UHPLC-MS Method for the Analysis of the Molecular Adjuvant Sulfavant A
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
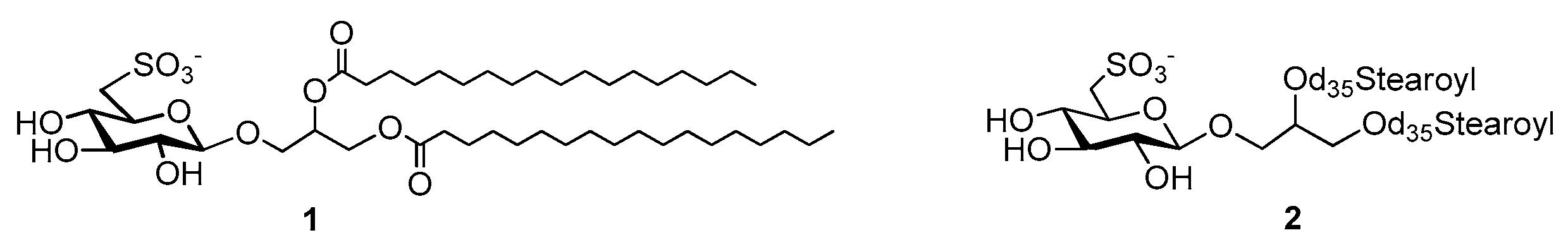
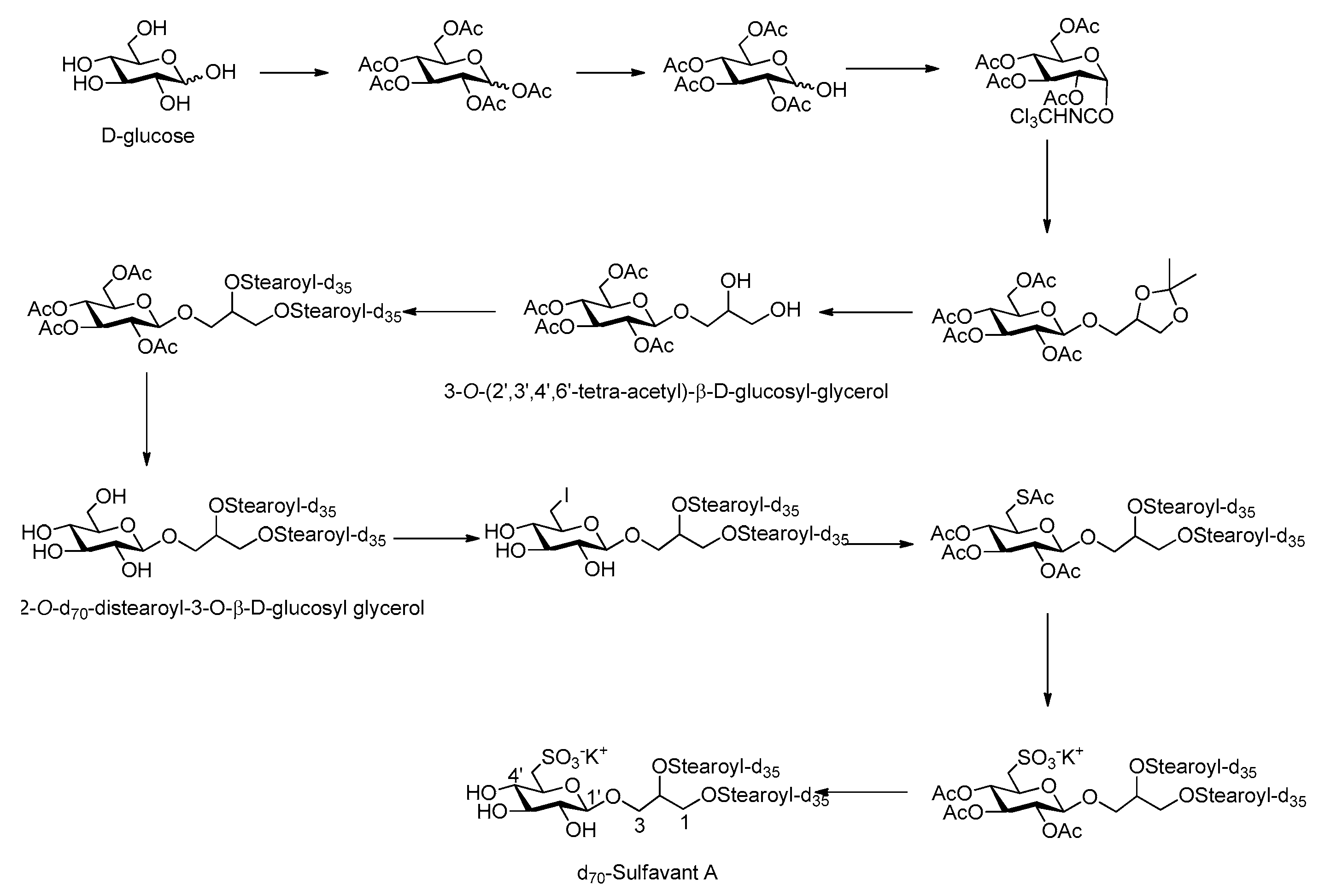
2.2. Synthetic Strategy of d70-Sulfavant A
2.3. Ultra-High-Performance Liquid Chromatography/High Resolution Mass Spectrometry (UHPLC/HRMS)
2.4. Standard Preparation and Stock Solution
2.5. Calibration Curve, Linearity and Detection and Quantitation Limits (LOD and LOQ)
2.6. Method Validation: Intra-and Inter-Day Accuracy and Precision
2.7. Evaluation of Matrix Effect on Mice Lung Extracts
3. Results and Discussion
3.1. Synthesis and Preparation of d70-Sulfavant A
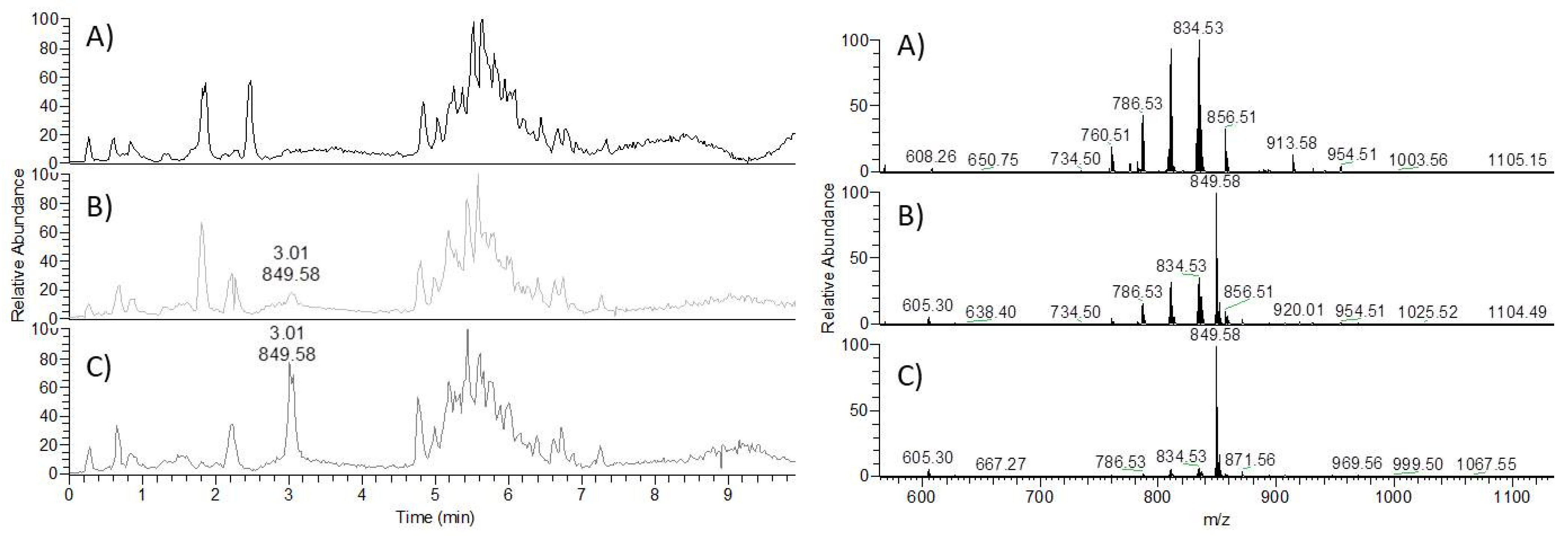
3.2. Method Development
3.3. Linearity, Accuracy, Precision and Matrix Effect
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nuzzo, G.; Gallo, C.; D’Ippolito, G.; Manzo, E.; Ruocco, N.; Russo, E.; Carotenuto, Y.; Costantini, M.; Zupo, V.; Sardo, A.; et al. UPLC–MS/MS Identification of Sterol Sulfates in Marine Diatoms. Mar. Drugs 2018, 17, 10. [Google Scholar] [CrossRef]
- Wood, P.L.; Siljander, H.; Knip, M. Lipidomics of human umbilical cord serum: Identification of unique sterol sulfates. Future Sci. OA 2017, 3, FSO193. [Google Scholar] [CrossRef]
- Dias, I.H.; Ferreira, R.; Gruber, F.; Vitorino, R.; Rivas-Urbina, A.; Sanchez-Quesada, J.L.; Silva, J.V.; Fardilha, M.; De Freitas, V.; Reis, A. Sulfate-based lipids: Analysis of healthy human fluids and cell extracts. Chem. Phys. Lipids 2019, 221, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Gallo, C.; Nuzzo, G.; D’Ippolito, G.; Manzo, E.; Sardo, A.; Fontana, A. Sterol Sulfates and Sulfotransferases in Marine Diatoms. In Computer Methods, Part C; Elsevier BV: Amsterdam, The Netherlands, 2018; Volume 605, pp. 101–138. [Google Scholar]
