Abstract
Many active ingredients currently prescribed show limited therapeutic efficacy, mainly due to their dissolution rate inadequate to treat the pathology of interest. A large drug particle size creates an additional problem if a specific site of action in the human body has to be reached. For this reason, active ingredient size reduction using micronization/nanonization techniques is a valid approach to improve the efficacy of active compounds. Supercritical carbon-dioxide-assisted technologies enable the production of different morphologies of different sizes, including nanoparticles and nanocrystals, by modulating operating conditions. Supercritical fluid-based processes have numerous advantages over techniques conventionally employed to produce nanosized particles or crystals, such as reduced use of toxic solvents, which are completely removed from the final product, ensuring safety for patients. Active compounds can be processed alone by supercritical techniques, although polymeric carriers are often added as stabilizers, to control the drug release on the basis of the desired therapeutic effect, as well as to improve drug processability with the chosen technology. This updated review on the application of supercritical micronization/nanonization techniques in the pharmaceutical field aims at highlighting the most effective current results, operating conditions, advantages, and limitations, providing future perspectives.
1. Introduction
In the medical and pharmaceutical fields, improving the therapies currently used for the treatment of various diseases is an ongoing challenge. An active pharmaceutical ingredient (API) can be administered via injection, orally, topically, or via other routes. The limited therapeutic efficacy of many pharmaceutical forms is often related to an inadequate drug release rate for a specific pathology, as well as to drug particle size not suitable for reaching a certain site of action [1,2]. For example, the drug particles have to be small enough (<200 nm) for subcutaneous, intramuscular, or intravenous injections [3]. Similarly, in the case of APIs contained in aerosolizable drugs, mean particle dimensions below 1 µm and controlled particle size distributions (PSDs) are desirable. It is also important to underline that many frequently prescribed drugs are characterized by low bioavailability, mainly caused by low solubility and poor dissolution rate in an aqueous environment [4,5,6,7,8,9]. Furthermore, the first-pass metabolism also affects the drug efficacy when it is orally administered [10,11]. Due to these disadvantageous characteristics, it is necessary to administer high and/or repeated dosages of drugs to achieve the desired therapeutic effect, which leads to several side effects on the patient’s health.
Over the years, various methods have been proposed to enhance the therapeutic efficacy of the APIs, such as the micronization of polymer/drug systems [12,13] or the application of nanoparticles or nanocrystals with controlled and/or targeted release [2,14,15]. In the latter case, the nanosized drug can effectively reach a specific site of action in the human body, as well as inducing an improvement in terms of dissolution rate and, consequently, bioavailability [16]. Indeed, the particle size reduction generally generates an increase in the specific surface of the API, leading to better contact with the water molecules surrounding the nanoparticles/nanocrystals of the drug. Since nanomaterials are similar in scale to biological molecules, they can be exploited for the prevention and treatment of various diseases [17]. Indeed, nanomaterials allow for the transport of diagnostic or therapeutic agents across biological barriers. Currently, more than 50 nanoparticle-based formulations have been approved for clinical use and marketed for the treatment of cancer, rare genetic diseases, and fungi, such as anesthetics and others [15,18,19,20].
Nanoparticles and nanocrystals containing APIs can be obtained with different techniques, such as spray-drying, freeze-drying, coacervation, jet-milling, and solvent evaporation [21,22,23,24,25,26,27]. However, toxic organic solvents are widely used in these traditional processes; high temperatures and long postprocess treatments are also employed to remove liquid solvents from the particles/crystals produced. High process temperatures can cause possible degradation of the active compound, in addition to the fact that the removal of the solvent from the final product is often not complete. Moreover, traditional micronization/nanonization techniques usually lead to the achievement of products characterized by irregular morphology and broad PSDs [21,22,23,24]. Many literature studies have demonstrated the possibility of overcoming all these drawbacks by using supercritical carbon dioxide (scCO2) for the drug micronization/nanonization [21,22,23,24,28,29,30]. A pure substance is in the supercritical fluid state if its temperature and pressure conditions are above the critical point value (Figure 1). In comparison to other supercritical fluids, scCO2 has mild critical temperature (Tc) and pressure (Pc), i.e., 31.1 °C and 7.3 MPa, respectively.

Figure 1.
Sketch of the phase diagram of a pure substance.
The versatility of scCO2 has made it possible to propose and develop different micronization/nanonization technologies over the years, exploiting the supercritical fluid as a solvent, antisolvent, co-solute, or co-solvent [31,32]. The combination of liquid-like properties (e.g., high density, high solvent power) and gas-like properties (e.g., high diffusivity, low viscosity, near-zero surface tension) of scCO2 allows the production of particles/crystals of smaller dimensions and with narrower PDSs than those obtained with conventional techniques. Nanomaterials with both amorphous and crystalline structures can be obtained by modulating the operating conditions during supercritical processing, particularly temperature and pressure. Operating in supercritical conditions, a proper comprehension of the mechanisms involved in the particles/crystals formation is possible only considering the high-pressure vapor–liquid phase equilibria (VLEs). According to van Konynenburg and Scott, binary mixtures can be classified into six basic types according to their VLEs [33,34]. Many binary systems consisting of an organic solvent and CO2 are classified as type I systems (Figure 2); they are characterized by a critical curve, namely, the vapor–liquid critical curve starting from the critical point of a component up to the critical point of the other component. Although the van Konynenburg and Scott classification describes the behavior of binary systems, it is often used to describe modifications of VLE behavior caused by the presence of the solute/solutes, i.e., in the case of ternary/quaternary mixtures [35].
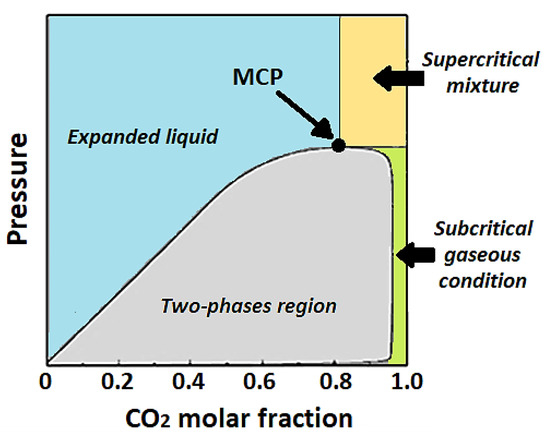
Figure 2.
Sketch of vapor–liquid phase equilibria (VLEs) of a type I binary system.
Until now, the microscopic size of numerous active compounds, both naturally occurring and chemically synthesized, have been successfully reduced by scCO2-assisted processes [36,37,38,39,40]. The APIs have been processed alone or using polymers/additives used as stabilizers, protecting agents, and/or as carriers to control the release of the active compound contained in the nanoparticles/nanocrystals [40,41,42,43,44,45]. Polymers are also used to improve the processability of pharmaceuticals in the presence of scCO2, in terms of recovery, morphology, and/or particle size.
Given the growing interest of the scientific community in nanotechnologies, this updated review is focused on the application of scCO2-assisted processes to produce pharmaceutical nanoparticles and nanocrystals, indicating the techniques used for their production and the differences so far. In this review, the focus is on literature studies that managed to prepare particles smaller than or at least equal to 250 nm. The advantages and drawbacks of using scCO2 in manufacturing nanosized drug delivery systems are highlighted. This review also provides guidelines for future perspectives on the use of supercritical technologies to prepare drug-loaded nanoparticles and nanocrystals for high therapeutic efficacy.
2. Production of Nanoparticles by Using CO2 as a Solvent
Over the years, several processes based on the use of scCO2 as a solvent have been developed to produce drug nanoparticles/nanocrystals. The following techniques belong to this category:
- -
- Rapid expansion of supercritical solution (RESS);
- -
- Rapid expansion of supercritical solutions with solid co-solvent (RESS-SC);
- -
- Rapid expansion of a supercritical solution into a liquid solvent (RESOLV), which, if the water is used as the receiving solvent, is called rapid expansion of supercritical solution into aqueous solutions (RESSAS).
Recently, some modified versions of the aforementioned technologies have been proposed, such as the ultrasonic-assisted RESOLV (US-RESOLV or US-RESSAS) [46,47].
These processes share the dissolution of the active compound in scCO2 alone [38,46,47,48,49,50,51,52,53,54,55,56] or mixed with liquid co-solvents, such as methanol and ethanol [57,58,59,60]. When an active compound is processed with the RESS-SC technique, solid co-solvents, generally menthol, have sometimes been used [61,62,63,64,65]. Co-solvents, surfactants, stabilizers, or even ultrasound are used to improve the dissolution/extraction of solutes. The solvent power of scCO2 and, consequently, the solubility of solute/solutes in the scCO2 itself are modulated by varying the operating temperature and pressure.
The RESS process (sketched in Figure 3) was the first proposed technique that uses scCO2 as a solvent. Briefly, it is based on the ability of the supercritical fluid to dissolve a certain amount of solute/solutes to be micronized, generally in saturation or near-saturation conditions. The scCO2 is fed to a saturation vessel/extractor containing the solutes to be dissolved and, therefore, micronized. Specific pressure and temperature conditions are employed in the extractor to dissolve a certain amount of solute/solutes. Then, the supercritical solution is delivered into a pre-expansion zone, which is usually at a higher temperature than that reached in the extractor. After the solutes have been solubilized in scCO2, a fast expansion of the mixture (solutes+ scCO2) occurs through a nozzle in a precipitation/collection chamber. Depressurization down to a lower pressure, generally atmospheric pressure, leads to supersaturation and, consequently, to the precipitation of solutes, while CO2 is vented out.
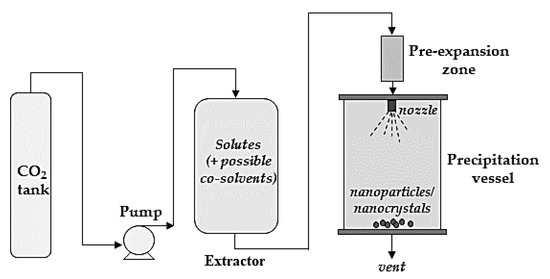
Figure 3.
A sketch of the rapid expansion of supercritical solution (RESS) process.
The size of the drug particles produced by the RESS process is affected by the extraction, pre-expansion, and expansion conditions, as well as the spray distance and the nozzle geometry. In general, the mean particle size is reduced by increasing the spray distance, which is often varied in the range of 5–10 cm [51,57]. Indeed, the residence time of the particles in the expansion vessel decreases the shorter the spray distance. Furthermore, by reducing the spray distance, the possibility of particle agglomeration due to a decrease in the angles between the particles increases. Pre-expansion and extraction temperatures and pressures are also among the most investigated parameters [51,57]. In particular, an increase in particle size is observed by increasing the pre-expansion temperature [57]. Close to the critical pressure, a moderate increase in temperature causes a large decrease in CO2 density; this leads to a reduction in the solubility of the solute/solutes in the supercritical fluid. This influence plays a key role in the supersaturation of the solution along the expansion path, causing early precipitation of the solute/solutes and the formation of larger particles when higher pre-expansion temperatures are employed. Conversely, a decrease in the mean particle size is often noted by increasing the extraction temperature, because the density and the solvent power of CO2 decrease [51]. On the other hand, as the temperature increases, the solubility of solute/solutes in the supercritical mixture increases, leading to an increase in the supersaturation of the solution. It is possible to state that, as supersaturation increases, there is a reduction in the critical dimensions of the nuclei and, therefore, smaller particles precipitate; this is the prevailing phenomenon during the extraction step [51]. Mean particle size can also be reduced by increasing the extraction pressure [51].
Until now, several APIs have been processed by the RESS technique to produce nanoparticles/nanocrystals, mainly nonsteroidal anti-inflammatory drugs (NSAIDs) [38,41,49,50,58]. In some cases, polymers/surfactants have also been used to prepare drug-loaded particles/crystals for different purposes, including the prevention of drug recrystallization or particle aggregation, as well as the control of drug dissolution [38,41]. For example, Gadermann et al. [41] avoided agglomeration of naproxen nanocrystals by using polylactic acid (PLA). Sharma et al. [38] attempted to produce ibuprofen nanoparticles using different surfactants. Sub-microparticles were obtained using polyethylene amine (PEI) with a molecular weight of 1300 Da and Tween-40, which led to the attainment of particles with the largest size. On the other hand, ibuprofen-loaded nanoparticles were produced using polyvinylpyrrolidone (PVP) and PEI with a molecular weight of 750,000 Da, which allowed reaching the smallest particle dimensions down to 7 nm. However, both PVP and 750,000 Da PEI assured drug particle sizes mostly below 100 nm. It was also shown that, in general, the particle size increased by increasing the surfactant concentration. The stability of the samples produced by RESS over time was also demonstrated.
