorption System
2.4. Instruments
3. Results and Discussion
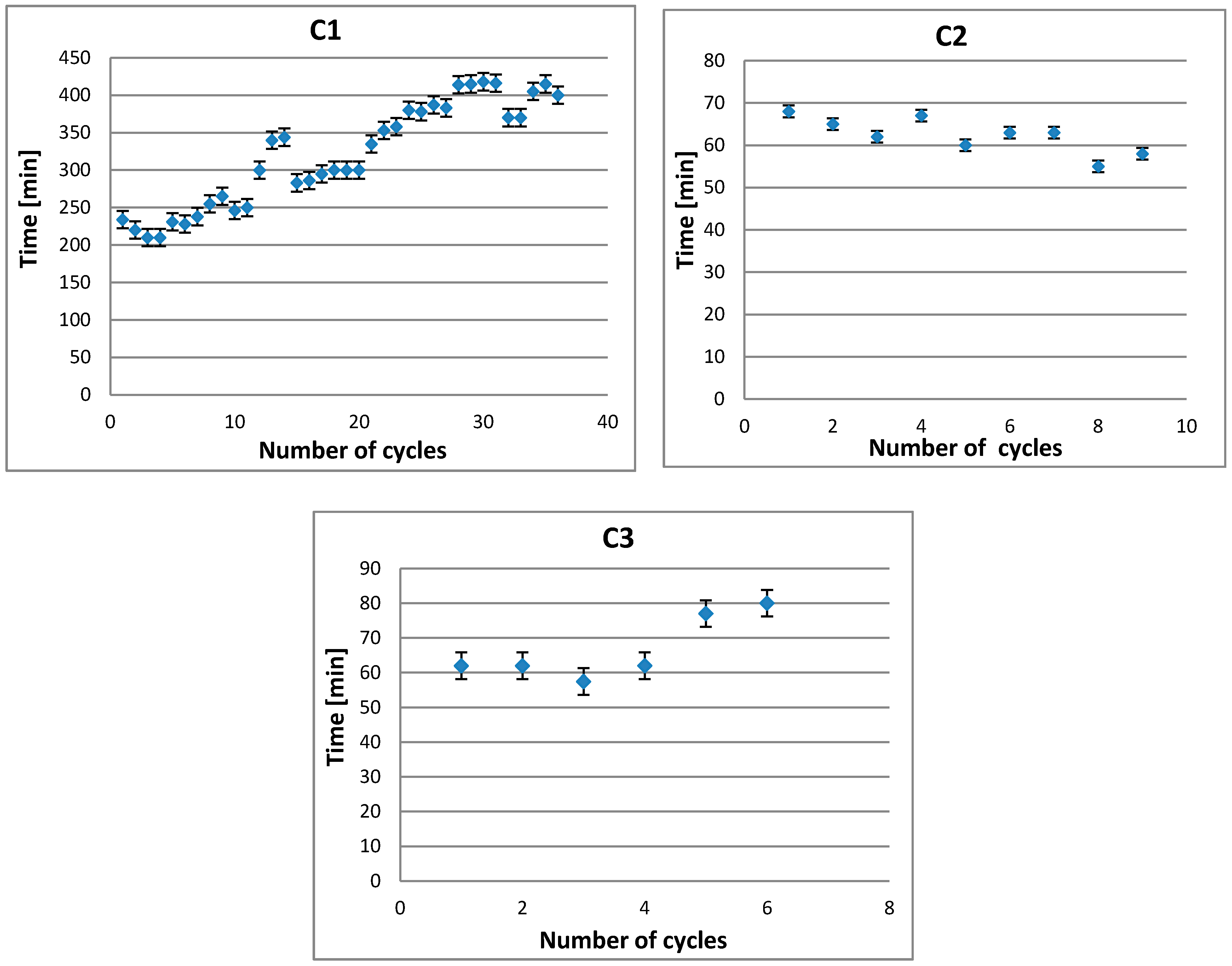
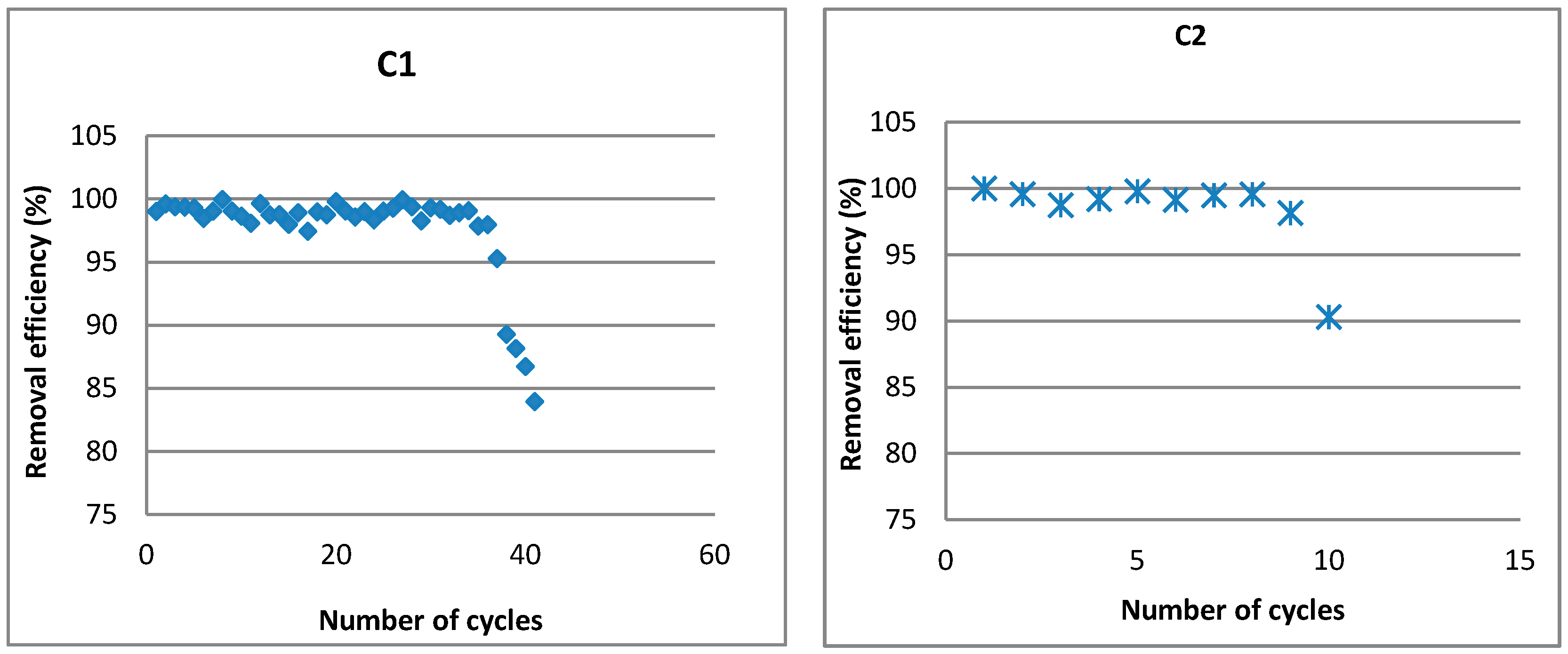
3.1. Evaluation of Hydraulic Parameters
3.2. Characterization of Permeate
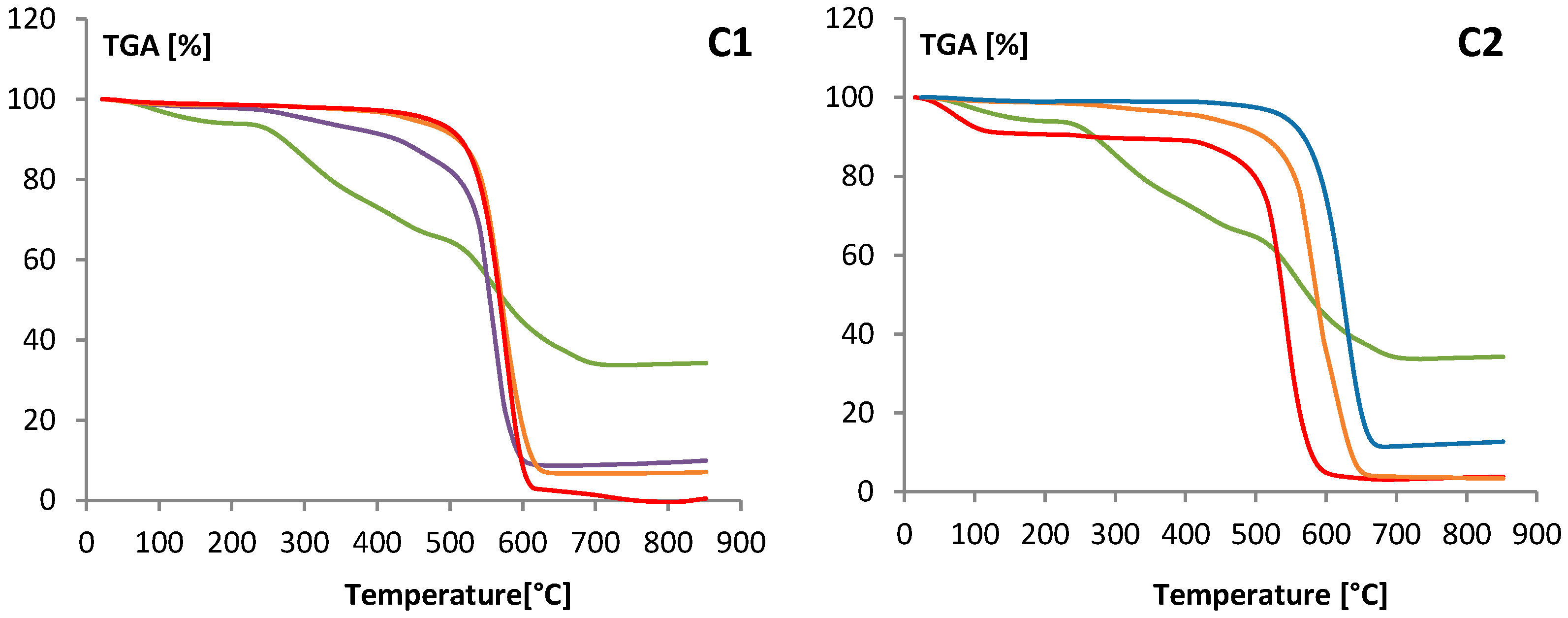
3.3. Characterization of MWCNTs Layer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bizuneh, A. Textile effluent treatment and decolorization techniques—A review. Bulg. J. Sci. Educ. 2012, 21, 434–456. [Google Scholar]
- Baughman, G.L.; Perenich, T.A. Fate of dyes in aquatic systems: I. Solubility and partitioning of some hydrophobic dyes and related compounds. Environ. Toxicol. Chem. 1988, 7, 183–199. [Google Scholar] [CrossRef]
- Srivastava, P.N.; Prakash, A. Bioaccumulation of heavy metals by algae and wheat plants fed by textile effluents. J. Water Pollut. Control Fed. 1991, 7, 25–30. [Google Scholar]
- Işık, M.; Sponza, D.T. Anaerobic/aerobic treatment of a simulated textile wastewater. Sep. Purif. Technol. 2008, 60, 64–72. [Google Scholar] [CrossRef]
- Asad, S.; Amoozegar, M.A.; Pourbabaee, A.A.; Sarbolouki, M.N.; Dastgheib, S.M. Decolorization of textile azo dyes by newly isolated halophilic and halotolerant bacteria. Bioresour. Technol. 2007, 98, 2082–2088. [Google Scholar] [CrossRef]
- Rajendran, R.; Karthik, S.; Sundaram Prabhavathi, P.; Sridevi, B.V.; Gopi, V. Comparative analysis of bioremediation potential of adapted and non-adapted fungi on azo dye containing textile effluent. Pak. J. Biol. Sci. 2011, 14, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Asamudo, N.U.; Daba, A.S.; Ezeronye, O.U. Bioremediation of textile effluent using Phanerochaete Chrysosporium. Afr. J. Biotechnol. 2005, 4, 1548–1553. [Google Scholar] [CrossRef]
- Karataş, M.; Dursun, S. Bio-decolourization of azo-dye under anaerobic batch conditions. J. Int. Environ. Appl. Sci. 2007, 2, 20–25. [Google Scholar]
- Dojcinovic, B.P.; Roglic, G.M.; Obradovic, B.M.; Kuraica, M.M.; Tosti, T.B.; Markovic, M.D.; Manojlovic, D.D. Decolorization of Reactive Black 5 using a dielectric barrier discharge in the presence of inorganic salts. J. Serb. Chem. Soc. 2012, 77, 535–548. [Google Scholar] [CrossRef]
- Pinheiro, H.M.; Touraud, E.; Thomas, O. Aromatic amines from azo dye reduction: Status review with emphasis on direct UV spectrophotometric detection in textile industry wastewaters. Dye. Pigment. 2004, 61, 121–139. [Google Scholar] [CrossRef]
- Carliell, C.M.; Barclay, S.J.; Naidoo, N.; Buckley, C.A.; Mulholland, D.A.; Senior, E. Microbial decolourisation of a reactive azo dye under anaerobic conditions. Water SA 1995, 21, 61–69. [Google Scholar]
