Hepatic Reirradiation for Patients with Recurrent Hepatocellular Carcinoma
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Radiotherapy
2.2. Follow-Up and Evaluation of Toxicity
2.3. Statistics
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bae, S.H.; Park, H.C.; Lim, D.H.; Lee, J.A.; Gwak, G.Y.; Choi, M.S.; Lee, J.H.; Koh, K.C.; Paik, S.W.; Yoo, B.C. Salvage treatment with hypofractionated radiotherapy in patients with recurrent small hepatocellular carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e603–e607. [Google Scholar] [CrossRef]
- Arnaoutakis, D.J.; Mavros, M.N.; Shen, F.; Alexandrescu, S.; Firoozmand, A.; Popescu, I.; Weiss, M.; Wolfgang, C.L.; Choti, M.A.; Pawlik, T.M. Recurrence Patterns and Prognostic Factors in Patients with Hepatocellular Carcinoma in Noncirrhotic Liver: A Multi-Institutional Analysis. Ann. Surg. Oncol. 2014, 23, 147–154. [Google Scholar] [CrossRef]
- Hashimoto, T.; Tokuuye, K.; Fukumitsu, N.; Igaki, H.; Hata, M.; Kagei, K.; Sugahara, S.; Ohara, K.; Matsuzaki, Y.; Akine, Y. Repeated proton beam therapy for hepatocellular carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Dawson, L.A. Overview: Where Does Radiation Therapy Fit in the Spectrum of Liver Cancer Local-Regional Therapies? Semin. Radiat. Oncol. 2011, 21, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Ohri, N.; Tomé, W.A.; Méndez Romero, A.; Mifften, M.; TenHaken, R.K.; Dawson, L.A.; Grimm, J.; Yorke, E.; Jackson, A. Local Control after Stereotactic Body Radiation Therapy for Liver Tumors. Int. J. Radiat. Oncol. Biol. Phys. 2018. [Google Scholar] [CrossRef] [PubMed]