Although, in several cases, the RESS process was effective in obtaining nanoparticles/nanocrystals, this micronization/nanonization technique has some drawbacks, mainly the very low solubility of polar drugs in scCO2 and the possible agglomeration of particles in the expansion zone [61]. The low solubility of polar active compounds in scCO2 severely limits the practical applicability of RESS technology on an industrial scale. To overcome this limitation, but also to further reduce the particle growth and agglomeration, several modifications to the RESS technique have been proposed over the years, including RESS-SC, namely, the use of solid co-solvents. The solid co-solvent is dissolved together with the active compound by scCO2 into the extractor, favoring the solubilization of the drug of interest. Through the subsequent rapid expansion, the solid co-solvent simultaneously precipitates around the drug molecules, thus avoiding the formation of surface-to-surface interactions between the drug particles, which generally cause their aggregations in the expansion chamber. Hence, the solid co-solvent can be removed from drug nanoparticles/nanocrystals by sublimation, generally performed by freeze-drying. Comparing the RESS and RESS-SC processes in obtaining phenytoin nanoparticles, Thakur et al. [61] proved that the use of menthol as a solid co-solvent not only increases the drug solubility in scCO2 but also leads to the obtainment of particles characterized by a smaller mean size.
Menthol has been mainly selected to produce nanoparticles/nanocrystals [61,62,63,64,65] as it has the main properties required of a solid co-solvent for the RESS-SC process. Indeed, a good solid co-solvent has to be soluble in scCO2 and must favor the solubility of the drug in the supercritical fluid; it has to be solid under the nozzle expansion conditions and characterized by a high vapor pressure to ensure easy sublimation, as well as being inert, nonflammable, nontoxic, and economical. However, Uchida et al. [65] proposed vanillin as an alternative solid co-solvent when aggregates are produced using menthol. The authors successfully prepared theophylline nanocrystals by processing them with vanillin.
In nearly all studies focused on RESS and RESS-SC techniques [38,41,48,49,50,57,58,61,62,63,64,65], the ultimate goal of drug size reduction at the nanoscale is to increase bioavailability and dissolution of the active compound of interest; however, dissolution tests are not performed to compare the dissolution kinetics of the drug in pure or processed form using scCO2 as a solvent. Only in the paper written by Keshavarz et al. [51] was it shown that nanoparticles of raloxifene, an anticancer drug, prepared by RESS accelerated the drug dissolution rate about sevenfold compared to untreated raloxifene.
Lastly, solid co-solvents are unable to redissolve the processed drug, which is possible when liquid co-solvents are used. However, the need for an additional postprocess for the final removal of the solid co-solvent has to be considered from an economic point of view.
The RESS technique was also compared with the RESOLV/RESSAS process for the attainment of nanosized pharmaceutical systems [52]. Unlike the RESS process, in the case of the RESOLV technology, the expansion of the supercritical mixture containing the solute/solutes to be nanonized takes place in a liquid solvent placed in the precipitation unit. Sometimes, organic solvents such as methanol (possibly containing stabilizers) are used [59,60]. However, the precipitation vessel is filled most frequently by water, which is often used as a receiving solvent since most of the treated active compounds are poorly soluble in water. In the latter case, the acronym RESOLV is replaced by RESSAS. The main purpose of using solvents in the precipitator, as well as polymers/stabilizers, is to inhibit the agglomeration of the nanoparticles/nanocrystals produced during/after expansion. In the paper of Paisana et al. [52], nanoparticles of olanzapine, which is a drug prescribed for the treatment of diseases such as schizophrenia or bipolar disorder, were prepared by both RESS and RESSAS processes. The particles produced by the two techniques showed similar dimensions. The dissolution rate of olanzapine was improved in the case of both nanoparticles obtained by RESS and those prepared by RESOLV. The influence of different polymers used as stabilizers, namely, hydroxypropylmethylcellulose (HPMC), poly(ethylene glycol) (PEG), and polysorbate, was also investigated. In particular, dissolution was improved with all three carriers selected; HPMC-based particles exhibited slower kinetics, despite the use of PEG- and polysorbate-caused particle growth.
Despite the advantages of using the RESOLV process to nanonize APIs, the nanoparticles/nanocrystals produced are often susceptible to aggregation in the receiving solvents/solutions [47,53]. To overcome this main drawback, ultrasonication has recently been coupled with RESOLV to improve drug nanonization [46,47]. Ultrasound generates pressure fluctuations in the liquid medium, leading to the formation of gas pockets that act as nuclei; as a result, the formed microbubbles grow and collapse rapidly, causing micro-implosions that provide high temperature and pressure [66,67]. Sodeifian et al. used the US-RESOLV technique to process an antihistamine drug and an antiarrhythmic drug, loratadine [46] and amiodarone hydrochloride [47], respectively. In the case of loratadine [46], employing RESSAS mean particle sizes smaller than those obtained by US-RESSAS were achieved under all process conditions investigated, in the ranges 124–680 nm and 26–271 nm, respectively. Indeed, the ultrasonic waves allowed preventing the agglomeration of the drug particles. As a result, loratadine nanoparticles obtained by US-RESSAS showed faster dissolution than the particles produced by RESSAS. Specifically, after 30 min of ultraviolet (UV)–visible light (vis) analysis, about 30% of the unprocessed drug dissolved in the aqueous medium, while approximately 70% and 80% of loratadine was released from RESSAS and US-RESSAS powders, respectively. In the other paper by Sodeifian et al. [47], the ultrasound-assisted RESOLV technique was compared with both conventional RESOLV and RESS to nanonize amiodarone hydrochloride. Two different hydrophilic polymers, namely, PVP and HPMC, were employed to stabilize the drug particles. Differential scanning calorimetry (DSC) and X-ray diffraction (XRD) analyses demonstrated a lower degree of crystallinity when amiodarone was nanonized by US-RESOLV compared to unprocessed and processed powders, especially to unprocessed and RESS-processed drug. All samples obtained using scCO2 as a solvent showed smaller particles and a faster dissolution rate than the pure antiarrhythmic drug. However, RESS-prepared particles had a mean size in the range 101–393 nm, while similar particle dimensions were obtained by US-RESOLV (in the range 48–255 nm) and RESOLV (in the range 51–265 nm) with a slightly faster dissolution in the case of the ultrasound-assisted process.
In summary, with the exception of the RESOLV/RESSAS technique which allows producing nanoparticles, the formation of agglomerates or particles/crystals with larger size and wider PSDs is observed by RESS-derived processes compared to other supercritical nanonization techniques. The main limiting factor in RESS-based processing is the low solubility of most active compounds in scCO2. The drug solubility can be increased by increasing the pressure, which leads to an increase in the scCO2 density and solvent power, but technological limitations have to be considered. In addition, although high temperatures in RESS technologies may promote drug solubility in scCO2, possible degradation of the active compound may occur.
Pharmaceutical nanoparticles and nanocrystals prepared using scCO2 as a solvent have been proposed for several applications, including oral [41,46,51,54,56], topical [56], intravenous injection, or infusion routes [55,59,61].
From an industrial point of view, there has not been a wide implementation of RESS-based technologies on a commercial scale (and, basically, of all supercritical micronization processes) as high productivity would entail high investment costs in equipment of adequate size for commercial production. The high costs due to energy consumption per unit of the product due to the high pressures involved in all supercritical methodologies have to be also considered [68]. However, the RESS technique can potentially be adapted to existing conventional spray-dryers in the pharmaceutical industry, reducing the investment costs and allowing operation on an industrial scale. Currently, RESS-based methodologies on a pilot/industrial scale are successfully applied by some companies, such as Thar, Phasex, and Applied Separations (United States of America, USA), Pierre Fabre and Hitex (France), and Feyecon (the Netherlands).
In Table 1, a summary of the active compounds processed to obtain nanoparticles/nanocrystals by techniques based on the use of scCO2 as a solvent is reported. The operating conditions that mainly affect the size of the drug particles/crystals produced are indicated, such as the pressure and temperature of the extraction, as well as pre-expansion or expansion steps. The morphology and size of the pharmaceutical product are also specified, as well as the use of a possible co-solvent.

Table 1.
An overview of nanoparticle production by using CO2 as a solvent. NP: nanoparticles; NC: nanocrystals; c: coalescing particles; AGG: aggregates; TPREEX: pre-expansion temperature; PPREEX: pre-expansion pressure; TEXT: extraction temperature; PEXT: extraction pressure; TEXP: expansion temperature; PEXP: expansion pressure; AH: amiodarone hydrochloride; TBTPP: fluorinated tetraphenylporphyrin; BSA: bovine serum albumin; HPMC: hydroxypropylmethylcellulose; PEG: poly(ethylene glycol); PEI: polyethylene amine; PLA: polylactic acid; PLLA: poly(l-lactide); PVA: poly(vinyl alcohol); PVP: polyvinylpyrrolidone; SDS: sodium dodecyl sulfate; DMSO: dimethyl sulfoxide; EtOH: ethanol; MeOH: methanol; RESS: rapid expansion of supercritical solution; SC: solid co-solvent; RESOLV: rapid expansion of a supercritical solution into a liquid solvent; RESSAS: rapid expansion of supercritical solution into aqueous solutions (RESSAS); US: ultrasound.
3. Production of Nanoparticles by Using CO2 as an Antisolvent
Supercritical CO2 is widely exploited as an antisolvent to produce pharmaceutical nanoparticles/nanocrystals using different techniques, including the following:
- -
- Aerosol solvent extraction system (ASES);
- -
- Supercritical gas antisolvent (GAS);
- -
- Precipitation with compressed fluid antisolvent (PCA);
- -
- Supercritical antisolvent precipitation (SAS);
- -
- Solution-enhanced dispersion by supercritical fluids (SEDS);
- -
- Supercritical fluid extraction of emulsions technology (SFEE).
Both conventional drugs [24,39,58,71,72,73] and active compounds of natural origin [23,74,75,76,77,78,79,80] have been nanonized using scCO2 as an antisolvent. Indeed, there is a recent trend in the production of pharmaceutical formulations using natural products or extracts, to exploit their beneficial properties on human health, including antioxidant, antimicrobial, anticancer, and anti-inflammatory ones [2]. Nanoparticles and nanocrystals based on different active compounds, such as antibiotics [24,71,72,81], NSAIDs [58,82,83], antifungals and antimicrobials [39,84,85], and drugs for prevention or cancer treatment [73,74,75,76,78,86], have been produced. Nanosized systems prepared using scCO2 as an antisolvent have been proposed for various pharmaceutical forms, such as those for inhalation [40], oral [39,71,87,88], topical [39,71,88], and injectable [71,78,89] routes.
The SAS process (schematized in Figure 4) is the most widely used technology in which scCO2 acts as an antisolvent. Liquid solvents that are completely miscible with scCO2 under process conditions are chosen, mainly dimethyl sulfoxide (DMSO), ethanol, methanol, and acetone. The solute to be nanonized has to be soluble in the selected liquid solvent but insoluble in the solvent–antisolvent binary mixture formed in the precipitation chamber. Therefore, when the liquid solution (solvent + solute) is injected through the nozzle into the precipitator filled with scCO2, the solute precipitates due to the supersaturation caused by the fast diffusion of scCO2 into the droplets formed after the jet breakup [90].
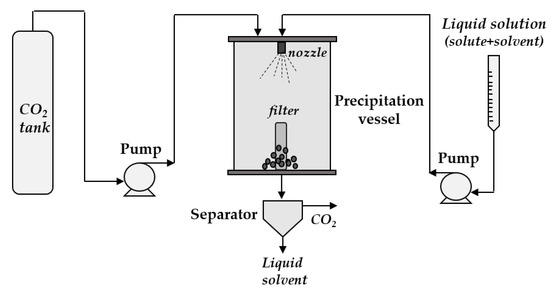
Figure 4.
A sketch of the supercritical antisolvent precipitation (SAS) process.
Nanoparticles are usually produced via the SAS process by working with low temperatures (in the range 35–50 °C) and pressures equal to or mostly higher than 9 MPa in the precipitation chamber. Indeed, the smallest particles have often been obtained by decreasing the operating temperature [42,82,91,92]. As a rule, the particle size also decreases by increasing the pressure, as the surface tension tends to zero faster, or by increasing the overall concentration of the solution, due to the increase of the viscosity [73,77,92,93,94]. Exemplificative scanning electron microscopy (SEM) images of large crystals of unprocessed diclofenac sodium and nanoparticles precipitated by the SAS process are reported in Figure 5a,b, respectively. Unlike nanoparticles, drug nanocrystals precipitated by the SAS process have been observed in very few papers [74,81].

