Abstract
The effects of nickel coating and heat treatment on the interfacial bonds of aluminum–iron (Al/Fe) alloys hybrid structures were investigated using microstructural analysis. The application of a nickel coating successfully suppressed the formation of defects such as gaps and oxide scale, improving the physical bonding of the interface. Optimizing the heat treatment conditions generated superior chemical bonding at the interface and facilitated the formation of a nickel-bearing phase in the Al matrix. Also, the types of nickel-bearing phase were influenced by solution treatment and proximity to the interface. By analyzing the isopleth phase diagram of the aluminum system for the ranges of nickel present in the Al, it was confirmed that the Ni:Cu ratio affected the precipitation characteristics of the system. However, when heated under conditions that were optimized for chemical bonding, the Al matrix decreased by approximately 40% (from 100 HV to 60 HV), due to grain growth. The effect of artificial aging increased the hardness of the Al matrix away from the interface by 35% (from 63 HV to 90 HV). On the other hand, this did not occur in the Al matrix near the interface. These results indicate that the nickel that diffused into the Al matrix interfered with the precipitation hardening effect.
1. Introduction
The demand for high-performance aluminum alloys has increased in the automotive industry in recent years due to their numerous advantages, including high strength-to-weight ratios (for reducing vehicle weight, which also improves fuel efficiency), excellent anti-corrosion characteristics, and superior thermal conductivities [1,2,3,4]. However, aluminum alloys are not suitable for certain automotive parts that require excellent mechanical properties and wear resistance, such as brake disks, engine blocks, and cylinder heads. This is because of their low Young’s modulus and vulnerability to high temperature [5]. To reduce the weight of specific parts and improve their heat dissipation characteristics, several studies have shifted their focus on aluminum–iron (Al/Fe) alloys hybrid structures combining aluminum and ferrous alloys [5,6,7]. These structures are being fabricated with the liquid–solid casting process based on the method of pouring liquid alloy onto a monoclinic insert of a steel plate as in many previous studies [8,9,10]. The fabricated structures in this method can combine the advantages of each metal [6,7].
A prerequisite for efficient hybrid structures is an excellent bond between the two metals. This is essential for achieving good mechanical properties and thermal conduction [11]. Two major complications, however, render the bonding of aluminum and ferrous alloys a quite challenging task. First, these alloys are susceptible to oxidation, leading to oxides that interfere with interfacial bonding. Second, the different physical/thermal properties of the two metals, such as the melting points and thermal expansion coefficients, induce poor wettability [5,6,7,11,12]. Therefore, a new process that overcomes these issues must be developed to achieve successful bonding.
The intermediate layer between ferrous and aluminum alloys has played a role in mitigating oxidation and improving joint performance, according to much previous research. The formation of the intermediate layer which contributes to obtaining an excellent bonding has been carried out by the hot dipping method. Coating materials have been selected from zinc, aluminum, and copper [2,5,7,11,13,14,15]. These coatings can effectively prevent direct interaction between steel and aluminum alloys and avert the oxidation of steel during the preheating stage, thus improving bonding [5,6,7,13,14]. There are few studies, however, on the effects of nickel coatings. Nickel coatings can enhance the corrosion resistance of metal objects [16,17], and nickel atoms can also augment the mechanical properties of aluminum alloys at elevated temperatures [18,19,20] due to their “impurity atom” behavior. Our research aimed to utilize the unique characteristics of nickel, with the aim of improving the interfacial bonding and mechanical properties of Al/Fe alloys hybrid structures.
In this work, we applied a nickel coating on an Al/Fe alloys hybrid structure, which was fabricated using a dissimilar casting technique. Then, various heat treatment conditions were implemented to evaluate the effects of nickel diffusion on the interfacial bonding and the microstructure of the Al matrix. We aimed to determine the optimized conditions to achieve superior chemical bonding. The chemical bonding mechanism under optimized heat treatment conditions was investigated and compared with thermodynamic calculations for an aluminum alloy system with a 1–10% nickel concentration range. Finally, hardness tests were performed to assess the combined effects of optimized heat treatment and nickel diffusion on the mechanical properties of the Al/Fe alloys hybrid structure.
2. Materials and Methods
A total of 316 stainless steel and A319 alloys were used to fabricate the Al/Fe alloys hybrid structures. The chemical composition of each alloy is shown in Table 1. Prior to the dissimilar casting process, the 316 stainless steel was coated with nickel by arc wire spraying (the detailed processing parameters are listed in Table 1 and Table 2). The aluminum alloy ingots were melted in an electric crucible at 750 °C and then degassed with argon gas for 10 min. Subsequently, nickel-coated steel plates preheated at 300 °C were placed on the metal mold. The molten aluminum was then poured into the metal mold, thereby fabricating the Al/Fe alloys hybrid structure after solidification.

Table 1.
Chemical composition of the A319, SS316, and Ni-5wt.% Al alloys (wt.%).

Table 2.
Processing parameters of arc wire spraying.
Heat treatment processes, involving solution treatment and artificial aging, were applied to the fabricated Al/Fe alloys hybrid structures. To determine the optimal heat treatment conditions for chemical bonding, different solution treatment temperatures/times and cooling rates were conducted in this work. As-cast samples were heat-treated at 500 °C, 530 °C, and 550 °C in an electric furnace. The solution treatment times varied from 1 h, to 3 h, to 8 h at each temperature, followed by either air- or furnace-cooling. Artificial aging was performed at 170 °C for 8 h, followed by air-cooling.