- Rezaee, A.; Ghaneian, M.T.; Hashemian, S.J.; Moussavi, G.; Khavanin, A.; Ghanizadeh, G. Decolorization of Reactive Blue 19 Dye from Textile Wastewater by the UV/H2O2 Process. J. Appl. Sci. 2008, 8, 1108–1112. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Prabakaran, G.; Pugalvendhan, R.; Jayaseelan, M. Studies on textile dye degradation by aspergillus niger from dye contaminated soil. Pollut. Res. 2009, 28, 555–560. [Google Scholar]
- Nehra, K.; Anju, M.; Malik, K. Isolation and optimization of conditions for maximum decolorization by textile-dye decolorizing bacteria. Pollut. Res. 2008, 27, 257–264. [Google Scholar]
- Andleeb, S.; Atiq, N.; Ali, M.I.; Razi-Ul-Hussnain, R.; Shafique, M.; Ahamad, B.; Ghumro, P.B.; Ahmad, S. Biological treatment of textile effluent in stirred tank bioreactor. Int. J. Agric. Biol. 2010, 12, 256–260. [Google Scholar]
- Pakshirajan, K.; Singh, S. Decolorization of synthetic wastewater containing azo dyes in a batch-operated rotating biological contactor reactor with the immobilized fungus phanerochaete chrysosporium. Ind. Eng. Chem. Res. 2010, 49, 7484–7487. [Google Scholar] [CrossRef]
- Bafana, A.; Krishnamurthi, K.; Devi, S.S.; Chakrabarti, T. Biological decolorization of C.I. Direct Black 38 by E. gallinarum. J. Hazard. Mater. 2008, 157, 187–193. [Google Scholar] [CrossRef]
- Dawkar, V.V.; Jadhav, U.U.; Jadhav, S.U.; Govindwar, S.P. Biodegradation of disperse textile dye brown 3REL by newly isolated Bacillus sp. VUS. J. Appl. Microbiol. 2008, 105, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Bauman, M.; Poberznik, M.; Lobnik, A. Textile wastewater cleaning with O3 and H2O2/O3 process. Tekstilec 2009, 52, 284–305. [Google Scholar]
- Mahamuni, N.N.; Adewuyi, Y.G. Advanced oxidation processes (AOPs) involving ultrasound for waste water treatment: A review with emphasis on cost estimation. Ultrason. Sonochem. 2010, 17, 990–1003. [Google Scholar] [CrossRef] [PubMed]
- De Luca, P.; Foglia, P.; Siciliano, C.; B.Nagy, J.; Macario, A. Water contaminated by industrial textile dye: Study on decolorization process. Environments 2019, 6, 101. [Google Scholar] [CrossRef]
- Huang, X.; Bo, X.; Zhao, Y.; Gao, B.; Wang, Y.; Sun, S.; Yue, Q.; Li, Q. Effects of compound bioflocculant on coagulation performance and floc properties for dye removal. Bioresour. Technol. 2014, 165, 116–121. [Google Scholar] [CrossRef]
- Bili’nska, L.; Blus, K.; Gmurek, M.; Ledakowicz, S. Coupling of electrocoagulation and ozone treatment for textile wastewater reuse. Chem. Eng. J. 2019, 358, 992–1001. [Google Scholar] [CrossRef]
- Dotto, J.; Fagundes-Klena, M.R.; Veita, M.T.; Palacio, S.M.; Bergamasco, R. Performance of different coagulants in the coagulation/flocculation process of textile wastewater. J. Clean. Prod. 2019, 208, 656–665. [Google Scholar] [CrossRef]
- Zhao, S.; Gao, B.; Yue, Q.; Wang, Y. Effect of Enteromorpha polysaccharides on coagulation performance and kinetics for dye removal. Colloids Surf. A Physicochem. Eng. Asp. 2014, 456, 253–260. [Google Scholar] [CrossRef]
