A Novel Singleton Giant Phage Yong-XC31 Lytic to the Pyropia Pathogen Vibrio mediterranei
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Antibiotic Susceptibility Test
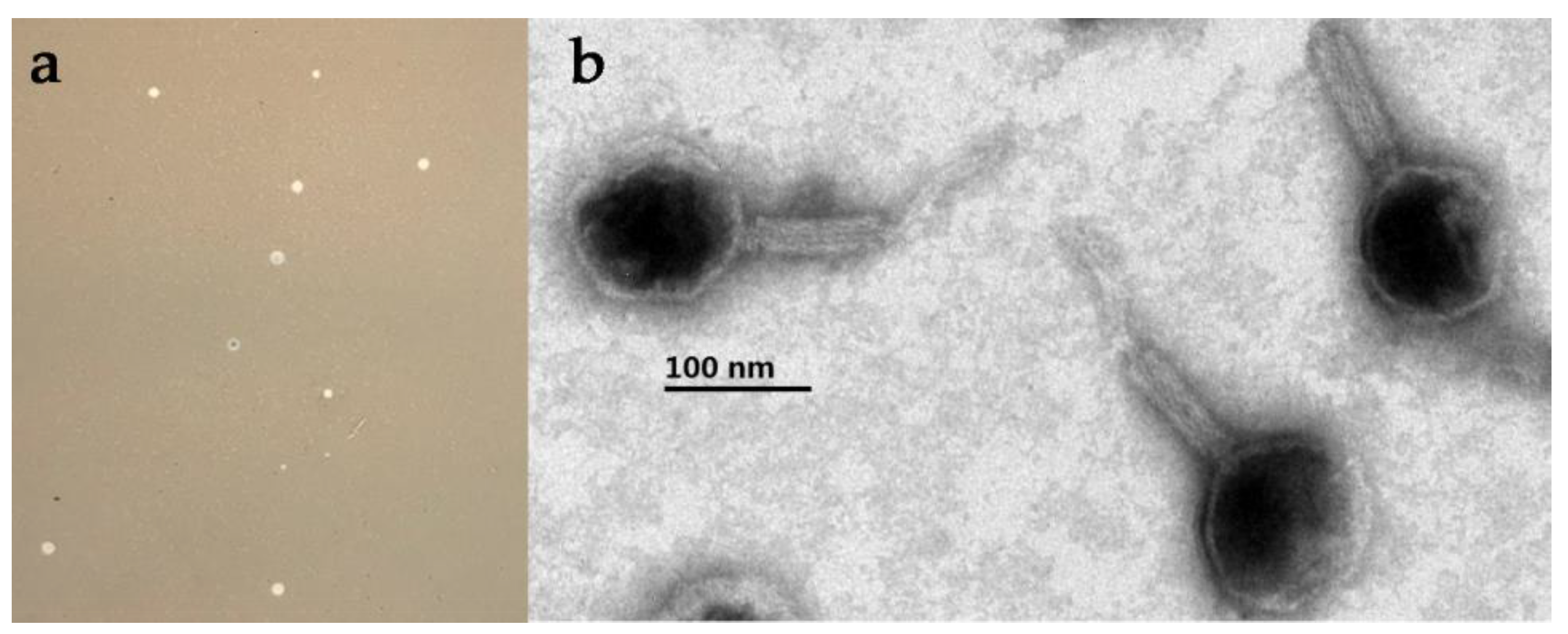
2.3. Phage Isolation and Morphological Observation
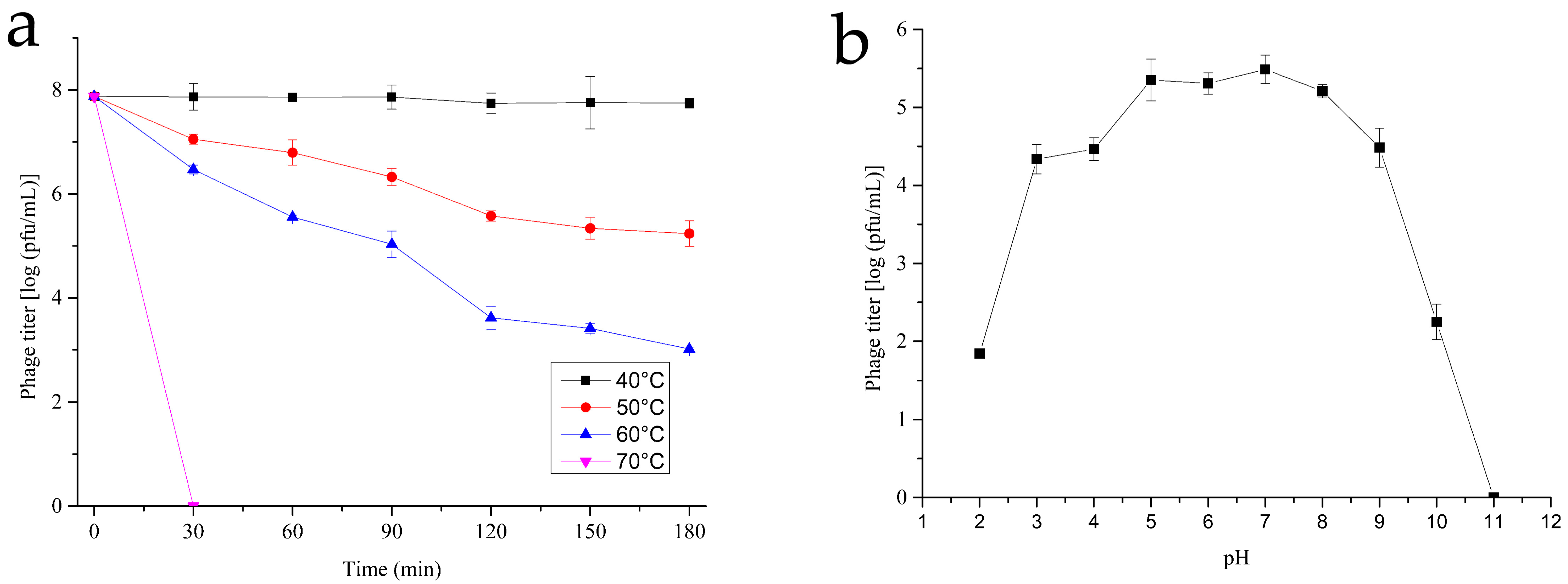
2.4. Thermal and pH Stability
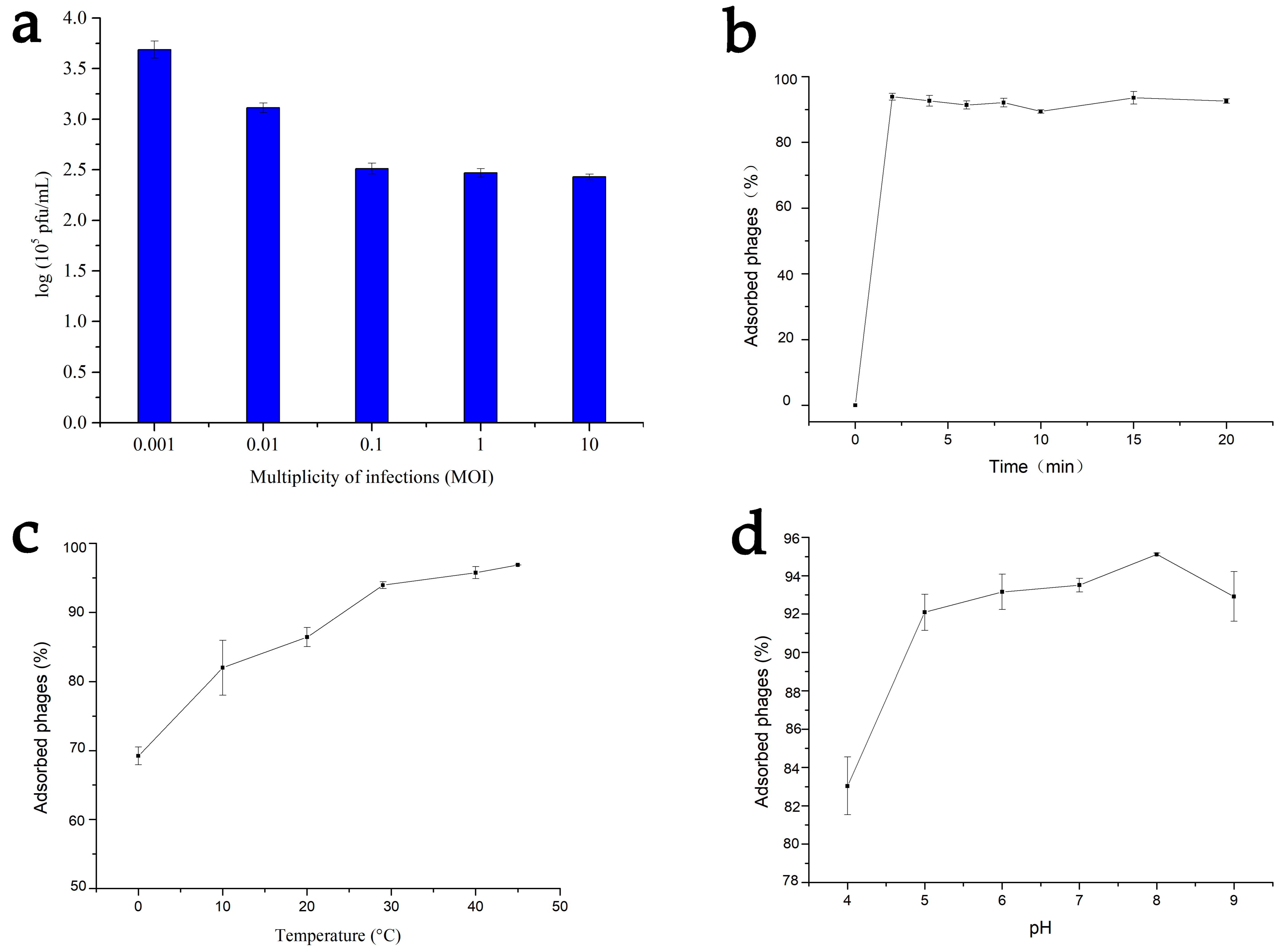
2.5. MOI Selection Experiments and Adsorption Test
2.6. One-Step Growth Experiment
2.7. Host Range Determination
2.8. Genome Extraction and Sequencing
2.9. Genome Annotation and Taxonomic Analyses
2.10. Genome Sequence Accession Number
3. Results
3.1. Antibiotic Susceptibility of V. mediterranei 117-T6
3.2. Phage Morphology
3.3. Thermal and pH Stability
3.4. Optimal MOI and Factors Influencing Adsorption
3.5. One-Step Growth Curve
3.6. Host Range Determination
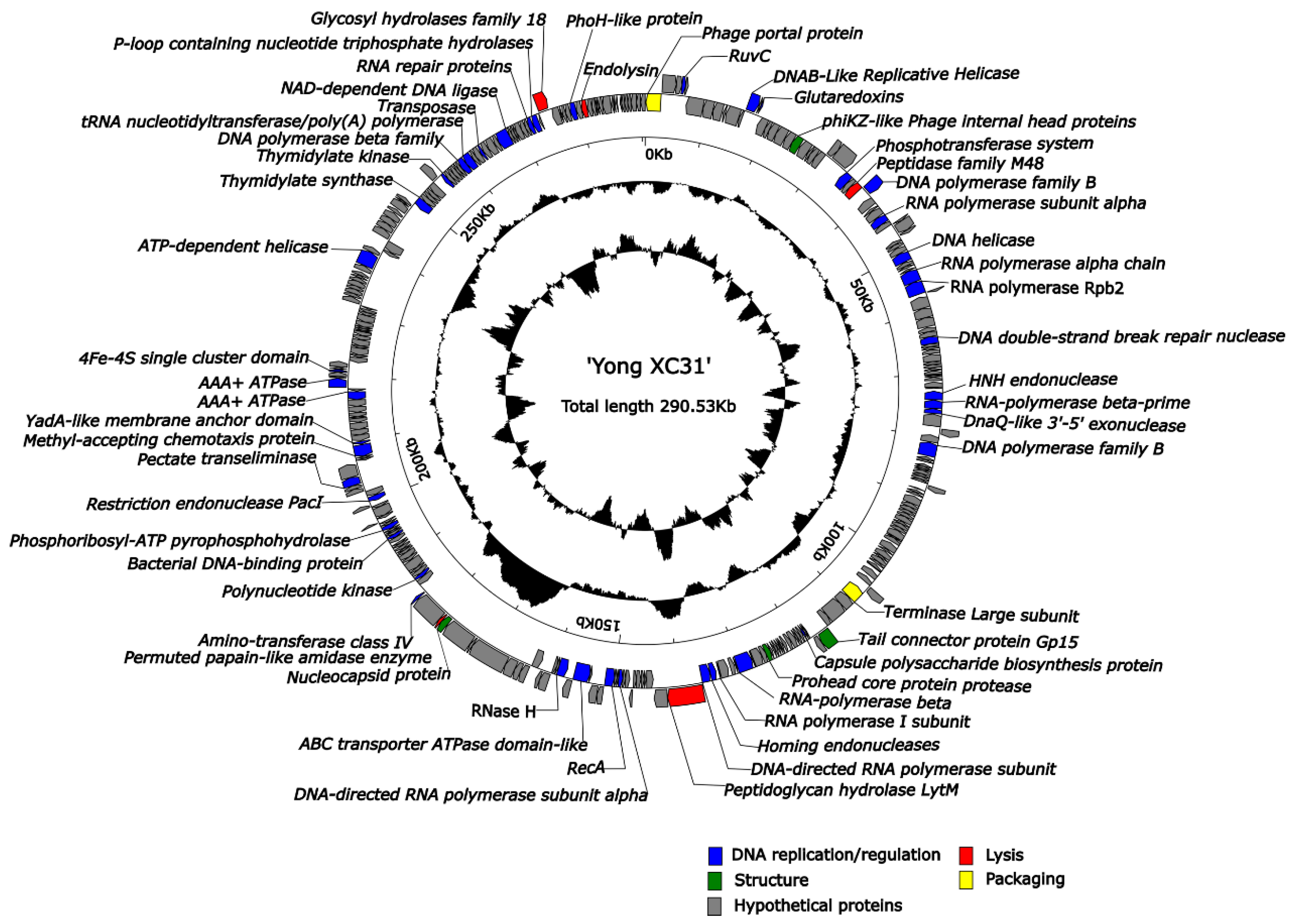
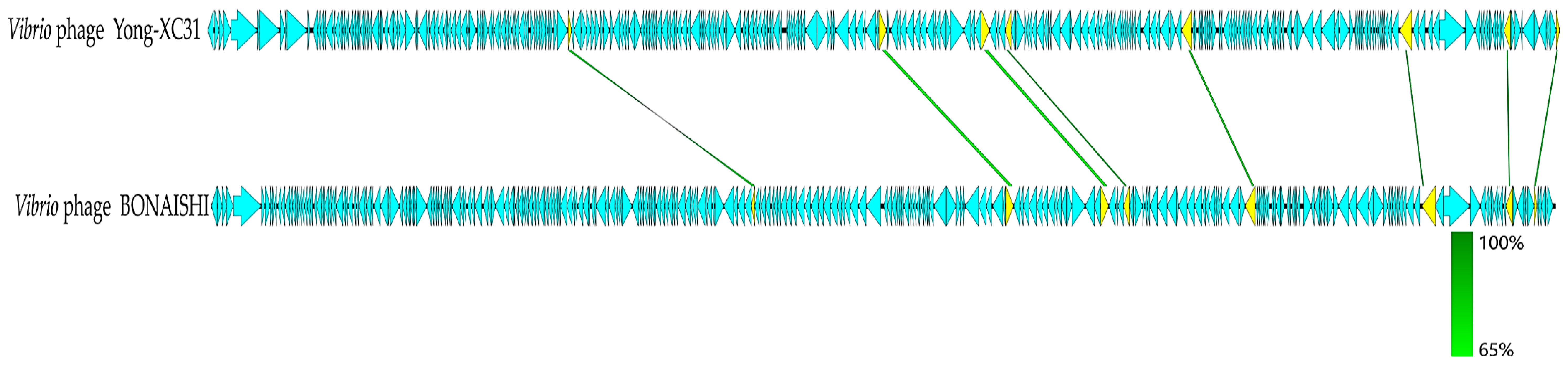
3.7. Genome Analysis of Yong-XC31
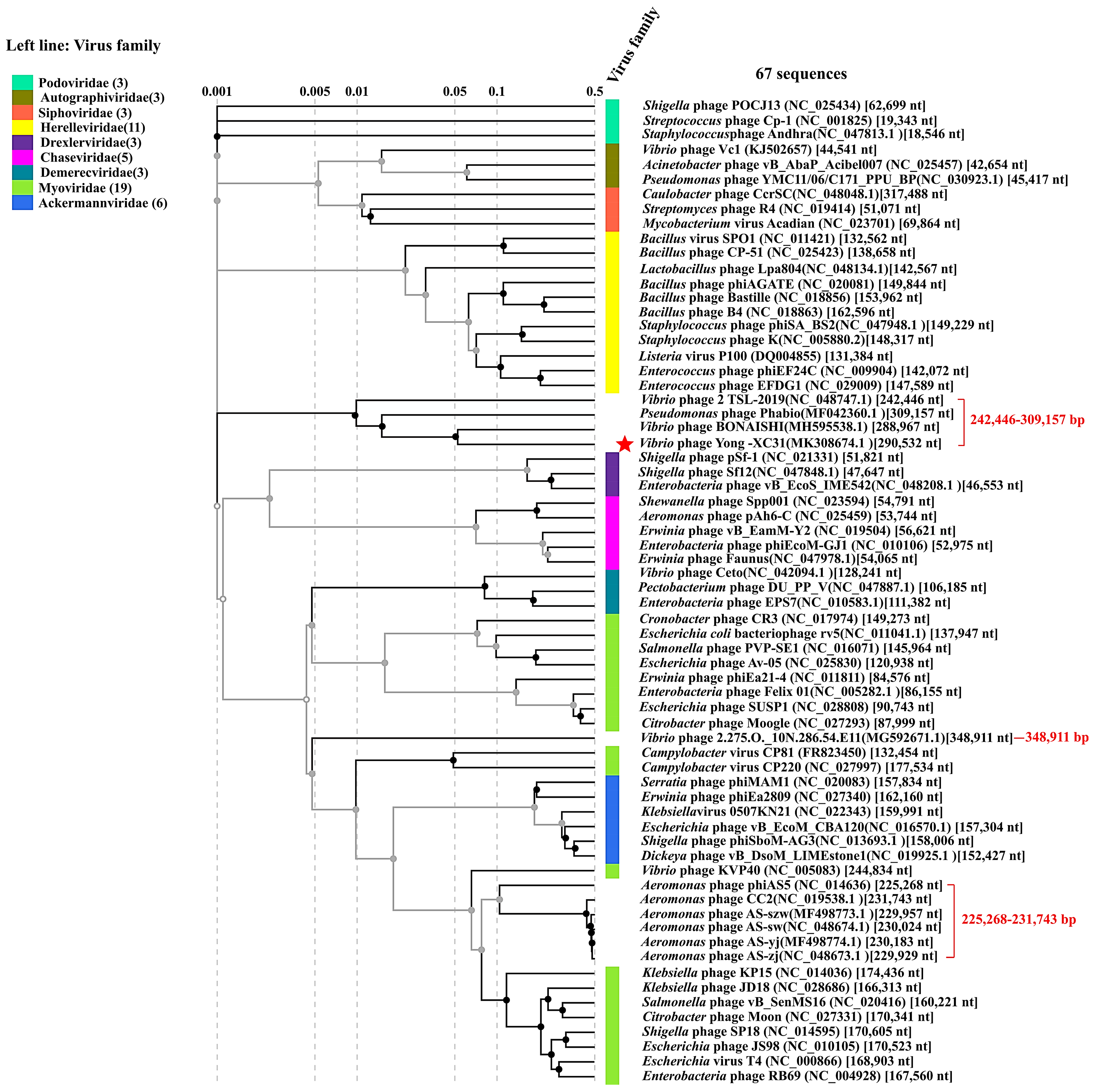
3.8. Taxonomy
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ben-Haim, Y.; Rosenberg, E. Vibrio coralliilyticus sp. nov., a temperature-dependent pathogen of the coral Pocillopora damicornis. Int. J. Syst. Evol. Microbiol. 2003, 53, 309–315. [Google Scholar] [CrossRef]
- Pang, L.; Zhang, X.H.; Zhong, Y.; Chen, J.; Li, Y.; Austin, B. Identification of Vibrio harveyi using PCR amplification of the toxR gene. Lett. Appl. Microbiol. 2006, 43, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Romalde, J.L.; Dieguez, A.L.; Lasa, A.; Balboa, S. New Vibrio species associated to molluscan microbiota: A review. Front. Microbiol. 2014, 4, e413. [Google Scholar] [CrossRef] [PubMed]
- Goudenège, D.; Travers, M.A.; Lemire, A.; Petton, B.; Haffner, P.; Labreuche, Y.; Tourbiez, D.; Mangenot, S. A single regulatory gene is sufficient to alter Vibrio aestuarianus pathogenicity in oysters. Environ. Microbiol. 2015, 17, 4189–4199. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Portillo, E.; Yarza, P.; Penalver, C.; Ramos-Esplá, A.A.; Anton, J. New insights into Oculina patagonica coral diseases and their associated Vibrio spp. communities. ISME J. 2014, 8, 1794–1807. [Google Scholar] [CrossRef] [PubMed]