The Al/Fe alloys hybrid structures were cut into samples using an electrical discharge machine. The samples were then polished with silicon carbide (SiC) polishing papers (up to grit #2400). The polishing process was then repeated using 0.25 μm diamond and 0.04 μm colloidal suspensions. The microstructure of the Al/Fe alloys hybrid structure was analyzed using field emission scanning electron microscopy (FE-SEM, MIRA3, Tescan), with an attached an energy-dispersive X-ray spectroscopy (EDS, EDAX) module. To study the effects of the nickel coating, the surface of the nickel-coated steel samples before and after the preheating process was analyzed with X-ray diffraction (XRD, D/MAX 2500 V L/PC, Rigaku) using Cu Kα radiation (λ =1.54 Å) generated at 40 kV and 30 mA. The chemical compositions of the intermetallic phases generated at the interface and in the Al matrix were studied by EDS. The element diffusions and chemical bonding in the Al/Fe alloys hybrid structure, under as-cast and optimized heat treatment conditions, were analyzed using an electron probe X-ray microanalyzer (EPMA, JXA-8230, JEOL).
To analyze the influence of nickel diffusion on the Al matrix during the heat treatment process, JmatPro® software was used, which calculates equilibrium phases as a function of temperature and input composition. Using this program, we investigated the formation of the nickel-bearing phase in relation to nickel addition in the A319 (Al-Si-Cu-Mg-Fe) alloy system. The equilibrium conditions of the various nickel-bearing phases in this alloy system were investigated at the 0–10% nickel range and are presented in corresponding isopleth phase diagrams.
The microhardness distribution of the Al/Fe alloys hybrid structure was measured using a Vickers hardness testing machine (HM-122, Akashi), with the preset parameters of load stress = 0.1 kgf and time duration = 15 s. By measuring the microhardness across the interface of the Al/Fe alloys hybrid structure, the change in hardness of each matrix in relation to heat treatment could be analyzed.
3. Results and Discussion
3.1. Effects of the Nickel Coating on the Interfacial Microstructures
Figure 1 shows the results of various analyses performed to investigate the effects of the nickel coating on the Al/Fe alloys hybrid structure. Figure 1a shows SEM micrographs of the Al/Fe alloys hybrid structure’s interface with nickel coating. The interfacial bonding exhibited superior physical bonding performance and was free from defects such as gaps and oxides. In conventional dissimilar casting processes used to fabricate Al/Fe alloys hybrid structures (such as diffusion bonding), solid-state steel is combined with a liquid-state aluminum alloy [21]. Considering the substantially different physicochemical properties of Fe and Al, however, severe oxidation occurs on the surface of steel during the pre-heating stage, thus hindering the bonding of solid-state steel and liquid-state aluminum alloy [5,6,7,12]. The oxide film prevents direct contact during the casting process, reducing the wettability between the metals, and thus results in poor bonding [6]. The nickel-coated steel in this study, however, enabled improved interfacial bonding by preventing oxide formation.
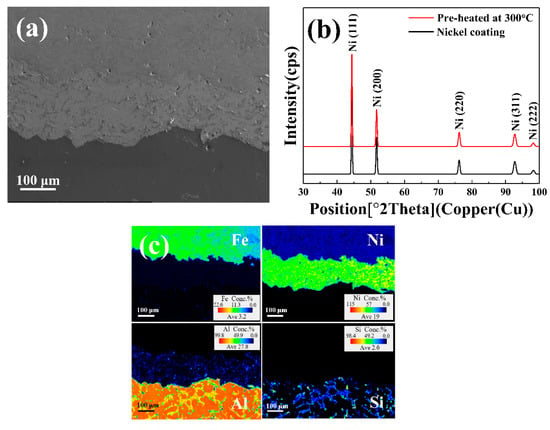
Figure 1.
(a) SEM micrographs of the Al/Fe alloys hybrid structure’s interface with nickel coating; (b) XRD patterns of nickel coated steel before and after heat treatment at 300 °C; (c) chemical element quantitative maps of (a): Fe, Ni, Al, and Si.
To examine the effects of the nickel coating in more detail, XRD patterns were obtained from the nickel-coated steel before and after the pre-heating step at 300 °C (Figure 1b). The XRD pattern from the surface of the nickel-coated steel did not change even after preheating at 300 °C, and no nickel oxide peak was detected. This shows that the nickel coating successfully suppressed the formation of an oxide film on the steel surface during preheating, and improved wettability between the aluminum melt and steel, leading to excellent physical bonding of the Al/Fe alloys hybrid structure [22]. It was therefore confirmed that preventing oxidation on the steel surface using a nickel coating is an effective way to improve the interfacial bonding of the Al/Fe alloys hybrid structure fabricated by dissimilar casting.
Figure 1c shows the chemical element quantitative maps of the Al/Fe alloys hybrid structure with a nickel coating. The boundaries between each metal, along with their morphological characteristics, were apparently discernible and indexed using different colors. No apparent distribution of Fe and Ni in the Al matrix was detected. The Si elements were located solely at the grain boundary of the Al matrix, and elements in the aluminum alloy did not move into the other metal matrix. These results indicated that the chemical bonding of the interface by elemental diffusion did not occur during dissimilar casting, implying that the nickel coating could theoretically have contributed to the physical bonding of the Al/Fe alloys hybrid structure.