Figure 5.
SEM images of (a) unprocessed diclofenac sodium and (b) nanoparticles of diclofenac sodium produced by the SAS process.
Several studies have shown that the SAS technique is more effective for obtaining small particles for pharmaceutical applications than conventional processes, including spray-drying [22,24], freeze-drying [21], milling technologies [23], and solvent evaporation [29]. For example, in the study by Park et al. [24], cefdinir nanoparticles prepared by SAS showed a narrower PSD (150 ± 70 nm) and a faster dissolution rate than cefdinir microparticles (2.32 ± 1.76 µm) produced by spray-drying. Similarly, resveratrol dissolution rate increased for both nanoparticles and sub-microparticles (in the range 150–500 nm) produced via SAS process by Ha et al. [23] compared to large crystals (18.75 µm) prepared by Fritz milling or crystals and irregular/coalescent particles (1.94 µm) obtained by jet-milling.
The SAS technique has been widely proposed both to process single active compounds and to coprecipitate drugs with polymeric carriers with different aims. The production of polymer-based nanoparticles allows to stabilize the active principle by reducing its recrystallization, to preserve it from oxidation and deactivation, or to influence the dissolution of the drug in aqueous media. Polymers are sometimes used to improve the SAS processability of active compounds in terms of morphology and/or powder recovery when they have been extracted by the solvent/antisolvent binary mixture [22,44,83]. To date, PVP is the most widely used carrier to produce pharmaceutical nanoparticles by the SAS process [44,83,93,94,95,96,97,98], followed by HPMC [29,88,89,99] and, to a lesser extent, Eudragit L100 [42] and ethyl cellulose [81,100]. The results of the dissolutions studies have shown that the dissolution rate of the active compound generally increases when it is released from PVP-based and HPMC-based nanoparticles; instead, Eudragit L100 delayed the drug release.
Montes et al. [58] compared RESS and SAS technologies in the preparation of naproxen particles. Coalescing and irregular nanoparticles of smaller size (60–220 nm) were mainly produced by RESS, sometimes even with the presence of large crystals; otherwise, the particles precipitated by SAS tend to be larger (in the range 80–1300 nm) but more homogeneous. From the XRD analyses, a lower degree of crystallinity was observed in the case of SAS powders.
In recent years, modified versions of the conventional SAS process have been proposed, aiming, in particular, to produce smaller nanoparticles with narrower PSDs. In this context, Chattopadhyay and Gupta [101,102] employed supercritical antisolvent precipitation with enhanced mass transfer (SAS-EM) to process an antifungal compound (griseofulvin) and an antibiotic (tetracycline), obtaining nanosized products. The main difference between the two SAS versions is that, in the case of the SAS-EM technique, the jet of the solution is deflected by a vibrating ultrasonic surface, and then it is atomized into smaller droplets than those obtained by conventional SAS. Furthermore, the ultrasound field generated by the vibrating surface increases the turbulence and the mixing of the solution and the supercritical antisolvent, resulting in a high mass transfer. Droplet agglomeration is also avoided with the improved and faster mixing promoted in SAS-EM, leading to the formation of droplets and, consequently, smaller particles than those produced by the conventional SAS process. Long et al. [103] used the supercritical CO2 antisolvent-assisted nano spray-drying (SASD) process to control the polymorphism of carbamazepine nanoparticles; in the SASD process, CO2 mixes with the solution containing the active principle in a small-volume high-pressure coaxial nozzle, in which supersaturation occurs. In this process, the atomization is involved in the method itself, resulting in the generation of a spray of ultrafine droplets in which the solvent is removed by thermal means.
ASES, GAS, and SEDS techniques are considered previous modifications of the SAS process. The ASES technique involves spraying the solution and scCO2 through an atomization nozzle into the precipitator filled with scCO2 at the desired pressure and temperature [39]. In the GAS process, the scCO2 is introduced into the precipitation vessel containing the liquid solution, i.e., the solute/solutes to be precipitated dissolve in the liquid solvent [84,85,86]. In the SEDS process [43,104,105], the scCO2 and the liquid solution are both injected through a coaxial nozzle, which causes a tangential impact between the two phases of the fluid, in a pre-mixing chamber. This impingement promotes better mixing between the solution and the antisolvent that can lead to the production of finer powders than those obtained by the conventional SAS process. However, for the production of lysozyme nanoparticles, Fusaro et al. [85] compared GAS technology with PCA, which is practically based on the same principles as the SAS process. The nanoparticles obtained by PCA, having dimensions in the range 18–197 nm, were smaller than those produced by GAS (particle size in the range 233–300 nm).
In summary, the morphology and the size of powders precipitated by SAS-derived technologies generally depend on the binary system high-pressure VLEs (Figure 2). Specifically, the position of the operating point with respect to the mixture critical point (MCP) of the binary mixture formed by the solvent and the antisolvent plays a key role [106]. Briefly, when the operating point pressure is lower than the MCP pressure, the operating point falls in the biphasic region, and crystals are generally observed. Operating above the MCP and at scCO2, high molar fractions of (preferentially greater than or equal to 0.95) spherical (and generally amorphous) particles are produced. The dimension of the precipitated particles is influenced by the distance between the operating point pressure and the MCP of the solvent/antisolvent binary system. By increasing the operating pressure, particle size generally decreases, switching from the formation of microparticles at pressures slightly above MCP pressure to the formation of nanoparticles a pressure far above the MCP. This outcome has been interpreted in terms of competition of two characteristic times: the jet breakup time and the surface tension vanishing time [107,108]. In SAS-based techniques, it has been observed that the jet breakup is the controlling mechanism at pressures close to the MCP of the solvent/scCO2 binary system; thus, micro-droplets are formed that are the native structure of the microparticles formed as a result of the scCO2 drying. On the other hand, at pressures far above the MCP, the surface tension vanishing occurs fast, prevailing on the jet breakup and leading to the precipitation of nanoparticles from a gaseous phase. Additionally, the switch from microparticles to nanoparticles strongly depends on the type of solvent. Some solvents, such as ethanol or dimethylsulfoxide (DMSO), present a broad transition zone from the two-phase to the single-phase mixing; microparticles can be produced using these solvents. On the other hand, solvents such as acetone have a narrow pressure range to switch from two-phase to single-phase mixing; in this case, nanoparticles generally precipitate [109,110]. The SFEE technique, which combines the advantages of traditional emulsion-based technologies (e.g., surface properties) with the advantages of scCO2-assisted processes (e.g., shorter processing time and final product high purity) allows easily obtaining nanoparticles [25,26,27,40,111,112]. The SFEE technology also allows processing of substances in continuous mode.
In short, in SFEE technology, scCO2 acts as an antisolvent to extract an organic solvent from the droplets of previously prepared oil-in-water (O/W) or water-in-oil (W/O) emulsions. O/W emulsions are more often used; in this case, the hydrophobic active compounds to be nanonized are dissolved in the oily phase, which typically consists of organic liquid solvents. The emulsion is generally fed from the top to an extraction column containing packing elements, while the scCO2 is delivered in countercurrent from the bottom of the tower [26,40]. The countercurrent flow between the emulsion and the supercritical fluid favors the extraction of the organic solvent from the emulsion droplets. Consequently, the compounds of interest precipitate in the form of nanosuspensions in water collected at the bottom of the column, while the solvent is recovered in a separator at the top. In other cases, although less frequently, the emulsion and the scCO2 are also fed in co-current mode [25]. The use of a batch SFEE apparatus was also adopted in some papers [27,111,112]. In this case, the emulsion is previously placed inside the extractor; then, scCO2 is fed to the extractor, at the desired temperature and pressure, until the selected operating time is reached. Finally, the vessel is depressurized and the suspension of nanoparticles in water is recovered. However, Prieto and Calvo [27] highlighted the possibility of reducing operation time and increasing production capacity by running a continuous mode in a packed column.
Two different SFEE apparatuses are sketched in Figure 6a,b, i.e., the continuous and countercurrent and the batch plant, respectively.
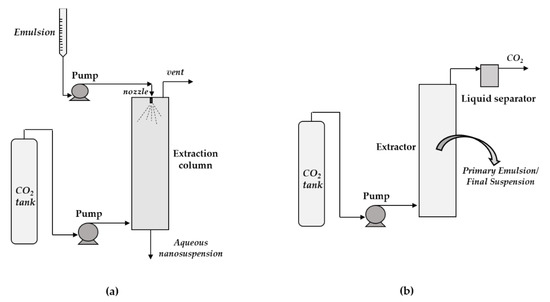
Figure 6.
A sketch of the Supercritical fluid extraction of emulsions (SFEE) technology: (a) continuous and countercurrent SFEE; (b) batch SFEE.
The superiority of the SFEE process over conventional emulsion solvent evaporation or other traditional technologies has been highlighted in some papers [25,26,27]. In the study by Giufrida et al. [25], medroxyprogesterone acetate, a poorly water-soluble hormone, WAS encapsulated into particles based on poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV), using both SFEE and conventional emulsion solvent evaporation techniques. The effect of the PHBV molecular weight on the morphology and size of produced particles was studied in detail. Using both technologies, a shift from micrometric to nanometric particle dimensions was observed by decreasing the PHBV molecular weight from 210,379 Da to 14,080 Da. In all cases examined, through the SFEE technique, smaller particles and higher encapsulation efficiencies are obtained than those achieved with the conventional emulsion solvent evaporation technique. Furthermore, in the case of the traditional emulsion solvent evaporation, longer times are required for the elimination of the solvent from the final product than those required by the supercritical technique. Similarly, in the paper by Prieto and Calvo [27], lower encapsulation efficiencies were reached by loading vitamin E into polycaprolactone (PCL) nanoparticles by conventional emulsion solvent evaporation compared to the SFEE technique. Moreover, a higher residual concentration of acetone was observed in the nanoparticles prepared with the traditional technique.
The scaleup of the processes based on the use of supercritical antisolvent may be easily achieved by increasing the batch volumes with respect to the bench scale while maintaining the antisolvent/solvent flow rates ratio and the pressure differentials unchanged [1,113]. However, there are still some challenges related to the scaleup of these techniques, including the possible nozzle clogging and the need for large precipitation vessels resistant to high pressures that require high investments for industrial production [114]. Moreover, the primary difficulty is related to the recovery and handling of dried nanoparticles produced. For this purpose, filters are mainly used in the analyzed papers, since they are efficient for the collection of low quantities of small particles, i.e., at lab scale. However, filters are burdensome and not easily adaptable to the industrial scale. The use of processes that allow producing nanoparticles in suspensions (e.g., SFEE technology) could be a possible answer to this issue; however, additional filtration and purification steps are required to obtain dried nanoparticles, resulting in additional costs and process time.
Nevertheless, several companies already employ pilot/industrial-scale plants involving the supercritical antisolvent, such as Thar (Pittsburgh, PA, USA), Phasex Corporation (North Andover, MA, USA), Crititech (Lawrence, KS, USA), Aphios (Woburn, MA, USA), and Applied Separations (Allentown, PA, USA), CrystecPharma (Bradford, UK), Cerbios-Pharma (Lugano, Switzerland), and FeyeCon (Weesp, The Netherlands).
Some papers include economic considerations on technologies based on the use of scCO2 as an antisolvent, since they are among the most applied in the industrial field [113,114,115], although the available literature focused on economic aspects is still limited for all supercritical techniques. The study by Kurniawansyah et al. [113] introduced a comparison from an economic point of view among GAS, SAS, and atomized rapid injection solvent extraction (ARISE) techniques; the latter one is a batch process to produce micron-sized particles using the supercritical antisolvent. Total production costs per unit of product were estimated in the ranges 6–13 USD/kg, 64–385 USD/kg, and 52–255 USD/kg for GAS, SAS, and ARISE processes, respectively. Micronization using the ARISE process was revealed to be more expensive than GAS, due to the need for additional vessels and pumps related to the use of an inert gas. On the other hand, the application of the GAS process usually leads to the precipitation of products with larger size and wider PSDs than those obtained by SAS and ARISE techniques, as well as a low recovery of the product. The SAS process was found to be more expensive than ARISE, which can generally operate with higher capacity, higher feed concentrations, and smaller antisolvent/solvent ratios, leading to lower capital investment and manufacturing costs. However, the application of supercritical micronization/nanonization technologies on a commercial scale is suitable for the processing of compounds with high added value, such as pharmaceuticals.