- Serrano, W.; Tarazona, U.I.; Olaechea, R.M.; Friedrich, M.W. Draft genome sequence of a new Vibrio strain with the potential to produce bacteriocin-like inhibitory substances, isolated from the gut microflora of scallop (Argopecten purpuratus). Genome Announc. 2018, 6, e00419-18. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Xu, M.Y.; He, Y.Y.; Tao, Z.; Yang, R.; Chen, H.M. Complete genome sequence of Vibrio mediterranei 117-T6, a potentially pathogenic bacterium isolated from the conchocelis of Pyropia spp. Microbiol. Resour. Announc. 2019, 8, e01569-01518. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.Y.; Yang, R.; Liu, Q.Q.; He, Y.Y.; Chen, H.M. Study on yellow spot disease in Pyropia species infected by Vibrio mediterranei 117-T6. J. Fish. China 2020, 44, 1–11. [Google Scholar] [CrossRef]
- Wang, X.L.; He, L.W.; Ma, Y.C.; Huan, L.; Wang, Y.Q.; Xia, B.M.; Wang, G.C. Economically important red algae resources along the Chinese coast: History, status, and prospects for their utilization. Algal Res. 2020, 46, 101817. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Trinanes, J.A.; Taylor, N.G.H.; Hartnell, R.; Siitonen, A.; Martinez-Urtaza, J. Emerging Vibrio risk at high latitudes in response to ocean warming. Nat. Clim. Chang. 2013, 3, 73–77. [Google Scholar] [CrossRef]
- Vezzulli, L.; Grande, C.; Reid, P.C.; Hélaouët, P.; Edwards, M.; Höfle, M.G.; Brettar, I.; Colwell, R.R.; Pruzzo, C. Climate infuence on Vibrio and associated human diseases during the past half-century in the coastal North Atlantic. Proc. Natl. Acad. Sci. USA 2016, 113, E5062–E5071. [Google Scholar] [CrossRef]
- Suttle, C.A. Marine viruses—major players in the global ecosystem. Nat. Rev. Microbio. 2007, 5, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Breitbart, M.; Bonnain, C.; Malki, K.; Sawaya, N.A. Phage puppet masters of the marine microbial realm. Nat. Microbiol. 2018, 3, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.H.; Gao, M.Y. Jumbo bacteriophages: An overview. Front. Microbiol. 2017, 8, 403. [Google Scholar] [CrossRef]
- Hendrix, R.W. Jumbo bacteriophages. Curr. Top. Microbiol. Immunol. 2009, 328, 229–240. [Google Scholar] [CrossRef]