- Mirzaian, M.; Kramer, G.; Poorthuis, B.J.H.M. Quantification of sulfatides and lysosulfatides in tissues and body fluids by liquid chromatography-tandem mass spectrometry. J. Lipid Res. 2015, 56, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Moyano, A.L.; Li, G.; Lopez-Rosas, A.; Månsson, J.-E.; Van Breemen, R.B.; Givogri, M.I. Distribution of C16:0, C18:0, C24:1, and C24:0 sulfatides in central nervous system lipid rafts by quantitative ultra-high-pressure liquid chromatography tandem mass spectrometry. Anal. Biochem. 2014, 467, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Pintado-Sierra, M.; García-Álvarez, I.; Bribián, A.; Medina-Rodríguez, E.; Lebrón-Aguilar, R.; Garrido, L.; De Castro, F.; Fernández-Mayoralas, A.; Quintanilla-López, J.E. A comprehensive profiling of sulfatides in myelin from mouse brain using liquid chromatography coupled to high-resolution accurate tandem mass spectrometry. Anal. Chim. Acta 2017, 951, 89–98. [Google Scholar] [CrossRef]
- Kongmanas, K.; Xu, H.; Yaghoubian, A.; Franchini, L.; Panza, L.; Ronchetti, F.; Faull, K.; Tanphaichitr, N. Quantification of seminolipid by LC-ESI-MS/MS-multiple reaction monitoring: Compensatory levels in Cgt mice. J. Lipid Res. 2010, 51, 3548–3558. [Google Scholar] [CrossRef]