Table 2 shows a list of the nanoparticles/nanocrystals of active compounds produced using scCO2 as an antisolvent by the different technologies mentioned. The polymeric carriers and the solvents employed, the operating pressures and temperatures, and the dimensions of the nanoparticles/nanocrystals produced are also indicated. For each technique, the results of studies focusing on the processing of active ingredients without any carrier/stabilizer are first reported, followed by coprecipitation/encapsulation studies.

Table 2.
An overview of nanoparticle production by using CO2 as an antisolvent. NP: nanoparticles; NC: nanocrystals; c: coalescing particles; AGG: aggregates; P: pressure; T: temperature; THF: tetrahydrofuran; β-CD: β-cyclodextrin; EC: ethyl cellulose; EL100: Eudragit L100; HP-β-CD: hydroxypropyl-β-cyclodextrin; HPMC: hydroxypropylmethyl cellulose; HPC: hydroxypropyl cellulose; HCO-60: polyoxyethylene (60) hydrogenated castor oil; PCL: polycaprolactone; PEG: poly(ethylene glycol); PHBV: poly(3-hydroxybutyrate-co-3-hydroxyvalerate; PMMA: poly(methyl methacrylate); PVA: poly(vinyl alcohol); PVM/MA: polymer poly (methyl vinyl ether-co-maleic anhydride); PVP: polyvinylpyrrolidone; AC: acetone; CHF: chloroform; DCM: dichloromethane; DMF: dimethylformamide; DMSO: dimethyl sulfoxide; EtAc: ethyl acetate; EtOH: ethanol; MeOH: methanol; NMP: N-methyl-2-pyrrolidone; ASES: aerosol solvent extraction system; GAS: supercritical gas antisolvent; PCA: precipitation with compressed fluid antisolvent; SAS: supercritical antisolvent precipitation; EM: enhanced mass transfer; SASD: supercritical CO2 antisolvent-assisted nano spray-drying; SEDS: solution-enhanced dispersion by supercritical fluids; SFEE: supercritical fluid extraction of emulsions technology.
4. Production of Nanoparticles by Using CO2 as a Co-Solute
To date, several active compounds have also been processed using scCO2 as a co-solute through the following technologies:
- -
- Particles from gas-saturated solutions (PGSS);
- -
- Supercritical-assisted atomization (SAA);
- -
- Supercritical-assisted injection in a liquid antisolvent (SAILA).
However, drug nanoparticles have been produced using CO2 as a co-solute in a reduced number of papers [36,37,131,132,133].
Some amount of scCO2 is soluble in some substances such as polymers, lipids, or high-molecular-weight drugs, particularly molten materials when considering the PGSS process (Figure 4). Briefly, the PGSS technique consists of mixing of scCO2 and molten substances (e.g., drug and polymer), usually into a static mixer. This mixture is rapidly expanded through a nozzle into a precipitation chamber generally at pressures close to atmospheric. The rapid expansion of the solution containing scCO2 causes a cooling effect on the small droplets formed, which are quickly blocked. Furthermore, the scCO2 solubilized in the molten substance acts as a plasticizing agent by depressing the melting and glass transition temperatures of the solute [134,135], thus promoting the breakup into small droplets during the depressurization. The produced particles are usually separated from the CO2 employing a filter or a cyclone.
Composite nanoparticles containing drugs have been mainly produced by the PGSS technique [37,131,132]. To this end, until now, PEG seems to be the most effective polymeric carrier for the PGSS process. Couto et al. [132] studied in detail the effect of PEG molecular weight (varied from 2000 to 35,000 Da) to prevent the aggregation of riboflavin-loaded particles when suspended in water. Using a PEG molecular weight equal to 2000 Da, the smallest nanoparticles (83 ± 0.36 nm) were produced, followed by PEG 35,000 Da (104 ± 5.7 nm) and PEG 10,000 Da (131 ± 40.2 nm). On the other hand, the encapsulation efficiency increased by increasing the PEG molecular weight from 10,000 Da to 20,000 Da, followed by a decrease at 35,000 Da.
The morphology and size of the PGSS product are influenced by different process parameters, including the scCO2 concentration in the molten substances, the precipitation temperature and pressure, and the nozzle geometry. However, the effect of the temperature and pressure conditions used to mix scCO2 and compounds to be nanonized is among the most studied [37,131,132]. The mixing pressure is often between 10 and 25 MPa to assure dissolution of scCO2 in the molten compounds. In general, particle size decreases with increasing mixing pressure, as better atomization is promoted, as well as a decrease in the melting temperature of compounds [37,131,132]. Moreover, from SEM analyses, Chen et al. [131] noted that irregular ibuprofen/PEG particles were obtained at the lower pressures investigated (in the range 10–15 MPa) compared to the smaller, spherical, and more dispersed nanoparticles produced at high pressures (up to 25 MPa). On the other hand, Couto et al. [132] observed that the highest encapsulation efficiency was reached at the lowest pressure studied (i.e., 10 MPa); hence, the latter can be considered the best, although the particle size is enlarged. As for the operating temperature [37,131], its increase reduces the viscosity of the melting mixture, which plays a key role in the formation of small droplets via PGSS. Nevertheless, a longer time is required to solidify the droplets when higher temperatures are used, leading to the formation of larger particles. Consequently, Chen et al. [131] noted only a slight increase in particle size with temperature. Conversely, in the study by Hu et al. [37], a decrease in average particle size was noted as the mixing temperature was increased, as the viscosity of the mixture decreased more rapidly as the temperature increased. In particular, according to the results obtained by the authors, nanoparticles containing coenzyme Q10 can be produced by operating at temperatures above 75 °C, which allowed them to reach an adequate viscosity for the formation of fine droplets by atomization. However, a further increase in temperature should be avoided due to the consequent difficult solidification of the atomized droplets.
Basically, in all articles focusing on the production of nanoparticles by PGSS, bimodal [132] or even multimodal PSDs [37,131] were mainly obtained under the tested process conditions. Although less pronounced, the other peaks in the reported PSDs also demonstrated the formation of sub-micrometric or even micrometric particles. This outcome can be considered a disadvantage in the preparation of particles via the PGSS technique. However, as stated by Hu et al. [37], the preferential production of nanoparticles can be promoted by increasing the pressure employed in the mixer. An enhancement in drug dissolution rate in the case of PGSS nanoparticles compared to the unprocessed drug was demonstrated only in the work of Chen et al. [131].
The main advantage of processing active compounds by PGSS is that no organic solvents are needed. On the other hand, the use of high temperatures to melt the solute is the main problem when thermosensitive materials such as pharmaceutical compounds are processed.
Better control of particle morphology and PSD with high drug entrapment efficiencies appears to be assured by the SAA process, although, in this case, limited use of liquid solvents is necessary [36,133]. Water, ethanol, methanol, and acetone are mainly employed, pure or mixed, to process active compounds using the SAA technique. However, until now, sub-micrometric particles have mostly been prepared by SAA [136,137,138,139], while pharmaceutical nanoparticles have been produced in few papers [36,133,140]. Briefly, SAA is based on the solubilization of a controlled amount of scCO2 in a liquid solution consisting of the solvents and solutes to be nanonized. Specifically, the scCO2 and the liquid solution are mixed in a saturator (or mixer), which is a packed column. The presence of packings guarantees a proper residence time and a large contact surface between scCO2 and the liquid solution, achieving complete mixing (i.e., saturation). Then, the expanded liquid obtained in the saturator is injected through a nozzle into a precipitation chamber, which is generally at pressures close to atmospheric or sometimes lower [36,133,141]. A gaseous thermal carrier, usually hot nitrogen, is fed to the precipitator during the injection of the liquid solution, for the complete evaporation of the solvents used. For this purpose, high temperatures (often between 80 and 90 °C) are also employed in the precipitator, depending on the selected solvents. Efficient atomization is assured by SAA (Figure 7) because the rapid expansion of scCO2 from the primary droplets formed by the jet breakup at the exit of the injector causes secondary atomization into smaller droplets [141]. To avoid the coalescence of the particles, it is necessary to perform a rapid and total removal of liquid solvents.
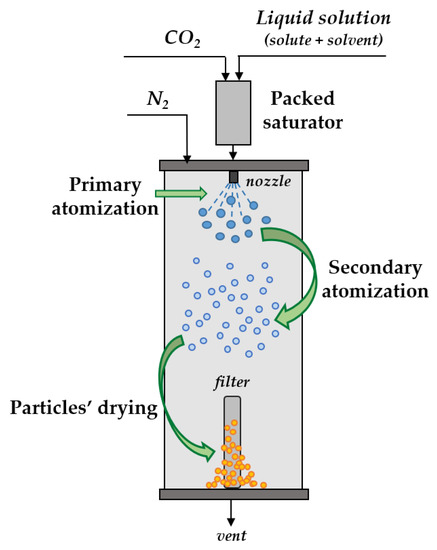
Figure 7.
A sketch of supercritical assisted atomization (SAA).
The mean size and PSDs of SAA-precipitated particles can be modulated by varying the operating conditions. One of the most important process parameters is the CO2/liquid solution mass feed ratio, also known as the “gas-to-liquid ratio” (GLR, w/w) [133,139,141]. The mass flow rates of the liquid solution and the CO2 have to guarantee an adequate residence time of the mixture in the saturator. When too high CO2 flow rates are used, the liquid could be solubilized in the scCO2, causing the so-called “antisolvent effect” and the consequent precipitation of the solute/solutes in the saturator. Typically, the particle size decreases and the PSD shrinks as the GLR increases, as more CO2 is solubilized in the liquid solution and, thus, the viscosity and surface tension of the mixture decrease. When operating at low GLR values, as well as at low temperatures in the precipitator, the time required for the complete evaporation of the liquid solvents increases; thus, the precipitation of the solute/solutes occurs slowly. A GLR value of 1.8 has often been found to be the most effective for the attainment of spherical particles by SAA [133,136,137,139,141]. The concentration of the solute/solutes in the liquid solution can also strongly influence the particle size [133,138,142]. For example, in the study by Reverchon and Spada [133], erythromycin nanoparticles were produced only by working with drug concentrations in the solution below 40 mg/mL. Therefore, as a rule, it is advisable to inject solutions at low concentrations to produce nanometric particles by SAA. Indeed, the size of the precipitated particles generally increases as the overall concentration of the liquid solution rises, due to an increase in the viscosity and surface tension of the solution [133,138,142]. When composite particles are prepared, the polymer/drug ratio in the solution can also influence the morphology and PSDs of SAA powders [36,137]. Labuschagne et al. [36] selected poly(d,l-lactide) (PDLLA) as the carrier to prepare rifampicin-loaded nanoparticles for a sustained release of the antibiotic. The effect of the PDLLA/rifampicin w/w ratio on the particle size and drug dissolution was investigated. In particular, as often observed, the dimensions of the nanoparticles decrease as the polymer/drug ratio decreases, while the antibiotic release is more prolonged the greater the quantity of polymeric carrier in the composite particles.
A limitation of the SAA process is certainly that it is difficult to process thermolabile materials due to the high process temperatures employed. For this reason, the use of lower than atmospheric operating pressures has been suggested for thermosensitive compounds [141].
Recently, a modified version of SAA with an enhanced mixer has been proposed, namely, supercritical assisted atomization introduced by a hydrodynamic cavitation mixer (SAA-HCM) [140,143,144,145]. A hydrodynamic cavitation mixer was introduced in the SAA process to intensify the mass transfer between the solvent and the scCO2 in the packed saturator. Mixing efficiency plays a key role in SAA particle production, as it affects secondary atomization due to the rapid expansion of scCO2 dissolved in the droplets. A homogeneous mixing allows obtaining, as desired, particles with narrow PSD. The cavitation is a rapid expansion that involves the collapse of bubbles or voids within a liquid medium [143]. The collision of liquid elements creates micro-jets, leading to an improvement of the mixing. The hydrodynamic cavitation mixer generates the collapse of bubbles and cavities through a localized pressure drop in a liquid stream. Peng et al. [140] managed to produce nanoparticles of a chemotherapy drug, namely, doxorubicin hydrochloride, using the SAA-HCM technique. The effect of the molecular weight of chitosan, selected as the polymeric carrier, on the morphology and particle size was investigated. However, this parameter did not have a significant influence, as the PSDs only shrank slightly by decreasing the molecular weight of chitosan. As expected, by decreasing the chitosan/doxorubicin ratio from 4/1 to 1/1 w/w, the particle size reduced, as a result of a decrease in viscosity and surface tension of the solution due to the lower molecular weight of the chemotherapy drug compared to the polymer. Dissolution tests conducted in liquid media at different pH values revealed that the doxorubicin-loaded nanoparticles were highly pH-sensitive. In particular, the drug release rate is greatly enhanced by decreasing the medium pH. The in vitro cytotoxicity profiles of the chitosan/doxorubicin nanoparticles were similar to the unprocessed drug, showing that the drug’s activity was preserved during the SAA-HCM process.