- Seol, S.W.; Yu, J.I.; Park, H.C.; Lim, D.H.; Oh, D.; Noh, J.M.; Cho, W.K.; Paik, S.W. Treatment outcome of hepatic re-irradiation in patients with hepatocellular carcinoma. Radiat. Oncol. J. 2015, 33, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, S.W.; Fan, C.C.; Ting, L.L.; Kuo, C.C.; Chiou, J.F. Clinical parameters for predicting radiation-induced liver disease after intrahepatic reirradiation for hepatocellular carcinoma. Radiat. Oncol. 2016, 11, 1–9. [Google Scholar] [CrossRef][Green Version]
- McDuff, S.G.R.; Remillard, K.A.; Zheng, H.; Raldow, A.C.; Wo, J.Y.; Eyler, C.E.; Drapek, L.C.; Goyal, L.; Blaszkowsky, L.S.; Clark, J.W.; et al. Liver reirradiation for patients with hepatocellular carcinoma and liver metastasis. Pract. Radiat. Oncol. 2018, 8, 414–421. [Google Scholar] [CrossRef]
- Owen, D.; Lukovic, J.; Hosni, A.; Crane, C.H.; Hong, T.S.; Dawson, L.A.; Velec, M.; Lawrence, T.S. Challenges in Reirradiation of Intrahepatic Tumors. Semin. Radiat. Oncol. 2020, 30, 242–252. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.; Ji, Y.; Cho, B.; Kim, S.S.; Jung, J.; Kwak, J.; Park, J.-H.; Lee, S.-W.; Kim, J.H.; et al. Evaluation of Hepatic Toxicity after Repeated Stereotactic Body Radiation Therapy for Recurrent Hepatocellular Carcinoma Using Deformable Image Registration. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Jun, B.G.; Kim, Y.D.; Kim, S.G.; Kim, Y.S.; Jeong, S.W.; Jang, J.Y.; Lee, S.H.; Kim, H.S.; Kang, S.H.; Kim, M.Y.; et al. Hepatitis B virus reactivation after radiotherapy for hepatocellular carcinoma and efficacy of antiviral treatment: A multicenter study. PLoS ONE 2018, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Park, H.; Lim, D.; Park, W. Predictive Factors for Child-Pugh Score Elevation in Hepatocellular Carcinoma Patients Treated with Conformal Radiation Therapy: Dose-Volume Histogram Analysis. Int. J. Radiat. Oncol. 2012, 84, S328–S329. [Google Scholar] [CrossRef]
- Rossi, S.; Ravetta, V.; Rosa, L.; Ghittoni, G.; Viera, F.T.; Garbagnati, F.; Silini, E.M.; Dionigi, P.; Calliada, F.; Quaretti, P.; et al. Repeated radiofrequency ablation for management of patients with cirrhosis with small hepatocellular carcinomas: A long-term cohort study. Hepatology 2011, 53, 136–147. [Google Scholar] [CrossRef]
- Yokoyama, K.; Anan, A.; Iwata, K.; Nishizawa, S.; Morihara, D.; Ueda, S.I.; Sakurai, K.; Iwashita, H.; Hirano, G.; Sakamoto, M.; et al. Limitation of repeated radiofrequency ablation in hepatocellular carcinoma: Proposal of a three (times) × 3 (years) index. J. Gastroenterol. Hepatol. 2012, 27, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Bhoori, S.; Sposito, C.; Mazzaferro, V. Repeated transarterial chemoembolization: An overfitting effort? J. Hepatol. 2015, 62, 1440–1442. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, N.; Kim, H.J.; Won, J.Y.; Kim, D.Y.; Han, K.H.; Jung, I.; Seong, J. Retrospective analysis of stereotactic body radiation therapy efficacy over radiofrequency ablation for hepatocellular carcinoma. Radiother. Oncol. 2019, 131, 81–87. [Google Scholar] [CrossRef]
- Kong, M.; Hong, S.E. Optimal follow-up duration for evaluating objective response to radiotherapy in patients with hepatocellular carcinoma: A retrospective study. Chin. J. Cancer 2014, 34, 79–85. [Google Scholar] [CrossRef][Green Version]
- Davenport, M.S.; Viglianti, B.L.; Al-Hawary, M.M.; Caoili, E.M.; Kaza, R.K.; Liu, P.S.C.; Maturen, K.E.; Chenevert, T.L.; Hussain, H.K. Comparison of acute transient dyspnea after intravenous administration of gadoxetate disodium and gadobenate dimeglumine: Effect on arterial phase image quality. Radiology 2013, 266, 452–461. [Google Scholar] [CrossRef]
- Lu, R.C.; She, B.; Gao, W.T.; Ji, Y.H.; Xu, D.D.; Wang, Q.S.; Wang, S.B. Positron-emission tomography for hepatocellular carcinoma: Current status and future prospects. World J. Gastroenterol. 2019, 25, 4682–4695. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; deOliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef]
- Zhu, A.X.; Kang, Y.K.; Yen, C.J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y.; et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 282–296. [Google Scholar] [CrossRef]
- Finn, R.S.; Ryoo, B.Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab As Second-Line Therapy in Patients with Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef]