- Gorski, A.; Miedzybrodzki, R.; Łobocka, M.; Glowackarutkowska, A.; Bednarek, A.; Borysowski, J.; Jonczykmatysiak, E.; Łusiakszelachowska, M.; Weberdąbrowska, B.; Baginska, N.J.V. Phage therapy: What have we learned? Viruses 2018, 10, 288. [Google Scholar] [CrossRef] [PubMed]
- Moye, Z.D.; Woolston, J.; Sulakvelidze, A. Bacteriophage applications for food production and processing. Viruses 2018, 10, 205. [Google Scholar] [CrossRef]
- Dy, R.L.; Rigano, L.A.; Fineran, P.C. Phage-based biocontrol strategies and their application in agriculture and aquaculture. Biochem. Soc. Trans. 2018, 46, 1605–1613. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Bukhari, S.M.A.U.S.; Andleeb, S.; Ali, M.; Raza, S.; Nawaz, M.A.; Hussain, T.; Rahman, S.U.; Shah, S.S.A. Bacteriophages: An overview of the control strategies against multiple bacterial infections in different fields. J. Basic Microbiol. 2019, 59, 123–133. [Google Scholar] [CrossRef]
- Van Etten, J.L.; Lane, L.C.; Dunigan, D.D. DNA viruses: The really big ones (Giruses). Annu. Rev. Microbiol. 2010, 64, 83–99. [Google Scholar] [CrossRef]
- Wang, Y.H.; Barton, M.; Elliott, L.; Li, X.X.; Abraham, S.; O’Dea, M.; Munro, J. Bacteriophage therapy for the control of Vibrio harveyi in greenlip abalone (Haliotis laevigata). Aquaculture 2017, 473, 251–258. [Google Scholar] [CrossRef]
- Prada-Peñaranda, C.; Salazar, M.; Güiza, L.; Pérez, M.I.; Leidy, C.; Vives-Florez, M.J. Phage preparation FBL1 prevents Bacillus licheniformis biofilm, bacterium responsible for the mortality of the Pacific white shrimp Litopenaeus vannamei. Aquaculture 2018, 484, 160–167. [Google Scholar] [CrossRef]
- Jun, J.W.; Kim, J.H.; Shin, S.P.; Han, J.E.; Chai, J.Y.; Park, S.C. Protective effects of the Aeromonas phages pAh1-C and pAh6-C against mass mortality of the cyprinid loach (Misgurnus anguillicaudatus) caused by Aeromonas hydrophila. Aquaculture 2013, 416, 289–295. [Google Scholar] [CrossRef]