- Cai, T.; Li, H.; Yang, R.; Wang, Y.; Li, R.; Yang, H.; Li, A.; Cheng, R. Efficient flocculation of an anionic dye from aqueous solutions using a cellulose-based flocculant. Cellulose 2015, 22, 1439–1449. [Google Scholar] [CrossRef]
- Altenbaher, B.; Turk, S.S. Treatment of textile processing wastewater with membrane filtrations. J. Text. Cloth. Technol. 2009, 58, 367–383. [Google Scholar]
- Rekha, R.; Chauhan, P.; Gangopadhyay, U.K. Zero effluent process by using membrane type solute separation systems for wet process house. Asian Text. J. 2009, 18, 65–69. [Google Scholar]
- Yun, J.; Jijn, D.; Lee, Y.-S.; Kim, H.-I. Photocatalytic treatment of acidic wastewater by electrospun composite nanofibers of pH-sensitive hydrogel and TiO2. Mater. Lett. 2010, 64, 2431–2434. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Lee, H.-S. Effects of TiO2 coating dosage and operational parameters on a TiO2/Ag photocatalysis system for decolorizing Procion red MX-5B. J. Hazard. Mater. 2010, 179, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-C.; Zhang, Y.; Tao, T.-X.; Zhang, L.; Fong, H. Silver nanoparticles on amidoxime fibers for photo-catalytic degradation of organic dyes in waste water. Appl. Surf. Sci. 2010, 257, 1092–1097. [Google Scholar] [CrossRef]
- De Luca, P.; Candamano, S.; Macario, A.; Crea, F.; B.Nagy, J. Preparation and characterization of plasters with photodegradative action. Buildings 2018, 8, 122. [Google Scholar] [CrossRef]
- Lodha, S.; Jain, A.; Punjabi, P.B. A novel route for waste water treatment: Photocatalytic degradation of Rhodamine B. Arab. J. Chem. 2010, 4, 383–387. [Google Scholar] [CrossRef]
- Mounteer, A.H.; Leite, T.A.; Lopes, A.C.; Medeiros, R.C. Removing textile mill effluent recalcitrant COD and toxicity using the H2O2/UV system. Water Sci. Technol. 2009, 60, 1895–1902. [Google Scholar] [CrossRef] [PubMed]
- Cantiello, A.; Candamano, S.; De Luca, P. Use of Zinc Ferrite for Photocatalytic Treatment of Water Contaminated with Organic Dye. IOP Conf. Ser. Mater. Sci. Eng. 2020, 739, 012054. [Google Scholar] [CrossRef]
- Perego, C.; Bagatin, R.; Tagliabue, M.; Vignola, R. Zeolites and related mesoporous materials for multi-talented environmental solutions. Micropor. Mesopor. Mater. 2013, 166, 37–49. [Google Scholar] [CrossRef]
- Martucci, A.; Leardini, L.; Nassi, M.; Sarti, E.; Bagatin, R.; Pasti, L. Removal of emerging organic contaminants from aqueous systems: Adsorption and location of methyl-tertiary-butylether on synthetic ferrierite. Mineral. Mag. 2014, 78, 1161–1175. [Google Scholar] [CrossRef]
- Northcott, K.A.; Bacus, J.; Taya, N.; Komatsu, Y.; Perera, J.M.; Stevens, G.W. Synthesis and characterization of hydrophobic zeolite for the treatment of hydrocarbon contaminated ground water. J. Hazard. Mater. 2010, 183, 434–440. [Google Scholar] [CrossRef] [PubMed]