- Huo, Y.R.; Eslick, G.D. Transcatheter arterial chemoembolization plus radiotherapy compared with chemoembolization alone for hepatocellular carcinoma a systematic review and meta-analysis. JAMA Oncol. 2015, 1, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.W.; Lin, L.C.; Kuo, Y.C.; Liang, J.A.; Kuo, C.C.; Chiou, J.F. Phase 2 study of combined sorafenib and radiation therapy in patients with advanced hepatocellular carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 1041–1047. [Google Scholar] [CrossRef]
| Characteristics | No. of Patients | % |
|---|---|---|
| Patient | 32 | |
| Age (years) | Mean: 66.0, (32–89) | |
| Gender | ||
| Male | 29 | 90.6 |
| Female | 3 | 9.4 |
| Tumor stage | ||
| AJCC 1 (7th ed., 2010) | ||
| T1 | 3 | 9.4 |
| T2 | 15 | 46.9 |
| T3 | 9 | 28.1 |
| T4 | 5 | 15.6 |
| BCLC 2 stage | ||
| A | 5 | 15.6 |
| B | 15 | 46.9 |
| C | 12 | 37.5 |
| Tumor numbers | ||
| One | 27 | 84.4 |
| Two | 5 | 15.6 |
| Follow-up (months) | Mean: 22.4, (1.5–127.0) | |
| Interval between two RT courses (months) | Mean: 16.5, (0.5–136.0) | |
| Child–Pugh score before the second RT | ||
| 5 | 22 | 68.8 |
| 6 | 6 | 18.8 |
| 7 | 4 | 12.5 |
| Hepatitis status | ||
| Hepatitis B | 19 | 59.4 |
| Hepatitis C | 5 | 15.6 |
| Both hepatitis B and C | 1 | 3.1 |
| Main portal vein tumor thrombosis | ||
| Present | 9 | 28.1 |
| Absent | 23 | 71.9 |
| Local therapies before RT | ||