- Zhang, Q.; Xing, S.Z.; Sun, Q.; Pei, G.Q.; Cheng, S.; Liu, Y.Y.; An, X.P.; Zhang, X.L.L.; Qu, Y.G.; Tong, Y.G. Characterization and complete genome sequence analysis of a novel virulent Siphoviridae phage against Staphylococcus aureus isolated from bovine mastitis in Xinjiang, China. Virus Genes 2017, 53, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Nikapitiya, C.; Dananjaya, S.H.S.; Chandrarathna, H.P.S.U.; Senevirathne, A.; De Zoysa, M.; Lee, J. Isolation and characterization of multidrug resistance Aeromonas salmonicida subsp. Salmonicida and its infecting novel phage ASP-1 from goldfish (Carassius auratus). Indian J. Microbiol. 2019, 59, 161–170. [Google Scholar] [CrossRef]
- Adams, M.H. Bacteriophages; Interscience Publishers Inc.: New York, NY, USA; London, UK, 1959. [Google Scholar]
- Lu, S.G.; Le, S.; Tan, Y.L.; Zhu, J.M.; Li, M.; Rao, X.C.; Zou, L.Y.; Li, S.; Wang, J.; Jin, X.L.; et al. Genomic and proteomic analyses of the terminally redundant genome of the Pseudomonas aeruginosa phage PaP1: Establishment of genus PaP1-like phages. PLoS ONE 2013, 8, e62933. [Google Scholar] [CrossRef]
- Zhang, X.L.L.; Wang, Y.H.; Li, S.S.; An, X.P.; Pei, G.Q.; Huang, Y.; Fan, H.; Mi, Z.Q.; Zhang, Z.Y.; Wang, W.; et al. A novel termini analysis theory using HTS data alone for the identification of Enterococcus phage EF4-like genome termini. BMC Genom. 2015, 16, 1–11. [Google Scholar] [CrossRef]
- Finn, R.D.; Clements, J.; Eddy, S.R. HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res. 2011, 39, W29–W37. [Google Scholar] [CrossRef]
- Söding, J.; Biegert, A.; Lupas, A.N. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 2005, 33, W244–W248. [Google Scholar] [CrossRef]
- Lowe, T.M.; Eddy, S.R. tRNAscan-SE: A program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997, 25, 955–964. [Google Scholar] [CrossRef]
- Johnson, M.; Zaretskaya, I.; Raytselis, Y.; Merezhuk, Y.; McGinnis, S.; Madden, T.L. NCBI BLAST: A better web interface. Nucleic Acids Res. 2008, 36, W5–W9. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Kim, Y.O.; Park, S.C.; Chun, J. OrthoANI: An improved algorithm and software for calculating average nucleotide identity. Int. J. Syst. Evol. Microbiol. 2016, 66, 1100–1103. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.P.; Göker, M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.L.; Xie, B.B.; Zhang, X.Y.; Chen, X.L.; Zhou, B.C.; Zhou, J.; Oren, A.; Zhang, Y.Z. A proposed genus boundary for the prokaryotes based on genomic insights. J. Bacteriol. 2014, 196, 2210–2215. [Google Scholar] [CrossRef]