The SAILA technique consists in the formation of an expanded liquid solution constituted by scCO2 and an organic solvent in which the solute of interest is dissolved. The expanded ternary solution formed inside a high-pressure saturator is depressurized into a receiving water phase (in which the solute is insoluble) through a nozzle. The water phase is continuously pumped inside the vessel, and the nanoparticle suspension is continuously recovered through a regulation valve [146,147]. The SAILA process is based on two pre-requisites: (1) the solute has to be soluble in the organic solvent and insoluble in water; (2) water and the organic solvent have to be miscible. In this process, carbon dioxide has the role of a co-solute, whereas water has the role of the antisolvent. SAILA technique has been successfully applied for the micro/nanonization of pure compounds [148,149,150,151,152] or for the attainment of polymer/drug coprecipitates [146,153,154]. The organic solvents commonly used for this process are acetone and ethanol; however, in some cases, isopropanol has also been used. The parameters mostly affecting morphology, mean size, and PSD are GLR and temperature. Indeed, in correspondence with different GLR, expanded liquid mixtures with different CO2 molar fractions are obtained. Particle size decreases with the increase in GLR and, consequently, the increase in XCO2. Regarding the effect of temperature, it is possible to observe that, when increasing the temperature, the surface tension of the liquid mixture containing scCO2 decreases and, as a consequence, a reduction in nanoparticle diameter is generally obtained. In summary, although the particles produced mainly through the SAA process are characterized by at least sub-micrometric dimensions, PSDs are narrower than those obtained with the PGSS method, in which difficult control of the shape/size of the precipitates is encountered. Undoubtedly, among the techniques based on the use of scCO2 as a co-solute, the smaller particles with a narrower and less polydisperse PSD are those obtained through the SAILA technique.
From a commercial point of view, some limitations are linked to the scale-up of the technologies assisted by the use of scCO2 as a co-solute, such as the possible nozzle blockage and any troubles related to the collection of ultrafine particles, among others. On the other hand, good adaptability to the spray-drying facilities still existing in the pharmaceutical industries can be encountered. However, Thar (USA), Cerbios-Pharma (Switzerland), and Feyecon (the Netherlands) are some of the companies with pilot/industrial facilities.
A list of the pharmaceutical nanoparticles produced by using scCO2 as a co-solute is reported in Table 3, indicating the solvent, the operating pressure, and temperature employed in the mixer or the precipitator. The morphology of the precipitated powder and the particle size are also specified.

Table 3.
An overview of nanoparticle production by using CO2 as a co-solute. NP: nanoparticles; PMIXER: pressure in the saturator/mixer; PPREC: pressure; TMIXER: temperature in the saturator/mixer; TPREC: precipitator temperature; FHCO: fully hydrogenated canola oil; PDLLA: poly(d,l-lactide); PEG: polyethylene glycol; AC: acetone; EtOH: ethanol; MeOH: methanol; PGSS: particles from gas-saturated solutions; SAA: supercritical-assisted atomization; HCM: hydrodynamic cavitation mixer; SAILA: supercritical-assisted injection in a liquid antisolvent (SAILA).
5. Conclusions
Pharmaceutical nanoparticles and nanocrystals can be successfully processed using scCO2-based techniques. The use of one supercritical technique rather than another depends on the processability of the active compounds, which is related to their solubility in scCO2 or organic solvents, as well as to behavior under certain conditions (e.g., process temperatures). All supercritical processes represent a valid alternative to conventional nanonization methods, generally obtaining smaller particles with narrower PSD and with fewer solvent residues.
Techniques based on the use of scCO2 as a solvent offer some advantages, such as a reduced use of organic solvents; on the other hand, several drawbacks have emerged, such as the possibility of processing only principles that are soluble in scCO2, the production of particles with broad PSDs or even agglomerates, and the need for high processing pressures, and sometimes, temperatures.
When scCO2 is used as an antisolvent, narrow PSDs are usually obtained and low process temperatures are typically employed. The recrystallization of the active principles can also be controlled using polymeric carriers; however, high concentrations of stabilizers may be required to ensure the desired morphology. Furthermore, it is necessary to use organic solvents, certainly in lower quantities than traditional nanonization technologies but sometimes higher than those required by other supercritical technologies. Processes based on the use of the supercritical fluid as a co-solute result in lower amounts of scCO2 than other supercritical technologies and, as a result, smaller volume vessels. The production of smaller particles and better control of PSDs compared to conventional nanonization techniques are assured by using scCO2 as a co-solute; however, the production of nanometric particles sometimes seems to be difficult. Moreover, in many cases, higher processing temperatures may be required than with other supercritical technologies.
From a commercial point of view, the main limitation for the scale-up of all the supercritical micronization/nanonization technologies is related to the high investment costs required for the high-pressure equipment and to the high energy consumption cost per unit of product. Nevertheless, several supercritical processes have already been implemented on a pilot/industrial scale.
Author Contributions
Conceptualization, P.F. and I.D.M.; methodology, P.F.; writing—original draft preparation, P.F.; writing—review and editing, I.D.M.; supervision, I.D.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Franco, P.; De Marco, I. Supercritical antisolvent coprecipitation in the pharmaceutical field: Different polymeric carriers for different drug releases. Can. J. Chem. Eng. 2020, 98, 1935–1943. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Dhand, C.; Prabhakaran, M.P.; Beuerman, R.W.; Lakshminarayanan, R.; Dwivedi, N.; Ramakrishna, S. Role of size of drug delivery carriers for pulmonary and intravenous administration with emphasis on cancer therapeutics and lung-targeted drug delivery. Rsc Adv. 2014, 4, 32673–32689. [Google Scholar] [CrossRef]
- Lipinski, C. Poor aqueous solubility—an industry wide problem in drug discovery. Am. Pharm. Rev. 2002, 5, 82–85. [Google Scholar]
- Heimbach, T.; Fleisher, D.; Kaddoumi, A. Overcoming poor aqueous solubility of drugs for oral delivery. In Prodrugs; Springer: Berlin/Heidelberg, Germany, 2007; pp. 157–215. [Google Scholar]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug solubility: Importance and enhancement techniques. Isrn Pharm. 2012, 2012, 195727–195736. [Google Scholar] [CrossRef]
- Vieth, M.; Siegel, M.G.; Higgs, R.E.; Watson, I.A.; Robertson, D.H.; Savin, K.A.; Durst, G.L.; Hipskind, P.A. Characteristic physical properties and structural fragments of marketed oral drugs. J. Med. Chem. 2004, 47, 224–232. [Google Scholar] [CrossRef]
- Rodriguez-Aller, M.; Guillarme, D.; Veuthey, J.-L.; Gurny, R. Strategies for formulating and delivering poorly water-soluble drugs. J. Drug Del. Sci.Tech. 2015, 30, 342–351. [Google Scholar] [CrossRef]
- Takagi, T.; Ramachandran, C.; Bermejo, M.; Yamashita, S.; Yu, L.X.; Amidon, G.L. A provisional biopharmaceutical classification of the top 200 oral drug products in the United States, Great Britain, Spain, and Japan. Mol. Pharm. 2006, 3, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.-Y.; Chen, Z.-Y.; Huang, C.-L.; Huang, B.; Zheng, Y.-R.; Zhang, Y.-F.; Lu, B.-Y.; He, L.; Liu, C.-S.; Long, X.-Y. A Non-Lipolysis Nanoemulsion Improved Oral Bioavailability by Reducing the First-Pass Metabolism of Raloxifene, and Related Absorption Mechanisms Being Studied. Int. J. Nanomed. 2020, 15, 6503. [Google Scholar] [CrossRef] [PubMed]
- S Darwich, A.; Neuhoff, S.; Jamei, M.; Rostami-Hodjegan, A. Interplay of metabolism and transport in determining oral drug absorption and gut wall metabolism: A simulation assessment using the “Advanced Dissolution, Absorption, Metabolism (ADAM)” model. Curr. Drug Metab. 2010, 11, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Prosapio, V.; De Marco, I.; Reverchon, E. Supercritical antisolvent coprecipitation mechanisms. J. Supercrit. Fluids 2018, 138, 247–258. [Google Scholar] [CrossRef]
- Varde, N.K.; Pack, D.W. Microspheres for controlled release drug delivery. Expert Opin. Biol. Ther. 2004, 4, 35–51. [Google Scholar] [CrossRef]
- Shi, Y.; van der Meel, R.; Chen, X.; Lammers, T. The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics 2020, 10, 7921. [Google Scholar] [CrossRef] [PubMed]
- Germain, M.; Caputo, F.; Metcalfe, S.; Tosi, G.; Spring, K.; Åslund, A.K.; Pottier, A.; Schiffelers, R.; Ceccaldi, A.; Schmid, R. Delivering the power of nanomedicine to patients today. J. Control. Release 2020, 326, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Amrutiya, J.; Bhatt, P.; Javia, A.; Jain, M.; Misra, A. Targeted delivery of monoclonal antibody conjugated docetaxel loaded PLGA nanoparticles into EGFR overexpressed lung tumour cells. J. Microencapsul. 2018, 35, 204–217. [Google Scholar] [CrossRef]
- Kim, B.Y.; Rutka, J.T.; Chan, W.C. Nanomedicine. N. Eng. J. Med. 2010, 363, 2434–2443. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.R.; Choo, Q.; Ashtikar, M.; Rocha, T.C.; Bremer-Hoffmann, S.; Wacker, M.G. Nanomedicines-Tiny particles and big challenges. Adv. Drug Deliv. Rev. 2019, 151, 23–43. [Google Scholar] [CrossRef] [PubMed]
- Weissig, V.; Pettinger, T.K.; Murdock, N. Nanopharmaceuticals (part 1): Products on the market. Int. J. Nanomed. 2014, 9, 4357. [Google Scholar] [CrossRef]
- Lee, C.-W.; Kim, S.-J.; Youn, Y.-S.; Widjojokusumo, E.; Lee, Y.-H.; Kim, J.; Lee, Y.-W.; Tjandrawinata, R.R. Preparation of bitter taste masked cetirizine dihydrochloride/β-cyclodextrin inclusion complex by supercritical antisolvent (SAS) process. J. Supercrit. Fluids 2010, 55, 348–357. [Google Scholar] [CrossRef]
- Wu, K.; Li, J.; Wang, W.; Winstead, D.A. Formation and characterization of solid dispersions of piroxicam and polyvinylpyrrolidone using spray drying and precipitation with compressed antisolvent. J. Pharm. Sci. 2009, 98, 2422–2431. [Google Scholar] [CrossRef]