| Nil | 11 | 34.4 |
| Operation | 2 | 6.3 |
| TACE | 7 | 21.9 |
| RFA | 2 | 6.3 |
| Operation and TACE | 6 | 18.8 |
| TACE and RFA | 3 | 9.4 |
| Operation, TACE and RFA | 1 | 3.1 |
| Radiation dose of the nd RT (BED 3, α/β = 10) | Mean: 52.3 Gy, (ranges, 12–109.5 Gy) | |
| Use of systemic therapy after the 2nd RT | ||
| Yes | 8 | 25.0 |
| No | 24 | 75.0 |
| Prognostic Factor | No. of Patient | Infield Progression-Free Survival (IF-PFS) | Overall Survival (OS) | ||||
|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | ||||
| n (%) | p Value | HR 1 (95% CI 2) | p Value | p Value | HR (95% CI) | p Value | |
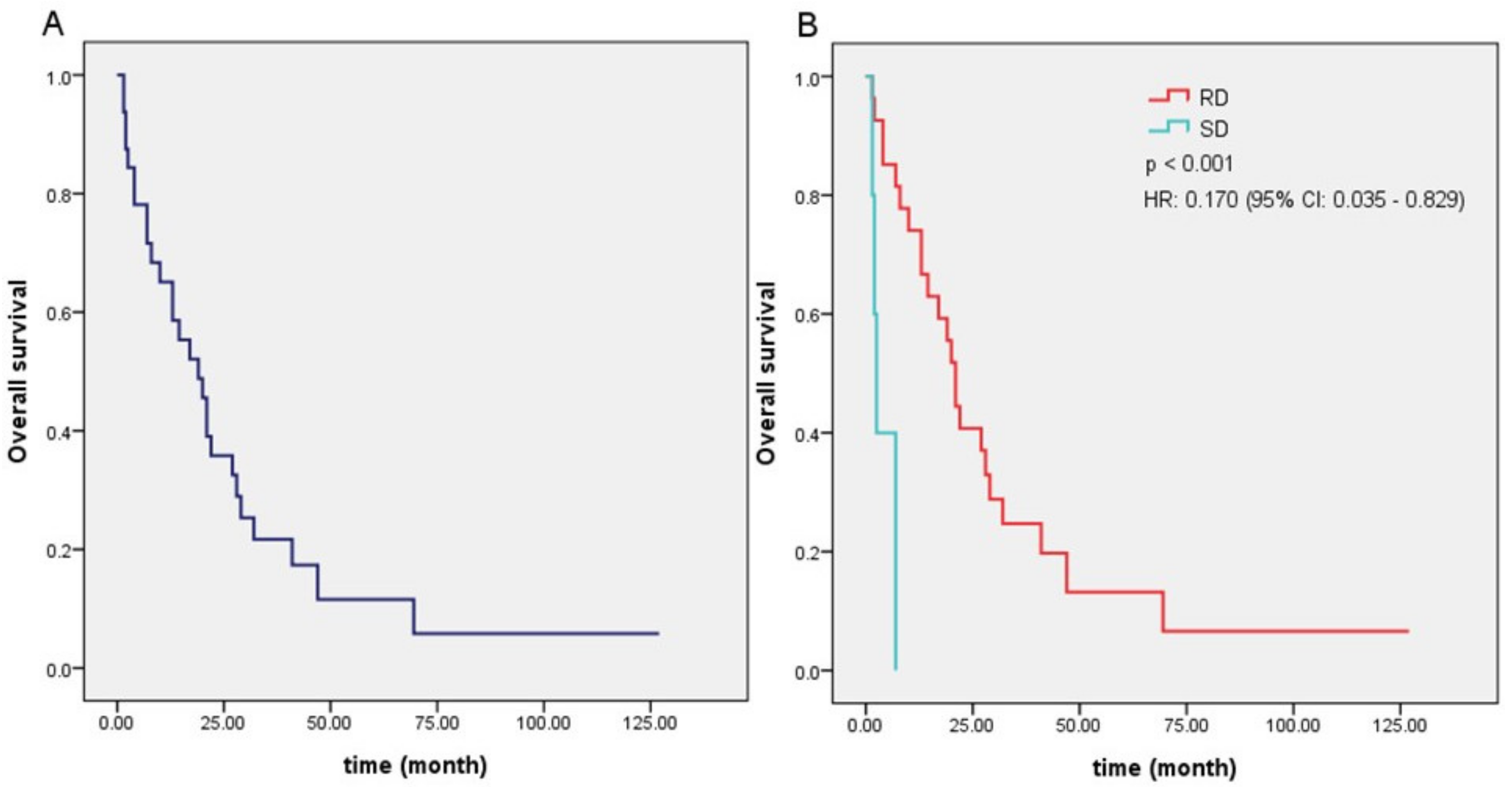
| Local control | <0.001 | 0.170 (0.035–0.829) | 0.028 | ||||
| Regression subgroup | 27 (84.4%) | ||||||
| Stable subgroup | 5 (15.6%) | ||||||
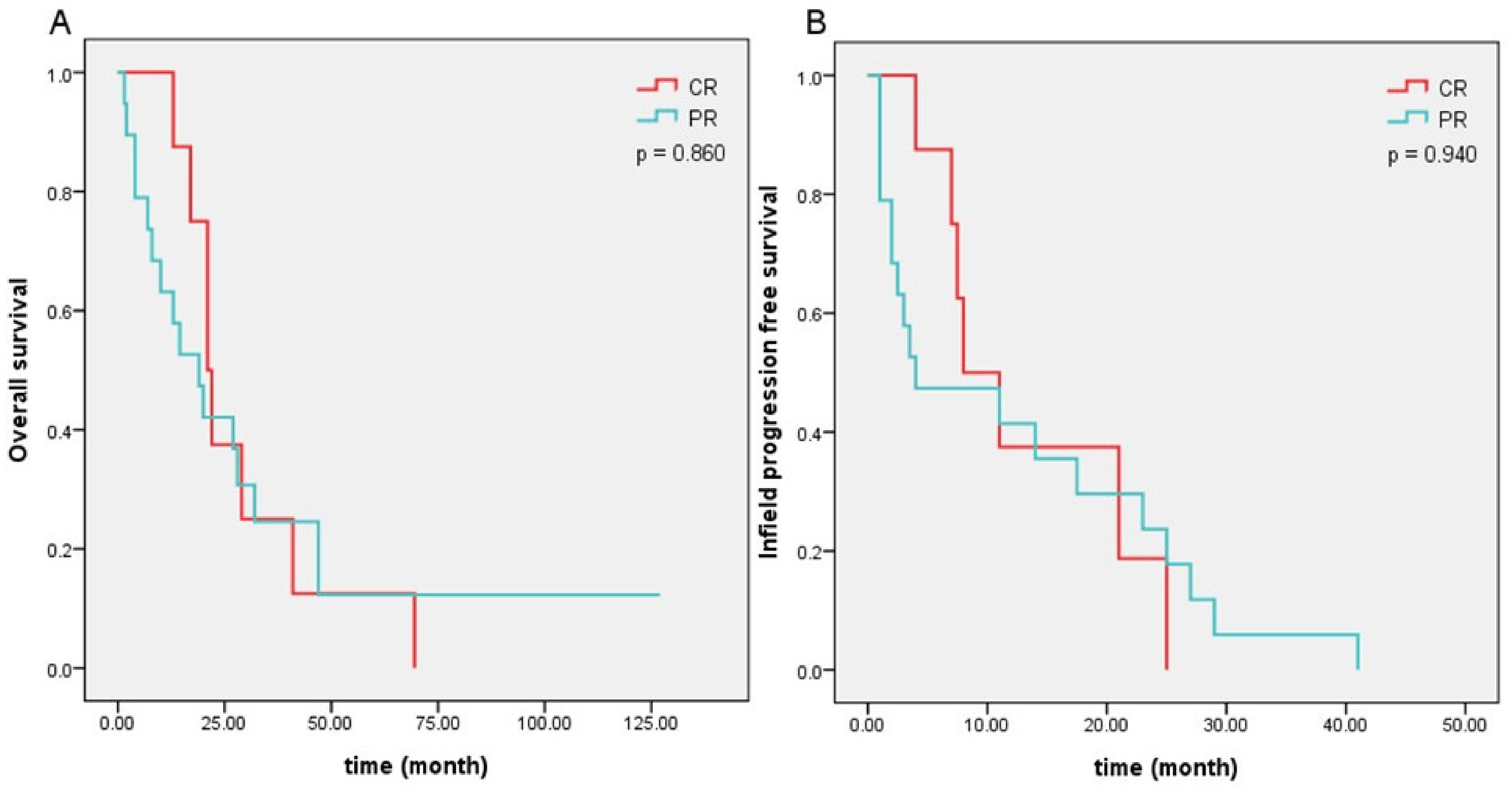
| Regression subgroup | 0.940 | 0.860 | |||||
| Complete remission | 8 (25%) | ||||||
| Partial remission | 19 (59.4%) | ||||||
| BCLC stage | 0.326 | 0.114 | |||||
| Stage A | 5 (15.6%) | ||||||
| Stage B | 15 (46.9%) | ||||||
| Stage C | 12 (37.5%) | ||||||
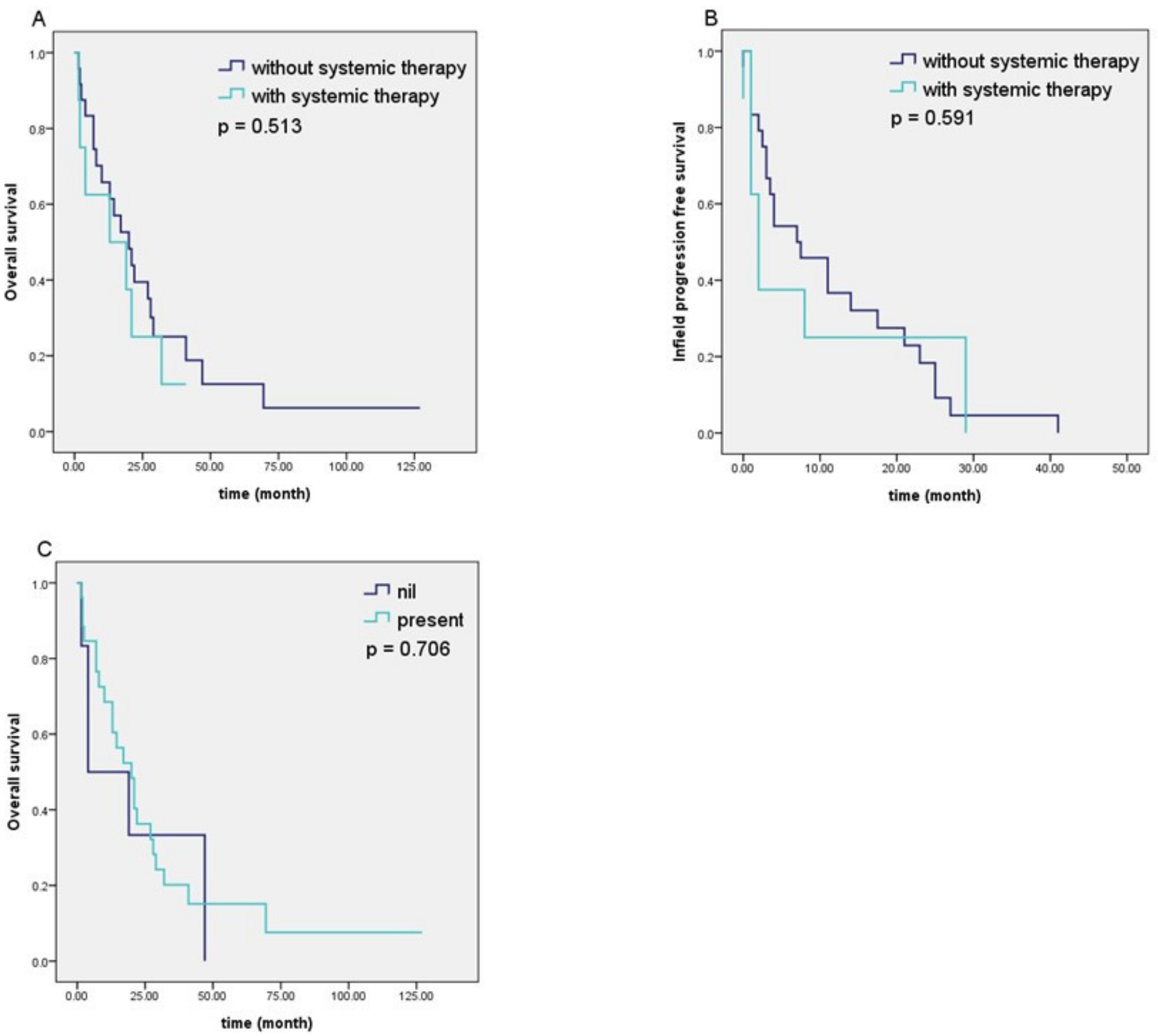
| Use of systemic therapy | 0.591 | 0.513 | |||||
| Use | 8 (25.0%) | ||||||
| Nonuse | 24 (75.0%) | ||||||
| Outfield recurrence and/or distant metastasis | 0.072 | 3.320 (0.933–11.812) | 0.064 | 0.706 | |||
| Nil | 6 (18.8%) | ||||||
| Presence | 26 (81.3%) | ||||||
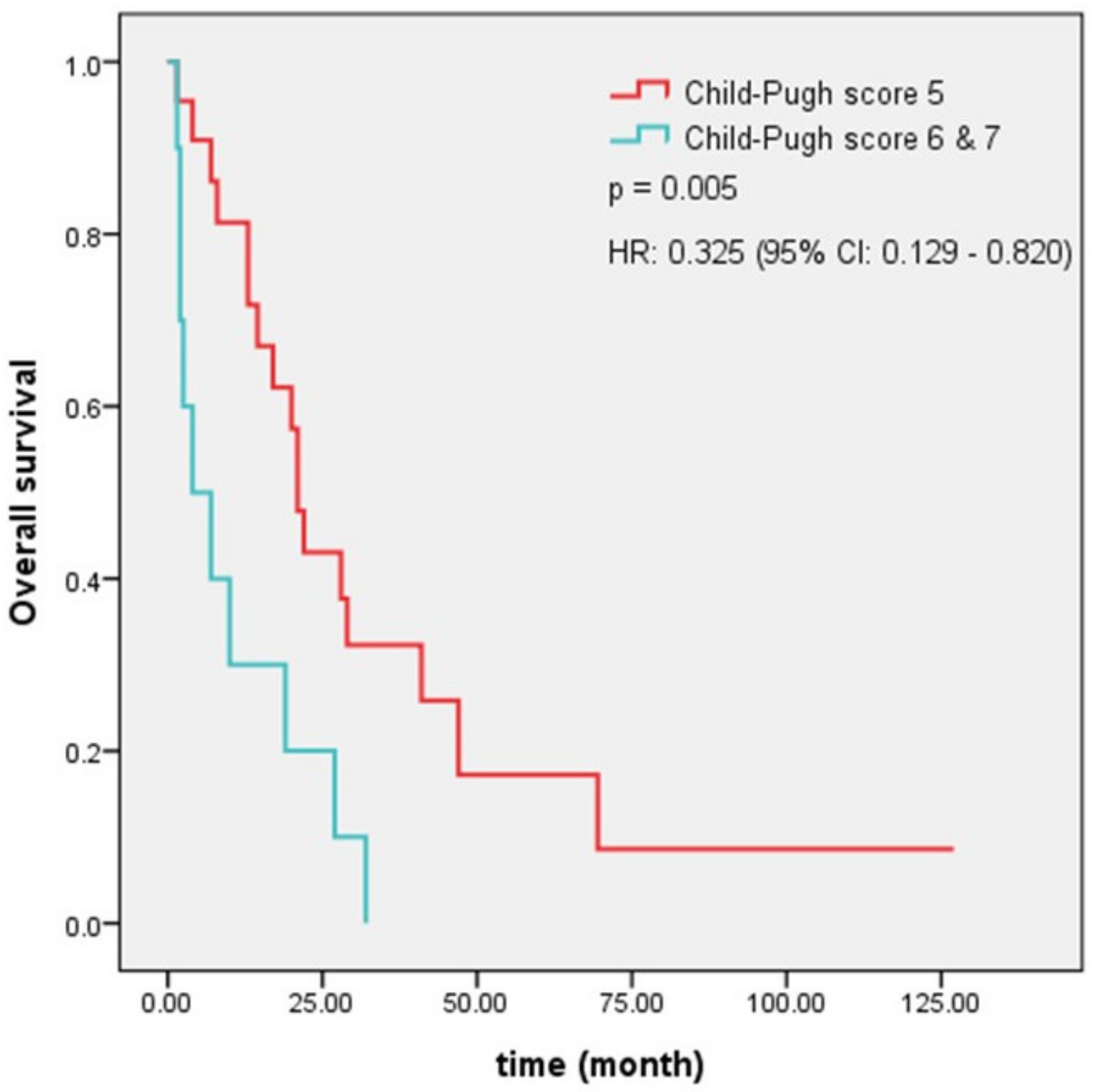