3.2. Effects of Heat Treatment on the Interfacial Microstructures
Figure 2 shows the interfacial microstructure of the Al/Fe alloys hybrid structure heat treated for 1 h at different solution temperatures, followed by furnace cooling. At a solution temperature of 500 °C (Figure 2a), the interface of the Al/Fe alloys hybrid structure demonstrated a similar microstructure to the as-cast condition without chemical bonding, and no intermetallic phase was formed in the Al matrix. When the solution temperature increased to 530 °C, the diffusion of atoms at the interface resulted in an intermetallic layer (Figure 2b). The nickel diffused into the Al matrix, and this led to formation of the intermetallic phase, due to the low solubility of nickel in aluminum [23]. There were no intermetallic phases in the Al matrix; however, as in the as-cast and 500 °C solution temperature conditions. Therefore, the temperature at which the inter-diffusion of atoms begins to occur at the interface is approximately 530 °C. When the solution temperature increased to 550 °C, the intermetallic phases at the interface formed a continuous layer and the thickness of the intermetallic layer increased significantly (Figure 2d). The chemical bonding that formed at the interface under this particular heat treatment condition was superior. The parabolic law correlates the solution temperature with the growth of the intermetallic layer [24,25,26], and is expressed as follows:
where is the intermetallic layer thickness, is the heat treatment time, is the coefficient of diffusion, is the diffusion constant, is the activation energy for intermetallic phase growth, is the absolute solution temperature, and is the gas constant. According to Equations (1) and (2), the solution temperature and diffusion coefficient are proportional, implying that an increase in solution temperature promotes the diffusion of atoms at the interface.
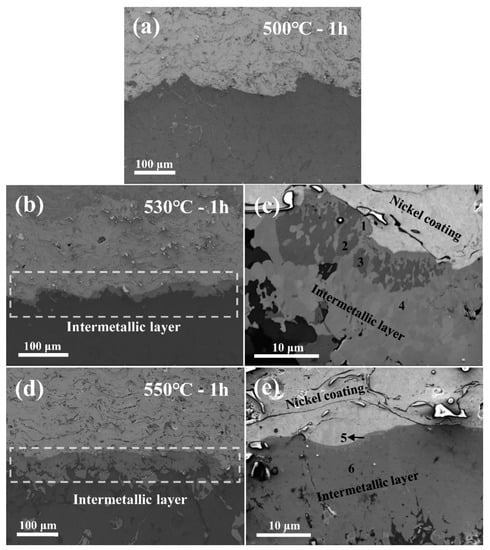
Figure 2.
SEM micrographs of the Al/Fe alloys hybrid structure’s interface under different solution temperatures followed by furnace cooling; (a) 500 °C, (b,c) 530 °C, and (d,e) 550 °C.
Figure 2c,e show the SEM micrographs of the intermetallic layers created at the interface, at different solution temperatures. Table 3 shows the results of the compositional analysis of the intermetallic layers formed at 530 and 550 °C. The compositions of the intermetallic layer after heat treatment were Al3CuNi / Al3Ni at 530 °C, and NiAl / Al3Ni at 550 °C. Thus, composition of the intermetallic layer varied with solution treatment temperature. The types of intermetallic layers formed through heat treatment were related to the ratio of diffused atoms. The higher solution temperature caused greater diffusion at the interface, affecting the types of intermetallic phase, which were determined by the Ni:Cu ratio in the Cu-containing Al matrix. As the Ni:Cu ratio increased, a nickel-bearing phase formed in the sequence of γ-Al7Cu4Ni, δ-Al3CuNi, and ε-Al3Ni [27]. Therefore, depending on the solution temperature, the intermetallic layer at the interface primarily comprised δ-Al3CuNi (at 530 °C), or ε-Al3Ni (at 550 °C).

Table 3.
The qualitative analysis of the intermetallic layer corresponding to the points in Figure 2c,e.
Figure 3a shows the changes in thickness of the intermetallic layer, at different solution treatment temperatures and annealing times. Heat treatment at 500 °C for 1 h and 3 h did not generate an intermetallic layer. The thickness of the layer formed after 8 h was approximately 10 μm, which was thinner than that for other solution treatment temperatures. The intermetallic layer formed at 530 °C continuously increased until 8 h, but remained thinner than the layer formed at 550 °C. After heat treatment at 550 °C for 1 h, the intermetallic layer at the interface was continuous and notably thicker than that for the other temperatures. At this heat treatment temperature, and compared with other temperatures, the thickness of the intermetallic layer increased sharply with heat treatment time. Hence, the solution temperature was determined to significantly promote the formation of thick and continuous intermetallic layers. The optimal solution temperature for the chemical bonding of the Al/Fe alloys hybrid structure was 550 °C.
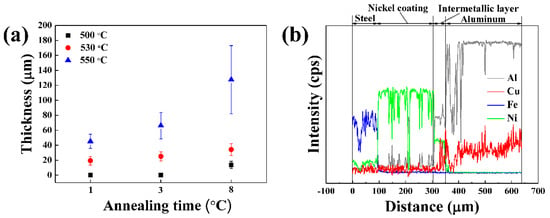
Figure 3.
(a) The quantitative analysis of intermetallic layer thickness under different solution temperature and annealing times and (b) electron probe X-ray microanalyzer (EPMA) line mapping of the Al/Fe alloys hybrid structure’s interface under 550 °C heat treatment, followed by furnace cooling.
Figure 3b shows the EPMA line mapping results of the Al/Fe alloys hybrid structure’s interface, after heat treatment at 550 °C. The diffusion of Ni and Al elements across the interface was evident, implying successful chemical bonding between the nickel and the aluminum following heat treatment. Furthermore, these results confirmed that the Fe elements from the steel did not actively diffuse into the Al matrix during the heat treatment process. This was due to the nickel coating.