- De Luca, P.; Bernaudo, I.; Elliani, R.; Tagarelli, A.; B.Nagy, J.; Macario, A. Industrial Waste Treatment by ETS-10 Ion Exchanger Material. Materials 2018, 11, 2316. [Google Scholar] [CrossRef]
- Vignola, R.; Bagatin, R.; Alessandra De Folly, D.; Flego, C.; Nalli, M.; Ghisletti, D.; Millini, R.; Sisto, R. Zeolites in a permeable reactive barrier (PRB): One year of field experience in a refinery groundwater—Part 1: The performances. Chem. Eng. J. 2011, 178, 204–209. [Google Scholar] [CrossRef]
- De Raffele, G.; Aloise, A.; De Luca, P.; Vuono, D.; Tagarelli, A.; B.Nagy, J. Kinetic and thermodynamic effects during the adsorption of heavy metals on ETS-4 and ETS-10 microporous materials. J. Porous Mater. 2016, 23, 389–400. [Google Scholar] [CrossRef]
- Maretto, M.; Blanchi, F.; Vignola, R.; Canepari, S.; Baric, M.; Iazzoni, R.; Tagliabue, M.; Papini, M.P. Microporous and mesoporous materials for the treatment of wastewater produced by petrochemical activities. J. Clean. Prod. 2014, 77, 22–34. [Google Scholar] [CrossRef]
- De Luca, P.; Mastroianni, C.; B.Nagy, J. Synthesis of self-bonded pellets of ETS-4 phase by new methodology of preparation. IOP Conf. Ser. Mater. Sci. Eng. 2018, 374, 012003. [Google Scholar] [CrossRef]
- Vuono, D.; De Luca, P.; B.Nagy, J.; Nastro, A. Synthesis and characterization of self-bonded ETS-4 and ETS-10 pellets. Micropor. Mesopor. Mater. 2008, 109, 118–137. [Google Scholar] [CrossRef]
- Veltri, M.; Vuono, D.; De Luca, P.; B.Nagy, J.; Nastro, A. Typical data of a new microporous material obtained from gels with titanium and silicon. J. Therm. Anal. Calorim. 2006, 84, 247–252. [Google Scholar] [CrossRef]
- De Luca, P.; Crea, F.; Fonseca, A.; B.Nagy, J. Direct formation of self-bonded pellets during the synthesisof mordenite and ZSM-11 zeolites from low water content systems. Micropor. Mesopor. Mater. 2001, 42, 37–48. [Google Scholar] [CrossRef]
- Cardoso, N.F.; Pinto, R.B.; Lima, E.C.; Calvete, T.; Amavisca, C.V.; Royer, B.; Cunha, M.L.; Fernandes, T.H.M.; Pinto, I.S. Removal of remazol black B textile dye from aqueous solution by adsorption. Desalination 2011, 269, 92–103. [Google Scholar] [CrossRef]
- Kannan, N.; Sundaram, M.M. Kinetics and mechanism of removal of methylene blue by adsorption on various carbons—A comparative study. Dye. Pigment. 2001, 51, 25–40. [Google Scholar] [CrossRef]
- Al-Degs, Y.S.; El-Barghouthi, M.I.; El-Sheikh, A.H.; Walker, G.M. Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dye. Pigment. 2008, 77, 16–23. [Google Scholar] [CrossRef]
- Pereira, M.F.R.; Soares, S.F.; Órfão, J.J.M.; Figueiredo, J.L. Adsorption of dyes on activated carbons: Influence of surface chemical groups. Carbon 2003, 41, 811–821. [Google Scholar] [CrossRef]
- Brooks, C.S. Mechanism of methylene blue dye adsorption on siliceous minerals. Kolloid Z. Z. Polym. 1964, 199, 31–36. [Google Scholar] [CrossRef]