- Sullivan, M.J.; Petty, N.K.; Beatson, S.A. Easyfig: A genome comparison visualizer. Bioinformatics 2011, 27, 1009–1010. [Google Scholar] [CrossRef]
- Bao, Y.; Chetvernin, V.; Tatusova, T. Improvements to pairwise sequence comparison (PASC): A genome-based web tool for virus classification. Arch. Virol. 2014, 159, 3293–3304. [Google Scholar] [CrossRef]
- Nishimura, Y.; Yoshida, T.; Kuronishi, M.; Uehara, H.; Ogata, H.; Goto, S. ViPTree: The viral proteomic tree server. Bioinformatics 2017, 33, 2379–2380. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Mupfunya, C.R.; Qekwana, D.N.; Naidoo, V. Antimicrobial use practices and resistance in indicator bacteria in communal cattle in the Mnisi community, Mpumalanga, South Africa. Vet. Med. Sci. 2020. [Google Scholar] [CrossRef]
- Walakira, J.K.; Carrias, A.A.; Hossain, M.J.; Jones, E.; Terhune, J.S.; Liles, M.R. Identification and characterization of bacteriophages specific to the catfish pathogen, Edwardsiella ictaluri. J. Appl. Microbiol. 2008, 105, 2133–2142. [Google Scholar] [CrossRef] [PubMed]
- Look, K.Y.; Moore, D.H.; Sutton, G.P.; Prajda, N.; Abonyi, M.; Weber, G. Increased thymidine kinase and thymidylate synthase activities in human epithelial ovarian carcinoma. Anticancer Res. 1997, 17, 2353–2356. [Google Scholar]
- Reuven, N.B.; Zhou, Z.; Deutscher, M.P. Functional overlap of tRNA nucleotidyltransferase, poly(A) polymerase I, and polynucleotide phosphorylase. J. Biol. Chem. 1997, 272, 33255–33259. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.; Rosselló-Móra, R. Shifting the genomic gold standard for the prokaryotic species definition. Proc. Natl. Acad. Sci. USA 2009, 106, 19126–19131. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, E.M.; Brister, J.R. How to name and classify your phage: An informal guide. Viruses 2017, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, E.M.; Sullivan, M.B.; Knezevic, P.; van Zyl, L.J.; Sarkar, B.L.; Dutilh, B.E.; Alfenas-Zerbini, P.; Łobocka, M.; Tong, Y.G.; Brister, J.R.; et al. Taxonomy of prokaryotic viruses: 2018-2019 update from the ICTV Bacterial and Archaeal Viruses Subcommittee. Arch. Virol. 2020, 165, 1253–1260. [Google Scholar] [CrossRef]