| Child–Pugh score before re-RT | <0.001 | 0.228 (0.085–0.612) | 0.003 | 0.005 | 0.325 (0.129–0.820) | 0.017 | |
| Child–Pugh score 5 | 22 (68.8%) | ||||||
| Child–Pugh score 6 and 7 | 10 (31.3%) | ||||||
| Tumor volume | 0.075 | 0.488 (0.172–1.383) | 0.177 | 0.059 | 0.782 (0.299–2.047) | 0.617 | |
| Tumor volume ≥ 100 (cm3) | 17 (53.1%) | ||||||
| Tumor volume < 100 (cm3) | 15 (46.9%) | ||||||
| Dose of radiotherapy | 0.458 | 0.708 | |||||
| BED < 50 (Gy) | 17 (53.1%) | ||||||
| BED ≥ 50 (Gy) | 15 (46.9%) | ||||||
| Main portal vein tumor thrombosis | 0.141 | 0.099 | |||||
| Nil | 23 (71.9%) | ||||||
| Presence | 9 (28.1%) | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Chen, P.-Y.; Cheng, T.-Y.; Chiou, J.-F. Hepatic Reirradiation for Patients with Recurrent Hepatocellular Carcinoma. Appl. Sci. 2021, 11, 1598. https://doi.org/10.3390/app11041598
Huang Y, Chen P-Y, Cheng T-Y, Chiou J-F. Hepatic Reirradiation for Patients with Recurrent Hepatocellular Carcinoma. Applied Sciences. 2021; 11(4):1598. https://doi.org/10.3390/app11041598
Chicago/Turabian StyleHuang, Yaoru, Po-Yung Chen, Tzu-Yen Cheng, and Jeng-Fong Chiou. 2021. "Hepatic Reirradiation for Patients with Recurrent Hepatocellular Carcinoma" Applied Sciences 11, no. 4: 1598. https://doi.org/10.3390/app11041598
APA StyleHuang, Y., Chen, P.-Y., Cheng, T.-Y., & Chiou, J.-F. (2021). Hepatic Reirradiation for Patients with Recurrent Hepatocellular Carcinoma. Applied Sciences, 11(4), 1598. https://doi.org/10.3390/app11041598