- Manzo, E.; Cutignano, A.; Pagano, D.; Gallo, C.; Barra, G.; Nuzzo, G.; Sansone, C.; Ianora, A.; Urbanek, K.; Fenoglio, D.; et al. A new marine-derived sulfoglycolipid triggers dendritic cell activation and immune adjuvant response. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Manzo, E.; Gallo, C.; Fioretto, L.; Nuzzo, G.; Barra, G.; Pagano, D.; Krauss, I.R.; Paduano, L.; Ziaco, M.; DellaGreca, M.; et al. Diasteroselective Colloidal Self-Assembly Affects the Immunological Response of the Molecular Adjuvant Sulfavant. ACS Omega 2019, 4, 7807–7814. [Google Scholar] [CrossRef]
- Manzo, E.; Fioretto, L.; Pagano, D.; Nuzzo, G.; Gallo, C.; De Palma, R.; Fontana, A. Chemical Synthesis of Marine-Derived Sulfoglycolipids, a New Class of Molecular Adjuvants. Mar. Drugs 2017, 15, 288. [Google Scholar] [CrossRef]
- Ziaco, M.; Fioretto, L.; Nuzzo, G.; Fontana, A.; Manzo, E. Short Gram-Scale Synthesis of Sulfavant A. Org. Process. Res. Dev. 2020, 24, 2728–2733. [Google Scholar] [CrossRef]
- Manzo, E.; Fioretto, L.; Gallo, C.; Ziaco, M.; Nuzzo, G.; D’Ippolito, G.; Borzacchiello, A.; Fabozzi, A.; De Palma, R.; Fontana, A. Preparation, Supramolecular Aggregation and Immunological Activity of the Bona Fide Vaccine Adjuvant Sulfavant S. Mar. Drugs 2020, 18, 451. [Google Scholar] [CrossRef]
- Manzo, E.; Ciavatta, M.L.; Pagano, D.; Fontana, A. An efficient and versatile chemical synthesis of bioactive glyco-glycerolipids. Tetrahedron Lett. 2012, 53, 879–881. [Google Scholar] [CrossRef]
- Manzo, E.; Ciavatta, M.L.; Pagano, D.; Fontana, A. Synthetic strategy for the preparation of bioactive galactoglycerolipids. Chem. J. Mold. 2011, 6, 27–29. [Google Scholar]
- Vial, J.; Jardy, A. Experimental Comparison of the Different Approaches to Estimate LOD and LOQ of an HPLC Method. Anal. Chem. 1999, 71, 2672–2677. [Google Scholar] [CrossRef]