- Ha, E.-S.; Park, H.; Lee, S.-K.; Sim, W.-Y.; Jeong, J.-S.; Baek, I.-H.; Kim, M.-S. Pure Trans-Resveratrol Nanoparticles Prepared by A Supercritical Antisolvent Process Using Alcohol and Dichloromethane Mixtures: Effect of Particle Size on Dissolution and Bioavailability in Rats. Antioxidants 2020, 9, 342. [Google Scholar] [CrossRef]
- Park, J.; Park, H.J.; Cho, W.; Cha, K.-H.; Kang, Y.-S.; Hwang, S.-J. Preparation and pharmaceutical characterization of amorphous cefdinir using spray-drying and SAS-process. Int. J. Pharm. 2010, 396, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Giufrida, W.M.; Cabral, V.F.; Cardoso-Filho, L.; dos Santos Conti, D.; de Campos, V.E.; da Rocha, S.R. Medroxyprogesterone-encapsulated poly (3-hydroxybutirate-co-3-hydroxyvalerate) nanoparticles using supercritical fluid extraction of emulsions. J. Supercrit. Fluids 2016, 118, 79–88. [Google Scholar] [CrossRef]
- Shekunov, B.Y.; Chattopadhyay, P.; Seitzinger, J.; Huff, R. Nanoparticles of poorly water-soluble drugs prepared by supercritical fluid extraction of emulsions. Pharm. Res. 2006, 23, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Prieto, C.; Calvo, L. Supercritical fluid extraction of emulsions to nanoencapsulate vitamin E in polycaprolactone. J. Supercrit. Fluids 2017, 119, 274–282. [Google Scholar] [CrossRef]
- da Fonseca Machado, A.P.; Rezende, C.A.; Rodrigues, R.A.; Barbero, G.F.; e Rosa, P.d.T.V.; Martínez, J. Encapsulation of anthocyanin-rich extract from blackberry residues by spray-drying, freeze-drying and supercritical antisolvent. Powder Technol. 2018, 340, 553–562. [Google Scholar] [CrossRef]
- Won, D.-H.; Kim, M.-S.; Lee, S.; Park, J.-S.; Hwang, S.-J. Improved physicochemical characteristics of felodipine solid dispersion particles by supercritical anti-solvent precipitation process. Int. J. Pharm. 2005, 301, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Seo, H.J.; Hong, S.-h.; Ha, E.-S.; Lee, S.; Kim, J.-S.; Baek, I.-h.; Kim, M.-S.; Hwang, S.-J. Characterization and therapeutic efficacy evaluation of glimepiride and L-arginine co-amorphous formulation prepared by supercritical antisolvent process: Influence of molar ratio and preparation methods. Int. J. Pharm. 2020, 119232. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.V.; Duarte, C.M. Dense CO2 as a solute, co-solute or co-solvent in particle formation processes: A review. Materials 2011, 4, 2017–2041. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.K.; Pathak, K. Supercritical fluid technology for solubilization of poorly water soluble drugs via micro-and naonosized particle generation. ADMET DMPK 2020, 8, 355–374. [Google Scholar] [CrossRef]
- Van Konynenburg, P.; Scott, R. Critical lines and phase equilibria in binary van der Waals mixtures. Philos. Trans. R. Soc. London. Ser. Amathematical Phys. Sci. 1980, 298, 495–540. [Google Scholar]
- Raeissi, S.; Florusse, L.; Peters, C. Scott–van Konynenburg phase diagram of carbon dioxide+ alkylimidazolium-based ionic liquids. J. Supercrit. Fluids 2010, 55, 825–832. [Google Scholar] [CrossRef]
- De Diego, Y.P.; Wubbolts, F.; Witkamp, G.; De Loos, T.W.; Jansens, P.J. Measurements of the phase behaviour of the system dextran/DMSO/CO2 at high pressures. J. Supercrit. Fluids 2005, 35, 1–9. [Google Scholar] [CrossRef]
- Labuschagne, P.W.; Adami, R.; Liparoti, S.; Naidoo, S.; Swai, H.; Reverchon, E. Preparation of rifampicin/poly (d, l-lactice) nanoparticles for sustained release by supercritical assisted atomization technique. J. Supercrit. Fluids 2014, 95, 106–117. [Google Scholar] [CrossRef]
- Hu, X.; Guo, Y.; Wang, L.; Hua, D.; Hong, Y.; Li, J. Coenzyme Q10 nanoparticles prepared by a supercritical fluid-based method. J. Supercrit. Fluids 2011, 57, 66–72. [Google Scholar] [CrossRef]
- Sharma, S.K.; Jagannathan, R. High throughput RESS processing of sub-10 nm ibuprofen nanoparticles. J. Supercrit. Fluids 2016, 109, 74–79. [Google Scholar] [CrossRef]
- Lee, S.; Nam, K.; Kim, M.S.; Jun, S.W.; Park, J.-S.; Woo, J.S.; Hwang, S.-J. Preparation and characterization of solid dispersions of itraconazole by using aerosol solvent extraction system for improvement in drug solubility and bioavailability. Arch. Pharmacal Res. 2005, 28, 866–874. [Google Scholar] [CrossRef]
- Chattopadhyay, P.; Shekunov, B.Y.; Yim, D.; Cipolla, D.; Boyd, B.; Farr, S. Production of solid lipid nanoparticle suspensions using supercritical fluid extraction of emulsions (SFEE) for pulmonary delivery using the AERx system. Adv. Drug Deliv. Rev. 2007, 59, 444–453. [Google Scholar] [CrossRef]
- Gadermann, M.; Kular, S.; Al-Marzouqi, A.H.; Signorell, R. Formation of naproxen–polylactic acid nanoparticles from supercritical solutions and their characterization in the aerosol phase. Phys. Chem. Chem. Phys. 2009, 11, 7861–7868. [Google Scholar] [CrossRef] [PubMed]
- Montes, A.; Gordillo, M.D.; Pereyra, C.; De los Santos, D.M.; Martínez de la Ossa, E.J. Ibuprofen–polymer precipitation using supercritical CO2 at low temperature. J. Supercrit. Fluids 2014, 94, 91–101. [Google Scholar] [CrossRef]
- Nerome, H.; Machmudah, S.; Fukuzato, R.; Higashiura, T.; Youn, Y.S.; Lee, Y.W.; Goto, M. Nanoparticle formation of lycopene/β-cyclodextrin inclusion complex using supercritical antisolvent precipitation. J. Supercrit. Fluids 2013, 83, 97–103. [Google Scholar] [CrossRef]
- Prosapio, V.; Reverchon, E.; De Marco, I. Coprecipitation of Polyvinylpyrrolidone/β-Carotene by Supercritical Antisolvent Processing. Ind. Eng. Chem. Res. 2015, 54, 11568–11575. [Google Scholar] [CrossRef]
- Bhatt, P.; Trehan, S.; Inamdar, N.; Mourya, V.K.; Misra, A. Polymers in Drug Delivery: An Update. In Applications of Polymers in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–42. [Google Scholar]
- Sodeifian, G.; Sajadian, S.A.; Ardestani, N.S.; Razmimanesh, F. Production of Loratadine drug nanoparticles using ultrasonic-assisted Rapid expansion of supercritical solution into aqueous solution (US-RESSAS). J. Supercrit. Fluids 2019, 147, 241–253. [Google Scholar] [CrossRef]
- Sodeifian, G.; Sajadian, S.A. Utilization of ultrasonic-assisted RESOLV (US-RESOLV) with polymeric stabilizers for production of amiodarone hydrochloride nanoparticles: Optimization of the process parameters. Chem. Eng. Res. Des. 2019, 142, 268–284. [Google Scholar] [CrossRef]
- Herrmann, M.; Förter-Barth, U.; Kröber, H.; Kempa, P.B.; Juez-Lorenzo, M.d.M.; Doyle, S. Co-Crystallization and Characterization of Pharmaceutical Ingredients. Part. Part. Syst. Charact. 2009, 26, 151–156. [Google Scholar] [CrossRef]
- Hermsdorf, D.; Jauer, S.; Signorell, R. Formation and stabilization of ibuprofen nanoparticles by pulsed rapid expansion of supercritical solutions. Mol. Phys. 2007, 105, 951–959. [Google Scholar] [CrossRef]
- Tuerk, M.; Bolten, D. Polymorphic properties of micronized mefenamic acid, nabumetone, paracetamol and tolbutamide produced by rapid expansion of supercritical solutions (RESS). J. Supercrit. Fluids 2016, 116, 239–250. [Google Scholar] [CrossRef]
- Keshavarz, A.; Karimi-Sabet, J.; Fattahi, A.; Golzary, A.; Rafiee-Tehrani, M.; Dorkoosh, F.A. Preparation and characterization of raloxifene nanoparticles using rapid expansion of supercritical solution (RESS). J. Supercrit. Fluids 2012, 63, 169–179. [Google Scholar] [CrossRef]
- Paisana, M.C.; Müllers, K.C.; Wahl, M.A.; Pinto, J.F. Production and stabilization of olanzapine nanoparticles by rapid expansion of supercritical solutions (RESS). J. Supercrit. Fluids 2016, 109, 124–133. [Google Scholar] [CrossRef]
- Yekefallah, M.; Raofie, F. Preparation of potent antioxidant nanosuspensions from olive leaves by Rapid expansion of supercritical solution into aqueous solutions (RESSAS). Ind. Crop. Prod. 2020, 155, 112756. [Google Scholar] [CrossRef]
- Pathak, P.; Meziani, M.J.; Desai, T.; Sun, Y.-P. Formation and stabilization of ibuprofen nanoparticles in supercritical fluid processing. J. Supercrit. Fluids 2006, 37, 279–286. [Google Scholar] [CrossRef]
- Pathak, P.; Prasad, G.L.; Meziani, M.J.; Joudeh, A.A.; Sun, Y.-P. Nanosized paclitaxel particles from supercritical carbon dioxide processing and their biological evaluation. Langmuir 2007, 23, 2674–2679. [Google Scholar] [CrossRef] [PubMed]
- Sane, A.; Limtrakul, J. Formation of retinyl palmitate-loaded poly (l-lactide) nanoparticles using rapid expansion of supercritical solutions into liquid solvents (RESOLV). J. Supercrit. Fluids 2009, 51, 230–237. [Google Scholar] [CrossRef]
- Atila, C.; Yıldız, N.; Çalımlı, A. Particle size design of digitoxin in supercritical fluids. J. Supercrit. Fluids 2010, 51, 404–411. [Google Scholar] [CrossRef]
- Montes, A.; Bendel, A.; Kürti, R.; Gordillo, M.; Pereyra, C.; de La Ossa, E.M. Processing naproxen with supercritical CO2. J. Supercrit. Fluids 2013, 75, 21–29. [Google Scholar] [CrossRef]
- Pathak, P.; Meziani, M.J.; Desai, T.; Foster, C.; Diaz, J.A.; Sun, Y.-P. Supercritical fluid processing of drug nanoparticles in stable suspension. J. Nanosci. Nanotechnol. 2007, 7, 2542–2545. [Google Scholar] [CrossRef]
- Pathak, P.; Meziani, M.J.; Desai, T.; Sun, Y.-P. Nanosizing drug particles in supercritical fluid processing. J. Am. Chem. Soc. 2004, 126, 10842–10843. [Google Scholar] [CrossRef]
- Thakur, R.; Gupta, R.B. Formation of phenytoin nanoparticles using rapid expansion of supercritical solution with solid cosolvent (RESS-SC) process. Int. J. Pharm. 2006, 308, 190–199. [Google Scholar] [CrossRef]
- Rostamian, H.; Lotfollahi, M.N. Production and characterization of ultrafine aspirin particles by rapid expansion of supercritical solution with solid co-solvent (RESS-SC): Expansion parameters effects. Part. Sci. Technol. 2019, 38, 617–625. [Google Scholar] [CrossRef]
- Pourasghar, M.; Fatemi, S.; Vatanara, A.; Najafabadi, A.R. Production of ultrafine drug particles through rapid expansion of supercritical solution; a statistical approach. Powder Technol. 2012, 225, 21–26. [Google Scholar] [CrossRef]
- Samei, M.; Vatanara, A.; Fatemi, S.; Najafabadi, A.R. Process variables in the formation of nanoparticles of megestrol acetate through rapid expansion of supercritical CO2. J. Supercrit. Fluids 2012, 70, 1–7. [Google Scholar] [CrossRef]
- Uchida, H.; Nishijima, M.; Sano, K.; Demoto, K.; Sakabe, J.; Shimoyama, Y. Production of theophylline nanoparticles using rapid expansion of supercritical solutions with a solid cosolvent (RESS-SC) technique. J. Supercrit. Fluids 2015, 105, 128–135. [Google Scholar] [CrossRef]
- Hielscher, K. Ultrasonic milling and dispersing technology for nano-particles. Mater. Res. Soc. Symp. Proc. 2012, 1479, 21–26. [Google Scholar] [CrossRef]