- Chiou, M.S.; Li, H.Y. Adsorption behavior of reactive dye in aqueous solution on chemical cross-linked chitosan beads. Chemosphere 2003, 50, 1095–1105. [Google Scholar] [CrossRef]
- Chiou, M.S.; Ho, P.Y.; Li, H.Y. Adsorption of anionic dyes in acid solutions using chemically cross-linked chitosan beads. Dye. Pigment. 2004, 60, 69–84. [Google Scholar] [CrossRef]
- Konaganti, V.K.; Kota, R.; Patil, S.; Madras, G. Adsorption of anionic dyes on chitosan grafted poly(alkyl methacrylate)s. Chem. Eng. J. 2010, 158, 393–401. [Google Scholar] [CrossRef]
- Annadurai, G.; Juang, R.S.; Lee, D.J. Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. J. Hazard. Mater. 2002, 92, 263–274. [Google Scholar] [CrossRef]
- Garg, V.K.; Gupta, R.; Yadav, A.B.; Kumar, R. Dye removal from aqueous solution by adsorption on treated sawdust. Bioresour. Technol. 2003, 89, 121–124. [Google Scholar] [CrossRef]
- McKay, G.; Poots, V.J.P. Kinetics and diffusion processes in colour removal from effluent using wood as an adsorbent. J. Chem. Technol. Biotechnol. 2007, 30, 279–292. [Google Scholar] [CrossRef]
- Lo, S.F.; Wang, S.Y.; Tsai, M.J.; Lin, L.D. Adsorption capacity and removal efficiency of heavy metal ions by Moso and Ma bamboo activated carbons. Chem. Eng. Res. Des. 2012, 90, 1397–1406. [Google Scholar] [CrossRef]
- Morais, S. Multi-Walled Carbon Nanotubes. Appl. Sci. 2019, 9, 2696. [Google Scholar] [CrossRef]
- Puch, F.; Hopmann, C. Morphology and tensile properties of unreinforced and short carbon fibre reinforced Nylon 6/multiwalled carbon nanotube-composites. Polymers 2014, 55, 3015–3025. [Google Scholar] [CrossRef]
- De Luca, P.; Nappo, G.; Siciliano, C.; B.Nagy, J. The role of carbon nanotubes and cobalt in the synthesis of pellets of titanium silicates. J. Porous Mater. 2018, 25, 283–296. [Google Scholar] [CrossRef]
- Ma, P.C.; Siddiqui, N.A.; Marom, G.; Kim, J.K. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1345–1367. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Li, W.Z.; Wang, D.Z.; Ren, Z.F.; Chou, T.W. Carbon nanotube/carbon fiber hybrid multiscale composites. J. Appl. Phys. 2002, 91, 6034–6037. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Rallini, M.; Ubertini, F.; Materazzi, A.L.; Kenny, J.M. Investigations on scalable fabrication procedures for self-sensing carbon nanotube cement-matrix composites for SHM applications. Cem. Concr. Compos. 2016, 65, 200–213. [Google Scholar] [CrossRef]
- Apul, O.G.; Karanfil, T. Adsorption of synthetic organic contaminants by carbon nanotubes: A critical review. Water Res. 2015, 68, 34–55. [Google Scholar] [CrossRef]
- Lico, D.; Vuono, D.; Siciliano, C.; B.Nagy, J.; De Luca, P. Removal on unleaded gasoline from water by multi-walled carbon nanotubes. J. Environ. Manag. 2019, 237, 636–643. [Google Scholar] [CrossRef]