- Cutignano, A.; Luongo, E.; Nuzzo, G.; Pagano, D.; Manzo, E.; Sardo, A.; Fontana, A. Profiling of complex lipids in marine microalgae by UHPLC/tandem mass spectrometry. Algal Res. 2016, 17, 348–358. [Google Scholar] [CrossRef]
- De Nicolò, A.; Cantù, M.; D’Avolio, A. Matrix effect management in liquid chromatography mass spectrometry: The internal standard normalized matrix effect. Bioanalysis 2017, 9, 1093–1105. [Google Scholar] [CrossRef]
- Schmidt, R.R.; Michel, J. Facile Synthesis ofα- andβ-O-Glycosyl Imidates; Preparation of Glycosides and Disaccharides. Angew. Chem. Int. Ed. 1980, 19, 731–732. [Google Scholar] [CrossRef]
- Schmidt, R.R. New Methods for the Synthesis of Glycosides and Oligosaccharides?Are There Alternatives to the Koenigs-Knorr Method? [New Synthetic Methods (56)]. Angew. Chem. Int. Ed. 1986, 25, 212–235. [Google Scholar] [CrossRef]
- Zhao, Y.-Y.; Wu, S.-P.; Liu, S.; Zhang, Y.; Lin, R. Ultra-performance liquid chromatography–mass spectrometry as a sensitive and powerful technology in lipidomic applications. Chem. Interact. 2014, 220, 181–192. [Google Scholar] [CrossRef]
- European Medicines Agency. An unacceptable choice. Prescrire Int. 2011, 20, 278. [Google Scholar]
- Analytical Procedures and Methods Validation for Drugs and Biologics Guidance for Industry Analytical Procedures and Methods Validation for Drugs and Biologics Guidance for Industry. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/analytical-procedures-and-methods-validation-drugs-and-biologics (accessed on 1 June 2020).