- Yasui, K. Acoustic Cavitation and Bubble Dynamics; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Santos, D.T.; Santana, Á.L.; Meireles, M.A.A.; Petenate, A.J.; Silva, E.K.; Albarelli, J.Q.; Johner, J.C.; Gomes, M.T.M.; Torres, R.A.D.C.; Hatami, T. Economical Effects of Supercritical Antisolvent Precipitation Process Conditions. In Supercritical Antisolvent Precipitation Process; Springer: Berlin/Heidelberg, Germany, 2019; pp. 75–82. [Google Scholar]
- Asghari, I.; Esmaeilzadeh, F. Formation of ultrafine deferasirox particles via rapid expansion of supercritical solution (RESS process) using Taguchi approach. Int. J. Pharm. 2012, 433, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Raofie, F. Micronization of vincristine extracted from Catharanthus roseus by expansion of supercritical fluid solution. J. Supercrit. Fluids 2019, 146, 172–179. [Google Scholar] [CrossRef]
- Elizondo, E.; Sala, S.; Imbuluzqueta, E.; González, D.; Blanco-Prieto, M.J.; Gamazo, C.; Ventosa, N.; Veciana, J. High loading of gentamicin in bioadhesive PVM/MA nanostructured microparticles using compressed carbon-dioxide. Pharm. Res. 2011, 28, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Yoon, T.J.; Son, W.-S.; Park, H.J.; Seo, B.; Kim, T.; Lee, Y.-W. Tetracycline nanoparticles precipitation using supercritical and liquid CO2 as antisolvents. J. Supercrit. Fluids 2016, 107, 51–60. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, X.; Zu, Y.; Jiang, R.; Zhao, D. Recrystallization and micronization of taxol using the supercritical antisolvent (SAS) process. Ind. Eng. Chem. Res. 2012, 51, 9591–9597. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Liu, D.; Gao, Y.; Qian, S. Preparation of apigenin nanocrystals using supercritical antisolvent process for dissolution and bioavailability enhancement. Eur. J. Pharm. Sci. 2013, 48, 740–747. [Google Scholar] [CrossRef]
- Zhao, X.; Zu, Y.; Li, Q.; Wang, M.; Zu, B.; Zhang, X.; Jiang, R.; Zu, C. Preparation and characterization of camptothecin powder micronized by a supercritical antisolvent (SAS) process. J. Supercrit. Fluids 2010, 51, 412–419. [Google Scholar] [CrossRef]
- Widjojokusumo, E.; Veriansyah, B.; Tjandrawinata, R.R. Supercritical anti-solvent (SAS) micronization of Manilkara kauki bioactive fraction (DLBS2347). J. CO2 Util. 2013, 3-4, 30–36. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, L.; Zu, Y.; Li, C.; Liu, S.; Yang, L.; Zhao, X.; Zu, B. Micronization of Ginkgo biloba extract using supercritical antisolvent process. Powder Technol. 2011, 209, 73–80. [Google Scholar] [CrossRef]
- Zhao, X.; Zu, Y.; Jiang, R.; Wang, Y.; Li, Y.; Li, Q.; Zhao, D.; Zu, B.; Zhang, B.; Sun, Z. Preparation and physicochemical properties of 10-hydroxycamptothecin (HCPT) nanoparticles by supercritical antisolvent (SAS) process. Int. J. Mol. Sci. 2011, 12, 2678–2691. [Google Scholar] [CrossRef] [PubMed]
- Montes, A.; Wehner, L.; Pereyra, C.; Martínez de la Ossa, E.J. Mangiferin nanoparticles precipitation by supercritical antisolvent process. J. Supercrit. Fluids 2016, 112, 44–50. [Google Scholar] [CrossRef]
- Zu, Y.; Zhang, Q.; Zhao, X.; Wang, D.; Li, W.; Sui, X.; Zhang, Y.; Jiang, S.; Wang, Q.; Gu, C. Preparation and characterization of vitexin powder micronized by a supercritical antisolvent (SAS) process. Powder Technol. 2012, 228, 47–55. [Google Scholar] [CrossRef]
- Djerafi, R.; Swanepoel, A.; Crampon, C.; Kalombo, L.; Labuschagne, P.; Badens, E.; Masmoudi, Y. Supercritical antisolvent co-precipitation of rifampicin and ethyl cellulose. Eur. J. Pharm. Sci. 2017, 102, 161–171. [Google Scholar] [CrossRef]
- Franco, P.; Reverchon, E.; De Marco, I. Zein/diclofenac sodium coprecipitation at micrometric and nanometric range by supercritical antisolvent processing. J. CO2 Util. 2018, 27, 366–373. [Google Scholar] [CrossRef]
- Zahran, F.; Cabañas, A.; Cheda, J.A.R.; Renuncio, J.A.R.; Pando, C. Dissolution rate enhancement of the anti-inflammatory drug diflunisal by coprecipitation with a biocompatible polymer using carbon dioxide as a supercritical fluid antisolvent. J. Supercrit. Fluids 2014, 88, 56–65. [Google Scholar] [CrossRef]
- Muhrer, G.; Mazzotti, M. Precipitation of lysozyme nanoparticles from dimethyl sulfoxide using carbon dioxide as antisolvent. Biotechnol. Progr. 2003, 19, 549–556. [Google Scholar] [CrossRef]
- Fusaro, F.; Kluge, J.; Mazzotti, M.; Muhrer, G. Compressed CO2 antisolvent precipitation of lysozyme. J. Supercrit. Fluids 2009, 49, 79–92. [Google Scholar] [CrossRef]
- Akbari, I.; Ghoreishi, S.; Habibi, N. Generation and precipitation of paclitaxel nanoparticles in basil seed mucilage via combination of supercritical gas antisolvent and phase inversion techniques. J. Supercrit. Fluids 2014, 94, 182–188. [Google Scholar] [CrossRef]
- Park, H.J.; Yoon, T.J.; Kwon, D.E.; Yu, K.; Lee, Y.-W. Coprecipitation of hydrochlorothiazide/PVP for the dissolution rate improvement by precipitation with compressed fluid antisolvent process. J. Supercrit. Fluids 2017, 126, 37–46. [Google Scholar] [CrossRef]
- Ha, E.-S.; Sim, W.-Y.; Lee, S.-K.; Jeong, J.-S.; Kim, J.-S.; Baek, I.-h.; Choi, D.H.; Park, H.; Hwang, S.-J.; Kim, M.-S. Preparation and evaluation of resveratrol-loaded composite nanoparticles using a supercritical fluid technology for enhanced oral and skin delivery. Antioxidants 2019, 8, 554. [Google Scholar] [CrossRef]
- Jin, H.Y.; Xia, F.; Zhao, Y.P. Preparation of hydroxypropyl methyl cellulose phthalate nanoparticles with mixed solvent using supercritical antisolvent process and its application in co-precipitation of insulin. Adv. Powder Technol. 2012, 23, 157–163. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. Supercritical Antisolvent Process for Pharmaceutical Applications: A Review. Processes 2020, 8, 938. [Google Scholar] [CrossRef]
- Franco, P.; Reverchon, E.; De Marco, I. Production of zein/antibiotic microparticles by supercritical antisolvent coprecipitation. J. Supercrit. Fluids 2019, 145, 31–38. [Google Scholar] [CrossRef]
- Kim, M.-S.; Jin, S.-J.; Kim, J.-S.; Park, H.J.; Song, H.-S.; Neubert, R.H.; Hwang, S.-J. Preparation, characterization and in vivo evaluation of amorphous atorvastatin calcium nanoparticles using supercritical antisolvent (SAS) process. Eur. J. Pharm. Biopharm. 2008, 69, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.-S.; Kim, J.-S.; Baek, I.-h.; Hwang, S.-J.; Kim, M.-S. Enhancement of dissolution and bioavailability of ezetimibe by amorphous solid dispersion nanoparticles fabricated using supercritical antisolvent process. J. Pharm. Investig. 2015, 45, 641–649. [Google Scholar] [CrossRef]
- Matos, R.L.; Lu, T.; Prosapio, V.; McConville, C.; Leeke, G.; Ingram, A. Coprecipitation of curcumin/PVP with enhanced dissolution properties by the supercritical antisolvent process. J. CO2 Util. 2019, 30, 48–62. [Google Scholar] [CrossRef]
- Chen, L.-F.; Xu, P.-Y.; Fu, C.-P.; Kankala, R.K.; Chen, A.-Z.; Wang, S.-B. Fabrication of Supercritical Antisolvent (SAS) Process-Assisted Fisetin-Encapsulated Poly (Vinyl Pyrrolidone)(PVP) Nanocomposites for Improved Anticancer Therapy. Nanomaterials 2020, 10, 322. [Google Scholar] [CrossRef]
- Chhouk, K.; Kanda, H.; Kawasaki, S.-I.; Goto, M. Micronization of curcumin with biodegradable polymer by supercritical anti-solvent using micro swirl mixer. Front. Chem. Sci. Eng. 2018, 12, 184–193. [Google Scholar] [CrossRef]
- Lestari, S.D.; Machmudah, S.; Winardi, S.; Kanda, H.; Goto, M. Particle micronization of Curcuma mangga rhizomes ethanolic extract/biopolymer PVP using supercritical antisolvent process. J. Supercrit. Fluids 2019, 146, 226–239. [Google Scholar] [CrossRef]
- Prosapio, V.; De Marco, I.; Scognamiglio, M.; Reverchon, E. Folic acid–PVP nanostructured composite microparticles by supercritical antisolvent precipitation. Chem. Eng. J. 2015, 277, 286–294. [Google Scholar] [CrossRef]
- Ha, E.-S.; Choo, G.-H.; Baek, I.-H.; Kim, J.-S.; Cho, W.; Jung, Y.S.; Jin, S.-E.; Hwang, S.-J.; Kim, M.-S. Dissolution and bioavailability of lercanidipine–hydroxypropylmethyl cellulose nanoparticles with surfactant. Int. J. Biol. Macromol. 2015, 72, 218–222. [Google Scholar] [CrossRef]
- Fernández-Ponce, M.T.; Masmoudi, Y.; Djerafi, R.; Casas, L.; Mantell, C.; de La Ossa, E.M.; Badens, E. Particle design applied to quercetin using supercritical anti-solvent techniques. J. Supercrit. Fluids 2015, 105, 119–127. [Google Scholar] [CrossRef]
- Chattopadhyay, P.; Gupta, R.B. Production of griseofulvin nanoparticles using supercritical CO2 antisolvent with enhanced mass transfer. Int. J. Pharm. 2001, 228, 19–31. [Google Scholar] [CrossRef]
- Chattopadhyay, P.; Gupta, R.B. Production of antibiotic nanoparticles using supercritical CO2 as antisolvent with enhanced mass transfer. Ind. Eng. Chem. Res. 2001, 40, 3530–3539. [Google Scholar] [CrossRef]
- Long, B.; Walker, G.M.; Ryan, K.M.; Padrela, L. Controlling Polymorphism of Carbamazepine Nanoparticles in a Continuous Supercritical-CO2-Assisted Spray Drying Process. Cryst. Growth Des. 2019, 19, 3755–3767. [Google Scholar] [CrossRef]
- Chen, A.-Z.; Li, Y.; Chau, F.-T.; Lau, T.-Y.; Hu, J.-Y.; Zhao, Z.; Mok, D.K.-W. Microencapsulation of puerarin nanoparticles by poly (L-lactide) in a supercritical CO2 process. Acta Biomater. 2009, 5, 2913–2919. [Google Scholar] [CrossRef]
- Kaga, K.; Honda, M.; Adachi, T.; Honjo, M.; Kanda, H.; Goto, M. Nanoparticle formation of PVP/astaxanthin inclusion complex by solution-enhanced dispersion by supercritical fluids (SEDS): Effect of PVP and astaxanthin Z-isomer content. J. Supercrit. Fluids 2018, 136, 44–51. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I. Mechanisms controlling supercritical antisolvent precipitate morphology. Chem. Eng. J. 2011, 169, 358–370. [Google Scholar] [CrossRef]
- Marra, F.; De Marco, I.; Reverchon, E. Numerical analysis of the characteristic times controlling supercritical antisolvent micronization. Chem. Eng. Sci. 2012, 71, 39–45. [Google Scholar] [CrossRef]
- Lengsfeld, C.S.; Delplanque, J.P.; Barocas, V.H.; Randolph, T.W. Mechanism Governing Microparticle Morphology during Precipitation by a Compressed Antisolvent: Atomization vs Nucleation and Growth. J. Phys. Chem. B 2000, 104, 2725–2735. [Google Scholar] [CrossRef]
- De Marco, I.; Knauer, O.; Cice, F.; Braeuer, A.; Reverchon, E. Interactions of phase equilibria, jet fluid dynamics and mass transfer during supercritical antisolvent micronization: The influence of solvents. Chem. Eng. J. 2012, 203, 71–80. [Google Scholar] [CrossRef]
- Reverchon, E.; Torino, E.; Dowy, S.; Braeuer, A.; Leipertz, A. Interactions of phase equilibria, jet fluid dynamics and mass transfer during supercritical antisolvent micronization. Chem. Eng. J. 2010, 156, 446–458. [Google Scholar] [CrossRef]
- Prieto, C.; Calvo, L. The encapsulation of low viscosity omega-3 rich fish oil in polycaprolactone by supercritical fluid extraction of emulsions. J. Supercrit. Fluids 2017, 128, 227–234. [Google Scholar] [CrossRef]