- Yang, R.; Liu, Q.Q.; He, Y.Y.; Tao, Z.; Xu, M.Y.; Luo, Q.J.; Chen, J.J.; Chen, H.M. Isolation and identification of Vibrio mediterranei 117-T6 as a pathogen associated with yellow spot disease of Pyropia (Bangiales, Rhodophyta). Aquaculture 2020, 526, 735372. [Google Scholar] [CrossRef]
- Ward, G.M.; Faisan, J.P., Jr.; Cottier-Cook, E.J.; Gachon, C.; Hurtado, A.Q.; Lim, P.E.; Matoju, I.; iMsuya, F.E.; Bass, D.; Brodie, J. A review of reported seaweed diseases and pests in aquaculture in Asia. J. World Aquac. Soc. 2020, 51, 815–828. [Google Scholar] [CrossRef]
- Zhu, J.K.; Xu, M.Y.; Liu, Q.Q.; Li, D.F.; Yang, R.; Chen, H.M. Bacteriophage therapy on the conchocelis of Pyropia haitanensis (Rhodophyta) infected by Vibrio mediterranei 117-T6. Aquaculture 2021, 531, 735853. [Google Scholar] [CrossRef]
- Jacquemot, L.; Bettarel, Y.; Monjol, J.; Corre, E.; Halary, S.; Desnues, C.; Bouvier, T.; Ferrier-Pagès, C.; Baudoux, A.C. Therapeutic potential of a new jumbo phage that infects Vibrio coralliilyticus, a widespread coral pathogen. Front. Microbiol. 2018, 9, 2501. [Google Scholar] [CrossRef]
- Lavysh, D.; Sokolova, M.; Minakhin, L.; Yakunina, M.; Artamonova, T.; Kozyavkin, S.; Makarova, K.S.; Koonin, E.V.; Severinov, K. The genome of AR9, a giant transducing Bacillus phage encoding two multisubunit RNA polymerases. Virology 2016, 495, 185–196. [Google Scholar] [CrossRef]
- Ceyssens, P.J.; Minakhin, L.; Van den Bossche, A.; Yakunina, M.; Klimuk, E.; Blasdel, B.; De Smet, J.; Noben, J.P.; Bläsi, U.; Severinov, K.; et al. Development of giant bacteriophage ϕKZ is independent of the host transcription apparatus. J. Virol. 2014, 88, 10501–10510. [Google Scholar] [CrossRef]
- Thomas, J.A.; Weintraub, S.T.; Wu, W.; Winkler, D.C.; Cheng, N.; Steven, A.C.; Black, L.W. Extensive proteolysis of head and inner body proteins by a morphogenetic protease in the giant Pseudomonas aeruginosa phage φKZ. Mol. Microbiol. 2012, 84, 324–339. [Google Scholar] [CrossRef]