Although the intermetallic layer thickness increased with increasing the solution treatment time and temperature, excessively long heat treatment times decreased the bonding strength of the Al/Fe alloys hybrid structure. Long-term heat treatment for 8 h caused the formation of cracks between the intermetallic layer and the aluminum substrate (Figure 4). We believe that there are two main reasons for this. First, the large difference in the coefficients of thermal expansion (CTE) between the aluminum alloy and the intermetallic layer induces excessive thermal stress, sufficient to form cracks at the interface [26,28,29]. The condition under which the crack is generated at the interface between the intermetallic layer and the aluminum alloy, is described by Equation (3) [30]:
where is the temperature change at which cracking and detachment are initiated at the interface between the aluminum alloy and the intermetallic phase, is the thickness of the intermetallic layer, is the difference in the coefficients of thermal expansion between the aluminum alloy and intermetallic phase, is Poisson’s ratio, and a is the length of the crack created at the interface as the temperature decreases during cooling. Among these parameters, and are intrinsic properties of the material and is a constant determined by heat treatment. Resulting from Equation (3), an increase in the intermetallic layer thickness induces a growth in the crack length [28]. Excessive heat treatment, including extreme solution temperatures and/or times, can trigger cracks at the interface, resulting in a decline in the bonding strength.

Figure 4.
SEM micrographs of the Al/Fe alloys hybrid structure’s interface under excessive heat treatment for 8h; (a) 500 °C, (b) 530 °C, and (c) 550 °C.
Second, the Kirkendall effect [31] generated by the difference in the diffusion ratio of the metals, causes the detachment of the intermetallic layer and Al substrate from the interface [32]. This phenomenon can trigger the formation of voids and pores at the interface, which assists detachment of the interface [28].
Figure 5 shows that the Ni elements diffused in the interface during heat treatment at 550 °C also contributed to the precipitation of the nickel-bearing phase in the Al matrix, in accordance with the distance from the interface. The EDS results (Table 4; Figure 5a,c) indicated that the type of intermetallic phases generated in the Al matrix near the interface differed between the as-cast and 550 °C heat treatment conditions. However, as indicated in Figure 5b,d, the identical types of intermetallic phases in the Al matrix away from interface were formed, regardless of the heat treatment. This implies that the nickel diffusion during the heat treatment process also affected the type of intermetallic phases in the Al matrix near the interface. The nickel reacted with the Al, Cu, and Fe atoms to precipitate the nickel-bearing phase, which was stable in this system. In particular, the types of phases containing Fe were different in the as-cast and 550 °C heat treatment conditions. Generally, the β-Al4.5FeSi formed by the presence of Fe in Al-Si-Cu alloys was presented as needle-shaped particles that causes stress concentration, and was weakly bonded to the Al matrix. This phase therefore compromises the mechanical properties of the alloys [33,34]. Accordingly, the presence of this phase at the interface could reduce the bonding strength of the Al/Fe alloys hybrid structure. However, the nickel coating at the interface prevented the diffusion of Fe into the Al matrix, and the diffused Ni atoms can also act as Fe absorbers. Thus, the formation of the β-Al4.5FeSi phase was suppressed, and instead the formation of Al9FeNi was promoted [27,35]. Due to the inability of nickel to diffuse into the Al matrix far from the interface during heat treatment, Al2Cu and β-Al4.5FeSi compounds were instead formed. (Figure 5d). The nickel-bearing phase (which possesses good thermal stability at elevated temperatures) that was formed at the α-Al grain boundary, improved the mechanical properties of the aluminum alloy at elevated temperatures. As mentioned above, among the nickel-bearing phases, the strip-like δ-Al3CuNi particularly enhance the mechanical properties of aluminum alloys at elevated temperatures [36].
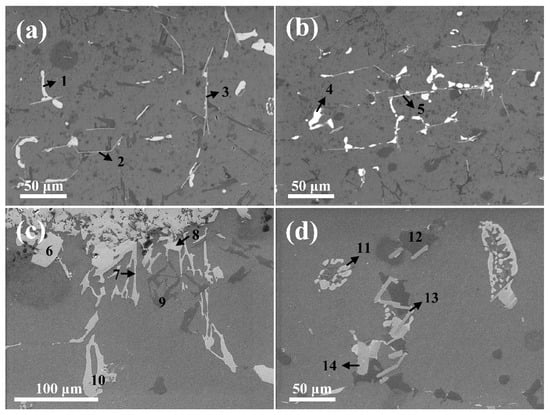
Figure 5.
SEM micrographs of the intermetallic phases in the Al matrix according to distance from the interface under as-cast and 550 ℃ heat treatment conditions; (a) as-cast (near interface), (b) as-cast (far from interface), (c) 550 °C heat treatment (near interface), and (d) 550 °C heat treatment (far from interface).

Table 4.
The qualitative analysis of the intermetallic phase corresponding to the points in Figure 5.