- Li, Y.; Du, Q.; Liu, T.; Peng, X.; Wang, J.; Sun, J.; Wang, Y.; Wu, S.; Wang, Z.; Xia, Y.; et al. Comparative study of methylene blue dye adsorption onto activated carbon, graphene oxide, and carbon nanotubes. Chem. Eng. Res. Des. 2013, 91, 361–368. [Google Scholar] [CrossRef]
- Aliyu, A.; Kariim, I.; Abdulkareem, S.A. Effects of aspect ratio of multi walled carbon nanotubes on coal washery waste water treatment. J. Environ. Manag. 2017, 202, 84–93. [Google Scholar] [CrossRef]
- Foglia, P.; Vuono, D.; Siciliano, C.; Napoli, A.; B.Nagy, J.; De Luca, P. Brackish water treatment with carbon nanotubes. IOP Conf. Ser. Mater. Sci. Eng. 2019, 572, 012047. [Google Scholar] [CrossRef]
- Gotovac, S.; Yang, C.M.; Hattori, Y.; Takahashi, K.; Kanoh, H.; Kaneko, K. Adsorption of polyaromatic hydrocarbons on single wall carbon nanotubes of different functionalities and diameters. J. Colloid Interf. Sci. 2007, 314, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Fard, A.K.; Rhadfi, T.; Mckay, G.; Al-Marri, M.; Abdala, A.; Hilal, N.; Hussein, M.A. Enhancing oil removal from water using ferric oxide nanoparticles doped carbon nanotubes adsorbents. Chem. Eng. J. 2016, 293, 90–101. [Google Scholar] [CrossRef]
- Chen, W.; Duan, L.; Zhu, D. Adsorption of polar and nonpolar organic chemicals to carbon nanotubes. Environ. Sci. Technol. 2007, 41, 8295–8300. [Google Scholar] [CrossRef] [PubMed]
- Machado, F.M.; Bergmann, C.P.; Fernandes, T.H.M.; Lima, E.C.; Royer, B.; Calvete, T.; Fagan, S.B. Adsorption of Reactive Red M-2BE dye from water solutions by multi-walled carbon nanotubes and activated carbon. J. Hazard. Mater. 2011, 192, 1122–1131. [Google Scholar] [CrossRef]
- Duman, O.; Tunç, S.; Polat, T.G.; Bozoğlan, B.K. Synthesis of magnetic oxidized multiwalled carbon nanotube-k-carrageenan-Fe3O4 nanocomposite adsorbent and its application in cationic Methylene Blue dye adsorption. Carbohydr. Polym. 2016, 147, 79–88. [Google Scholar] [CrossRef]
- Duman, O.; Tunc, S.; Bozoglan, B.K.; Polat, T.G. Removal of triphenylmethane and reactive azo dyes from aqueous solution by magnetic carbon nanotube-k-carrageenan-Fe3O4 nanocomposite. J. Alloys Compd. 2016, 687, 370–383. [Google Scholar] [CrossRef]
- De Benedetto, C.; Macario, A.; Siciliano, C.; B.Nagy, J.; De Luca, P. Adsorption of Reactive Blue 116 Dye and Reactive Yellow 81 Dye from Aqueous Solutions by Multi-Walled Carbon Nanotubes. Materials 2020, 13, 2757. [Google Scholar] [CrossRef] [PubMed]
- De Luca, P.; B.Nagy, J. Treatment of Water Contaminated with Reactive Black-5 Dye by Carbon Nanotubes. Materials 2020, 13, 5508. [Google Scholar] [CrossRef]
- Pellizzari Wielewski, L.; Zuccolotto, T.; Soares, M.; Tentler Prola, L.D.; de Liz, M.V. Degradation of the Textile Dye Reactive Black 5 by Basidiomycetes. Rev. Ambient. Água 2020, 15, 1. [Google Scholar] [CrossRef]