- Lévai, G.; Martín, Á.; de Paz, E.; Rodríguez-Rojo, S.; Cocero, M.J. Production of stabilized quercetin aqueous suspensions by supercritical fluid extraction of emulsions. J. Supercrit. Fluids 2015, 100, 34–45. [Google Scholar] [CrossRef]
- Kurniawansyah, F.; Mammucari, R.; Tandya, A.; Foster, N.R. Scale− Up and economic evaluation of the atomized rapid injection solvent extraction process. J. Supercrit. Fluids 2017, 127, 208–216. [Google Scholar] [CrossRef]
- Rosa, M.T.M.G.; Alvarez, V.H.; Albarelli, J.Q.; Santos, D.T.; Meireles, M.A.A.; Saldaña, M.D.A. Supercritical anti-solvent process as an alternative technology for vitamin complex encapsulation using zein as wall material: Technical-economic evaluation. J. Supercrit. Fluids 2019. [Google Scholar] [CrossRef]
- Weber, A.; Tschernjaew, J.; Berger, T.; Bork, M. A production plant for gas antisolvent crystallization. In Proceedings of the 5th Meeting on Supercritical Fluids, Nice, France, 23–25 March 1998; pp. 281–285. [Google Scholar]
- Esfandiari, N.; Ghoreishi, S.M. Ampicillin Nanoparticles Production via Supercritical CO2 Gas Antisolvent Process. Aaps Pharmscitech 2015, 16, 1263–1269. [Google Scholar] [CrossRef]
- Kim, M.S.; Song, H.S.; Park, H.J.; Hwang, S.J. Effect of solvent type on the nanoparticle formation of atorvastatin calcium by the supercritical antisolvent process. Chem. Pharm. Bull. 2012, 60, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.Y.; Fu, C.P.; Kankala, R.K.; Wang, S.B.; Chen, A.Z. Supercritical carbon dioxide-assisted nanonization of dihydromyricetin for anticancer and bacterial biofilm inhibition efficacies. J. Supercrit. Fluids 2020, 161. [Google Scholar] [CrossRef]
- Adami, R.; Reverchon, E.; Järvenpää, E.; Huopalahti, R. Supercritical AntiSolvent micronization of nalmefene HCl on laboratory and pilot scale. Powder Technol. 2008, 182, 105–112. [Google Scholar] [CrossRef]
- Yang, L.; Sun, Z.; Zu, Y.; Zhao, C.; Sun, X.; Zhang, Z.; Zhang, L. Physicochemical properties and oral bioavailability of ursolic acid nanoparticles using supercritical anti-solvent (SAS) process. Food Chem. 2012, 132, 319–325. [Google Scholar] [CrossRef]
- Vallejo, R.; Gonzalez-Valdivieso, J.; Santos, M.; Rodriguez-Rojo, S.; Arias, F.J. Production of elastin-like recombinamer-based nanoparticles for docetaxel encapsulation and use as smart drug-delivery systems using a supercritical anti-solvent process. J. Ind. Eng. Chem. 2021, 93, 361–374. [Google Scholar] [CrossRef]
- Fu, C.; Wei, R.; Xu, P.; Luo, S.; Zhang, C.; Kankala, R.K.; Wang, S.; Jiang, X.; Wei, X.; Zhang, L.; et al. Supercritical fluid-assisted fabrication of diselenide-bridged polymeric composites for improved indocyanine green-guided photodynamic therapy. Chem. Eng. J. 2021, 407. [Google Scholar] [CrossRef]
- Ha, E.S.; Choo, G.H.; Baek, I.H.; Kim, M.S. Formulation, characterization, and in vivo evaluation of celecoxib-PVP solid dispersion nanoparticles using supercritical antisolvent process. Molecules 2014, 19, 20325–20339. [Google Scholar] [CrossRef]
- Matos, R.L.; Lu, T.; Leeke, G.; Prosapio, V.; McConville, C.; Ingram, A. Single-step coprecipitation and coating to prepare curcumin formulations by supercritical fluid technology. J. Supercrit. Fluids 2020, 159. [Google Scholar] [CrossRef]
- Ha, E.S.; Kim, J.S.; Baek, I.H.; Yoo, J.W.; Jung, Y.; Moon, H.R.; Kim, M.S. Development of megestrol acetate solid dispersion nanoparticles for enhanced oral delivery by using a supercritical antisolvent process. Drug Des. Dev. Ther. 2015, 9, 4269–4277. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, F.; Guo, Z.; Zhao, Y. Preparation and characterization of resveratrol/hydroxypropyl-β-cyclodextrin inclusion complex using supercritical antisolvent technology. J. Food Process Eng. 2012, 35, 677–686. [Google Scholar] [CrossRef]
- Adeli, E. The use of supercritical anti-solvent (SAS) technique for preparation of Irbesartan-Pluronic® F-127 nanoparticles to improve the drug dissolution. Powder Technol. 2016, 298, 65–72. [Google Scholar] [CrossRef]
- Junior, O.V.; Cardoso, F.A.R.; Giufrida, W.M.; de Souza, M.F.; Cardozo-Filho, L. Production and computational fluid dynamics-based modeling of PMMA nanoparticles impregnated with ivermectin by a supercritical antisolvent process. J. CO2 Util. 2020, 35, 47–58. [Google Scholar] [CrossRef]
- Saad, S.; Ahmad, I.; Kawish, S.M.; Khan, U.A.; Ahmad, F.J.; Ali, A.; Jain, G.K. Improved cardioprotective effects of hesperidin solid lipid nanoparticles prepared by supercritical antisolvent technology. Colloids Surf. B 2020, 187, 110628. [Google Scholar] [CrossRef] [PubMed]
- Tirado, D.F.; Latini, A.; Calvo, L. The encapsulation of hydroxytyrosol-rich olive oil in Eudraguard® protect via supercritical fluid extraction of emulsions. J. Food Eng. 2021, 290. [Google Scholar] [CrossRef]
- Chen, W.; Hu, X.; Hong, Y.; Su, Y.; Wang, H.; Li, J. Ibuprofen nanoparticles prepared by a PGSS™-based method. Powder Technol. 2013, 245, 241–250. [Google Scholar] [CrossRef]
- Couto, R.; Alvarez, V.; Temelli, F. Encapsulation of Vitamin B2 in solid lipid nanoparticles using supercritical CO2. J. Supercrit. Fluids 2017, 120, 432–442. [Google Scholar] [CrossRef]
- Reverchon, E.; Spada, A. Erythromycin micro-particles produced by supercritical fluid atomization. Powder Technol. 2004, 141, 100–108. [Google Scholar] [CrossRef]
- Kikic, I.; Vecchione, F. Supercritical impregnation of polymers. Curr. Opin. Solid State Mater. Sci. 2003, 7, 399–405. [Google Scholar] [CrossRef]
- Lian, Z.; Epstein, S.A.; Blenk, C.W.; Shine, A.D. Carbon dioxide-induced melting point depression of biodegradable semicrystalline polymers. J. Supercrit. Fluids 2006, 39, 107–117. [Google Scholar] [CrossRef]
- Wu, H.-T.; Chen, H.-C.; Lee, H.-K. Controlled release of theophylline-chitosan composite particles prepared using supercritical assisted atomization. Braz. J. Chem. Eng. 2019, 36, 895–904. [Google Scholar] [CrossRef]
- Wu, H.-T.; Yang, C.-P.; Huang, S.-C. Dissolution enhancement of indomethacin-chitosan hydrochloride composite particles produced using supercritical assisted atomization. J. Taiwan Inst. Chem. Eng. 2016, 67, 98–105. [Google Scholar] [CrossRef]
- Wu, H.-T.; Yang, M.-W. Precipitation kinetics of PMMA sub-micrometric particles with a supercritical assisted-atomization process. J. Supercrit. Fluids 2011, 59, 98–107. [Google Scholar] [CrossRef]
- Reverchon, E.; Della Porta, G. Micronization of antibiotics by supercritical assisted atomization. J. Supercrit. Fluids 2003, 26, 243–252. [Google Scholar] [CrossRef]
- Peng, H.-H.; Hong, D.-X.; Guan, Y.-X.; Yao, S.-J. Preparation of pH-responsive DOX-loaded chitosan nanoparticles using supercritical assisted atomization with an enhanced mixer. Int. J. Pharm. 2019, 558, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, E.; Antonacci, A. Polymer microparticles production by supercritical assisted atomization. J. Supercrit. Fluids 2007, 39, 444–452. [Google Scholar] [CrossRef]
- Wu, H.-T.; Tsai, H.-M.; Li, T.-H. Formation of Polyethylene Glycol Particles Using a Low-Temperature Supercritical Assisted Atomization Process. Molecules 2019, 24, 2235. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.-Q.; Guan, Y.-X.; Yao, S.-J.; Zhu, Z.-Q. Supercritical fluid assisted atomization introduced by hydrodynamic cavitation mixer (SAA-HCM) for micronization of levofloxacin hydrochloride. J. Supercrit. Fluids 2008, 43, 524–534. [Google Scholar] [CrossRef]
- Hong, D.-X.; Yun, Y.-L.; Guan, Y.-X.; Yao, S.-J. Preparation of micrometric powders of parathyroid hormone (PTH1–34)-loaded chitosan oligosaccharide by supercritical fluid assisted atomization. Int. J. Pharm. 2018, 545, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-B.; Du, Z.; Tang, C.; Guan, Y.-X.; Yao, S.-J. Formulation of insulin-loaded N-trimethyl chitosan microparticles with improved efficacy for inhalation by supercritical fluid assisted atomization. Int. J. Pharm. 2016, 505, 223–233. [Google Scholar] [CrossRef]
- Campardelli, R.; Reverchon, E. Instantaneous coprecipitation of polymer/drug microparticles using the supercritical assisted injection in a liquid antisolvent. J. Supercrit. Fluids 2017, 120, 151–160. [Google Scholar] [CrossRef]
- Campardelli, R.; Adami, R.; Della Porta, G.; Reverchon, E. Nanoparticle precipitation by Supercritical Assisted Injection in a Liquid Antisolvent. Chem. Eng. J. 2012, 192, 246–251. [Google Scholar] [CrossRef]
- Campardelli, R.; Adami, R.; Reverchon, E. Preparation of stable aqueous nanodispersions of β-carotene by supercritical assisted injection in a liquid antisolvent C3—Procedia Engineering. Procedia Eng. 2012, 42, 1493–1501. [Google Scholar] [CrossRef]
- Campardelli, R.; Oleandro, E.; Adami, R.; Reverchon, E. Polymethylmethacrylate (PMMA) sub-microparticles produced by Supercritical Assisted Injection in a Liquid Antisolvent. J. Supercrit. Fluids 2014, 92, 93–99. [Google Scholar] [CrossRef]
- Campardelli, R.; Oleandro, E.; Reverchon, E. Supercritical assisted injection in a liquid antisolvent for PLGA and PLA microparticle production. Powder Technol. 2016, 287, 12–19. [Google Scholar] [CrossRef]
- Trucillo, P.; Campardelli, R. Production of solid lipid nanoparticles with a supercritical fluid assisted process. J. Supercrit. Fluids 2019, 143, 16–23. [Google Scholar] [CrossRef]
- Campardelli, R.; Reverchon, E. α-Tocopherol nanosuspensions produced using a supercritical assisted process. J. Food Eng. 2015, 149, 131–136. [Google Scholar] [CrossRef]
- Palazzo, I.; Campardelli, R.; Scognamiglio, M.; Reverchon, E. Zein/luteolin microparticles formation using a supercritical fluids assisted technique. Powder Technol. 2019, 356, 899–908. [Google Scholar] [CrossRef]
- Palazzo, I.; Trucillo, P.; Campardelli, R.; Reverchon, E. Antioxidants entrapment in polycaprolactone microparticles using supercritical assisted injection in a liquid antisolvent. Food Bioprod. Process. 2020, 123, 312–321. [Google Scholar] [CrossRef]
- Tandya, A.; Dehghani, F.; Foster, N.R. Micronization of cyclosporine using dense gas techniques. J. Supercrit. Fluids 2006, 37, 272–278. [Google Scholar] [CrossRef]
- Salmaso, S.; Bersani, S.; Elvassore, N.; Bertucco, A.; Caliceti, P. Biopharmaceutical characterisation of insulin and recombinant human growth hormone loaded lipid submicron particles produced by supercritical gas micro-atomisation. Int. J. Pharm. 2009, 379, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.A.; Li, J.; Padrela, L.; Almeida, A.; Matos, H.A.; de Azevedo, E.G. Anti-solvent effect in the production of lysozyme nanoparticles by supercritical fluid-assisted atomization processes. J. Supercrit. Fluids 2009, 48, 253–260. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).