The different types of nickel-bearing phases produced by nickel diffusion at the interface and Al matrix could be explained by the results of the thermodynamic calculations, which reveal the equilibrium nickel concentration range of each phase. Figure 6a,b show calculated phase diagrams of the A319 (Al-Si-Cu-Mg-Fe) alloy system at different nickel contents (0–10%). The red line indicates the specific nickel concentration that affected the transformation of the nickel-bearing phase. Table 5 presents the nickel-bearing phase transformation stages of this alloy system under equilibrium conditions, at each nickel concentration range. The results show that the types of nickel-bearing phases and nickel concentrations in the alloy system were interdependent, and that based on the nickel content ranges, the equilibrium phases of the system differed. At nickel contents above 5.14%, ε-Al3Ni starts to form in this system, but then transforms to δ-Al3CuNi, until the nickel concentration reached 6.16%. In this reaction, the liquids reacted with ε-Al3Ni to produce δ-Al3CuNi. Therefore, for ε-Al3Ni to be stable in this system, the nickel concentration must exceed 6.16%; this is the minimum amount of nickel required to diffuse into the Al matrix, and form ε-Al3Ni during heat treatment. At critically low nickel concentrations, the Al and Cu atoms combined with Ni to form δ-Al3CuNi, and attained equilibrium in the absence of ε-Al3Ni, until the nickel concentration reached 5.14%. The δ-Al3CuNi remained in equilibrium over a wide range of nickel concentrations because the A319 alloy system contained significant amounts of Cu. It should be noted that ε-Al3Ni and δ-Al3CuNi attained equilibrium at different ranges of nickel concentration. The analysis of the phase diagram indicated that the Ni:Cu ratio determined which nickel-bearing phase would form in this alloy system. Although δ-Al3CuNi attained the equilibrium state at almost every nickel concentration range, ε-Al3Ni was solely formed when the Ni:Cu ratio in the alloy system exceeded 2.2. These results indicate that the formation of the nickel-bearing phases, influenced by the solution temperature and proximity to the interface, can vary. The Τ-Al9FeNi attained the equilibrium state over a wide range of nickel concentrations in this system; when the nickel concentration surpassed 0.15%, Τ-Al9FeNi started to form; however, it did not attain equilibrium during solidification until the 0.73%. On the range of 0.73% to 5.14% nickel, Τ-Al9FeNi formed through the peritectic reaction between the liquids and β-Al4.5FeSi. Especially, β-Al4.5FeSi was completely transformed into Τ-Al9FeNi at 2.32% nickel and above. When the nickel concentration exceeded 5.14%, the Τ-Al9FeNi phase was formed without the peritectic reaction, while maintaining its equilibrium state as the nickel concentration continued to increase. At this concentration range, Ni reacted with Fe to selectively form Τ-Al9FeNi, whereas β-Al4.5FeSi was not formed, confirming the theory that nickel can act as an Fe absorber and effectively suppress the formation of β-Al4.5FeSi in this system. Since no β-Al4.5FeSi were observed near the interface of the Al matrix during the heat treatment process, this theory was confirmed experimentally.
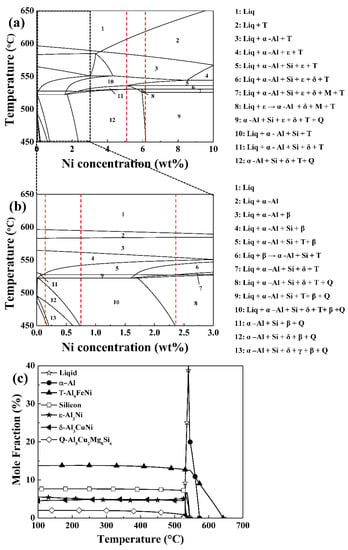
Figure 6.
(a,b) Calculated phase diagram of the Al–Si–Cu–Mg–Fe system for different nickel ranges (0–10%), and (c) mole fraction of different phases as a temperature function in the Al-7.98Si-2.8Cu-0.64Mg-0.74Fe-8.62Ni system.

Table 5.
Nickel-bearing phase transformation of the Al–Si–Cu–Mg–Fe system under equilibrium conditions at different nickel ranges.
Figure 6c illustrates the mole fractions of different intermetallic phases as a function of temperature in the Al–Si–Cu–Mg–Fe–Ni system containing 8.62% Ni. Due to the considerable amount of nickel required to form the ε-Al3Ni phase in the A319 alloy system with high Cu, the mole fraction of the ε-Al3Ni phase started to escalate from above the 6.16% nickel, and was higher than that of δ-Al3CuNi at 8.62% nickel. Therefore, for the intermetallic layer comprising the ε-Al3Ni phase to be generated at the interface, more than 8.62% nickel was required in the Al matrix during the heat treatment process.
Figure 7 illustrates the interfacial microstructures of the Al/Fe alloys hybrid structures observed at different solution temperatures, followed by air cooling. Cracks occurred between the nickel coating layer and the Al matrix at 500 °C temperature (Figure 7a). At temperatures of 530 °C and 550 °C, cracks formed between the intermetallic layer and the Al matrix, as shown in Figure 7b,c. Hence, cracks occurred at the interface due to air cooling, regardless of the heat treatment temperature. We believe this was due to a large thermal gradient created by air cooling. Thermal stress during heat treatment is created by the difference in CTE between the two materials in contact at the interface [29]. The CTEs in the two materials in the hybrid materials are different, resulting in disparate contraction rates during rapid cooling. This disparity caused severe thermal shock at the interface, which in turn escalated the thermal stress applied to the interface. The results show that furnace cooling was vastly superior to air cooling for promotion of superior chemical bonding at the interface of the Al/Fe alloys hybrid structure. Furthermore, the Al3Ni phase was precipitated in the Al matrix close to the interface of the Al/Fe alloys hybrid structure, after heat treatment at 550 °C followed by air-cooling (Figure 7d). However, as shown in Figure 5c, which shows the furnace cooling conditions after the same heat treatment process, Al3Ni was not observed in the Al matrix, indicating that the cooling rate also affects the type of intermetallic phase formed. Under these particular heat treatment conditions, the Al3Ni that was formed in the Al matrix, transformed into Al3CuNi during the furnace cooling stage. In contrast, under a faster air-cooling rate, the reaction could not be completed and Al3Ni was precipitated in the Al matrix instead.
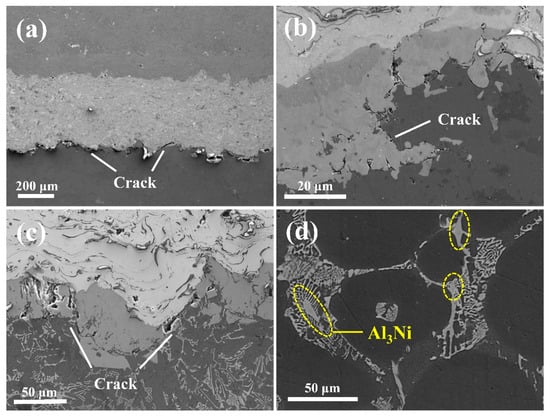
Figure 7.