- Policicchio, A.; Vuono, D.; Rugiero, T.; De Luca, P.; B.Nagy, J. Study of MWCNTs adsorption perfomances in gas processes. J. CO2 Util. 2015, 10, 30–39. [Google Scholar] [CrossRef]
- Sarvestani, M.R.J.; Doroudi, Z. Removal of Reactive Black 5 from Waste Waters by Adsorption: A Comprehensive Review. J. Water Environ. Nanotechnol. 2020, 5, 180–190. [Google Scholar]
- Mengelizadeh, N.; Pourzamani, H. Adsorption of Reactive Black 5 Dye from Aqueous Solutions by Carbon Nanotubes and its Electrochemical Regeneration Process. Health Scope 2020. [Google Scholar] [CrossRef]
- Naghizadeh, A.; Nasseri, S.; Mahvi, A.H.; Nabizadeh, R.; Kalantary, R.R.; Rashidi, A. Continuous adsorption of natural organic matters in a column packed with carbon nanotubes. J. Environ. Health Sci. Eng. 2013, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Badran, I.; Qut, O.; Manasrah, A.D.; Abualhasan, M. Continuous adsorptive removal of glimepiride using multi-walled carbon nanotubes in fixed-bed column. Environ. Sci. Pollut. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hayati, B.; Maleki, A.; Najafi, F.; Gharibia, F.; McKay, G.; Gupta, V.K.; Puttaiah, S.H.; Marzban, N. Heavy metal adsorption using PAMAM/CNT nanocomposite from aqueous solution in batch and continuous fixed bed systems. Chem. Eng. J. 2018, 346, 258–270. [Google Scholar] [CrossRef]
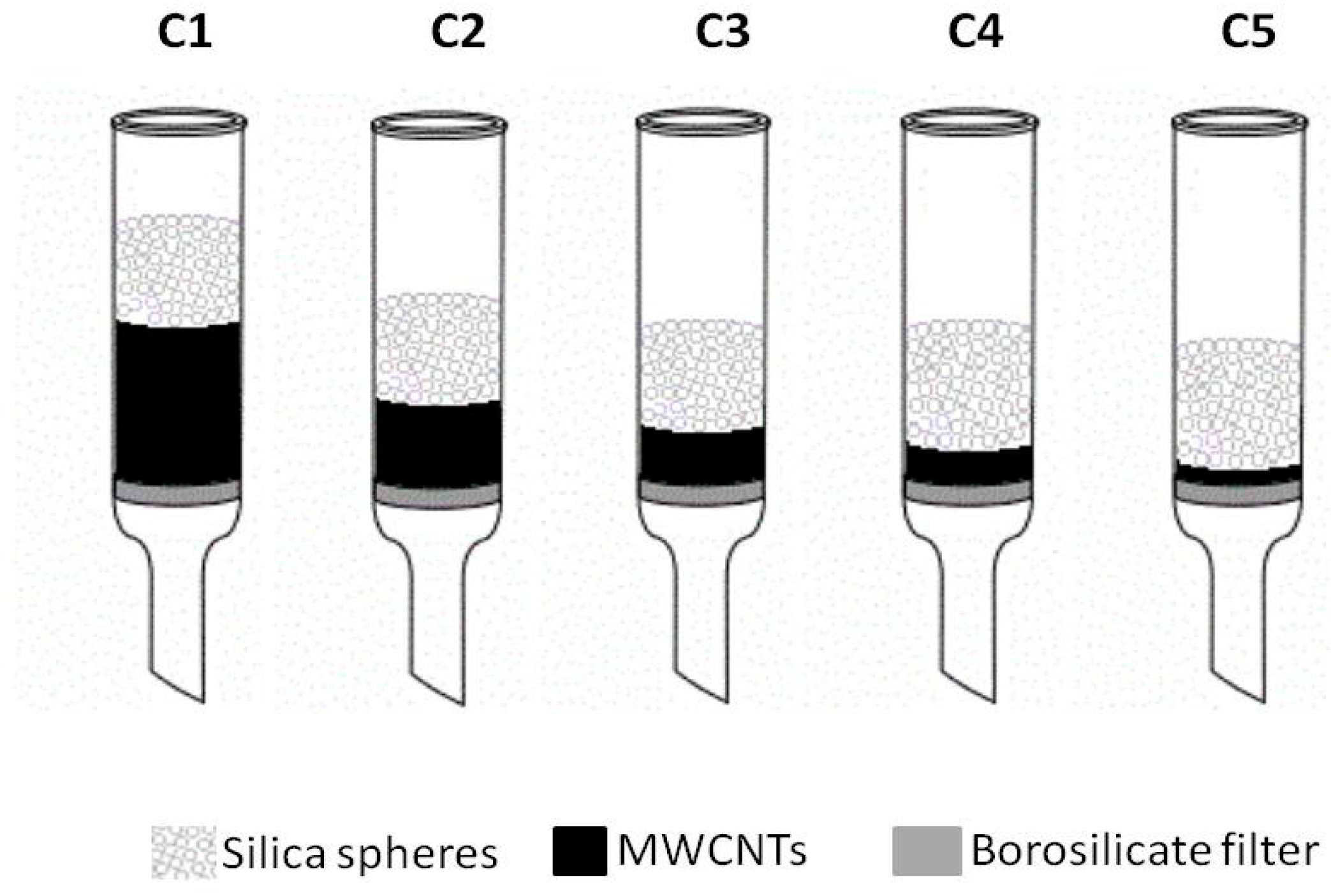
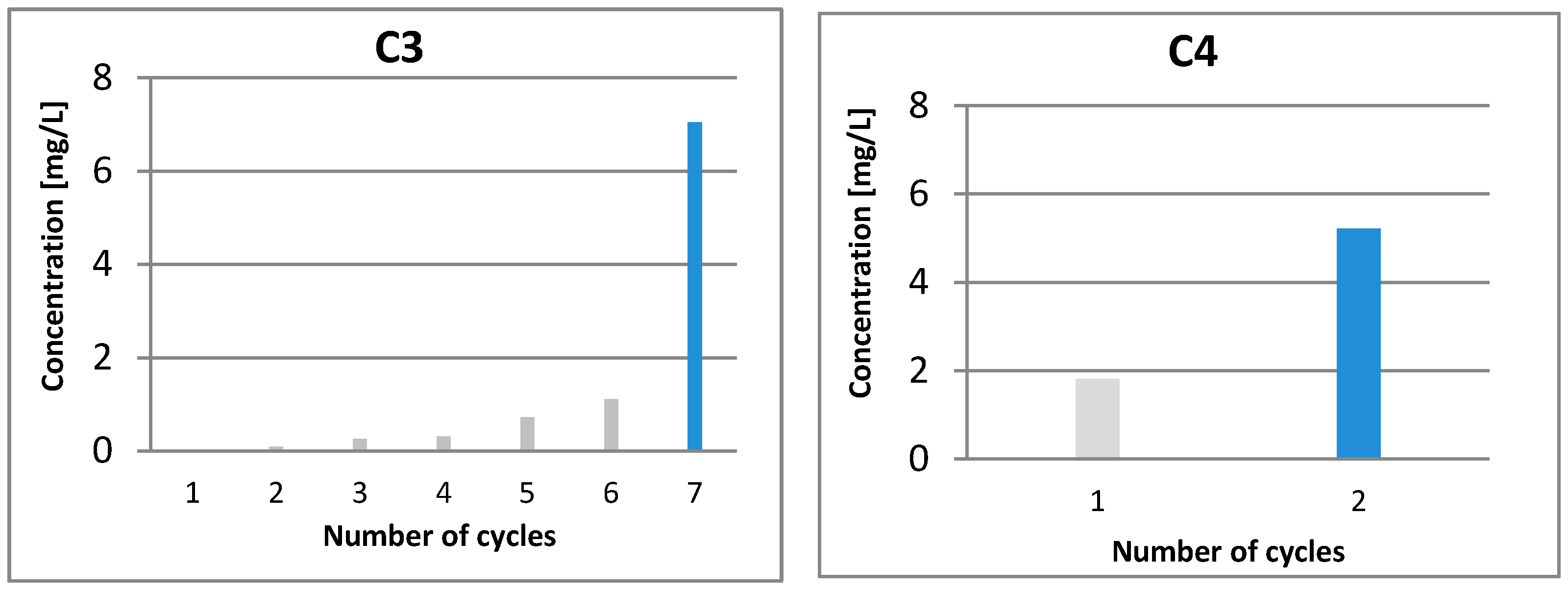
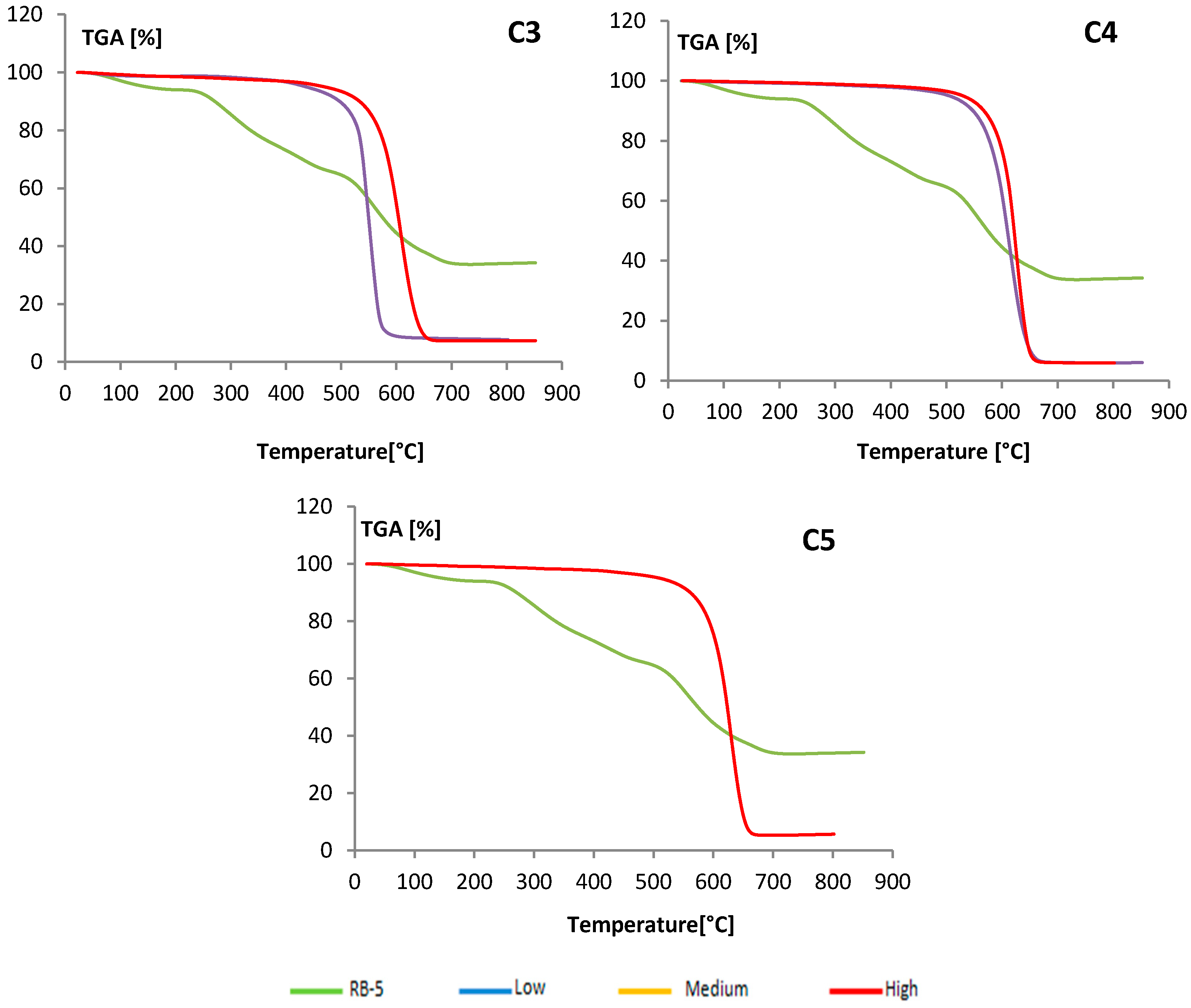
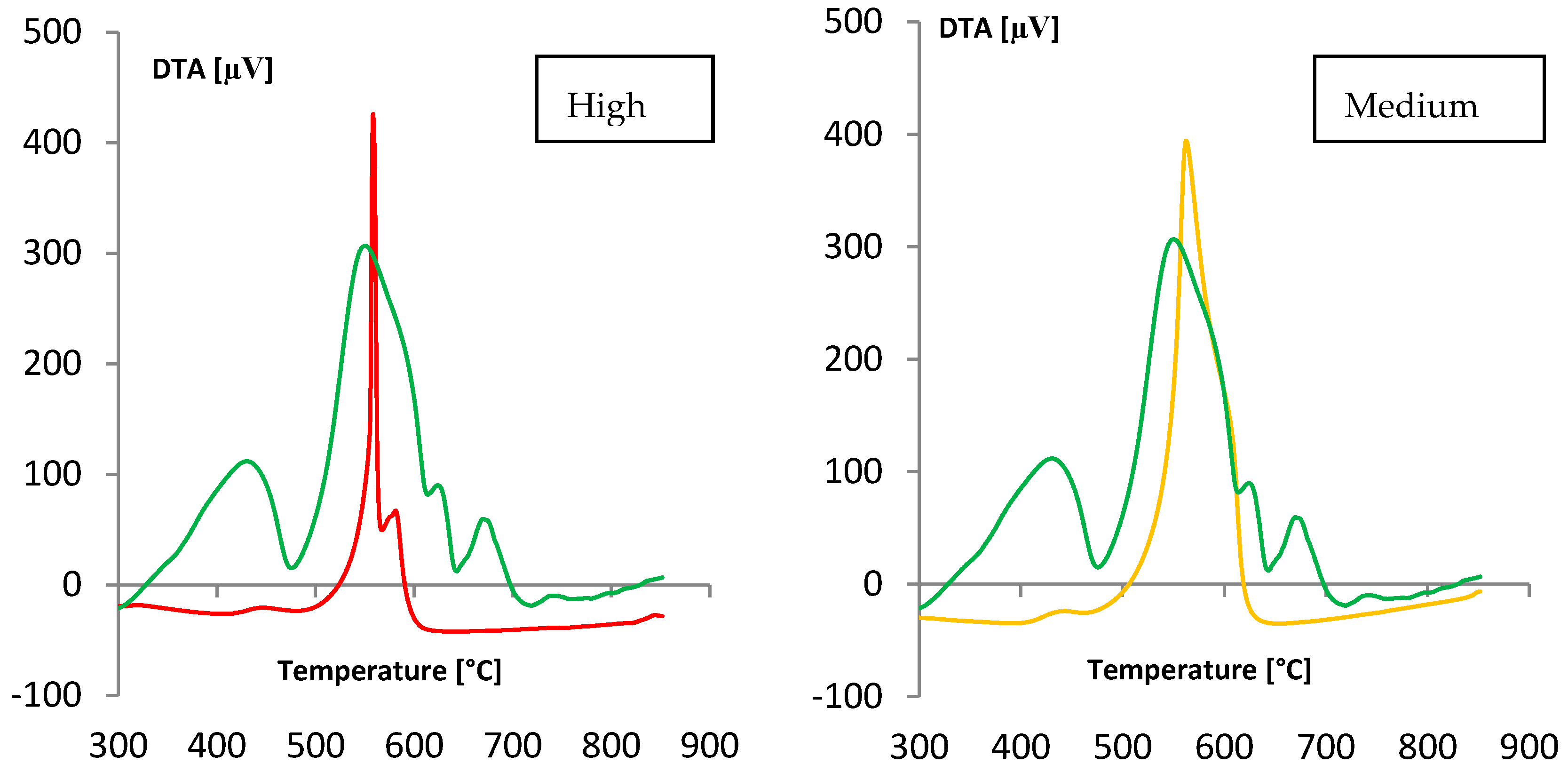
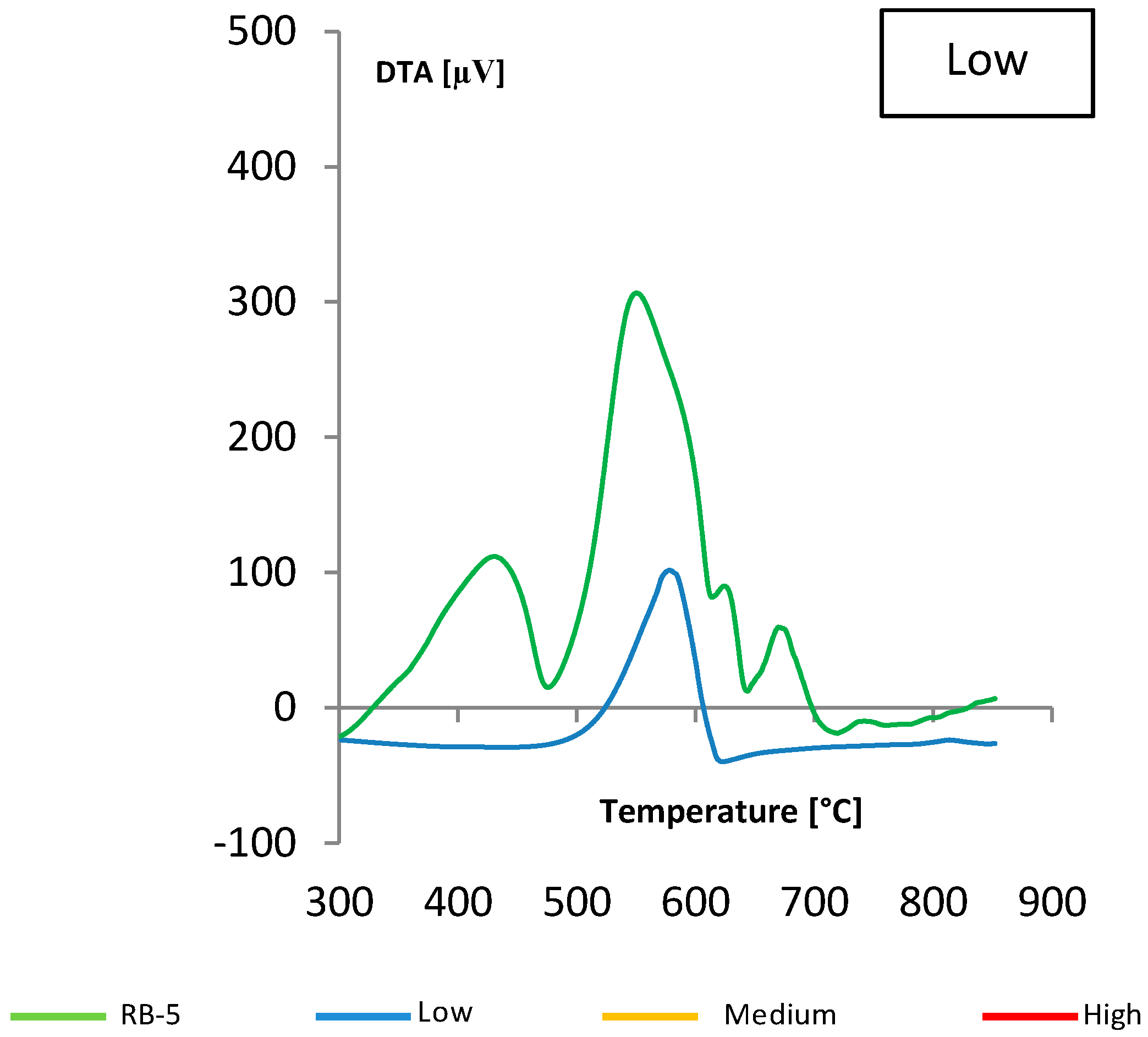
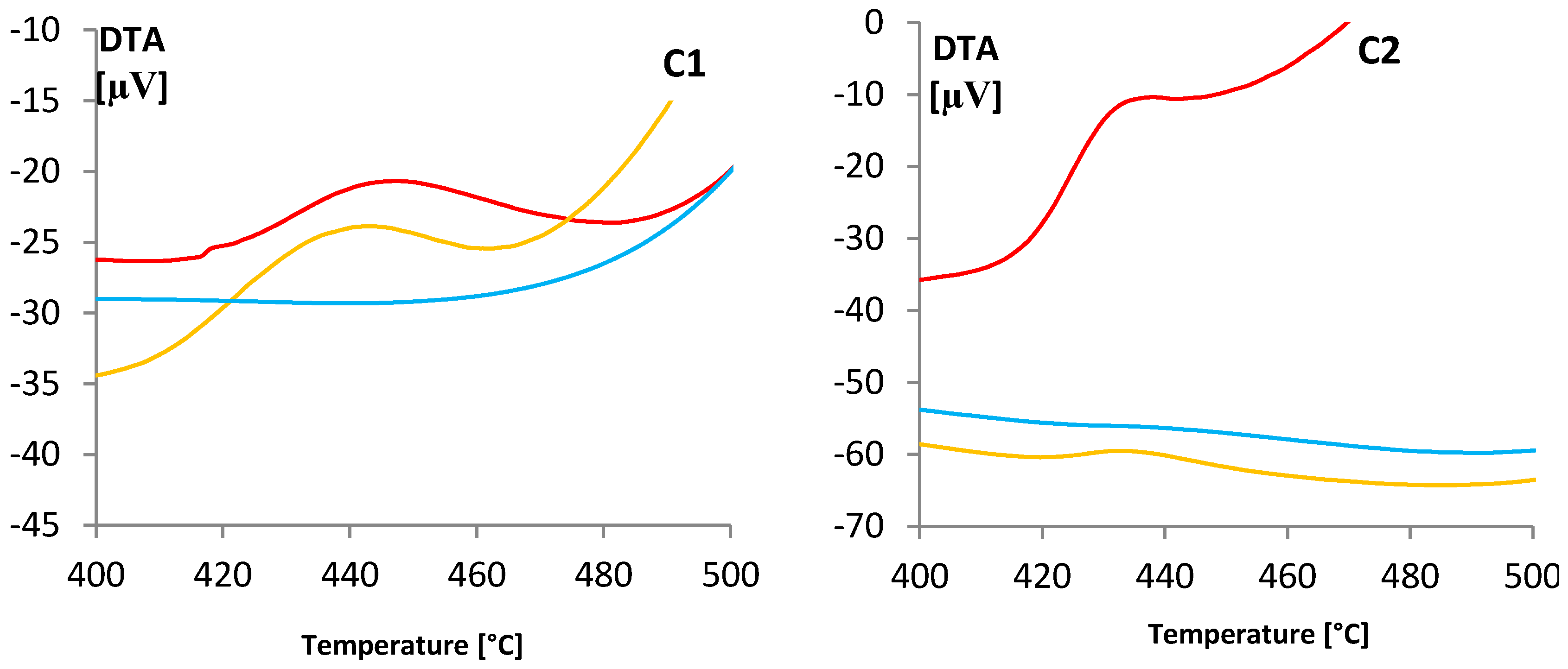
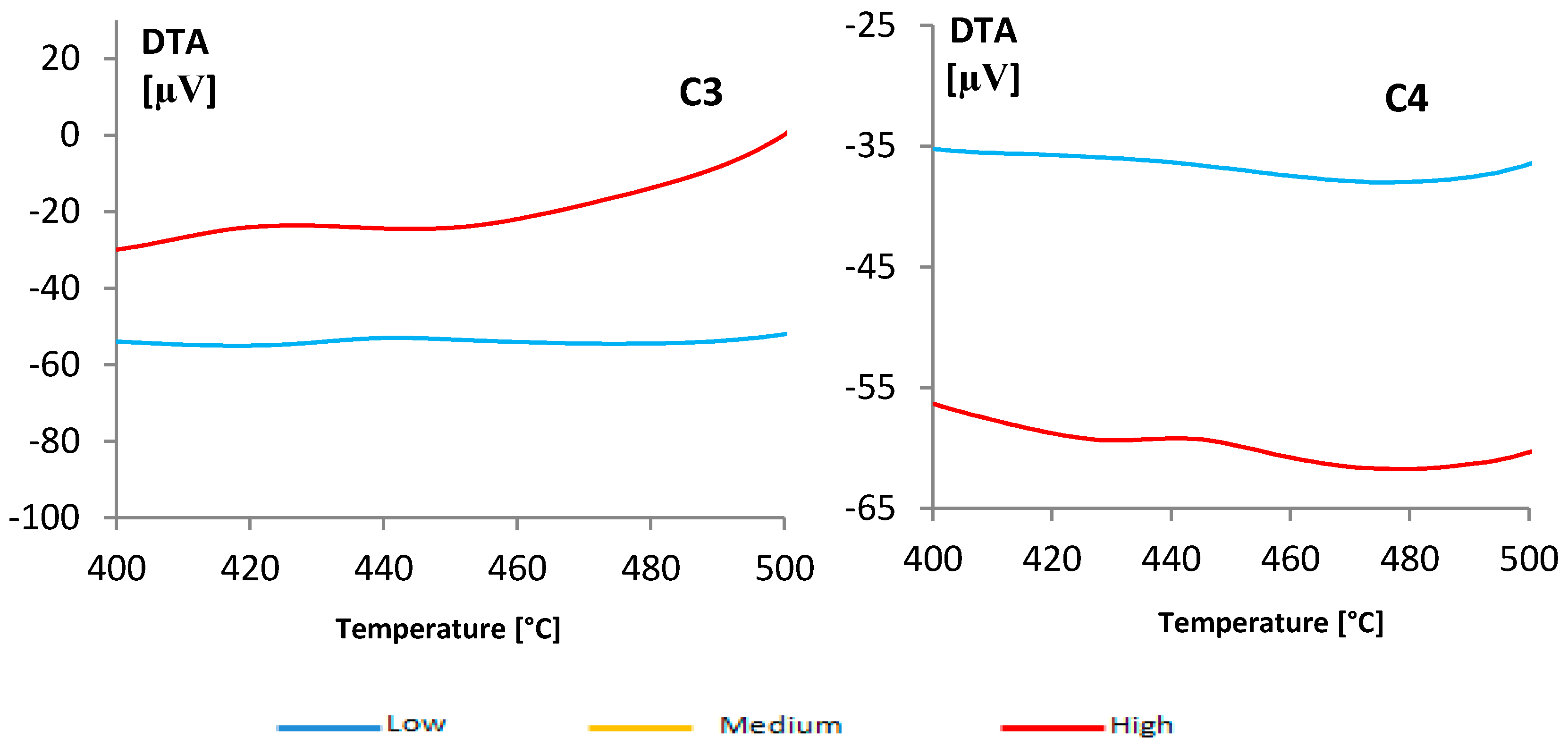
 (gray) and colored
(gray) and colored  (blue) solutions are shown.
(blue) solutions are shown.
 (gray) and colored
(gray) and colored  (blue) solutions are shown.
(blue) solutions are shown.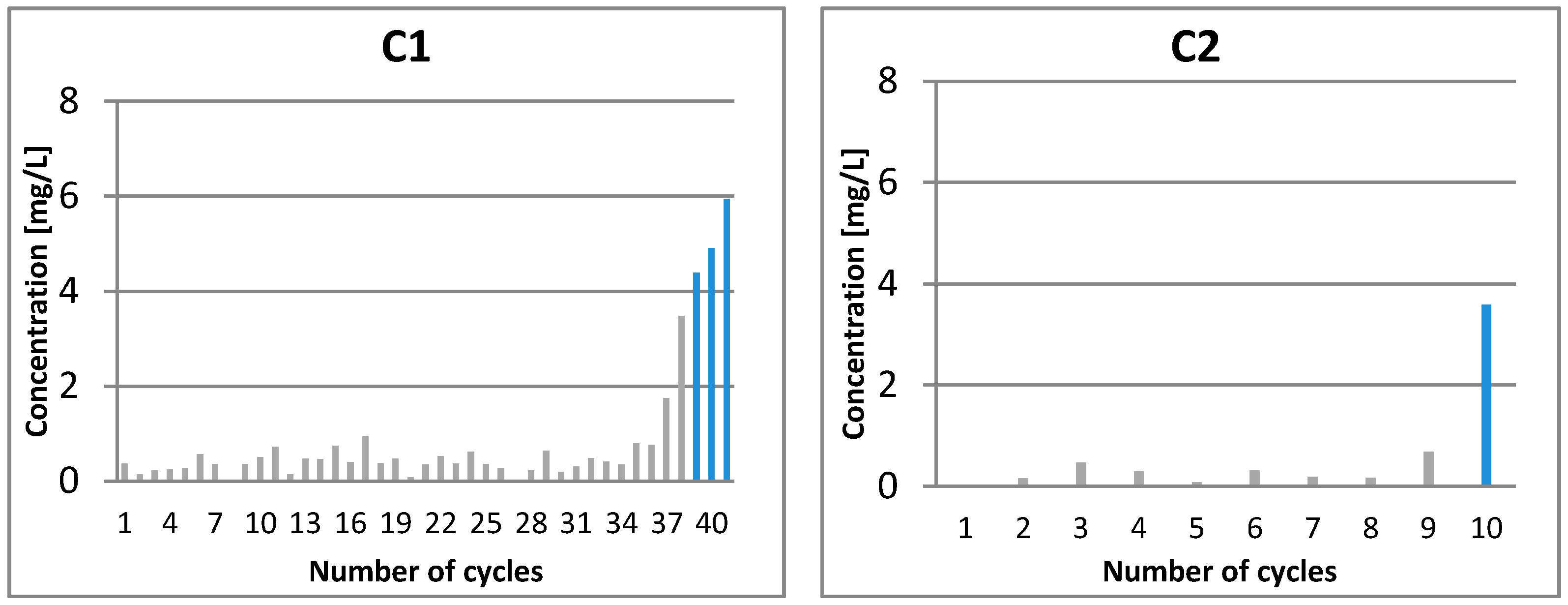
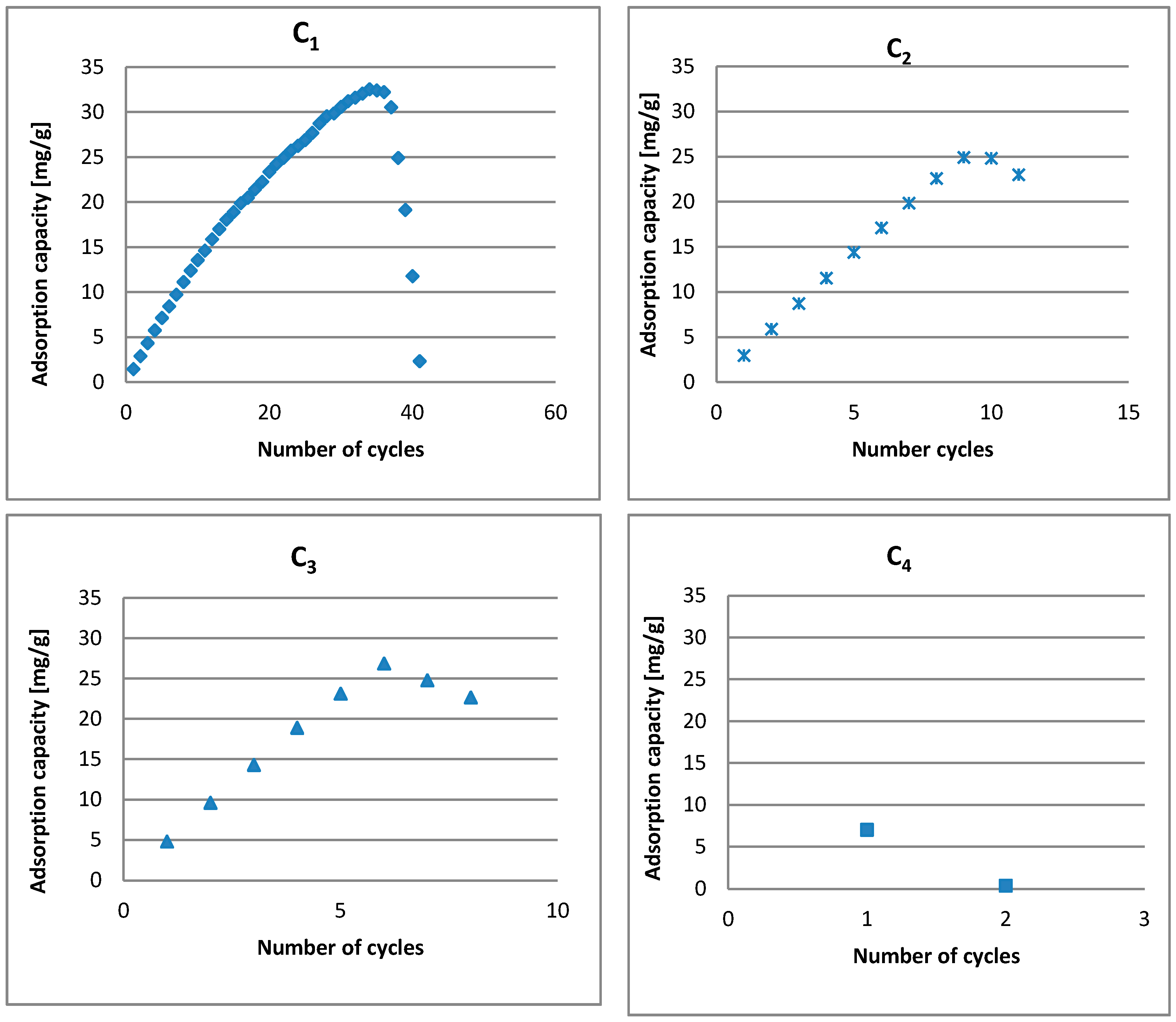


| C1 | C2 | C3 | C4 | C5 | |
|---|---|---|---|---|---|
| Column diameter [cm] | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Height of silica spheres layer [cm] | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 |
| Height of MWCNT layer [cm] | 4.50 | 2.25 | 1.40 | 1.00 | 0.40 |
| Volume of MWCNT layer [cm3] | 14.14 | 7.07 | 4.40 | 3.14 | 1.26 |
| Weight of MWCNT layer [g] | 2.50 | 1.25 | 0.75 | 0.50 | 0.20 |
| Adsorption Column | Number of Adsorption Cycles | Total Volume Treated [L] |
|---|---|---|
| C1 | 38 | 3.8 |
| C2 | 9 | 0.9 |
| C3 | 6 | 0.6 |
| C4 | 1 | 0.1 |
| C5 | 0 | 0.0 |
| Column | Mads [g] | VTreated [L] | V ads [m3] | A [m2] | Q [L/h] | Time [h] | CUR [g/L] | J [m3/m2·h] | EBCT [min] |
|---|---|---|---|---|---|---|---|---|---|
| C1 | 2.5 | 3.8 | 14.14 × 10−6 | 3.14 × 10−4 | 0.0194 | 195.7 | 0.66 | 0.066 | 43.687 |
| C2 | 1.25 | 0.9 | 7.07 × 10−6 | 3.14 × 10−4 | 0.0947 | 9.5 | 1.39 | 0.301 | 4.477 |