- Avataneo, V.; D’Avolio, A.; Cusato, J.; Cantù, M.; De Nicolò, A. LC-MS application for therapeutic drug monitoring in alternative matrices. J. Pharm. Biomed. Anal. 2019, 166, 40–51. [Google Scholar] [CrossRef] [PubMed]


| Time (min) | Mobile Phase A (%) a | Mobile Phase B (%) b |
|---|---|---|
| 0 | 85 | 15 |
| 1 | 85 | 15 |
| 6 | 98 | 2 |
| 8 | 100 | 0 |
| 10 | 100 | 0 |
| 11 | 85 | 15 |
| 15 | 85 | 15 |
| T0 | T48 | |||
|---|---|---|---|---|
| MeOH | Matrix | MeOH | Matrix | |
| SULF A | y = 40229x R2 = 0.9996 | y = 47981x R2 = 0.9997 | y = 50537x R2 = 0.9991 | y = 46508x R2 = 0.9993 |
| d70-SULF A | y = 23689x R2 = 0.9998 | y = 24546x R2 = 0.9991 | y = 28250x R2 = 1 | y = 24185x R2 = 0.9996 |
| SULF A in MeOH | ||||
|---|---|---|---|---|
| Nominal Concentration (ngmL−1) | Mean ± SD a | Accuracy (%) b | Precision (CV %) c | |
| T0 | 30 | 27.8 ± 2.2 | 92.6 | 8.0 |
| 300 | 315.0 ± 19.4 | 105 | 6.1 | |
| 1500 | 1247.8 ± 69.2 | 84.2 | 5.5 | |
| T12 (Intra-day) | 30 | 27.7 ± 1.14 | 92.4 | 4.1 |
| 300 | 313.9 ± 17.6 | 104.6 | 5.6 | |
| 1500 | 1265.2 ± 27.6 | 84.3 | 2.2 | |
| T48 (Inter-day) | 30 | 30.7 ± 1.5 | 102.2 | 4.9 |
| 300 | 327.7 ± 16.3 | 109.2 | 5.0 | |
| 1500 | 1353.6 ± 15.0 | 90.2 | 1.1 | |
| SULF A in Matrix | ||||
| Nominal Concentration (ngmL−1) | Mean ± SD a | Accuracy (%) b | Precision (CV %) c | |
| T0 | 30 | 26.0 ± 2.2 | 86.6 | 8.6 |
| 300 | 283.1 ± 6.5 | 94.4 | 2.3 | |
| 1500 | 1459.9 ± 79.7 | 97.3 | 5.4 | |
| T12 (Intra-day) | 30 | 28.6 ± 2.0 | 95.2 | 7.1 |
| 300 | 274.4 ± 16.6 | 91.5 | 6.1 | |
| 1500 | 1457.3 ± 84.1 | 97.1 | 5.8 | |
| T48 (Inter-day) | 30 | 27.0 ± 1.2 | 90.0 | 4.3 |
| 300 | 279.8 ± 22.0 | 93.3 | 7.9 | |
| 1500 | 1432.5 ± 46.2 | 95.5 | 10.2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuzzo, G.; Manzo, E.; Ziaco, M.; Fioretto, L.; Campos, A.M.; Gallo, C.; d’Ippolito, G.; Fontana, A. UHPLC-MS Method for the Analysis of the Molecular Adjuvant Sulfavant A. Appl. Sci. 2021, 11, 1451. https://doi.org/10.3390/app11041451
Nuzzo G, Manzo E, Ziaco M, Fioretto L, Campos AM, Gallo C, d’Ippolito G, Fontana A. UHPLC-MS Method for the Analysis of the Molecular Adjuvant Sulfavant A. Applied Sciences. 2021; 11(4):1451. https://doi.org/10.3390/app11041451
Chicago/Turabian StyleNuzzo, Genoveffa, Emiliano Manzo, Marcello Ziaco, Laura Fioretto, Ana Margarida Campos, Carmela Gallo, Giuliana d’Ippolito, and Angelo Fontana. 2021. "UHPLC-MS Method for the Analysis of the Molecular Adjuvant Sulfavant A" Applied Sciences 11, no. 4: 1451. https://doi.org/10.3390/app11041451
APA StyleNuzzo, G., Manzo, E., Ziaco, M., Fioretto, L., Campos, A. M., Gallo, C., d’Ippolito, G., & Fontana, A. (2021). UHPLC-MS Method for the Analysis of the Molecular Adjuvant Sulfavant A. Applied Sciences, 11(4), 1451. https://doi.org/10.3390/app11041451