- Yakunina, M.; Artamonova, T.; Borukhov, S.; Makarova, K.S.; Severinov, K.; Minakhin, L. A non-canonical multisubunit RNA polymerase encoded by a giant bacteriophage. Nucleic Acids Res. 2015, 43, 10411–20104. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.; Clokie, M.R.J.; Millard, A. Cyanophage infection and photoinhibition in marine cyanobacteria. Res. Microbiol. 2004, 155, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Bragg, J.G.; Chisholm, S.W. Modeling the fitness consequences of a Cyanophage-encoded photosynthesis gene. PLoS ONE 2008, 3, e3550. [Google Scholar] [CrossRef] [PubMed]
- Salah Ud-Din, A.I.M.; Roujeinikova, A. Methyl-accepting chemotaxis proteins: A core sensing element in prokaryotes and archaea. Cell. Mol. Life Sci. 2017, 74, 3293–3303. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Bauer, C.E. Chemosensory signaling systems that control bacterial survival. Trends Microbiol. 2014, 22, 389–398. [Google Scholar] [CrossRef]
- Pickering, B.S.; Lopilat, J.E.; Smith, D.R.; Watnick, P.I. The transcription factor Mlc promotes Vibrio cholerae biofilm formation through repression of phosphotransferase system components. J. Bacteriol. 2014, 196, 2423–2430. [Google Scholar] [CrossRef]
- Shin, D.H. A preliminary X-ray study of a refolded PTS EIIBfruc protein from Escherichia coli. Protein Pept. Lett. 2008, 15, 630–632. [Google Scholar] [CrossRef] [PubMed]


| No. Bacteria Strains | Lytic Ability | No. Bacteria Strains | Lytic Ability |
|---|---|---|---|
| Vibrio mediterranei 117-T6 | + | Escherichia coli DH5α | - |
| Vibrio hispanicus XXY | - | Citrobacter freundii | - |
| Vibrio fluvialis XXY1 | - | Shewanella putrefaciens | - |
| Vibrio unlnificus XXY | - | Streptococcus iniae | - |
| Vibrio fluvialis XXY2 | - | Alternate pseudomonas XY | - |
| Vibrio parahaemolyticus 1A08161 | - | Shigella dysenteriae | - |
| Vibrio parahaemolyticus 1A11655 | - | Proteus vulgaris | - |
| Vibrio alginolyticus LDF | - | Proteus mirabilis | - |
| Vibrio alginolyticus XY | - | Aeromonas hydrophila | - |
| Vibrio alginolyticus WY | - | Shigella sonnei | - |
| Vibrio alginolyticus SZT | - | Pseudomonas aeruginosa LDF | - |