| C3 | 0.75 | 0.6 | 4.40 × 10−6 | 3.14 × 10−4 | 0.0869 | 6.9 | 1.25 | 0.278 | 3.036 |
| C4 | 0.50 | 0.1 | 3.14 × 10−6 | 3.14 × 10−4 | 0.5 | 0.2 | 5.00 | 1.591 | 0.377 |
| Column | V treated [L] | Total Weight of RB5 Utilized [mg] | Total Permeate RB5 [mg] | Total Retentate RB5 [mg] | R [mgRet/gMWCNTs] |
|---|---|---|---|---|---|
| C1 | 3.8 | 140.6 | 1.978 | 138.622 | 55.44 |
| C2 | 0.9 | 33.3 | 0.234 | 33.066 | 26.45 |
| C3 | 0.6 | 22.2 | 0.250 | 21.950 | 29.27 |
| C4 | 0.1 | 3.7 | 0.181 | 3.519 | 7.04 |
| Sample | TC [mg/L] | IC [mg/L] | TOC [mg/L] |
|---|---|---|---|
| RB5 | 9.539 | 0.247 | 9.292 |
| C1-01 | 13.190 | 7.333 | 5.859 |
| C1-15 | 3.522 | 1.508 | 2.014 |
| C1-24 | 3.508 | 1.909 | 1.600 |
| C1-36 | 2.864 | 1.237 | 1.627 |
| C1-37 | 3.079 | 1.123 | 1.956 |
| C1-38 | 3.532 | 1.342 | 2.190 |
| C1-39 | 3.749 | 1.395 | 2.354 |
| C1-41 | 5.458 | 1.380 | 4.078 |
| C2-01 | 3.890 | 3.292 | 0.599 |
| C2-09 | 3.421 | 1.815 | 1.606 |
| C2-10 | 3.890 | 1.970 | 1.920 |
| C3-01 | 4.836 | 2.953 | 1.883 |
| C3-06 | 5.463 | 1.374 | 4.089 |
| C3-07 | 4.260 | 0.713 | 3.548 |
| C4-01 | 2.018 | 1.228 | 0.790 |
| C4-02 | 3.150 | 1.006 | 2.144 |
| C5-01 | 5.391 | 0.589 | 4.812 |
| Layer | Total Weight Loss [%] | Heigh of Total MWCNT Layer in the Column [cm] | Difference Weight Loss between High and Low Layer (%) |
|---|---|---|---|
| C1-Low | 82.05 | ||
| C1-Medium | 90.43 | 4.50 | 11.96 |
| C1-High | 94.01 | ||
| C2-Low | 86.58 | ||
| C2-Medium | 87.25 | 2.25 | 7.88 |
| C2-High | 94.46 | ||
| C3-Low | 89.51 | 0.24 | |
| C3-High | 89.75 | 1.4 | |
| C4-Low | 92.12 | 0.13 | |
| C4-High | 92.25 | ||
| C5 | 92.42 | 0.4 | --- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Luca, P.; Chiodo, A.; Macario, A.; Siciliano, C.; B.Nagy, J. Semi-Continuous Adsorption Processes with Multi-Walled Carbon Nanotubes for the Treatment of Water Contaminated by an Organic Textile Dye. Appl. Sci. 2021, 11, 1687. https://doi.org/10.3390/app11041687
De Luca P, Chiodo A, Macario A, Siciliano C, B.Nagy J. Semi-Continuous Adsorption Processes with Multi-Walled Carbon Nanotubes for the Treatment of Water Contaminated by an Organic Textile Dye. Applied Sciences. 2021; 11(4):1687. https://doi.org/10.3390/app11041687
Chicago/Turabian StyleDe Luca, Pierantonio, Antonio Chiodo, Anastasia Macario, Carlo Siciliano, and Jànos B.Nagy. 2021. "Semi-Continuous Adsorption Processes with Multi-Walled Carbon Nanotubes for the Treatment of Water Contaminated by an Organic Textile Dye" Applied Sciences 11, no. 4: 1687. https://doi.org/10.3390/app11041687
APA StyleDe Luca, P., Chiodo, A., Macario, A., Siciliano, C., & B.Nagy, J. (2021). Semi-Continuous Adsorption Processes with Multi-Walled Carbon Nanotubes for the Treatment of Water Contaminated by an Organic Textile Dye. Applied Sciences, 11(4), 1687. https://doi.org/10.3390/app11041687