(a–c) SEM micrographs of the Al/Fe alloys hybrid structure’s interface under different solution temperature, followed by air cooling; (a) 500 °C, (b) 530 °C, and (c) 550 °C. (d) The precipitated Al3Ni at the matrix after 550° C heat treatment, followed by air cooling.
Figure 8 summarizes the chemical bonding mechanism of the Al/Fe alloys hybrid structure with nickel coating under optimized heat treatment conditions. During the 550 °C heat treatment process, inter-diffusion of Al and Ni occurred at the interface of the Al/Fe alloys hybrid structure during chemical bonding, whereas the Fe elements were obstructed by the nickel coating and did not diffuse into the Al matrix (Figure 8a). Figure 8b shows the intermetallics formed by the diffusion of elements at the interface and in the Al matrix during the heat treatment process. The type of intermetallics was dependent on the Ni:Cu ratio and generated a continuous layer at the interface. Finally, Figure 8c shows that the precipitated Al3Ni in the Al matrix transformed into Al3CuNi during the furnace cooling process, and superior chemical bonding was achieved at the interface.
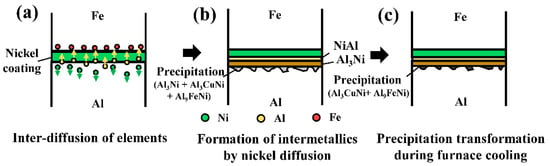
Figure 8.
Schematic illustration of the chemical bonding process of the Al/Fe alloys hybrid structure under optimized heat treatment condition.
3.3. Micro-Hardness Distribution in the Al/Fe Alloys Hybrid Structure
Under optimized heat treatment conditions, multiple layers were formed at the Al/Fe alloys hybrid structure’s interface. The effects of heat treatment and elemental diffusion on the hardness of each layer and matrix were analyzed using the Vickers hardness test. Figure 9 illustrates the microhardness distribution across the Al/Fe alloys hybrid structure interface for as-cast and optimized heat treatment conditions. The hardness of the steel matrix and the nickel coating layer did not change after the heat treatment. The intermetallic layer, composed of the hard and brittle nickel-bearing phase, exhibited the highest hardness in the Al/Fe alloys hybrid structure. Furthermore, it was confirmed that heat treatment significantly reduced the hardness of the Al matrix (40%, from 100 HV to 60 HV). We believe that the heat treatment parameters, such as 550 °C solution temperature and furnace cooling, were sufficient for grains to develop in the Al matrix, thus impacting its hardness [37]. Furthermore, the hardness of the Al matrix remained similar, regardless of the distance from the interface. In the microstructural analysis, the intermetallic phases that formed differed depending on the distance from the interface, implying that the nickel-bearing phase created by the diffusion of nickel did not affect the hardness of the Al matrix at room temperature. The effect of artificial aging (AG) was investigated for alleviating the hardness reduction of the Al matrix. The artificial aging process only affected the hardness of the Al matrix in the Al/Fe alloys hybrid structure; however, the effect was dependent on the distance from the interface. The hardness of the Al matrix far from the interface increased by approximately 35% (from 60 HV to 93 HV), whereas that of the Al matrix near the interface did not increase. During artificial aging, the Al-Si-Cu aluminum alloy precipitates nanosized θ-Al2Cu in the Al matrix. The precipitation hardening effect from these precipitates increased the hardness of the Al matrix far from the interface [38]; however, the hardness of the Al matrix near the interface did not increase. Thus, precipitation hardening did not occur near the interface, even after artificial aging. The explanation for this could involve diffusion of nickel. The nickel moved into the Al matrix and combined with Al and Cu atoms to form the nickel-bearing phase. During this reaction, the Cu atoms in the Al matrix near the interface were depleted. Therefore, the θ-Al2Cu phase could not form and precipitation hardening did not occur [27]. To summarize, the nickel that diffused into the Al matrix contributed to the formation of the nickel-bearing phase but interfered with the precipitation hardening process.
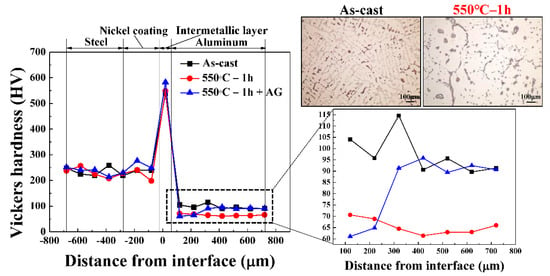
Figure 9.
Micro-hardness distribution across the Al/Fe alloys hybrid structure’s interface under as-cast and heat treatment conditions; AG: Artificial aging.
4. Conclusions
This study investigated a methodology to improve the interfacial bonding between aluminum and ferrous alloys, which has previously been difficult. Our results indicated that the application of a nickel coating, combined with an optimized heat treatment process, effectively enhanced the interfacial bonding of the Al/Fe alloys hybrid structure.
(1) The Al/Fe alloys hybrid structure was fabricated using a dissimilar casting process and utilized a nickel coating on steel. The interface of the Al/Fe alloys hybrid structure with the nickel coating was free from defects and exhibited improved mechanical bonding. However, chemical bonding did not occur at the interface during dissimilar casting.
(2) The heat treatment process at 550 °C, followed by furnace cooling, improved the interfacial bonding through the formation of a continuous and relatively thick intermetallic layer. However, an excessively long heat treatment and a fast cooling rate caused cracks at the interface.
(3) Depending on the solution temperature, different types of nickel-bearing phases formed at the interface of the Al/Fe alloys hybrid structure and in the Al matrix. The types of these phases were determined by Ni:Cu ratios. This ratio was affected by the heat treatment temperature.