| Vibrio harveyi 1–5 | - | Pseudomonas aeruginosa SZT | - |
| Vibrio harveyi LDF | - | Aeromonas sobria LY-23 | - |
| Vibrio pacinii XY | - | Aeromonas sobria ATCC43979 | - |
| Vibrio anguillarum | - | Pseudoalteromonas issachenkonii XY | - |
| Aeromonas bivalvium XXY | - | Salmonella paratyphi B | - |
| Edwardsiella tarda SZT | - | Enterobacter cloacae | - |
| Edwardsiella tarda LW | - | Marinomonas sp.XY | - |
| Enterobacter sakazakii | - | Pseudomonas sp. XXY | - |
| Strain | Accession no. | ANI, % | isDDH, % | POCP, % |
|---|---|---|---|---|
| Vibrio phage BONAISHI | MH595538 | 58.65 | 0 | 6.774 |
| Aeromonas phage phiAS5 | HM452126 | 0 | 0 | 0.606 |
| Aeromonas phage CC2 | JX123262 | 0 | 0 | 0.268 |
| Aeromonas phage AS-yj | MF498774 | 0 | 0 | 0.278 |
| Aeromonas phage AS-szw | MF498773 | 0 | 0 | 0.273 |
| Aeromonas phage AS-zj | MF448340 | 0 | 0 | 0.272 |
| Aeromonas phage AS-sw | MF498775 | 0 | 0 | 0.271 |
| Pseudomonas phage Phabio | MF042360 | 0 | 0 | 0.26 |
| Vibrio phage 2.275.O._10N.286.54.E11 | MG592671 | 0 | 0 | 0.245 |
| Vibrio phage 2 TSL-2019 | MF063068 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Li, D.; Tong, Y.; Fang, J.; Yang, R.; Qin, W.; Lin, W.; Pan, L.; Liu, W. A Novel Singleton Giant Phage Yong-XC31 Lytic to the Pyropia Pathogen Vibrio mediterranei. Appl. Sci. 2021, 11, 1602. https://doi.org/10.3390/app11041602
Xu L, Li D, Tong Y, Fang J, Yang R, Qin W, Lin W, Pan L, Liu W. A Novel Singleton Giant Phage Yong-XC31 Lytic to the Pyropia Pathogen Vibrio mediterranei. Applied Sciences. 2021; 11(4):1602. https://doi.org/10.3390/app11041602
Chicago/Turabian StyleXu, Lihua, Dengfeng Li, Yigang Tong, Jing Fang, Rui Yang, Weinan Qin, Wei Lin, Lingtin Pan, and Wencai Liu. 2021. "A Novel Singleton Giant Phage Yong-XC31 Lytic to the Pyropia Pathogen Vibrio mediterranei" Applied Sciences 11, no. 4: 1602. https://doi.org/10.3390/app11041602
APA StyleXu, L., Li, D., Tong, Y., Fang, J., Yang, R., Qin, W., Lin, W., Pan, L., & Liu, W. (2021). A Novel Singleton Giant Phage Yong-XC31 Lytic to the Pyropia Pathogen Vibrio mediterranei. Applied Sciences, 11(4), 1602. https://doi.org/10.3390/app11041602