(4) The nickel coating prevented the diffusion of Fe elements between the steel matrix and the Al matrix during heat treatment. Additionally, the diffused nickel, which acted as an Fe absorber during heat treatment, promoted the formation of T-Al9FeNi (called the nickel-bearing phase), instead of β-Al4.5FeSi.
(5) The optimized heat treatment conditions for the chemical bonding of the Al/Fe alloys hybrid structure’s interface induced a 40% decrease (from 100 HV to 60 HV) in the hardness of the Al matrix, whereas artificial aging increased the Al matrix hardness by 35% (from 63 HV to 90 HV), through precipitation hardening. Artificial aging had no effect in the Al matrix near the interface, however, due to the diffusion of nickel.
Author Contributions
Conceptualization, G.M. and E.L.; validation, G.M. and E.L.; formal analysis, G.M. and E.L.; investigation, G.M. and E.L.; data curation. G.M.; methodology, G.M.; visualization, G.M.; resources, G.M.; writing—original draft preparation, G.M.; supervision, E.L.; project administration, E.L.; funding acquisition, E.L.; writing—review and editing, E.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Research Foundation of Korea (NRF) granted by the Korean Government (NRF-2019R1I1A3A01062863).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Grosselle, F.; Timelli, G.; Bonollo, F. Doe applied to microstructural and mechanical properties of Al-Si-Cu-Mg casting alloys for automotive applications. Mater. Sci. Eng. A 2010, 527, 3536–3545. [Google Scholar] [CrossRef]
- Liu, Y.; Bian, X.; Zhang, K.; Yang, C.; Feng, L.; Kim, H.S.; Guo, J. Interfacial microstructures and properties of aluminum alloys/galvanized low-carbon steel under high-pressure torsion. Mater. Des. 2014, 64, 287–293. [Google Scholar] [CrossRef]
- Lee, E.; Mishra, B. Effect of solidification cooling rate on mechanical properties and microstructure of Al-Si-Mn-Mg alloy. Mater. Trans. 2017, 58, 1624–1627. [Google Scholar] [CrossRef]
- Ahn, C.; Jo, I.; Ji, C.; Cho, S.; Mishra, B.; Lee, E. Creep behavior of high-pressure die-cast AlSi10MnMg aluminum alloy. Mater. Charact. 2020, 167, 110495. [Google Scholar] [CrossRef]
- Khoonsari, E.; Jalilian, F.; Paray, F.; Emadi, D.; Drew, R.A.L. Interaction of 308 stainless steel insert with A319 aluminium casting alloy. Mater. Sci. Technol. 2010, 26, 833–841. [Google Scholar] [CrossRef]
- Jiang, W.; Fan, Z.; Li, G.; Li, C. Effects of zinc coating on interfacial microstructures and mechanical properties of aluminum/steel bimetallic composites. J. Alloy Compd. 2016, 678, 249–257. [Google Scholar] [CrossRef]
- Jiang, W.; Fan, Z.; Li, G.; Liu, X.; Liu, F. Effects of hot-dip galvanizing and aluminizing on interfacial microstructures and mechanical properties of aluminum/iron bimetallic composites. J. Alloy Compd. 2016, 688, 742–751. [Google Scholar] [CrossRef]
- Wróbel, T.; Wiedermann, J.; Skupień, P. Bimetallic castings in a chromium–nickel stainless steel working surface layer configuration with a grey cast iron base. Trans. Indian Inst. Met. 2015, 68, 571–580. [Google Scholar] [CrossRef]
- Mola, R.; Bucki, T. Characterization of the bonding zone in AZ91/AlSi12 bimetals fabricated by liquid-solid compound casting using unmodified and thermally modified AlSi12 alloy. Stroj. Vestn. J. Mech. Eng. 2020, 66, 439–448. [Google Scholar] [CrossRef]
- Ramadan, M.; Alghamdi, A.S.; Hafez, K.M.; Subhani, T.; Abdel Halim, K.S. Development and optimization of tin/flux mixture for direct tinning and interfacial bonding in aluminum/steel bimetallic compound casting. Materials 2020, 13, 5642. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bian, X.; Yang, J.; Zhang, K.; Feng, L.; Yang, C. An investigation of metallurgical bonding in Al-7Si/gray iron bimetal composites. J. Mater. Res. 2013, 28, 3190. [Google Scholar] [CrossRef]
- Springer, H.; Kostka, A.; Payton, E.J.; Raabe, D.; Kaysser-Pyzalla, A.; Eggeler, G. On the formation and growth of intermetallic phases during interdiffusion between low-carbon steel and aluminum alloys. Acta Mater. 2011, 59, 1586–1600. [Google Scholar] [CrossRef]
- Bakke, A.O.; Arnberg, L.; Løland, J.-O.; Jørgensen, S.; Kvinge, J.; Li, Y. Formation and evolution of the interfacial structure in al/steel compound castings during solidification and heat treatment. J. Alloy Compd. 2020, 849, 156685. [Google Scholar] [CrossRef]
- Jiang, W.; Fan, Z.; Li, C. Improved steel/aluminum bonding in bimetallic castings by a compound casting process. J. Mater. Process. Technol. 2015, 226, 25–31. [Google Scholar] [CrossRef]
- Szymczak, T. The structure of connection of the AlSi5–HS6-5-2 compound casting obtained by alphinising. Arch. Foundry Eng. 2011, 11, 175–184. [Google Scholar]
- Kundu, S.; Chatterjee, S. Characterization of diffusion bonded joint between titanium and 304 stainless steel using a Ni interlayer. Mater. Charact. 2008, 59, 631–637. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, G.-Q.; Shen, Q.; Zhang, L.-M.; Huang, Z.-J. Characterization of diffusion-bonded joint between Al and Mg using a Ni interlayer. Rare Met. 2016, 35, 537–542. [Google Scholar] [CrossRef]
- Meng, F.; Wu, Y.; Hu, K.; Li, Y.; Sun, Q.; Liu, X. Evolution and strengthening effects of the heat-resistant phases in Al–Si Piston alloys with different Fe/Ni ratios. Materials 2019, 12, 2506. [Google Scholar] [CrossRef]
- Awe, S.A.; Seifeddine, S.; Jarfors, A.E.W.; Lee, Y.C.; Dahle, A.K. Development of new Al-Cu-Si alloys for high temperature performance. Adv. Mater. Lett. 2017, 8, 695–701. [Google Scholar] [CrossRef]
- Medrano-Prieto, H.; Garaz-Reyes, C.G.; Gomez-Esparza, C.D.; Estrada-Guel, I.; Aguilar-Santillan, J.; Maldonado-Orozco, M.C.; Martinez-Sanchez, R. Effect of Nickel addition and solution treatment time on microstructure and hardness of Al-Si-Cu aged alloys. Mater. Charact. 2016, 120, 168–174. [Google Scholar] [CrossRef]
- Te, A.; Harrison, R.A.; Olson, S.D. Dissimilar Metal Casting; Worcester Polytechnic Institute: Worcester, MA, USA, 2016. [Google Scholar]
- Pakzaman, H.; Divandari, M.; Khavandi, A. Effect of nickel coating on steel wire reinforcement on mechanical properties of aluminum matrix composites produced via lost foam casting. In Proceedings of the Iran International Aluminum Conference (IIAC2012), Arak, Iran, 15–16 May 2012. [Google Scholar]
- Zolotorevsky, V.S.; Belov, N.A.; Glazoff, M.V. Casting Aluminum Alloys; Elsevier: Amsterdam, The Netherlands, 2007; Volume 12. [Google Scholar]
- Seeger, A. The mechanisms of diffusion in metals and alloys. J. Less Common Met. 1972, 28, 387–418. [Google Scholar] [CrossRef]
- Kobayashi, S.; Yakou, T. Control of intermetallic compound layers at interface between steel and aluminum by diffusion-treatment. Mater. Sci. Eng. A 2002, 338, 44–53. [Google Scholar] [CrossRef]
- Jiang, W.; Li, G.; Wu, Y.; Liu, X.; Fan, Z. Effect of heat treatment on bonding strength of aluminum/steel bimetal produced by a compound casting. J. Mater. Process. Technol. 2018, 258, 239–250. [Google Scholar] [CrossRef]
- Farkoosh, A.; Javidani, M.; Hoseini, M.; Larouche, D.; Pekguleryuz, M. Phase formation in as-solidified and heat-treated Al-Si-Cu-Mg-Ni alloys: Thermodynamic assessment and experimental investigation for alloy design. J. Alloy Compd. 2013, 551, 596–606. [Google Scholar] [CrossRef]
- Adabi, M.; Amadeh, A.A. Formation mechanisms of Ni-Al intermetallics during heat treatment of Ni coating on 6061 Al substrate. Trans. Nonferrous Met. Soc. China 2015, 25, 3959–3966. [Google Scholar] [CrossRef]
- Mondolfo, L.F. Aluminum Alloys: Structure and Properties; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Evans, H.; Lobb, R. Conditions for the initiation of oxide-scale cracking and spallation. Corros. Sci. 1984, 24, 209–222. [Google Scholar] [CrossRef]
- Paul, A.; Laurila, T.; Vuorinen, V.; Divinski, S.V. Interdiffusion and the Kirkendall effect in binary systems. In Thermodynamics, Diffusion and the Kirkendall Effect in Solids; Springer: Cham, Switzerland, 2014; pp. 239–298. [Google Scholar]
- Tavoosi, M. The Kirkendall void formation in Al/Ti interface during solid-state reactive diffusion between Al and Ti. Surf. Interfaces 2017, 9, 196–200. [Google Scholar] [CrossRef]
- Ma, Z.; Samuel, A.M.; Samuel, F.H.; Doty, H.W.; Valtierra, S. A study of tensile properties in Al-Si-Cu and Al-Si-Mg alloys: Effect of β-iron intermetallics and porosity. Mater. Sci. Eng. A 2008, 490, 36–51. [Google Scholar] [CrossRef]
- Puncreobutr, C.; Lee, P.D.; Kareh, K.M.; Connolley, T.; Fife, J.L.; Phillion, A.B. Influence of Fe-rich intermetallics on solidification defects in Al–Si–Cu alloys. Acta Mater. 2014, 68, 42–51. [Google Scholar] [CrossRef]
- Mbuya, T.; Odera, B.; Ng’ang’a, S. Influence of iron on castability and properties of aluminium silicon alloys: Literature review. Int. J. Cast Met. Res. 2003, 16, 451–465. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Wu, Y.; Wang, L.; Liu, X. Quantitative comparison of three Ni-containing phases to the elevated-temperature properties of Al-Si piston alloys. Mater. Sci. Eng. A 2010, 527, 7132–7137. [Google Scholar] [CrossRef]
- Furukawa, M.; Horita, Z.; Nemoto, M.; Valiev, R.Z.; Langdon, T.G. Microhardness measurements and the Hall-Petch relationship in an Al-3% Mg alloy with submicrometer grain size. Acta Mater. 1996, 44, 4619–4629. [Google Scholar] [CrossRef]
- Krupiński, M.; Król, M.; Maniara, R. Heat treatment of Al-Si-Cu alloys. In Solid State Phenomena; Trans Tech Publications: Zurich, Switzerland, 2018. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).